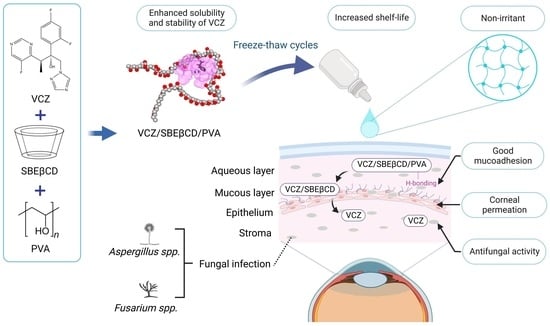
Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes
Abstract
1. Introduction
2. Results and Discussion
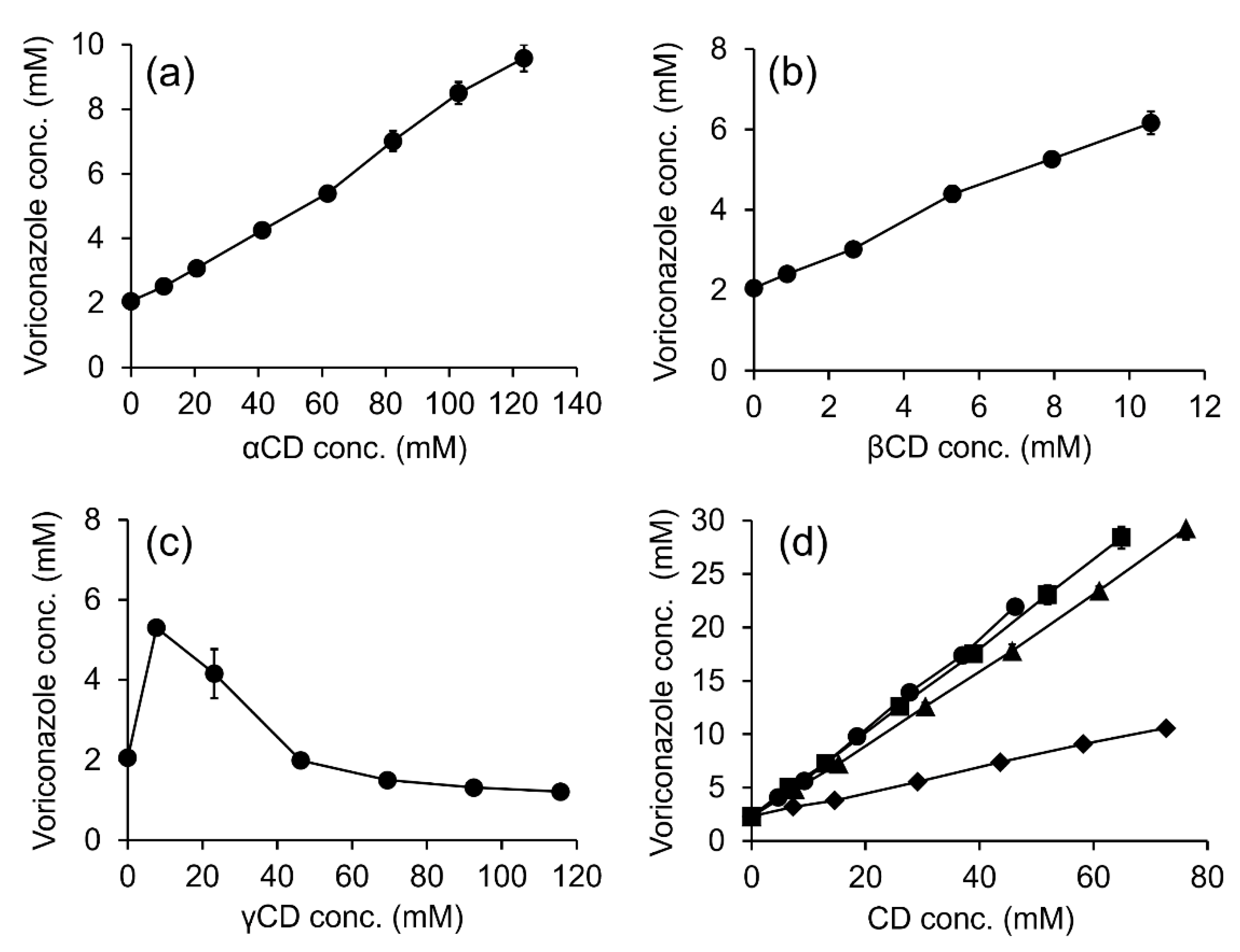
2.1. Solubility Determinations
2.2. Particle Size, Size Distribution and Zeta Potential of Binary VCZ/SBEβCD and Ternary VCZ/SBEβCD/Polymer Complexes
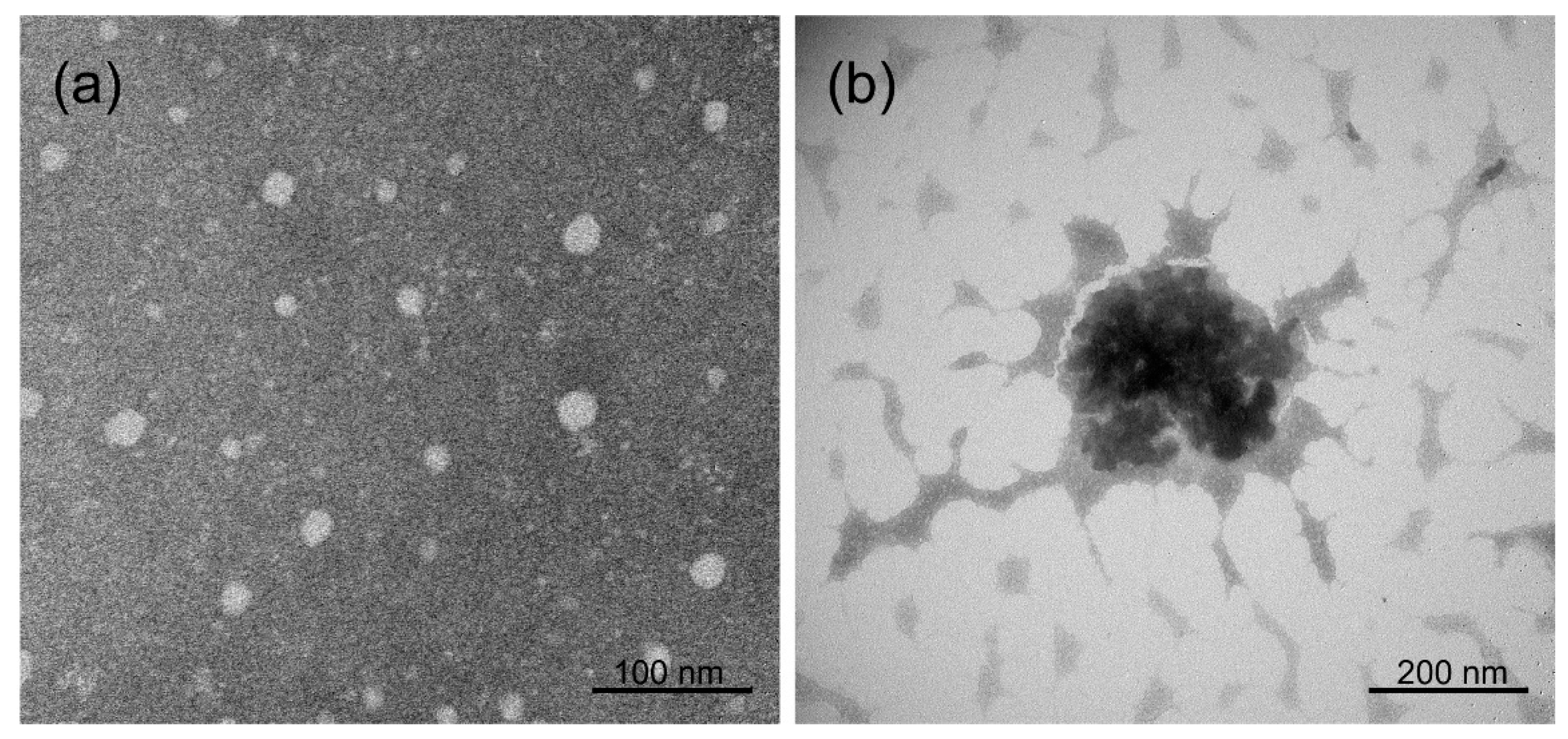
2.3. Transmission Electron Microscopy (TEM) Analysis
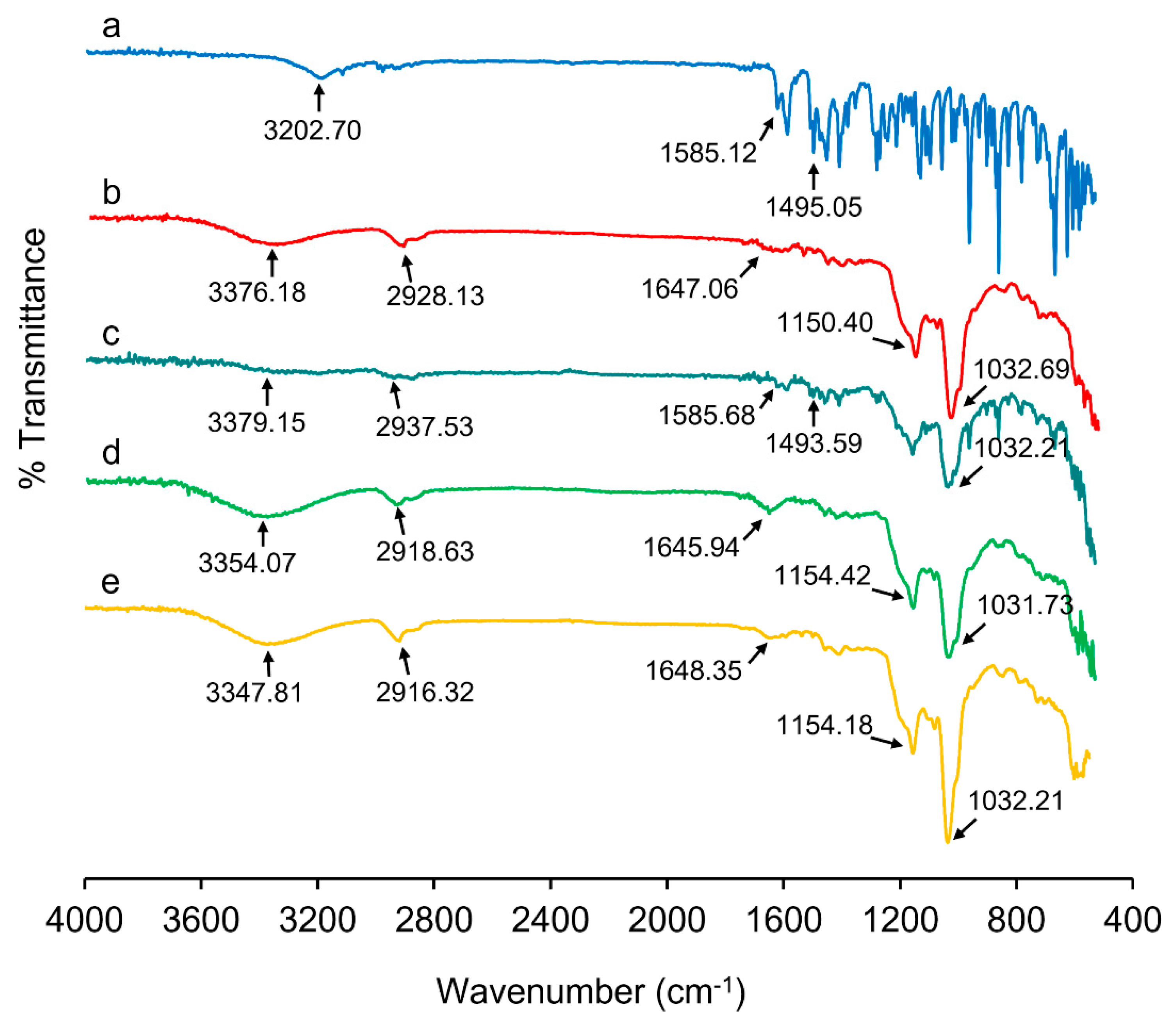
2.4. Fourier-Transform Infrared (FT-IR) Spectroscopy
2.5. Proton Nuclear Magnetic Resonance (1H-NMR) Spectroscopy
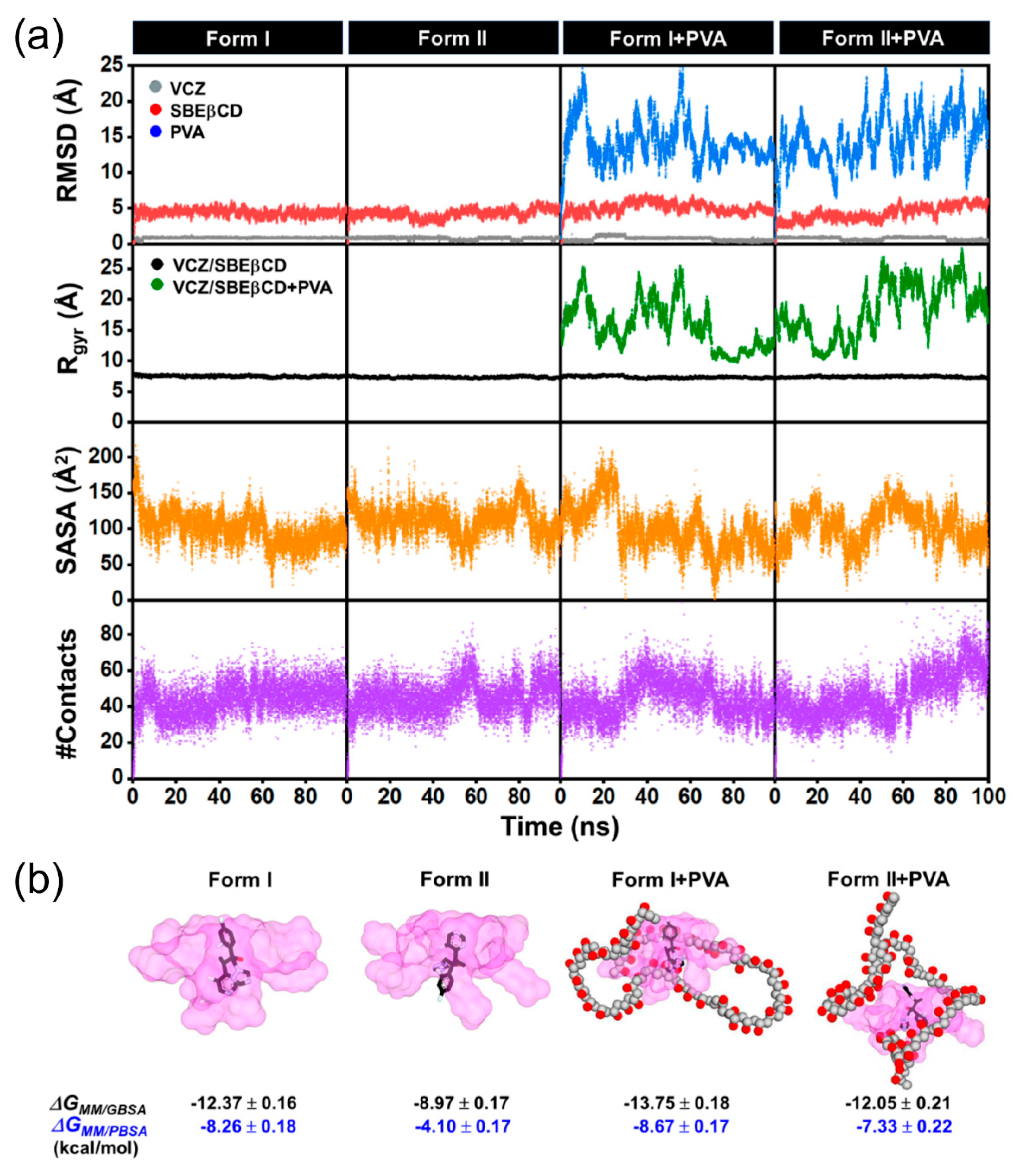
2.6. Molecular Dynamics (MD) Simulations
2.7. Kinetic Degradation Studies of VCZ
2.8. Physicochemical and Chemical Characterizations of VCZ Eye Drop Formulations
2.9. In Vitro Mucoadhesion Studies
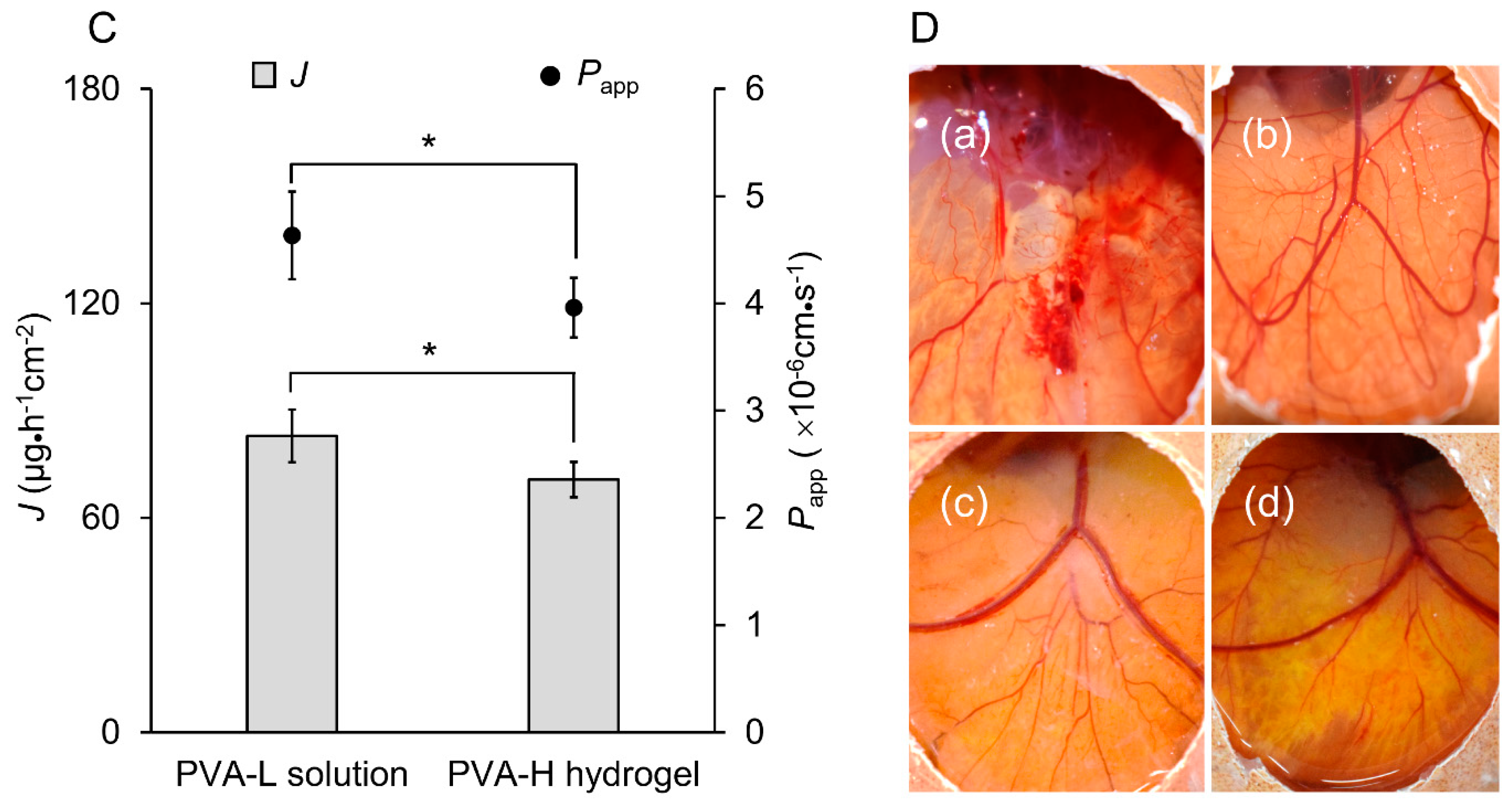
2.10. In Vitro Drug Release and Ex Vivo Permeation Studies
2.11. Irritation Study by Hen’s Egg Test–Chorioallantoic Membrane (HET-CAM)
2.12. In Vitro Antifungal Activity
2.13. Stability Study
3. Materials and Methods
3.1. Materials
3.2. Solubility Determinations
3.3. Quantitative Determinations
3.4. Particle Size, Size Distribution, Zeta Potential Analysis and Morphological Characterization
3.4.1. DLS Measurement
3.4.2. TEM Analysis
3.5. Preparation and Characterization of the Binary VCZ/SBEβCD and Ternary VCZ/SBEβCD/PVA Complexes
3.5.1. FT-IR Spectroscopy
3.5.2. 1H-NMR Spectroscopy
3.6. MD Simulations
3.7. Kinetic Degradation Studies
3.8. Preparation of VCZ Eye Drop Formulations
3.9. Characterizations of VCZ Eye Drop Formulations
3.9.1. pH, Viscosity and Osmolality
3.9.2. Determination of Drug Content
3.10. In Vitro Mucoadhesion Study
3.11. In Vitro Drug-Release Study
3.12. Ex Vivo Permeation Study
3.13. HET-CAM Test
3.14. In Vitro Antifungal Activity
3.15. Stability Study
3.16. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acharya, Y.; Acharya, B.; Karki, P. Fungal keratitis: Study of increasing trend and common determinants. Nepal J. Epidemiol. 2017, 7, 685–693. [Google Scholar] [CrossRef]
- Sharma, N.; Bagga, B.; Singhal, D.; Nagpal, R.; Kate, A.; Saluja, G.; Maharana, P.K. Fungal keratitis: A review of clinical presentations, treatment strategies and outcomes. Ocul. Surf. 2022, 24, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, B.L.; Lalitha, P.; Loh, A.R.; Fothergill, A.W.; Prajna, N.V.; Srinivasan, M.; Kabra, A.; Chidambaram, J.; Acharya, N.R.; Lietman, T.M. Susceptibility testing and clinical outcome in fungal keratitis. Br. J. Ophthalmol. 2010, 94, 384. [Google Scholar] [CrossRef]
- Donovan, C.; Arenas, E.; Ayyala, R.S.; Margo, C.E.; Espana, E.M. Fungal keratitis: Mechanisms of infection and management strategies. Surv. Ophthalmol. 2022, 67, 758–769. [Google Scholar] [CrossRef]
