Equine Muscle Derived Mesenchymal Stem Cells Loaded with Water-Soluble Curcumin: Modulation of Neutrophil Activation and Enhanced Protection against Intracellular Oxidative Attack
Abstract
1. Introduction
2. Results
2.1. NDS27 Cell Loading, Cell Viability and Metabolism after Loading
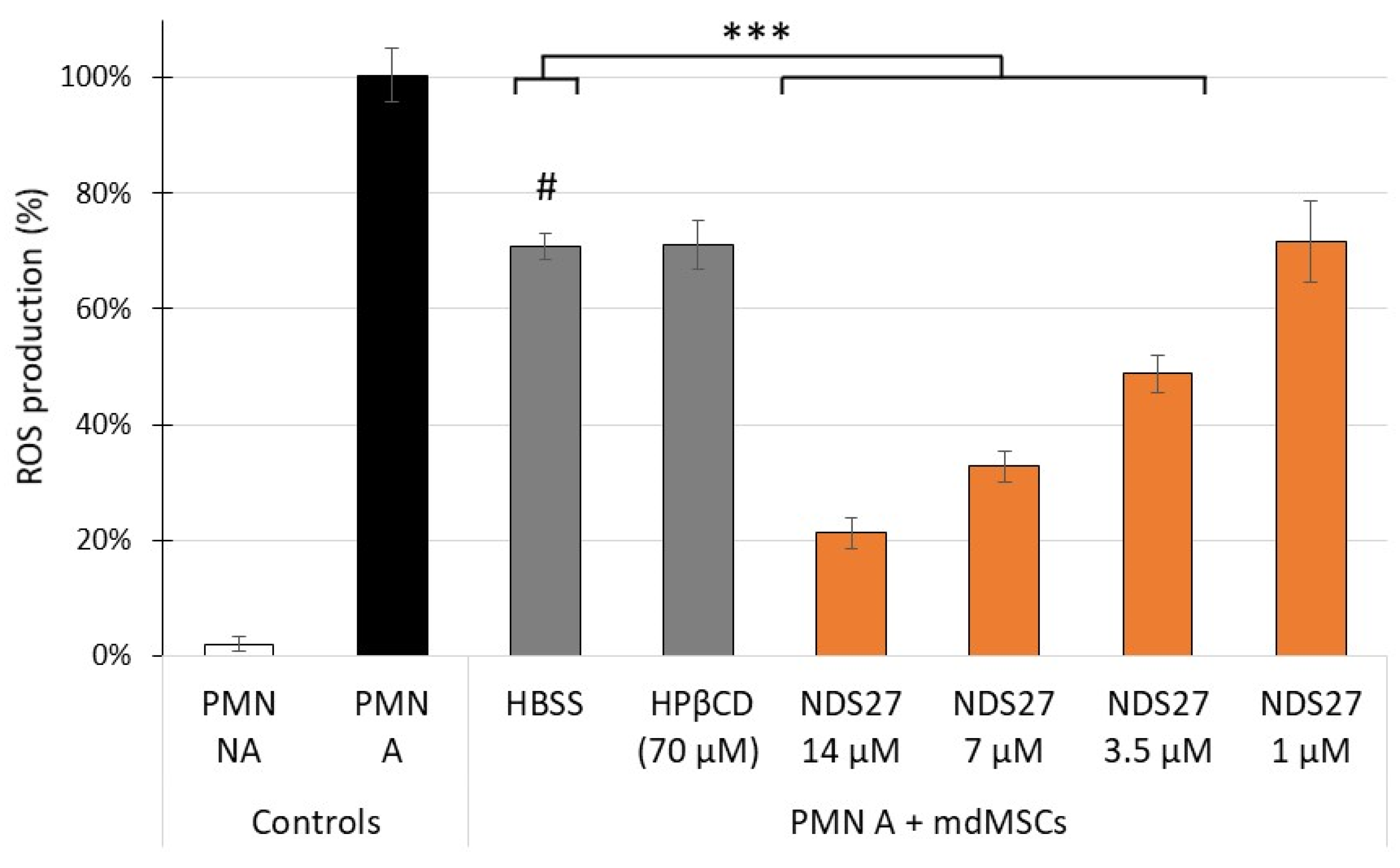
2.2. Effect of NDS27 Loaded mdMSCs on the Stimulated Neutrophils: ROS Production, Total and Active MPO Release and NET Formation
2.2.1. ROS Production
2.2.2. Total and Active MPO Release
2.2.3. NET-MPO Formation
2.3. Protective Effect of NDS27 against Intracellular ROS Production
3. Material and Methods
3.1. Chemicals and Reagents
3.2. mdMSC Culture
3.3. Cell Loading with NDS27 and HPβCD
3.4. Cell Viability and Metabolism of MSCs after a Loading
3.5. HPLC Quantification of Curcumin from NDS27 Incorporated into the Cells
3.6. Effects of Non-Loaded and Loaded mdMSCs on Stimulated Neutrophils: ROS Production, Total or Active MPO Released by Degranulation, and NET Formation
3.6.1. Measurement of ROS Production
3.6.2. Measurement of Active and Total MPO Release by Neutrophil Degranulation
3.6.3. Measurement of the Active and Total MPO Bound to the NET (NET-MPO)
3.7. Intracellular mdMSCs ROS Production under Cumene Attack
3.8. Statistical Analysis
4. Discussion
4.1. mdMSCs Loading with NDS27
4.2. NDS27 Effects on mdMSCs Metabolism and Viability
4.3. Protective Effects of NDS27 Loaded mdMSCs against the Oxidant Activity of Neutrophils
4.4. Protective Effect of ND27 against Intracellular Oxidant Stress on mdMSCs
4.5. Conclusions
- We have developed a quick and efficient method to load, with a good yield (20%), equine mdMSCs suspensions with the water-soluble curcumin derivative NDS27. The loading decreases the cell metabolism but does not induce cytotoxicity;
- The loading with NDS27 increases the anti-inflammatory activity of mdMSCs against stimulated neutrophils, as measured by the lowering of ROS production and the significant inhibition of the catalytic activity of degranulated or NET- bound MPO;
- The loaded mdMSCs acquire a major capacity to decrease the intracellular ROS production induced by an oxidant stress while decreasing cell metabolism.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, W.; Xu, J. Immune Modulation by Mesenchymal Stem Cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.K.; Mashaghi, A.; Amouzegar, A.; Li, M.; Foulsham, W.; Sahu, S.K.; Chauhan, S.K. Mesenchymal Stromal Cells Inhibit Neutrophil Effector Functions in a Murine Model of Ocular Inflammation. Invest. Ophthal. Mol. Vis. Sci. 2018, 59, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Ceusters, J.; Lejeune, J.P.; Sandersen, C.; Niesten, A.; Lagneaux, L.; Serteyn, D. From Skeletal Muscle to Stem Cells: An Innovative and Minimally-Invasive Process for Multiple Species. Sci. Rep. 2017, 7, 696. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Ceusters, J.; Graide, H.; Mouithys-Mickalad, A.; Serteyn, D. Muscle Derived Mesenchymal Stem Cells Inhibit the Activity of the Free and the Neutrophil Extracellular Trap (NET)-Bond Myeloperoxidase. Cells 2021, 10, 3486. [Google Scholar] [CrossRef] [PubMed]
