Derivation of Limbal Stem Cells from Human Adult Mesenchymal Stem Cells for the Treatment of Limbal Stem Cell Deficiency
Abstract
1. Introduction
2. Results
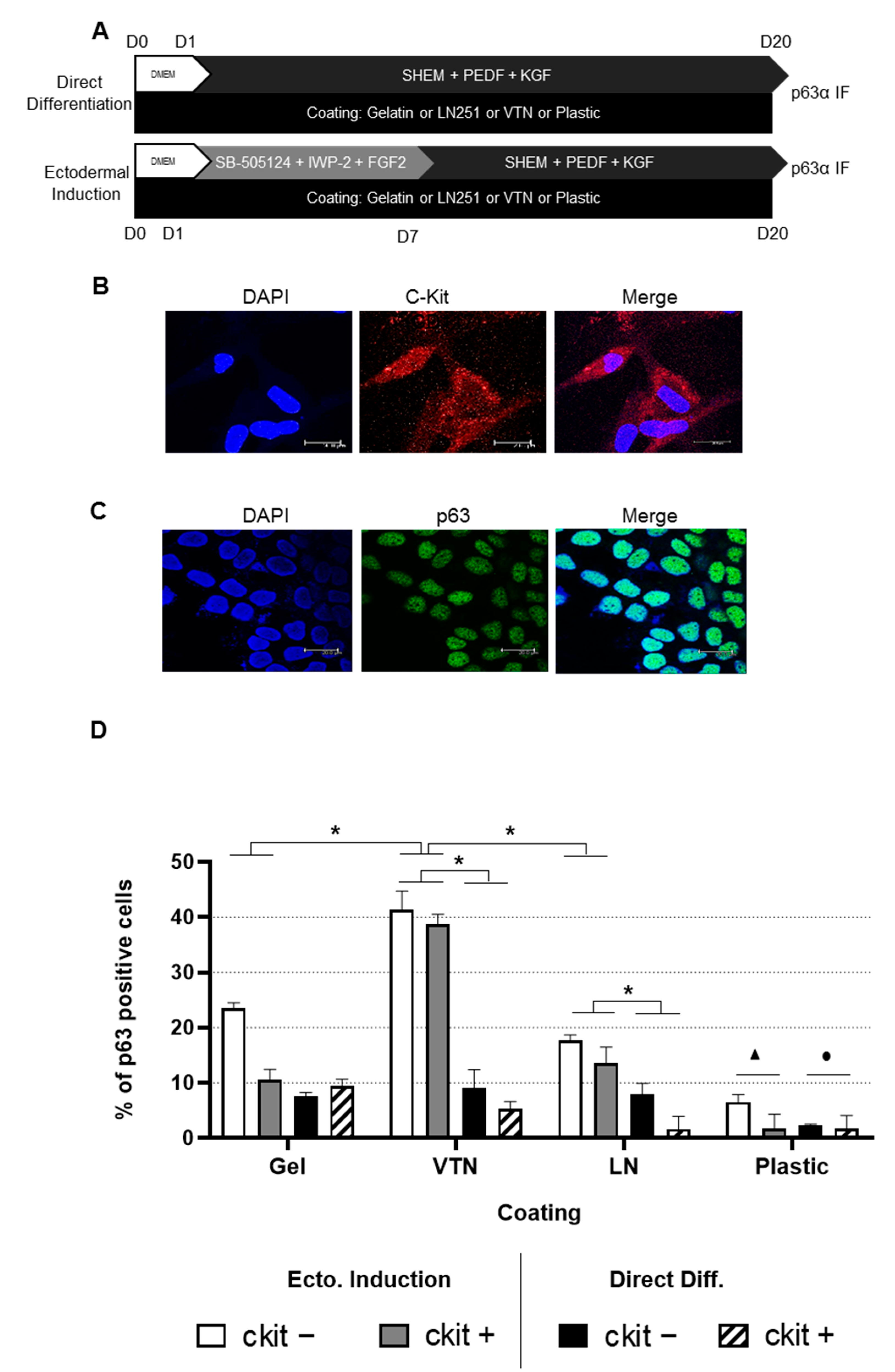
2.1. Differentiation into LSCs Is Achieved in Human ADSC Subpopulations Independently ofc-Kit Expression
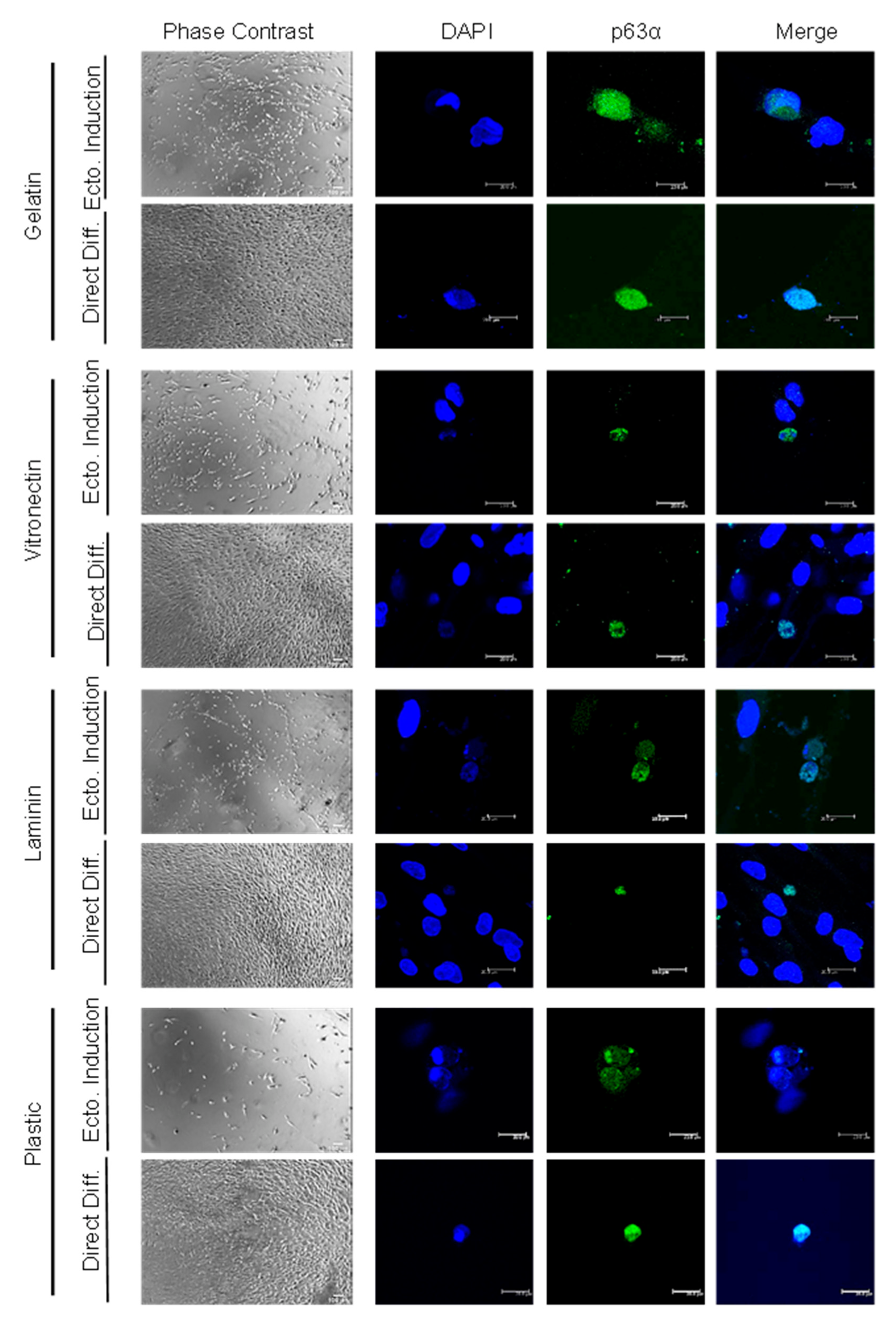
2.2. High Differentiation Efficiency of hADSCs into LSCs Is Achieved by Previous Ectodermal Induction and Vitronectin Coating, Independent of c-Kit Expression
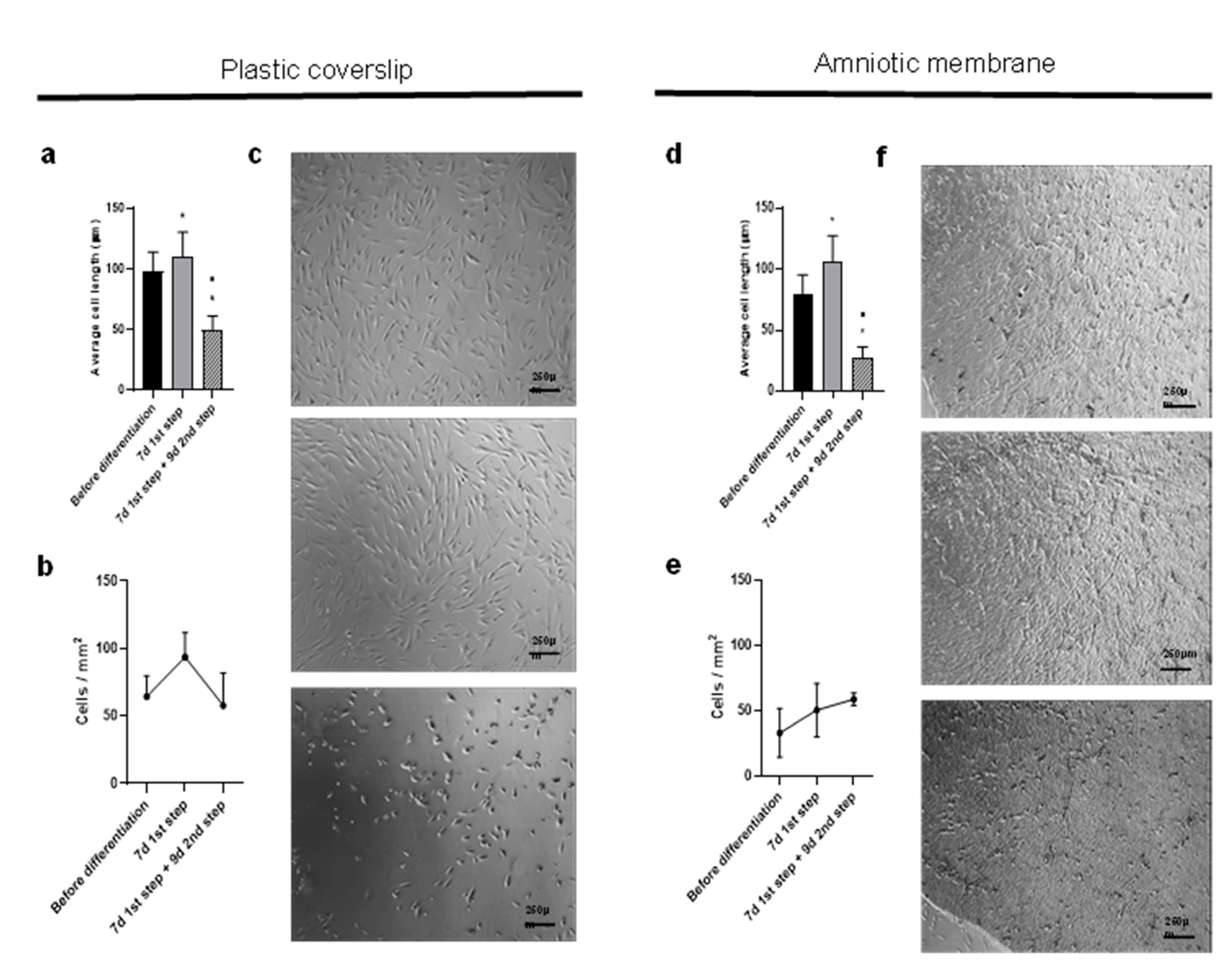
2.3. Differentiation over Amniotic Membrane Shows Morphological Changes, Accelerated Kinetics and Increased Cell Counts
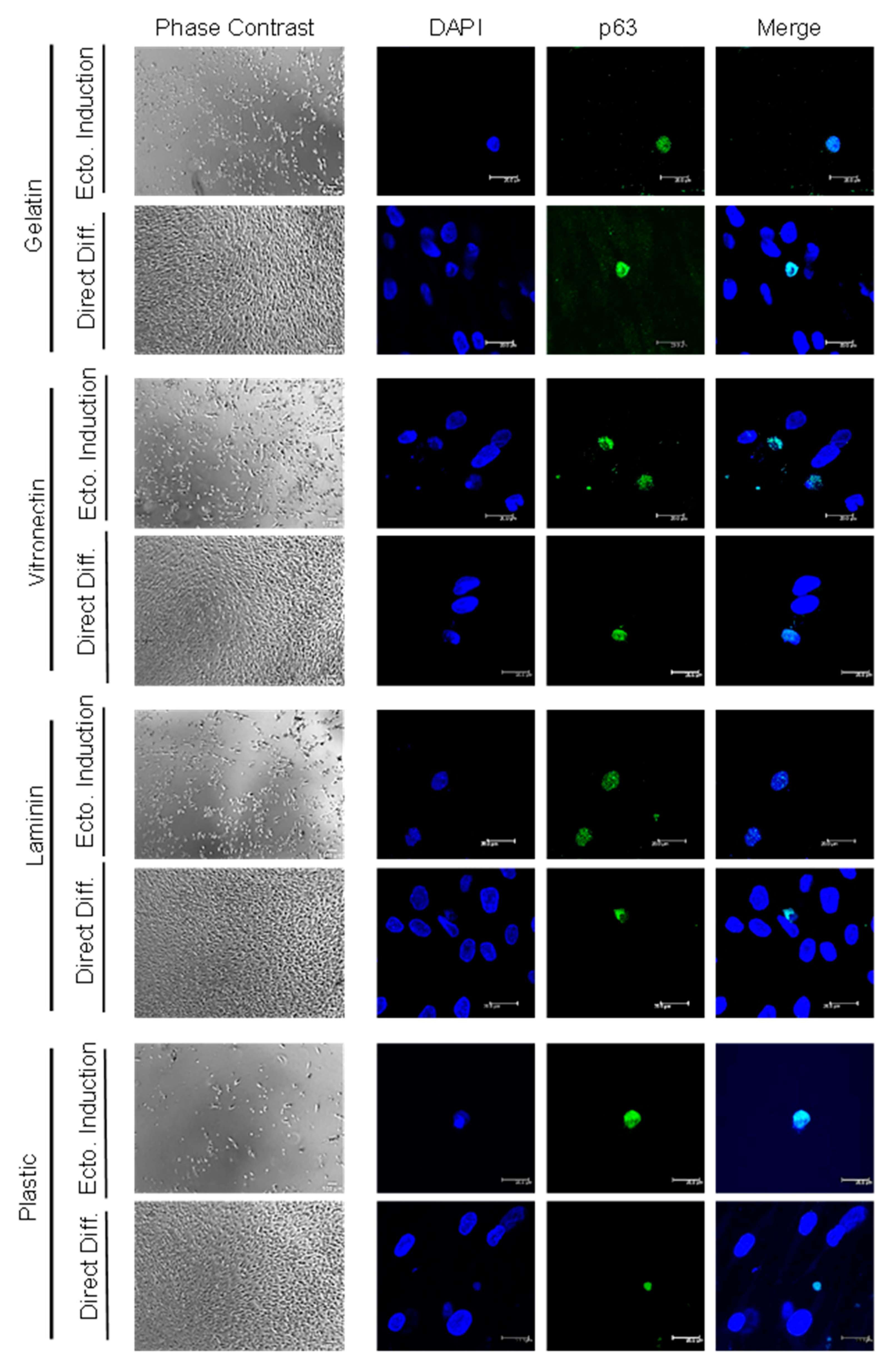
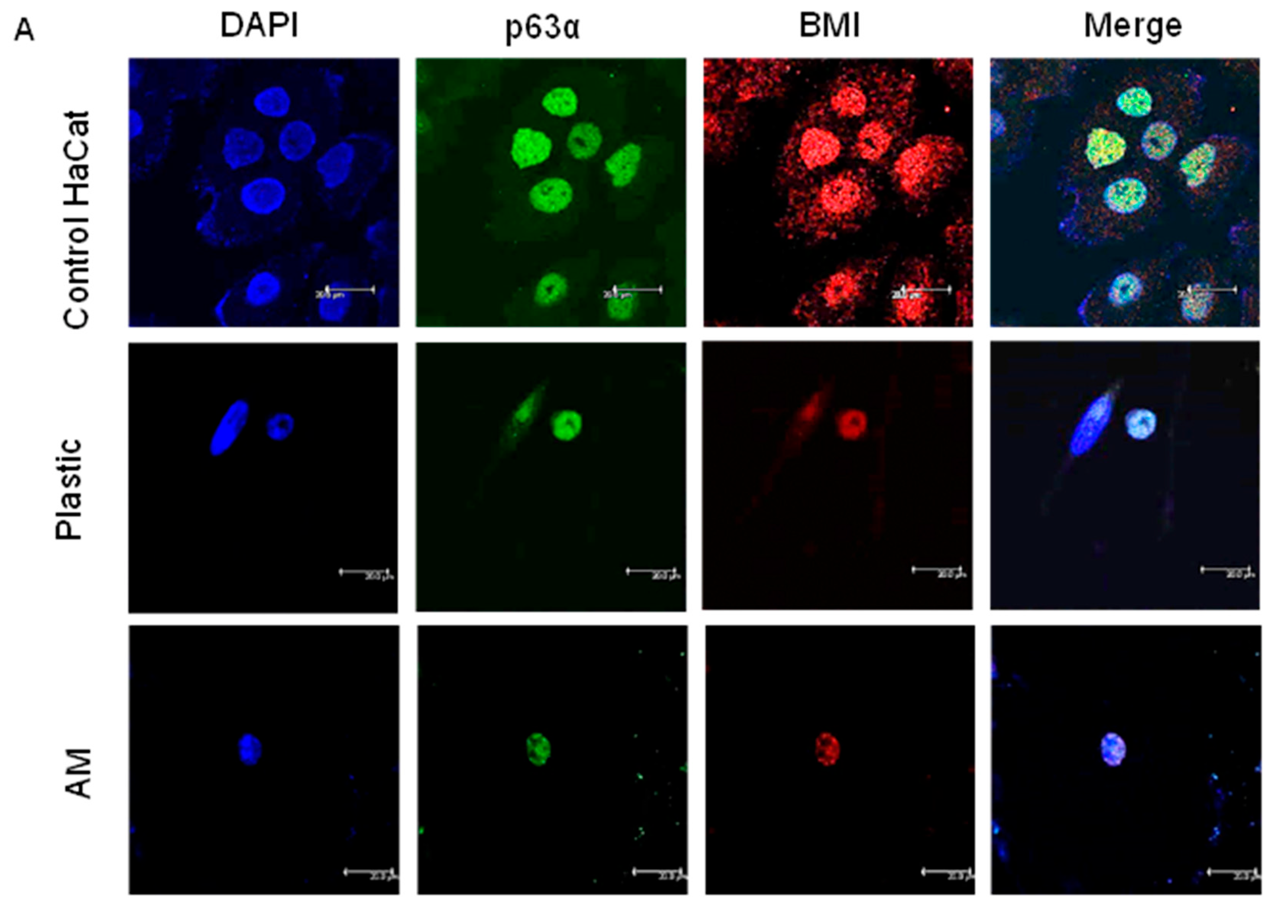
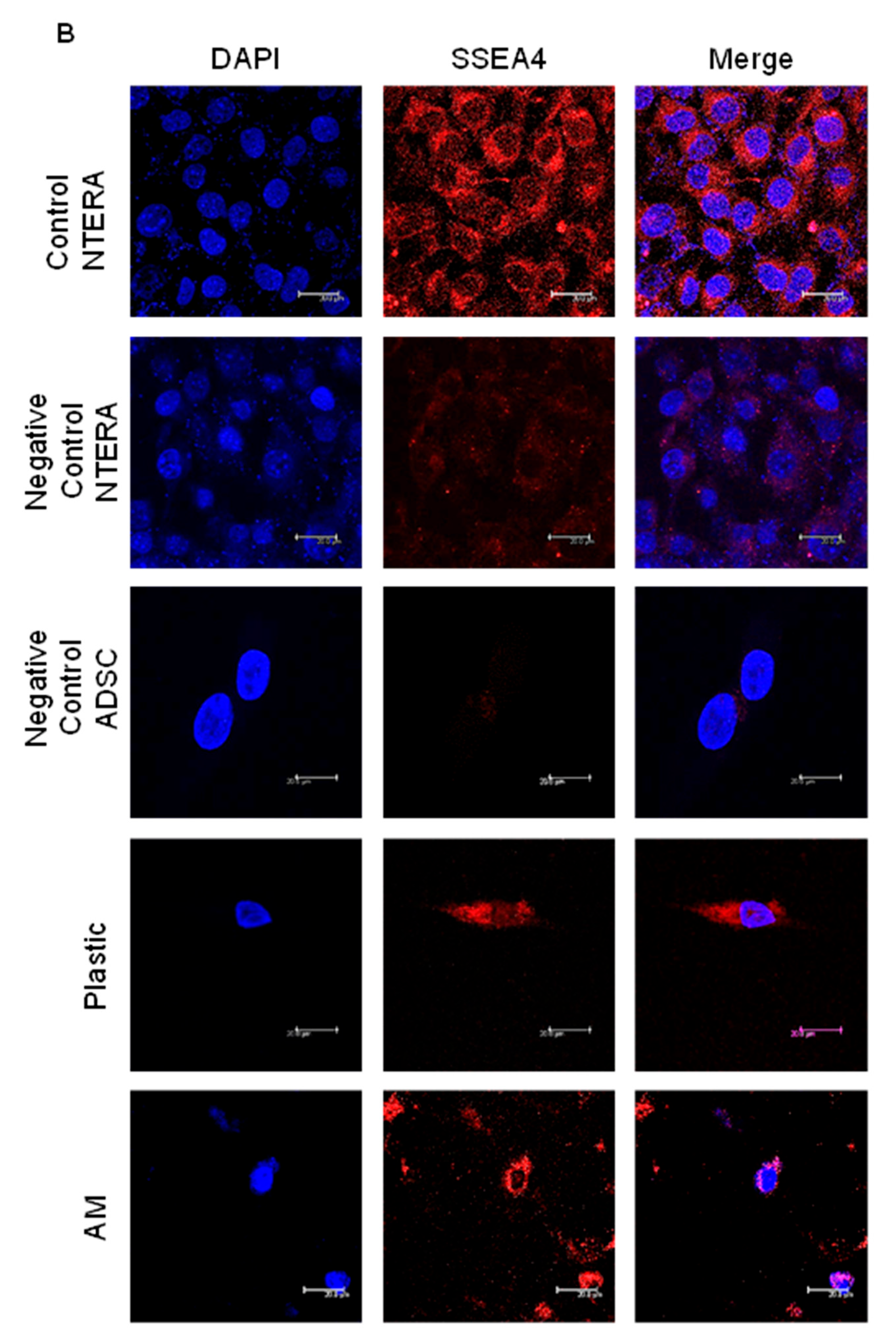
2.4. The Percentage of ADSC-Derived LSCs Positive for LSC Markers Peaks at 17 Days of Differentiation
3. Discussion
4. Materials and Methods
4.1. Immunomagnetic Separation of hADSC Subpopulations
4.2. In Vitro Directed Transdifferentiation into Limbal Stem Cells
4.3. Limbal Stem Cell Marker Characterization
4.4. Amniotic Membrane Preparation
4.5. Statistics
Author Contributions
Funding

Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Resnikoff, S.; Pascolini, D.; Etya’ale, D.; Kocur, I.; Pararajasegaram, R.; Pokharel, G.P.; Mariotti, S.P. Global Data on Visual Impairment in the Year 2002. Bull. World Health Organ. 2004, 82, 844–851. [Google Scholar]
- Bourne, R.R.A.; Steinmetz, J.D.; Flaxman, S.; Briant, P.S.; Taylor, H.R.; Resnikoff, S.; Casson, R.J.; Abdoli, A.; Abu-Gharbieh, E.; Afshin, A.; et al. Trends in Prevalence of Blindness and Distance and near Vision Impairment over 30 Years: An Analysis for the Global Burden of Disease Study. Lancet Glob. Heal. 2021, 9, e130–e143. [Google Scholar] [CrossRef]
- Whitcher, J.P.; Srinivasan, M.; Upadhyay, M.P. Corneal Blindness: A Global Perspective. Bull. World Health Organ. 2001, 79, 214–221. [Google Scholar]
- Nuzzi, A.; Pozzo Giuffrida, F.; Luccarelli, S.; Nucci, P. Corneal Epithelial Regeneration: Old and New Perspectives. Int. J. Mol. Sci. 2022, 23, 13114. [Google Scholar] [CrossRef]
- Miesfeld, J.B.; Brown, N.L. Eye Organogenesis: A Hierarchical View of Ocular Development, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 132, ISBN 9780128104897. [Google Scholar]
- Holland, E.J. Management of Limbal Stem Cell Deficiency: A Historical Perspective, Past, Present, and Future. Cornea 2015, 34, S9–S15. [Google Scholar] [CrossRef]