- Brown, L.; Leck, A.K.; Gichangi, M.; Burton, M.J.; Denning, D.W. The global incidence and diagnosis of fungal keratitis. Lancet Infect. Dis. 2021, 21, e49–e57. [Google Scholar] [CrossRef] [PubMed]
- Sanati, H.; Belanger, P.; Fratti, R.; Ghannoum, M. A new triazole, voriconazole (UK-109,496), blocks sterol biosynthesis in Candida albicans and Candida krusei. Antimicrob. Agents Chemother. 1997, 41, 2492–2496. [Google Scholar] [CrossRef] [PubMed]
- Saravolatz, L.D.; Johnson, L.B.; Kauffman, C.A. Voriconazole: A new triazole antifungal agent. Clin. Infect. Dis. 2003, 36, 630–637. [Google Scholar]
- Al-Badriyeh, D.; Neoh, C.F.; Stewart, K.; Kong, D.C. Clinical utility of voriconazole eye drops in ophthalmic fungal keratitis. Clin. Ophthalmol. 2010, 4, 391–405. [Google Scholar]
- Lau, D.; Leung, L.; Fullinfaw, R.; Davies, G.E. Chemical stability of voriconazole 1% eye drops. J. Pharm. Pract. Res. 2008, 38, 179–182. [Google Scholar] [CrossRef]
- Al-Badriyeh, D.; Li, J.; Stewart, K.; Kong, D.C.M.; Leung, L.; Davies, G.E.; Fullinfaw, R. Stability of extemporaneously prepared voriconazole ophthalmic solution. Am. J. Health Syst. Pharm. 2009, 66, 1478–1483. [Google Scholar] [CrossRef]
- Adams, A.I.H.; Gosmann, G.; Schneider, P.H.; Bergold, A.M. LC stability studies of voriconazole and structural elucidation of its major degradation product. Chromatographia 2009, 69, 115–122. [Google Scholar] [CrossRef]
- Popielec, A.; Loftsson, T. Effects of cyclodextrins on the chemical stability of drugs. Int. J. Pharm. 2017, 531, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Jarho, P.; Masson, M.; Järvinen, T. Cyclodextrins in drug delivery. Expert Opin. Drug Deliv. 2005, 2, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, M.; Eiriksson, F.F.; Loftsson, T. Stability characterization, kinetics and mechanism of tacrolimus degradation in cyclodextrin solutions. Int. J. Pharm. 2020, 586, 119579. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, H.; Gu, J.; Guo, T.; Yang, S.; Guo, Z.; Zhang, X.; Zhu, W.; Zhang, J. Ternary system of dihydroartemisinin with hydroxypropyl-β-cyclodextrin and lecithin: Simultaneous enhancement of drug solubility and stability in aqueous solutions. J. Pharm. Biomed. Anal. 2013, 83, 141–148. [Google Scholar] [CrossRef]
- Jain, D.; Carvalho, E.; Banthia, A.K.; Banerjee, R. Development of polyvinyl alcohol-gelatin membranes for antibiotic delivery in the eye. Drug Dev. Ind. Pharm. 2011, 37, 167–177. [Google Scholar] [CrossRef]
- Chaudhari, P.; Birangal, S.; Mavlankar, N.; Pal, A.; Mallela, L.S.; Roy, S.; Kodoth, A.K.; Ghate, V.; Nampoothiri, M.; Lewis, S.A. Oil-free eye drops containing cyclosporine A/cyclodextrin/PVA supramolecular complex as a treatment modality for dry eye disease. Carbohydr. Polym. 2022, 297, 120007. [Google Scholar] [CrossRef]