- Frangie, C.; Daher, J. Role of Myeloperoxidase in Inflammation and Atherosclerosis (Review). Biomed. Rep. 2022, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Hypochlorous Acid Chemistry in Mammalian Cells-Influence on Infection and Role in Various Pathologies. Int. J. Mol. Sci. 2022, 23, 10735. [Google Scholar] [CrossRef] [PubMed]
- Parker, H.; Albrett, A.M.; Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase Associated with Neutrophil Extracellular Traps Is Active and Mediates Bacterial Killing in the Presence of Hydrogen Peroxide. J. Leukoc. Biol. 2012, 91, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Davies, M.J. Role of Myeloperoxidase and Oxidant Formation in the Extracellular Environment in Inflammation-Induced Tissue Damage. Free Radic. Biol. Med. 2021, 172, 633–651. [Google Scholar] [CrossRef]
- Siraki, A.G. The Many Roles of Myeloperoxidase: From Inflammation and Immunity to Biomarkers, Drug Metabolism and Drug Discovery. Redox. Biol. 2021, 46, 102109. [Google Scholar] [CrossRef]
- Noronha Nc, N.D.C.; Mizukami, A.; Caliári-Oliveira, C.; Cominal, J.G.; Rocha, J.L.M.; Covas, D.T.; Swiech, K.; Malmegrim, K.C.R. Priming Approaches to Improve the Efficacy of Mesenchymal Stromal Cell-Based Therapies. Stem. Cell Res. Ther. 2019, 10, 131. [Google Scholar] [CrossRef]
- Shokat, Z.; Ali, A.; Ahmed, U.; Javed, M.R.; Qasim, M.; Tariq, M.; Ahmed, M.R.; Masoud, M.S. Mesenchymal Stem Cells: From Regeneration to Drug Delivery Systems. Crit. Rev. Ther. Drug Carrier Syst. 2021, 38, 33–73. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Wu, Y.; Yang, X.; Huang, S.; Yu, F.; Deng, H.; Zhang, S.; Xiang, Q. Stem Cell and Its Derivatives as Drug Delivery Vehicles: An Effective New Strategy of Drug Delivery System. All Life 2021, 14, 782–798. [Google Scholar] [CrossRef]
- Cherniack, E.P.; Chekuri, S.; Lee, H.F. Potential Non-Neoplastic Applications for Polyphenols in Stem Cell Utilization. Curr. Drug Targets 2019, 20, 347–353. [Google Scholar] [CrossRef]
- Wang, N.; Wang, F.; Gao, Y.; Yin, P.; Pan, C.; Liu, W.; Zhou, Z.; Wang, J. Curcumin Protects Human Adipose-Derived Mesenchymal Stem Cells against Oxidative Stress-Induced Inhibition of Osteogenesis. J. Pharmacol. Sci. 2016, 132, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, K.; Wang, J.; Liu, K.; Wu, G.; Li, Y.; Luo, L.; Zheng, Z.; Hu, D. Hypoxic Preconditioning Combined with Curcumin Promotes Cell Survival and Mitochondrial Quality of Bone Marrow Mesenchymal