- Guo, Z.H.; Zhang, W.; Jia, Y.Y.S.; Liu, Q.X.; Li, Z.F.; Lin, J.S. An Insight into the Difficulties in the Discovery of Specific Biomarkers of Limbal Stem Cells. Int. J. Mol. Sci. 2018, 19, 1982. [Google Scholar] [CrossRef]
- Bardag-Gorce, F.; Diaz, A.; Niihara, R.; Stark, J.; Cortez, D.; Lee, A.; Hoft, R.; Niihara, Y. Aldehyde Dehydrogenases Expression in Corneal Epithelial Cells with Limbal Stem Cell Deficiency. Int. J. Mol. Sci. 2022, 23, 4032. [Google Scholar] [CrossRef]
- Li, W.; Hayashida, Y.; Chen, Y.T.; Tseng, S.C. Niche Regulation of Corneal Epithelial Stem Cells at the Limbus. Cell Res. 2007, 17, 26–36. [Google Scholar] [CrossRef]
- Grieve, K.; Ghoubay, D.; Georgeon, C.; Thouvenin, O.; Bouheraoua, N.; Paques, M.; Borderie, V.M. Three-Dimensional Structure of the Mammalian Limbal Stem Cell Niche. Exp. Eye Res. 2015, 140, 75–84. [Google Scholar] [CrossRef]
- Seyed-Safi, A.G.; Daniels, J.T. The Limbus: Structure and Function. Exp. Eye Res. 2020, 197, 108074. [Google Scholar] [CrossRef]
- Pellegrini, G.; Dellambra, E.; Golisano, O.; Martinelli, E.; Fantozzi, I.; Bondanza, S.; Ponzin, D.; McKeon, F.; De Luca, M. P63 Identifies Keratinocyte Stem Cells. Proc. Natl. Acad. Sci. USA 2001, 98, 3156–3161. [Google Scholar] [CrossRef]
- De Miguel, M.P.; Casaroli-Marano, R.P.; Nieto-Nicolau, N.; Martínez-Conesa, E.M.; Alió del Barrio, J.L.; Alió, J.L.; Fuentes, S.; Arnalich-Montiel, F. Frontiers in Regenerative Medicine for Cornea and Ocular Surface. Front. Stem Cell Regen. Med. Res. 2015, 1, 92–138. [Google Scholar]
- Collin, J.; Queen, R.; Zerti, D.; Bojic, S.; Dorgau, B.; Moyse, N.; Molina, M.M.; Yang, C.; Dey, S.; Reynolds, G.; et al. A Single Cell Atlas of Human Cornea That Defines Its Development, Limbal Progenitor Cells and Their Interactions with the Immune Cells. Ocul. Surf. 2021, 21, 279–298. [Google Scholar] [CrossRef]
- Cheng, C.C.; Wang, D.Y.; Kao, M.H.; Chen, J.K. The Growth-Promoting Effect of KGF on Limbal Epithelial Cells Is Mediated by Upregulation of ΔNp63α through the P38 Pathway. J. Cell Sci. 2009, 122, 4473–4480. [Google Scholar] [CrossRef]
- Baylis, O.; Figueiredo, F.; Henein, C.; Lako, M.; Ahmad, S. 13 Years of Cultured Limbal Epithelial Cell Therapy: A Review of the Outcomes. J. Cell. Biochem. 2011, 112, 993–1002. [Google Scholar] [CrossRef]
- Kolli, S.A.I.; Ahmad, S.; Lako, M.; Figueiredo, F. Successful Clinical Implementation of Corneal Epithelial Stem Cell Therapy for Treatment of Unilateral Limbal Stem Cell Deficiency. Stem Cells 2010, 28, 597–610. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal Stem-Cell Therapy and Long-Term Corneal Regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- De Miguel, M.P.; Alio, J.L.; Arnalich-Montiel, F.; Fuentes-Julian, S.; de Benito-Llopis, L.; Amparo, F.; Bataille, L. Cornea and Ocular Surface Treatment. Curr. Stem. Cell Res. Ther. 2010, 5, 195–204. [Google Scholar] [CrossRef]