- Kayaci, F.; Ertas, Y.; Uyar, T. Enhanced thermal stability of eugenol by cyclodextrin inclusion complex encapsulated in electrospun polymeric nanofibers. J. Agric. Food Chem. 2013, 61, 8156–8165. [Google Scholar] [CrossRef]
- Cerchiara, T.; Luppi, B.; Bigucci, F.; Zecchi, V. Effect of chitosan on progesterone release from hydroxypropyl-β-cyclodextrin complexes. Int. J. Pharm. 2003, 258, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Soe, H.M.S.H.; Chamni, S.; Mahalapbutr, P.; Kongtaworn, N.; Rungrotmongkol, T.; Jansook, P. The investigation of binary and ternary sulfobutylether-β-cyclodextrin inclusion complexes with asiaticoside in solution and in solid state. Carbohydr. Res. 2020, 498, 108190. [Google Scholar] [CrossRef] [PubMed]
- Hargittai, I.; Hargittai, M. Molecular structure of hyaluronan: An introduction. Struct. Chem. 2008, 19, 697–717. [Google Scholar] [CrossRef]
- Jansook, P.; Kulsirachote, P.; Loftsson, T. Cyclodextrin solubilization of celecoxib: Solid and solution state characterization. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 75–88. [Google Scholar] [CrossRef]
- Suvarna, P.; Chaudhari, P.; Birangal, S.; Mallela, L.S.; Roy, S.; Koteshwara, A.; Aranjani, J.M.; Lewis, S.A. Voriconazole–cyclodextrin supramolecular ternary complex-loaded ocular films for management of fungal keratitis. Mol. Pharm. 2021, 19, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Schönbeck, C.; Madsen, T.L.; Peters, G.H.; Holm, R.; Loftsson, T. Soluble 1:1 complexes and insoluble 3:2 complexes–Understanding the phase-solubility diagram of hydrocortisone and γ-cyclodextrin. Int. J. Pharm. 2017, 531, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Wenz, G. Influence of intramolecular hydrogen bonds on the binding potential of methylated β-cyclodextrin derivatives. Beilstein J. Org. Chem. 2012, 8, 1890–1895. [Google Scholar] [CrossRef]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef]
- Zia, V.; Rajewski, R.A.; Bornancini, E.R.; Luna, E.A.; Stella, V.J. Effect of alkyl chain length and degree of substitution on the complexation of sulfoalkyl ether β-cyclodextrins with steroids. J. Pharm. Sci. 1997, 86, 220–224. [Google Scholar] [CrossRef]
- Zia, V.; Rajewski, R.A.; Stella, V.J. Effect of cyclodextrin charge on complexation of neutral and charged substrates: Comparison of (SBE)7M-β-CD to HP-β-CD. Pharm. Res. 2001, 18, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Faucci, M.T.; Mura, P. Effect of water-soluble polymers on naproxen complexation with natural and chemically modified β-cyclodextrins. Drug Dev. Ind. Pharm. 2001, 27, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Frikdriksdóttir, H.; Sigurkdardóttir, A.M.; Ueda, H. The effect of water-soluble polymers on drug-cyclodextrin complexation. Int. J. Pharm. 1994, 110, 169–177. [Google Scholar] [CrossRef]