Stem Cells, and Accelerates Cutaneous Wound Healing via PGC-1α/SIRT3/HIF-1α Signaling. Free Radic. Biol. Med. 2020, 159, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The Indian Solid Gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar] [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Padmavathi, G.; Monisha, J.; Roy, N.K.; Prasad, S.; Aggarwal, B.B. Curcumin, the Golden Nutraceutical: Multitargeting for Multiple Chronic Diseases. Br. J. Pharmacol. 2017, 174, 1325–1348. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple Biological Activities of Curcumin: A Short Review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Barzegar, A.; Moosavi-Movahedi, A.A. Intracellular ROS Protection Efficiency and Free Radical-Scavenging Activity of Curcumin. PLoS ONE 2011, 6, e26012. [Google Scholar] [CrossRef]
- Franck, T.; Kohnen, S.; Grulke, S.; Neven, P.; Goutman, Y.; Peters, F.; Pirotte, B.; Deby-Dupont, G.; Serteyn, D. Inhibitory Effect of Curcuminoids and Tetrahydrocurcuminoids on Equine Activated Neutrophils and Myeloperoxidase Activity. Physiol. Res. 2008, 57, 577–587. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, P.; Song, P.; Xiong, W.; Chen, H.; Peng, W.; Wang, S.; Li, S.; Fu, Z.; Wang, Y.; et al. Pretreatment of Adipose Derived Stem Cells with Curcumin Facilitates Myocardial Recovery via Antiapoptosis and Angiogenesis. Stem Cells Int. 2015, 2015, 638153. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. Photophysics, Photochemistry and Photobiology of Curcumin: Studies from Organic Solutions, Bio-Mimetics and Living Cells. J. Photochem. Photobiol. C Photochem. Rev. 2009, 10, 81–95. [Google Scholar] [CrossRef]
- Derochette, S.; Mouithys-Mickalad, A.; Franck, T.; Collienne, S.; Ceusters, J.; Deby-Dupont, G.; Neven, P.; Serteyn, D. NDS27 Combines the Effect of Curcumin Lysinate and Hydroxypropyl-β-Cyclodextrin to Inhibit Equine PKCδ and NADPH Oxidase Involved in the Oxidative Burst of Neutrophils. FEBS Open Bio 2014, 4, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Aldib, I.; Zouaoui Boudjeltia, K.; Furtmüller, P.G.; Obinger, C.; Neven, P.; Prévost, M.; Soubhye, J.; van Antwerpen, P.; Mouithys-Mickalad, A.; et al. The Soluble Curcumin Derivative NDS27 Inhibits Superoxide Anion Production by Neutrophils and Acts as Substrate and Reversible Inhibitor of Myeloperoxidase. Chem. Biol. Interact. 2019, 297, 34–43. [Google Scholar] [CrossRef]
- Derochette, S.; Serteyn, D.; Mouithys-Mickalad, A.; Ceusters, J.; Deby-Dupont, G.; Neven, P.; Franck, T. EquiNox2: A New Method to Measure NADPH Oxidase Activity and to Study Effect of Inhibitors and Their Interactions with the Enzyme. Talanta 2015, 144, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Sandersen, C.; Serteyn, D.; Olejnik, D.; Art, T.; Franck, T.; Neven, P. Inhalation with NDS27 Attenuates Pulmonary Neutrophilic Inflammation in Recurrent Airway Obstruction. Vet. Rec. 2011, 169, 100. [Google Scholar] [CrossRef]