- Dobrowolski, D.; Orzechowska-Wylegala, B.; Wowra, B.; Wróblewska-Czajka, E.; Grolik, M.; Wylegala, E. An Analysis of the Progression of Conjunctivalisation after Transplantation of Cultivated Corneal Epithelium. J. Ophthalmol. 2021, 2021, 8499640. [Google Scholar] [CrossRef]
- Yin, J.; Jurkunas, U. Limbal Stem Cell Transplantation and Complications. Semin. Ophthalmol. 2018, 33, 134–141. [Google Scholar] [CrossRef]
- Cabral, J.V.; Jackson, C.J.; Utheim, T.P.; Jirsova, K. Ex Vivo Cultivated Oral Mucosal Epithelial Cell Transplantation for Limbal Stem Cell Deficiency: A Review. Stem Cell Res. Ther. 2020, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Prabhasawat, P.; Chirapapaisan, C.; Jiravarnsirikul, A.; Ekpo, P.; Uiprasertkul, M.; Thamphithak, R.; Matamnan, S.; Boonwong, C. Phenotypic Characterization of Corneal Epithelium in Long-Term Follow-Up of Patients Post-Autologous Cultivated Oral Mucosal Epithelial Transplantation. Cornea 2021, 40, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Conesa, E.M.; Espel, E.; Reina, M.; Casaroli-Marano, R.P. Characterization of Ocular Surface Epithelial and Progenitor Cell Markers in Human Adipose Stromal Cells Derived from Lipoaspirates. Investig. Ophthalmol. Vis. Sci. 2012, 53, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Miguel, T.; Galindo, S.; Reinoso, R.; Corell, A.; Martino, M.; Pérez-Simón, J.A.; Calonge, M. In Vitro Simulation of Corneal Epithelium Microenvironment Induces a Corneal Epithelial-like Cell Phenotype from Human Adipose Tissue Mesenchymal Stem Cells. Curr. Eye Res. 2013, 38, 933–944. [Google Scholar] [CrossRef]
- Galindo, S.; Herreras, J.M.; López-Paniagua, M.; Rey, E.; de la Mata, A.; Plata-Cordero, M.; Calonge, M.; Nieto-Miguel, T. Therapeutic Effect of Human Adipose Tissue-Derived Mesenchymal Stem Cells in Experimental Corneal Failure Due to Limbal Stem Cell Niche Damage. Stem Cells 2017, 35, 2160–2174. [Google Scholar] [CrossRef]
- Calonge, M.; Pérez, I.; Galindo, S.; Nieto-Miguel, T.; López-Paniagua, M.; Fernández, I.; Alberca, M.; García-Sancho, J.; Sánchez, A.; Herreras, J.M. A Proof-of-Concept Clinical Trial Using Mesenchymal Stem Cells for the Treatment of Corneal Epithelial Stem Cell Deficiency. Transl. Res. 2019, 206, 18–40. [Google Scholar] [CrossRef]