- Messner, M.; Kurkov, S.V.; Jansook, P.; Loftsson, T. Self-assembled cyclodextrin aggregates and nanoparticles. Int. J. Pharm. 2010, 387, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Ryzhakov, A.; Do Thi, T.; Stappaerts, J.; Bertoletti, L.; Kimpe, K.; Sá Couto, A.R.; Saokham, P.; Van den Mooter, G.; Augustijns, P.; Somsen, G.W.; et al. Self-assembly of cyclodextrins and their complexes in aqueous solutions. J. Pharm. Sci. 2016, 105, 2556–2569. [Google Scholar] [CrossRef]
- Cortés, H.; Hernández-Parra, H.; Bernal-Chávez, S.A.; Prado-Audelo, M.L.D.; Caballero-Florán, I.H.; Borbolla-Jiménez, F.V.; González-Torres, M.; Magaña, J.J.; Leyva-Gómez, G. Non-ionic surfactants for stabilization of polymeric nanoparticles for biomedical uses. Materials 2021, 14, 3197. [Google Scholar] [CrossRef]
- Baptista, J.G.C.; Rodrigues, S.P.J.; Matsushita, A.F.Y.; Vitorino, C.; Maria, T.M.R.; Burrows, H.D.; Pais, A.A.C.C.; Valente, A.J.M. Does poly(vinyl alcohol) act as an amphiphilic polymer? An interaction study with simvastatin. J. Mol. Liq. 2016, 222, 287–294. [Google Scholar] [CrossRef]
- Sonntag, H.; Ehmke, B.; Miller, R.; Knapschinski, L. Steric stabilization of polyvinyl alcohol adsorbed on silica/water and water/oil interfaces. Adv. Colloid Interface Sci. 1982, 16, 381–390. [Google Scholar] [CrossRef]
- Miletic, T.; Kyriakos, K.; Graovac, A.; Ibric, S. Spray-dried voriconazole–cyclodextrin complexes: Solubility, dissolution rate and chemical stability. Carbohydr. Polym. 2013, 98, 122–131. [Google Scholar] [CrossRef]
- Liu, F.; Antoniou, J.; Li, Y.; Majeed, H.; Liang, R.; Ma, Y.; Ma, J.; Zhong, F. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for tea polyphenol encapsulation. Food Hydrocoll. 2016, 57, 291–300. [Google Scholar] [CrossRef]
- Mura, P.; Faucci, M.T.; Bettinetti, G.P. The influence of polyvinylpyrrolidone on naproxen complexation with hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Sci. 2001, 13, 187–194. [Google Scholar] [CrossRef]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Pessine, F.B.; Calderini, A.; Alexandrino, G.L. Cyclodextrin inclusion complexes probed by NMR techniques. In Magnetic Resonance Spectroscopy; Books on Demand: Norderstedt, Germany, 2012; Volume 3, p. 264. [Google Scholar]
- Coles, W.H.; Jaros, P.A. Dynamics of ocular surface pH. Br. J. Ophthalmol. 1984, 68, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Kramer, I.; Haber, M.; Duis, A. Formulation requirements for the ophthalmic use of antiseptics. Dev. Ophthalmol. 2002, 33, 85–116. [Google Scholar] [PubMed]
- Frisch, D.; Eyring, H.; Kincaid, J.F. Pressure and temperature effects on the viscosity of liquids. J. Appl. Phys. 1940, 11, 75–80. [Google Scholar] [CrossRef]