- Sandersen, C.; Bienzle, D.; Cerri, S.; Franck, T.; Derochette, S.; Neven, P.; Mouytis-Mickalad, A.; Serteyn, D. Effect of Inhaled Hydrosoluble Curcumin on Inflammatory Markers in Broncho-Alveolar Lavage Fluid of Horses with LPS-Induced Lung Neutrophilia. Multidiscip. Respir. Med. 2015, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Colin, M.; Dechêne, L.; Ceusters, J.; Niesten, A.; Demazy, C.; Lagneaux, L.; Zouaoui Boudjeltia, K.; Franck, T.; van Antwerpen, P.; Renard, P.; et al. Priming of Mesenchymal Stem Cells with a Hydrosoluble Form of Curcumin Allows Keeping Their Mesenchymal Properties for Cell-Based Therapy Development. J. Cell Mol. Med. 2021, 25, 4877–4881. [Google Scholar] [CrossRef] [PubMed]
- Franck, T.; Grulke, S.; Deby-Dupont, G.; Deby, C.; Duvivier, H.; Peters, F.; Serteyn, D. Development of an Enzyme-Linked Immunosorbent Assay for Specific Equine Neutrophil Myeloperoxidase Measurement in Blood. J. Vet. Diagn. Investig. 2005, 17, 412–419. [Google Scholar] [CrossRef]
- Neven, P.; Serteyn, D.; Delarge, J.; Kiss, R.; Mathieu, V.; Cataldo, D.; Rocks, N. Water Soluble Curcumin Compositions for Use in Anti-Cancer and Anti-Inflammatory Therapy. Patent: WO/2009/144220, 3 December 2009. [Google Scholar]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2001, 21, A.3B.1–A.3B.2. [Google Scholar] [CrossRef]
- Pycock, J.F.; Allen, W.E.; Morris, T.H. Rapid, Single-Step Isolation of Equine Neutrophils on a Discontinuous Percoll Density Gradient. Res. Vet. Sci. 1987, 42, 411–412. [Google Scholar] [CrossRef] [PubMed]
- Thålin, C.; Daleskog, M.; Göransson, S.P.; Schatzberg, D.; Lasselin, J.; Laska, A.-C.; Kallner, A.; Helleday, T.; Wallén, H.; Demers, M. Validation of an Enzyme-Linked Immunosorbent Assay for the Quantification of Citrullinated Histone H3 as a Marker for Neutrophil Extracellular Traps in Human Plasma. Immunol. Res. 2017, 65, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Parrado, J.; Bougria, M.; Machado, A. Effect of Oxidative Stress, Produced by Cumene Hydroperoxide, on the Various Steps of Protein Synthesis. Modifications of Elongation Factor-2. J. Biol. Chem. 1996, 271, 23105–23110. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Hou, Q.; Zhao, Y.; Zhong, L.; Fu, X. Potential Pre-Activation Strategies for Improving Therapeutic Efficacy of Mesenchymal Stem Cells: Current Status and Future Prospects. Stem Cell Res. Ther. 2022, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Hameeda, P.; Katti, S.; Jammalamadugu, R.; Bhatt, K.; Peram, M.R.; Kumbar, V. Comparison of Effects of Curcumin and Nano-Curcumin on the Survival of Human-Derived Mesenchymal Stem Cells: An Experimental Study. J. Adv. Oral Res. 2020, 11, 148–155. