Abstract
The native role of extracellular vesicles (EVs) in mediating the transfer of biomolecules between cells has raised the possibility to use them as therapeutic vehicles. The development of therapies based on EVs is now expanding rapidly; here we will describe the current knowledge on different key points regarding the use of EVs in a clinical setting. These points are related to cell sources of EVs, isolation, storage, and delivery methods, as well as modifications to the releasing cells for improved production of EVs. Finally, we will depict the application of EVs therapies in clinical trials, considering the impact of the COVID-19 pandemic on the development of these therapies, pointing out that although it is a promising therapy for human diseases, we are still in the initial phase of its application to patients.
1. Introduction
In the last three decades, a new mechanism of intercellular communication, based on the release into the extracellular medium of vesicles surrounded by a lipid bilayer by different cells, has begun to be studied in depth. The first mention of these vesicles appeared in 1967 when Peter Wolf defined platelet powder as the sediment obtained by ultracentrifuging platelet-free plasma [1]. Later, apoptotic bodies, which are vesicles released during the process of apoptosis, capable of functioning as signaling particles for cells of the immune system, were described [2]. In recent years, it has become clear that virtually all cells, healthy or not, release different types of vesicles into the extracellular environment that mediate intercellular communication across several tissues. The different types of vesicles have been given various nomenclatures according to their size and releasing cell: microvesicles, exosomes, ectosomes, endosomes, oncosomes, or microparticles [3].
Extracellular vesicles (EVs) serve as vehicles for the transfer of different molecules between cells (proteins, lipids, DNA, RNA, miRNAs, etc.) and are involved in numerous physiological and pathological processes such as the removal of unwanted proteins, antigen presentation, genetic exchange, immune response, angiogenesis, inflammation, tumor metastasis, and dissemination of pathogens or oncogenes [3,4,5].
The most studied types of EVs are exosomes and microvesicles (MVs). Exosomes are smaller, as they present a size between 30 and 200 nm, are formed inside the cell and accumulate in multivesicular bodies (MVBs). Later, these exosomes are released by the fusion of MVBs with the plasma membrane. On the other hand, MVs are larger, up to 1000 nm in size, although smaller MVs can also be found. MVs are formed directly from the plasma membrane, so theoretically the membrane of these EVs has the same composition as that of the cell of origin [3,6,7]. This nomenclature, however, is not completely accurate from an experimental point of view, as most vesicle isolation methods are based on vesicle size, which does not allow complete differentiation between the origin of the vesicles. More recently, the ISEV (International Society of Extracellular Vesicles) has proposed a nomenclature based on vesicle size: small EVs (sEVs) for EVs traditionally referred to as exosomes (diameter up to 200 nm) and large EVs (lEVs) for EVs traditionally referred to as microvesicles (diameter between 200–1000 nm) [6]. This boundary defining small EVs has varied over the last years between 100 and 200 nm.
The clinical use of EVs has been boosted by the encouraging results of studies in preclinical models of different fields, such as cancer, immune diseases, or regenerative medicine, that have shown the potential use of these vesicles as biomarkers, treatments, or drug delivery systems [8]. These results have led to the development of clinical-grade EVs, and clinical trials using EVs have gained momentum in the last 3–4 years, greatly conditioned by the COVID-19 pandemic [9,10,11]. Here, we will focus on the use of EVs from different sources as a therapeutic approach for human diseases, reviewing the current state-of-the-art regarding clinical trials, and discussing the challenges and limitations that this new avenue of research presents.
2. Cell Sources of EVs for Clinical Purposes
2.1. EVs Obtained from Body Fluids
EVs can be isolated from body fluids such as semen, urine, milk, bronchoalveolar lavage fluid, and plasma, for therapeutic applications (Figure 1).
Figure 1.
Most used cell sources for EVs obtention and their possible clinical applications.
Seminal EVs are mainly used for the treatment of infertility as they enhance sperm motility and modulate the implantation process of the embryo [12].
Urinal EVs have a major role in the prediction of the response to therapy in urogenital system diseases [13]. Indeed, the identification of specific biomarkers, including protein, lipids, and miRNAs, within urinary EVs unveils the prognosis of the prostate, bladder, and renal cancers [14].
Human milk EVs (hMEVs) are safe for oral consumption, which supports their use as a nutraceutical/therapeutic agent [15]. hMEVs are mainly employed in human milk fortification to promote the beneficial effects of maternal breast milk or to improve infant milk formula [16,17]. Moreover, hMEVs have shown a therapeutic role in intestinal inflammation as they enhance tissue repair in a mouse model of preterm infants with Necrotizing Enterocolitis [18]. hMEVs also display bioactive molecules with anti-viral effects, such as dengue [19], and anti-tumor effects through miR-148a-3p, which hampers colon cancer development [20].