- Rahman, M.Q.; Chuah, K.S.; Macdonald, E.C.A.; Trusler, J.P.M.; Ramaesh, K. The effect of pH, dilution, and temperature on the viscosity of ocular lubricants-shift in rheological parameters and potential clinical significance. Eye 2012, 26, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Recent advances in mucoadhesive interface materials, mucoadhesion characterization, and technologies. Adv. Mater. Interfaces 2022, 9, 2200211. [Google Scholar] [CrossRef]
- Jones, D.S.; Laverty, T.P.; Morris, C.; Andrews, G.P. Statistical modelling of the rheological and mucoadhesive properties of aqueous poly(methylvinylether-co-maleic acid) networks: Redefining biomedical applications and the relationship between viscoelasticity and mucoadhesion. Colloids Surf. B Biointerfaces 2016, 144, 125–134. [Google Scholar] [CrossRef]
- Shaikh, R.; Raj Singh, T.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 2011, 3, 89–100. [Google Scholar]
- Roy, S.; Pal, K.; Anis, A.; Pramanik, K.; Prabhakar, B. Polymers in mucoadhesive drug-delivery systems: A brief note. Des. Monomers Polym. 2009, 12, 483–495. [Google Scholar] [CrossRef]
- Fumio, U.; Hiroshi, Y.; Kumiko, N.; Sachihiko, N.; Kenji, S.; Yasunori, M. Swelling and mechanical properties of poly(vinyl alcohol) hydrogels. Int. J. Pharm. 1990, 58, 135–142. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Long, J.; Nand, A.V.; Bunt, C.; Seyfoddin, A. Controlled release of dexamethasone from poly(vinyl alcohol) hydrogel. Pharm. Dev. Technol. 2019, 24, 839–848. [Google Scholar] [CrossRef]
- Malhotra, S.; Khare, A.; Grover, K.; Singh, I.; Pawar, P. Design and evaluation of voriconazole eye drops for the treatment of fungal keratitis. J. Pharm. 2014, 2014, 490595. [Google Scholar] [CrossRef]
- Gilleron, L.; Coecke, S.; Sysmans, M.; Hansen, E.; van Oproy, S.; Marzin, D.; van Cauteren, H.; Vanparys, P. Evaluation of the HET-CAM-TSA method as an alternative to the draize eye irritation test. Toxicol. In Vitro 1997, 11, 641–644. [Google Scholar] [CrossRef]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.M.; Cairns, D. The hen’s egg chorioallantoic membrane (HET-CAM) test to predict the ophthalmic irritation potential of a cysteamine-containing gel: Quantification using Photoshop® and ImageJ. Int. J. Pharm. 2015, 490, 1–8. [Google Scholar] [CrossRef]
- Maw, P.D.; Pienpinijtham, P.; Pruksakorn, P.; Jansook, P. Cyclodextrin-based Pickering nanoemulsions containing amphotericin B: Part II. Formulation, antifungal activity, and chemical stability. J. Drug Deliv. Sci. Technol. 2022, 69, 103174. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The complexation efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2020, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Goetz, A.W.; Homeyer, N.; et al. Amber 2016 Reference Manual; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Oo, A.; Kerdpol, K.; Mahalapbutr, P.; Rungrotmongkol, T. Molecular encapsulation of emodin with various β-cyclodextrin derivatives: A computational study. J. Mol. Liq. 2021, 347, 118002. [Google Scholar] [CrossRef]