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Weng, Q.; Xiao, L.; Li, Q. Curcumin Analogues Attenuate Aβ 25-35 -Induced Oxidative Stress in PC12 Cells via Keap1/Nrf2/HO-1 Signaling Pathways. Chem. Biol. Interact. 2019, 305, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; He, C.; He, J.; Chu, J.; Liu, H.; Deng, X. Curcumin-Mediated Bone Marrow Mesenchymal Stem Cell Sheets Create a Favorable Immune Microenvironment for Adult Full-Thickness Cutaneous Wound Healing. Stem Cell Res. Ther. 2018, 9, 21. [Google Scholar] [CrossRef]
- Azam, M.; Ghufran, H.; Butt, H.; Mehmood, A.; Ashfaq, R.; Ilyas, A.M.; Ahmad, M.R.; Riazuddin, S. Curcumin Preconditioning Enhances the Efficacy of Adipose-Derived Mesenchymal Stem Cells to Accelerate Healing of Burn Wounds. Burns Trauma 2021, 9, tkab021. [Google Scholar] [CrossRef]
- Perteghella, S.; Crivelli, B.; Catenacci, L.; Sorrenti, M.; Bruni, G.; Necchi, V.; Vigani, B.; Sorlini, M.; Torre, M.L.; Chlapanidas, T. Stem Cell-Extracellular Vesicles as Drug Delivery Systems: New Frontiers for Silk/Curcumin Nanoparticles. Int. J. Pharm. 2017, 520, 86–97. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Hajighasemi, S.; Jamialahmadi, T.; Majeed, M.; Sahebkar, A. The Effect of Curcumin on the Differentiation of Mesenchymal Stem Cells into Mesodermal Lineage. Molecules 2019, 24, 4029. [Google Scholar] [CrossRef]
- Tripodo, G.; Chlapanidas, T.; Perteghella, S.; Vigani, B.; Mandracchia, D.; Trapani, A.; Galuzzi, M.; Tosca, M.C.; Antonioli, B.; Gaetani, P.; et al. Mesenchymal Stromal Cells Loading Curcumin-INVITE-Micelles: A Drug Delivery System for Neurodegenerative Diseases. Colloids Surf. B Biointerfaces 2015, 125, 300–308. [Google Scholar] [CrossRef]
- Paultre, K.; Cade, W.; Hernandez, D.; Reynolds, J.; Greif, D.; Best, T.M. Therapeutic Effects of Turmeric or Curcumin Extract on Pain and Function for Individuals with Knee Osteoarthritis: A Systematic Review. BMJ. Open Sport Exerc. Med. 2021, 7, e000935. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Devel Ther. 2021, 15, 4503. [Google Scholar] [CrossRef]
- Zielińska, A.; Alves, H.; Marques, V.; Durazzo, A.; Lucarini, M.; Alves, T.F.; Morsink, M.; Willemen, N.; Eder, P.; Chaud, M.V.; et al. Properties, Extraction Methods, and Delivery Systems for Curcumin as a Natural Source of Beneficial Health Effects. Medicina 2020, 56, 336. [Google Scholar] [CrossRef]
- Moustapha, A.; Pérétout, P.A.; Rainey, N.E.; Sureau, F.; Geze, M.; Petit, J.-M.; Dewailly, E.; Slomianny, C.; Petit, P.X. Curcumin Induces Crosstalk between Autophagy and Apoptosis Mediated by Calcium Release from the Endoplasmic Reticulum, Lysosomal Destabilization and Mitochondrial Events. Cell Death Discov. 2015, 1, 15017. [Google Scholar] [CrossRef] [PubMed]