- Nieto-Nicolau, N.; Martínez-Conesa, E.M.; Fuentes-Julián, S.; Arnalich-Montiel, F.; García-Tuñón, I.; De Miguel, M.P.; Casaroli-Marano, R.P. Priming Human Adipose-Derived Mesenchymal Stem Cells for Corneal Surface Regeneration. J. Cell. Mol. Med. 2021, 25, 5124–5137. [Google Scholar] [CrossRef]
- Blazejewska, E.A.; Schlötzer-Schrehardt, U.; Zenkel, M.; Bachmann, B.; Chankiewitz, E.; Jacobi, C.; Kruse, F.E. Corneal Limbal Microenvironment Can Induce Transdifferentiation of Hair Follicle Stem Cells into Corneal Epithelial-like Cells. Stem Cells 2009, 27, 642–652. [Google Scholar] [CrossRef]
- Yang, X.; Moldovan, N.I.; Zhao, Q.; Mi, S.; Zhou, Z.; Chen, D.; Gao, Z.; Tong, D.; Dou, Z. Reconstruction of Damaged Cornea by Autologous Transplantation of Epidermal Adult Stem Cells. Mol. Vis. 2008, 14, 1064–1074. [Google Scholar]
- Casaroli-Marano, R.P.; Martínez-Conesa, E.M.; Nieto-Nicolau, N.; Arnalich-Montiel, F.; Fuentes-Julian, S.; De Miguel, M.P. Adipose Derived Stem Cells (ADS) for Ocular Surface Regeneration. Invest. Ophthalmol. Vis. Sci. 2014, 55, 5183. [Google Scholar]
- Kacham, S.; Bhure, T.S.; Eswaramoorthy, S.D.; Naik, G.; Rath, S.N.; Parcha, S.R.; Basu, S.; Sangwan, V.S.; Shukla, S. Human Umbilical Cord-derived Mesenchymal Stem Cells Promote Corneal Epithelial Repair in Vitro. Cells 2021, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Yazdanpanah, G.; Haq, Z.; Kang, K.; Jabbehdari, S.; Rosenblatt, M.L.; Djalilian, A.R. Strategies for Reconstructing the Limbal Stem Cell Niche. Ocul. Surf. 2019, 17, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; González, S.; Roberts, J.A.S.; Robertson, S.Y.T.; Ruiz, M.; Zheng, J.; Deng, S.X. Human Limbal Epithelial Stem Cell Regulation, Bioengineering and Function. Prog. Retin. Eye Res. 2021, 85, 1100956. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Stewart, R.; Yung, S.; Kolli, S.; Armstrong, L.; Stojkovic, M.; Figueiredo, F.; Lako, M. Differentiation of Human Embryonic Stem Cells into Corneal Epithelial-Like Cells by In Vitro Replication of the Corneal Epithelial Stem Cell Niche. Stem Cells 2007, 25, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ishikawa, Y.; Ito, M.; Kageyama, T.; Takashiba, K.; Fujioka, T.; Tsujikawa, M.; Miyoshi, H.; Yamato, M.; Nakamura, Y.; et al. Generation of Corneal Epithelial Cells from Induced Pluripotent Stem Cells Derived from Human Dermal Fibroblast and Corneal Limbal Epithelium. PLoS ONE 2012, 7, e45435. [Google Scholar] [CrossRef]
- Shalom-Feuerstein, R.; Serror, L.; De La Forest Divonne, S.; Petit, I.; Aberdam, E.; Camargo, L.; Damour, O.; Vigouroux, C.; Solomon, A.; Gaggioli, C.; et al. Pluripotent Stem Cell Model Reveals Essential Roles for MiR-450b-5p and MiR-184 in Embryonic Corneal Lineage Specification. Stem Cells 2012, 30, 898–909. [Google Scholar] [CrossRef]