- Kaiyawet, N.; Rungrotmongkol, T.; Hannongbua, S. Effect of halogen substitutions on dump to stability of thymidylate synthase/dUMP/mTHF ternary complex using molecular dynamics simulation. J. Chem. Inf. Model. 2013, 53, 1315–1323. [Google Scholar] [CrossRef] [PubMed]
- Mahalapbutr, P.; Chusuth, P.; Kungwan, N.; Chavasiri, W.; Wolschann, P.; Rungrotmongkol, T. Molecular recognition of naphthoquinone-containing compounds against human DNA topoisomerase IIα ATPase domain: A molecular modeling study. J. Mol. Liq. 2017, 247, 374–385. [Google Scholar] [CrossRef]
- Mahalapbutr, P.; Wonganan, P.; Charoenwongpaiboon, T.; Prousoontorn, M.; Chavasiri, W.; Rungrotmongkol, T. Enhanced solubility and anticancer potential of mansonone g by β-cyclodextrin-based host-guest complexation: A computational and experimental study. Biomolecules 2019, 9, 545. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Kirschner, K.N.; Yongye, A.B.; Tschampel, S.M.; González-Outeiriño, J.; Daniels, C.R.; Foley, B.L.; Woods, R.J. GLYCAM06: A generalizable biomolecular force field. Carbohydrates. J. Comput. Chem. 2008, 29, 622–655. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Luty, B.A.; van Gunsteren, W.F. Calculating electrostatic interactions using the particle−particle particle−mesh method with nonperiodic long-range interactions. J. Phys. Chem. 1996, 100, 2581–2587. [Google Scholar] [CrossRef]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef]
- Croitoru, C.; Roata, I.C.; Pascu, A.; Stanciu, E.M. Diffusion and controlled release in physically crosslinked poly (vinyl alcohol)/iota-carrageenan hydrogel blends. Polymers 2020, 12, 1544. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, S.; Sahoo, P.K. Topical delivery of acetazolamide by encapsulating in mucoadhesive nanoparticles. Asian J. Pharm. Sci. 2017, 12, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.L. (Ed.) 5–Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Bruschi, M.L. (Ed.) Woodhead Publishing: Cambridge, UK, 2015; pp. 63–86. [Google Scholar]
- ICCVAM. ICCVAM-Recommended Test Method Protocol, “Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Test Method”; Technical Report 10-7553; NIH: Bethesda, MD, USA, 2010. [Google Scholar]
- Luepke, N. Hen’s egg chorioallantoic membrane test for irritation potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibiliy Testing of Filamentous Fingi; Approved Standard-Second Edition. CLSI Document M38-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008; Volume 28. [Google Scholar]