- Sala de Oyanguren, F.J.; Rainey, N.E.; Moustapha, A.; Saric, A.; Sureau, F.; O’Connor, J.E.; Petit, P.X. Highlighting Curcumin-Induced Crosstalk between Autophagy and Apoptosis as Supported by Its Specific Subcellular Localization. Cells 2020, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Itaya, M.; Miyazawa, T.; Khalifa, S.; Shimizu, N.; Nakagawa, K. The Inhibition of Interaction with Serum Albumin Enhances the Physiological Activity of Curcumin by Increasing Its Cellular Uptake. Food Funct. 2022, 13, 639–648. [Google Scholar] [CrossRef]
- Wang, Y.; Nguyen, D.T.; Yang, G.; Anesi, J.; Kelly, J.; Chai, Z.; Ahmady, F.; Charchar, F.; Golledge, J. A Modified MTS Proliferation Assay for Suspended Cells to Avoid the Interference by Hydralazine and β-Mercaptoethanol. Assay Drug Dev. Technol. 2021, 19, 184–190. [Google Scholar] [CrossRef]
- Trujillo, J.; Granados-Castro, L.F.; Zazueta, C.; Andérica-Romero, A.C.; Chirino, Y.I.; Pedraza-Chaverrí, J. Mitochondria as a Target in the Therapeutic Properties of Curcumin. Arch. Pharm. 2014, 347, 873–884. [Google Scholar] [CrossRef]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Rafiee, R.; Sathyapalan, T.; Sahebkar, A. Effects of Curcumin on Mitochondria in Neurodegenerative Diseases. BioFactors 2020, 46, 5–20. [Google Scholar] [CrossRef]
- Ghufran, H.; Mehmood, A.; Azam, M.; Butt, H.; Ramzan, A.; Yousaf, M.A.; Ejaz, A.; Tarar, M.N.; Riazuddin, S. Curcumin Preconditioned Human Adipose Derived Stem Cells Co-Transplanted with Platelet Rich Plasma Improve Wound Healing in Diabetic Rats. Life Sci. 2020, 257, 118091. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhai, Z.; Ying, H.; Lu, L.; Zhang, J.; Zeng, Y. Curcumin PriMed ADMSCs Derived Small Extracellular Vesicle Exert Enhanced Protective Effects on Osteoarthritis by Inhibiting Oxidative Stress and Chondrocyte Apoptosis. J. Nanobiotechnol. 2022, 20, 123. [Google Scholar] [CrossRef]
- Rogers, N.M.; Stephenson, M.D.; Kitching, A.R.; Horowitz, J.D.; Coates, P.T.H. Amelioration of Renal Ischaemia-Reperfusion Injury by Liposomal Delivery of Curcumin to Renal Tubular Epithelial and Antigen-Presenting Cells. Br. J. Pharmacol. 2012, 166, 194–209. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Pardillo-Díaz, R.; Carrascal, L.; Ayala, A.; Nunez-Abades, P. Oxidative Stress Induced by Cumene Hydroperoxide Evokes Changes in Neuronal Excitability of Rat Motor Cortex Neurons. Neuroscience 2015, 289, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.; Caro, P.; Gómez, J.; Barja, G. Testing the Vicious Cycle Theory of Mitochondrial ROS Production: Effects of H2O2 and Cumene Hydroperoxide Treatment on Heart Mitochondria. J. Bioenerg. Biomembr. 2006, 38, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Lambeth, J.D.; Kalyanaraman, B. On the Use of L-012, a Luminol-Based Chemiluminescent Probe, for Detecting Superoxide and Identifying Inhibitors of NADPH Oxidase: A Reevaluation. Free Radic. Biol. Med. 2013, 65, 1310–1314. [Google Scholar] [CrossRef]