- Aberdam, E.; Petit, I.; Sangari, L.; Aberdam, D. Induced Pluripotent Stem Cell-Derived Limbal Epithelial Cells (LiPSC) as a Cellular Alternative for in Vitro Ocular Toxicity Testing. PLoS ONE 2017, 12, e0179913. [Google Scholar] [CrossRef]
- Watanabe, S.; Hayashi, R.; Sasamoto, Y.; Tsujikawa, M.; Ksander, B.R.; Frank, M.H.; Quantock, A.J.; Frank, N.Y.; Nishida, K. Human IPS Cells Engender Corneal Epithelial Stem Cells with Holoclone-Forming Capabilities. iScience 2021, 24, 102688. [Google Scholar] [CrossRef]
- Hongisto, H.; Vattulainen, M.; Ilmarinen, T.; Mikhailova, A.; Skottman, H. Efficient and Scalable Directed Differentiation of Clinically Compatible Corneal Limbal Epithelial Stem Cells from Human Pluripotent Stem Cells. J. Vis. Exp. 2018, 2018, e58279. [Google Scholar] [CrossRef]
- Blazquez-Martinez, A.; Chiesa, M.; Arnalich, F.; Fernandez-Delgado, J.; Nistal, M.; De Miguel, M.P. C-Kit Identifies a Subpopulation of Mesenchymal Stem Cells in Adipose Tissue with Higher Telomerase Expression and Differentiation Potential. Differentiation 2014, 87, 147–160. [Google Scholar] [CrossRef]
- Holtzinger, A.; Streeter, P.R.; Sarangi, F.; Hillborn, S.; Niapour, M.; Ogawa, S.; Keller, G. New Markers for Tracking Endoderm Induction and Hepatocyte Differentiation from Human Pluripotent Stem Cells. Development 2015, 142, 4253–4265. [Google Scholar] [CrossRef]
- Dietrich-Ntoukas, T.; Hofmann-Rummelt, C.; Kruse, F.E.; Schlötzer-Schrehardt, U. Comparative Analysis of the Basement Membrane Composition of the Human Limbus Epithelium and Amniotic Membrane Epithelium. Cornea 2012, 31, 564–569. [Google Scholar] [CrossRef]
- Dziasko, M.A.; Daniels, J.T. Anatomical Features and Cell-Cell Interactions in the Human Limbal Epithelial Stem Cell Niche. Ocul. Surf. 2016, 14, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Polisetti, N.; Sorokin, L.; Okumura, N.; Koizumi, N.; Kinoshita, S.; Kruse, F.E.; Schlötzer-Schrehardt, U. Laminin-511 and -521-Based Matrices for Efficient Ex Vivo-Expansion of Human Limbal Epithelial Progenitor Cells. Sci. Rep. 2017, 7, 5152. [Google Scholar] [CrossRef] [PubMed]
- Echevarria, T.J.; Chow, S.; Watson, S.; Wakefield, D.; Di Girolamo, N. Vitronectin: A Matrix Support Factor for Human Limbal Epithelial Progenitor Cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8138–8147. [Google Scholar] [CrossRef]