- ICH. Stability Testing of New Drug Substances and Products (Q1AR2); European Medicines Agency: Amsterdam, The Netherlands, 2003. [Google Scholar]

| Physicochemical Properties | Voriconazole |
|---|---|
| Chemical structure | 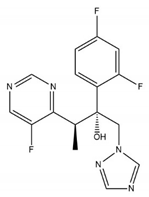 |
| Molecular Weight (g/mol) | 349.3 |
| Melting point (°C) a | 132.4 |
| pKa | 1.76 |
| logPo/w b | 1.65 |
| S0 in water (at RT c) | 0.5 mg/mL |
| Cyclodextrin | Polymers | Type | R2 | K1:1 (M−1) | CE | CE ratio b |
|---|---|---|---|---|---|---|
| αCD | - | AL | 0.9947 | 30.3 | 0.066 | - |
| βCD | - | AL | 0.9946 | 301.8 | 0.658 | - |
| γCD | - | BS | - a | - a | - a | - |
| RMβCD | - | AL | 0.9993 | 250.1 | 0.545 | - |
| HPβCD | - | AL | 0.9996 | 306.6 | 0.668 | - |
| CMβCD | - | AL | 0.9989 | 59.6 | 0.130 | - |
| SBEβCD | - | AL | 0.9987 | 338.1 | 0.737 | 1.00 |
| SBEβCD | 1% PVA | AL | 0.9957 | 412.4 | 0.950 | 1.30 |
| SBEβCD | 0.01% HA | AL | 0.9987 | 339.3 | 0.781 | 1.06 |
| SBEβCD | 0.1% CS | AL | 0.9918 | 355.1 | 0.818 | 1.12 |
| Cyclodextrin (%w/v) | Polymer (%w/v) | Mean Particle Size (nm) | Intensity (%) | PDI a | Zeta Potential (mV) |
|---|---|---|---|---|---|
| 10% SBEβCD | - | 1.34 ± 0.43 | 58.30 ± 33.44 | 0.21 ± 0.07 | −1.01 ± 2.35 |
| 11.24 ± 8.55 | 23.16 ± 18.48 | ||||
| 719.92 ± 315.74 | 18.53 ± 12.98 | ||||
| 0.1% CS | 22.70 ± 6.87 | 9.98 ± 4.63 | 0.53 ± 0.02 | +2.75 ± 1.33 | |
| 240.73 ± 81.13 | 89.98 ± 7.04 | ||||
| 4269 ± 656.91 | 2.87 ± 1.59 | ||||
| 0.01% HA | 1.17 ± 0.10 | 48.74 ± 22.01 | 0.68 ± 0.35 | −1.73 ± 4.70 | |
| 977.22 ± 321.61 | 46.10 ± 24.08 | ||||
| 1546.17 ± 222.57 | 7.71 ± 4.54 | ||||
| 1% PVA | 1.14 ± 0.10 | 14.33 ± 1.34 | 0.60 ± 0.06 | −3.15 ± 2.46 | |
| 23.55 ± 2.56 | 63.20 ± 5.10 | ||||
| 366.20 ± 97.59 | 20.44 ± 4.72 |
 | ||
|---|---|---|
| 1H Assignment | Δδ* = (δcomplex − δfree) | |
| VCZ/SBEβCD | VCZ/SBEβCD/PVA | |
| VCZ | ||
| H-1 | −0.006 | −0.006 |
| H-2 | −0.004 | −0.004 |
| H-3 | −0.004 | −0.001 |
| H-4 | −0.005 | −0.005 |
| H-5 | −0.005 | −0.005 |
| H-6 | −0.005 | −0.011 |
| H-7 | −0.002 | −0.002 |
| H-9 | −0.003 | −0.003 |
| H-10 | +0.003 | +0.003 |
| SBEβCD | ||
| H-1 | +0.001 | +0.001 |
| H-2,4 | +0.002 | −0.005 |
| H-3 | +0.002 | +0.009 |
| H-5 | +0.004 | +0.022 |
| H-6 | - a | - a |
| H-2′,H-3′ | - a | +0.001 |
 | |||
|---|---|---|---|
| Drug | Cyclodextrin | Polymer | kobs (h−1) at 40 °C |
| Voriconazole | - | - | 3.53 × 10−3 |
| SBEβCD | - | 6.70 × 10−4 | |
| 1% PVA | 5.79 × 10−4 | ||
| 2% PVA | 5.55 × 10−4 | ||
| 5% PVA | 5.27 × 10−4 | ||
| Parameter | Formulation | ||
|---|---|---|---|
| PVA-L Solution | PVA-L Hydrogel | PVA-H Hydrogel | |
| pH | 6.98 ± 0.35 | 6.71 ± 0.30 | 6.70 ± 0.07 |
| Osmolality (mOsmol/kg) | 302.3 ± 3.5 | 306.7 ± 10.0 | 294.7 ± 2.51 |
| Viscosity at 25 °C (cPs) | 8.46 ± 0.78 | 26.73 ± 1.05 a | 160.00 ± 13.00 a |
| Viscosity at 35 °C (cPs) | 5.36 ± 0.12 | 19.56 ± 2.38 a | 109.13 ± 25.30 a |