- Ghufran, H.; Azam, M.; Mehmood, A.; Ashfaq, R.; Baig, M.T.; Malik, K.; Shahid, A.A.; Riazuddin, S. Tumoricidal Effects of UnpriMed and Curcumin-PriMed Adipose-Derived Stem Cells on Human Hepatoma HepG2 Cells under Oxidative Conditions. Tissue Cell 2022, 79, 101968. [Google Scholar] [CrossRef]




| Incubation Time (min) | [Mean Curcumin] f (µM) | SD | CV (%) | Absorbed Curcumin (%) |
|---|---|---|---|---|
| 2 | 3.12 | 0.20 | 6.29 | 22.3 ± 1.4 |
| 5 | 2.84 | 0.13 | 4.62 | 20.3 ± 0.9 |
| 10 | 3.03 | 0.23 | 7.72 | 21.6 ± 1.7 |
| 20 | 3.03 | 0.49 | 16.09 | 21.7 ± 3.5 |
| [Curcumin] i (µM) | [Mean Curcumin] f (µM) | SD | CV (%) | Absorbed Curcumin (%) |
|---|---|---|---|---|
| 14 | 3.38 | 0.20 | 5.81 | 25.2 ± 1.4 |
| 7 | 1.53 | 0.16 | 10.53 | 22.3 ± 2.3 |
| 3.5 | 0.69 | 0.11 | 16.43 | 18.2 ± 3.2 |
| 1 | 0.13 | 0.05 | 40.01 | 11.5 ± 5.4 |
| mdMSCs with | Viability (%) | |
|---|---|---|
| Before loading | HBSS | 87.46 ± 7.86 |
| After loading (10 min) | HBSS | 87.39 ± 9.91 |
| HPβCD (70 µM) | 85.82 ± 11.76 | |
| NDS27 (7 µM) | 86.87 ± 7.07 | |
| NDS27 (14 µM) | 82.26 ± 11.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franck, T.; Ceusters, J.; Graide, H.; Niesten, A.; Duysens, J.; Mickalad, A.M.; Serteyn, D. Equine Muscle Derived Mesenchymal Stem Cells Loaded with Water-Soluble Curcumin: Modulation of Neutrophil Activation and Enhanced Protection against Intracellular Oxidative Attack. Int. J. Mol. Sci. 2023, 24, 1030. https://doi.org/10.3390/ijms24021030
Franck T, Ceusters J, Graide H, Niesten A, Duysens J, Mickalad AM, Serteyn D. Equine Muscle Derived Mesenchymal Stem Cells Loaded with Water-Soluble Curcumin: Modulation of Neutrophil Activation and Enhanced Protection against Intracellular Oxidative Attack. International Journal of Molecular Sciences. 2023; 24(2):1030. https://doi.org/10.3390/ijms24021030
Chicago/Turabian StyleFranck, Thierry, Justine Ceusters, Hélène Graide, Ariane Niesten, Julien Duysens, Ange Mouithys Mickalad, and Didier Serteyn. 2023. "Equine Muscle Derived Mesenchymal Stem Cells Loaded with Water-Soluble Curcumin: Modulation of Neutrophil Activation and Enhanced Protection against Intracellular Oxidative Attack" International Journal of Molecular Sciences 24, no. 2: 1030. https://doi.org/10.3390/ijms24021030
APA StyleFranck, T., Ceusters, J., Graide, H., Niesten, A., Duysens, J., Mickalad, A. M., & Serteyn, D. (2023). Equine Muscle Derived Mesenchymal Stem Cells Loaded with Water-Soluble Curcumin: Modulation of Neutrophil Activation and Enhanced Protection against Intracellular Oxidative Attack. International Journal of Molecular Sciences, 24(2), 1030. https://doi.org/10.3390/ijms24021030