- Hayashi, R.; Ishikawa, Y.; Sasamoto, Y.; Katori, R.; Nomura, N.; Ichikawa, T.; Araki, S.; Soma, T.; Kawasaki, S.; Sekiguchi, K.; et al. Co-Ordinated Ocular Development from Human IPS Cells and Recovery of Corneal Function. Nature 2016, 531, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Duan, H.; Wang, X.; Zhao, C.; Li, W.; Dong, C.; Li, Z.; Zhou, Q. Transplantation of Human Induced Pluripotent Stem Cell-Derived Neural Crest Cells for Corneal Endothelial Regeneration. Stem Cell Res. Ther. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Chen, B.; Dodge, M.E.; Tang, W.; Lu, J.; Ma, Z.; Fan, C.-W.; Wei, S.; Hao, W.; Kilgore, J.; Williams, N.S.; et al. Small Molecule-Mediated in Tissue Regeneration and Cancer. Nat. Chem. Biol. 2009, 5, 100–107. [Google Scholar] [CrossRef]
- DaCosta Byfield, S.; Major, C.; Laping, N.J.; Roberts, A.B. SB-505124 Is a Selective Inhibitor of Transforming Growth Factor-β Type I Receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2004, 65, 744–752. [Google Scholar] [CrossRef]
- Tsai, T.H.; Sun, M.H.; Ho, T.C.; Ma, H.I.; Liu, M.Y.; Tsao, Y.P. Notch Prevents Transforming Growth Factor-Beta-Assisted Epithelial-Mesenchymal Transition in Cultured Limbal Progenitor Cells through the Induction of Smad7. Mol. Vis. 2014, 20, 522–534. [Google Scholar]
- Mikhailova, A.; Ilmarinen, T.; Uusitalo, H.; Skottman, H. Small-Molecule Induction Promotes Corneal Epithelial Cell Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Rep. 2014, 2, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Vattulainen, M.; Ilmarinen, T.; Viheriälä, T.; Jokinen, V.; Skottman, H. Corneal Epithelial Differentiation of Human Pluripotent Stem Cells Generates ABCB5+ and ∆Np63α+ Cells with Limbal Cell Characteristics and High Wound Healing Capacity. Stem Cell Res. Ther. 2021, 12, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, N.; Suh, T.C.; Ali, K.M.; Sefat, E.; Jahan, U.M.; Huang, Y.; Gilger, B.C.; Gluck, J.M. Induced Pluripotent Stem Cell-Derived Corneal Cells: Current Status and Application. Stem Cell Rev. Rep. 2022, 18, 2817–2832. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chen, S.L.; Wu, J.Y.; Ho, M.Y.; Chen, L.J.; Hsieh, J.W.; Cheng, H.C.; Tsao, Y.P. PEDF Promotes Self-Renewal of Limbal Stem Cell and Accelerates Corneal Epithelial Wound Healing. Stem Cells 2013, 31, 1775–1784. [Google Scholar] [CrossRef]
- Peterson, J.L.; Ceresa, B.P. Epidermal Growth Factor Receptor Expression in the Corneal Epithelium. Cells 2021, 10, 2409. [Google Scholar] [CrossRef]