| Drug content (%) | 100.17 ± 1.46 | 100.47 ± 2.13 | 100.57 ± 3.17 |
| Sample | A. flavus | F. solani | ||
|---|---|---|---|---|
| MIC a | MFC b | MIC a | MFC b | |
| VCZ solubilized in DMSO | 0.78 | 0.78 | 12.5 | 12.5 |
| Vfend®-eye drops | 0.78 | 3.10 | 25.0 | 25.0 |
| PVA-L solution | 0.39 | 0.70 | 12.5 | 12.5 |
| PVA-H hydrogel | 0.39 | 3.10 | 25.0 | 25.0 |
| Formulations | VCZ Content | Degradation Rate Constant (kobs, Day−1) | t90 (Days) | |
|---|---|---|---|---|
| Initial | 6 Months | |||
| 2–8 °C | ||||
| Vfend® eye drops | 102.15 ± 5.08 | 92.49 ± 5.08 | 5.62 × 10−4 | 183 |
| PVA-L solution | 100.17 ± 1.46 | 94.62 ± 1.44 | 3.48 × 10−4 | 301 |
| PVA-H hydrogel | 100.56 ± 3.17 | 98.65 ± 1.41 | 8.95 × 10−5 | 1172 |
| Long term (30 ± 2 °C, 60 ± 5% RH a) | ||||
| Vfend® eye drops | 102.15 ± 5.08 | 86.39 ± 2.43 | 1.00 × 10−3 | 105 |
| PVA-L solution | 100.17 ± 1.46 | 93.72 ± 1.13 | 3.90 × 10−4 | 267 |
| PVA-H hydrogel | 100.56 ± 3.17 | 98.36 ± 0.59 | 1.03 × 10−4 | 1015 |
| Accelerated (40 ± 2 °C, 75 ± 5% RH a) | ||||
| Vfend® eye drops | 102.15 ± 5.08 | 68.27 ± 0.86 | 2.40 × 10−3 | 44 |
| PVA-L solution | 100.17 ± 1.46 | 87.53 ± 3.11 | 8.60 × 10−4 | 123 |
| PVA-H hydrogel | 100.56 ± 3.17 | 98.18 ± 0.91 | 1.16 × 10−4 | 903 |
| Formulations | Compositions (% w/v) a | |||
|---|---|---|---|---|
| VCZ | SBEβCD | PVA (LMW) b | PVA (HMW) c | |
| PVA-L solution | 0.5 | 6 | 5 | - |
| PVA-L hydrogel | 0.5 | 6 | 10 | - |
| PVA-H hydrogel | 0.5 | 6 | - | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soe, H.M.S.H.; Kerdpol, K.; Rungrotmongkol, T.; Pruksakorn, P.; Autthateinchai, R.; Wet-osot, S.; Loftsson, T.; Jansook, P. Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes. Int. J. Mol. Sci. 2023, 24, 2343. https://doi.org/10.3390/ijms24032343
Soe HMSH, Kerdpol K, Rungrotmongkol T, Pruksakorn P, Autthateinchai R, Wet-osot S, Loftsson T, Jansook P. Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes. International Journal of Molecular Sciences. 2023; 24(3):2343. https://doi.org/10.3390/ijms24032343
Chicago/Turabian StyleSoe, Hay Man Saung Hnin, Khanittha Kerdpol, Thanyada Rungrotmongkol, Patamaporn Pruksakorn, Rinrapas Autthateinchai, Sirawit Wet-osot, Thorsteinn Loftsson, and Phatsawee Jansook. 2023. "Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes" International Journal of Molecular Sciences 24, no. 3: 2343. https://doi.org/10.3390/ijms24032343
APA StyleSoe, H. M. S. H., Kerdpol, K., Rungrotmongkol, T., Pruksakorn, P., Autthateinchai, R., Wet-osot, S., Loftsson, T., & Jansook, P. (2023). Voriconazole Eye Drops: Enhanced Solubility and Stability through Ternary Voriconazole/Sulfobutyl Ether β-Cyclodextrin/Polyvinyl Alcohol Complexes. International Journal of Molecular Sciences, 24(3), 2343. https://doi.org/10.3390/ijms24032343