- Pellegrini, G.; Rama, P.; Matuska, S.; Lambiase, A.; Bonini, S.; Pocobelli, A.; Colabelli, R.G.; Spadea, L.; Fasciani, R.; Balestrazzi, E.; et al. Biological Parameters Determining the Clinical Outcome of Autologous Cultures of Limbal Stem Cells. Regen. Med. 2013, 8, 553–567. [Google Scholar] [CrossRef]
- Barbaro, V.; Testa, A.; Di Iorio, E.; Mavilio, F.; Pellegrini, G.; De Luca, M. C/EBPδ Regulates Cell Cycle and Self-Renewal of Human Limbal Stem Cells. J. Cell Biol. 2007, 177, 1037–1049. [Google Scholar] [CrossRef]
- Truong, T.T.; Huynh, K.; Nakatsu, M.N.; Deng, S.X. SSEA4 Is a Potential Negative Marker for the Enrichment of Human Corneal Epithelial Stem/Progenitor Cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6315–6320. [Google Scholar] [CrossRef]
- Maddox, J.R.; Ludlow, K.D.; Li, F.; Niyibizi, C. Breast and Abdominal Adipose Multipotent Mesenchymal Stromal Cells and Stage-Specific Embryonic Antigen 4 Expression. Cells Tissues Organs 2012, 196, 107–116. [Google Scholar] [CrossRef]
- Guasti, L.; Prasongchean, W.; Kleftouris, G.; Mukherjee, S.; Thrasher, A.J.; Bulstrode, N.W.; Ferretti, P. High Plasticity of Pediatric Adipose Tissue-Derived Stem Cells: Too Much for Selective Skeletogenic Differentiation? Stem Cells Transl. Med. 2012, 1, 384–395. [Google Scholar] [CrossRef]
- Varghese, J.; Griffin, M.; Mosahebi, A.; Butler, P. Systematic Review of Patient Factors Affecting Adipose Stem Cell Viability and Function: Implications for Regenerative Therapy. Stem Cell Res. Ther. 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Baharvand, H.; Heidari, M.; Ebrahimi, M.; Valadbeigi, T.; Salekdeh, G.H. Proteomic Analysis of Epithelium-Denuded Human Amniotic Membrane as a Limbal Stem Cell Niche. Mol. Vis. 2007, 13, 1711–1721. [Google Scholar] [PubMed]
- Rendal-Vázquez, M.E.; San-Luis-Verdes, A.; Yebra-Pimentel-Vilar, M.T.; López-Rodríguez, I.; Domenech-García, N.; Andión-Núñez, C.; Blanco-García, F. Culture of Limbal Stem Cells on Human Amniotic Membrane. Cell Tissue Bank. 2012, 13, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, A.; Ilmarinen, T.; Ratnayake, A.; Petrovski, G.; Uusitalo, H.; Skottman, H.; Rafat, M. Human Pluripotent Stem Cell-Derived Limbal Epithelial Stem Cells on Bioengineered Matrices for Corneal Reconstruction. Exp. Eye Res. 2016, 146, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Koivusalo, L.; Kauppila, M.; Samanta, S.; Parihar, V.S.; Ilmarinen, T.; Miettinen, S.; Oommen, O.P.; Skottman, H. Tissue Adhesive Hyaluronic Acid Hydrogels for Sutureless Stem Cell Delivery and Regeneration of Corneal Epithelium and Stroma. Biomaterials 2019, 225, 119516. [Google Scholar] [CrossRef]
- Haagdorens, M.; Cėpla, V.; Melsbach, E.; Koivusalo, L.; Skottman, H.; Griffith, M.; Valiokas, R.; Zakaria, N.; Pintelon, I.; Tassignon, M.J. In Vitro Cultivation of Limbal Epithelial Stem Cells on Surface-Modified Crosslinked Collagen Scaffolds. Stem Cells Int. 2019, 2019. [Google Scholar] [CrossRef]
- Rico-Sánchez, L.; Garzón, I.; González-Andrades, M.; Ruíz-García, A.; Punzano, M.; Lizana-Moreno, A.; Muñoz-Ávila, J.I.; Sánchez-Quevedo, M.d.C.; Martínez-Atienza, J.; Lopez-Navas, L.; et al. Successful Development and Clinical Translation of a Novel Anterior Lamellar Artificial Cornea. J. Tissue Eng. Regen. Med. 2019, 13, 2142–2154. [Google Scholar] [CrossRef]
- Anton-Sales, I.; Koivusalo, L.; Skottman, H.; Laromaine, A.; Roig, A. Limbal Stem Cells on Bacterial Nanocellulose Carriers for Ocular Surface Regeneration. Small 2021, 17, 2003937. [Google Scholar] [CrossRef]
- Galindo, S.; de la Mata, A.; López-Paniagua, M.; Herreras, J.M.; Pérez, I.; Calonge, M.; Nieto-Miguel, T. Subconjunctival Injection of Mesenchymal Stem Cells for Corneal Failure Due to Limbal Stem Cell Deficiency: State of the Art. Stem Cell Res. Ther. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Marta, C.M.; Adrian, M.; Jorge, F.D.; Francisco, A.M.; De Miguel, M.P. Improvement of an Effective Protocol for Directed Differentiation of Human Adipose Tissue-Derived Adult Mesenchymal Stem Cells to Corneal Endothelial Cells. Int. J. Mol. Sci. 2021, 22, 11982. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadenas-Martin, M.; Arnalich-Montiel, F.; Miguel, M.P.D. Derivation of Limbal Stem Cells from Human Adult Mesenchymal Stem Cells for the Treatment of Limbal Stem Cell Deficiency. Int. J. Mol. Sci. 2023, 24, 2350. https://doi.org/10.3390/ijms24032350
Cadenas-Martin M, Arnalich-Montiel F, Miguel MPD. Derivation of Limbal Stem Cells from Human Adult Mesenchymal Stem Cells for the Treatment of Limbal Stem Cell Deficiency. International Journal of Molecular Sciences. 2023; 24(3):2350. https://doi.org/10.3390/ijms24032350
Chicago/Turabian StyleCadenas-Martin, Marta, Francisco Arnalich-Montiel, and Maria P De Miguel. 2023. "Derivation of Limbal Stem Cells from Human Adult Mesenchymal Stem Cells for the Treatment of Limbal Stem Cell Deficiency" International Journal of Molecular Sciences 24, no. 3: 2350. https://doi.org/10.3390/ijms24032350
APA StyleCadenas-Martin, M., Arnalich-Montiel, F., & Miguel, M. P. D. (2023). Derivation of Limbal Stem Cells from Human Adult Mesenchymal Stem Cells for the Treatment of Limbal Stem Cell Deficiency. International Journal of Molecular Sciences, 24(3), 2350. https://doi.org/10.3390/ijms24032350