Airway EVs can be isolated from bronchoalveolar lavage fluid (BALF). BALF-EVs are famous for their inflammatory modulatory properties, which make them valuable tools for the treatment of different lung diseases [21]. As an example, BALF-EVs increase interleukins IL-1β and IL-6, Tumor Necrosis Factor α (TNFα), and CC motif chemokine Ligand 2 (CCL2) in sarcoidosis disease [22,23] and acute respiratory distress syndrome (ARDS) [24]. Further, BALF-EVs improve leukotriene and IL-8 production in asthma patients [25].
Human plasma has been used as a therapy since several decades ago [26,27]. Plasma-derived EVs are released from different cells and have been analyzed as therapeutics for several conditions, such as cardiac and bone diseases, wound healing, aging, and muscle atrophy due to their anti-inflammatory, antioxidant, pro-angiogenic, and anti-apoptotic properties [27]. These beneficial effects of plasma-EVs derive from their intrinsic capacity to modulate cell signaling cascades such as Toll-Like Receptor 4 (TLR4), Extracellular signal-Regulated Kinase 1/2 (ERK1/2), and p38 Mitogen-activated protein kinase (P38MAPK) [28], to increase the expression of collagen type III, and to decrease the expression of TGF-β and IL-6 [29]. In addition to regular plasma, modified versions of the regular plasma have also been investigated as a source for therapeutic EVs. Among them, plasma containing additional blood components (platelets), plasma from donors who have undergone specific procedures (ischemic pre-conditioning or exercise training), and plasma from donors with specific characteristics (young age or infection survivors) have been studied [27].
2.2. EVs Obtained from in vitro Cell Cultures
EVs can also be isolated from cell cultures for therapeutical purposes. The majority of EVs obtained in vitro come from mesenchymal stem cell (MSC) cultures, and among them, the most widely used are bone marrow MSC, umbilical cord MSC, and adipose-derived MSC (Figure 1).
Human bone marrow MSC-derived EVs (hBM-MSC-EVs) are mostly involved in the treatment of diseases of connective tissues such as cartilage and tendon defects, or osteoarthritis [30]. hBM-MSC-EVs can promote cartilage regeneration through the stimulation of chondrocytes to produce proteoglycans and type II collagen [31]. hBM-MSC-EVs enhance tendon healing by stimulating tendon resident stem cells [32]. In addition, EVs derived from BM-MSCs contain miR-146a, which is a famous anti-inflammatory microRNA. miR-146a suppresses the transcriptional activity of the Nuclear Factor Kappa-light-chain-enhancer of activated B cells (NF-κB) pathway, reducing the expression of the downstream inflammatory factors IL-1β, IL-6, and TNF-α [33].
The therapeutic efficacy of human umbilical cord MSC-derived EVs (hUC-MSC-EVs) has been investigated in many diseases. hUC-MSC-EVs promote nerve regeneration and motor function in the injured areas [34]. Indeed, it has been proved that hUC-MSC-EVs play a neuroprotective role in perinatal brain damage [35,36]. hUC-MSC-EVs are also useful for cartilage injury since they can significantly improve cartilage repair through miRNA-181c-5p [37]. In hepatic pathologies, hUC-MSC-EVs are capable to reduce hepatic inflammation, collagen deposition, oxidative stress, and apoptosis in liver ischemia/reperfusion injury [38,39]. Moreover, hUC-MSC-EVs have shown positive effects on renal disease as they improve kidney function in grade III-IV patients [40].
EVs from human adipose tissue-derived mesenchymal stem cells (hAD-MSC-EVs) are effective in a wide range of diseases [41], mainly due to their beneficial properties for tissue repair, reduction in injuries, and antiinflammation. Additionally, this source has advantages in its high availability. Therefore, EVs from this source have a broad application prospect. In a study of osteoarthritis, hAD-MSC-EVs reduced the production of IL-6 and PGE2 [42]. Similarly, a study of allergic asthma showed a reduction in IL-5 levels in lung tissue treated with hAD-MSC-EVs [43]. In a study of atopic dermatitis, hAD-MSC-EVs reduced mRNA expression of various inflammatory cytokines such as IL-4, IL-23, IL-31, and TNF-α [44]. In the aspect of wound healing, hAD-MSC-EVs were able to increase the gene expression of N-cadherin, cyclin-1, Proliferating Cell Nuclear Antigen (PCNA), and collagen I, III [45,46].
Although performed using mouse AD-MSC-EVs, the following studies display the versatility of these EVs. In one study, AD-MSC-EVs administration proved to modulate the apoptotic function of mutant Huntingtin aggregates, thereby hampering Huntington’s disease (HD) progression [47]. In an Alzheimer’s disease (AD) mouse model, it was also revealed that AD-MSC-EVs reduced b-amyloid deposits and neuronal death [48]. Finally, we have recently shown the beneficial effect of young mice-derived AD-MSC-EVs for antiaging purposes as they recovered muscle and kidney functions [49].
On the other hand, EVs can also be isolated in vitro from neural cells (such as neurons, oligodendrocytes, microglia, and astrocytes) and immune cells (macrophages, dendritic cells, T cells, and natural killer cells) for therapy (Figure 1).
Neural cells including neurons, astrocytes, oligodendrocytes, and microglia release EVs for neural communication but are also involved in the propagation of various central nervous system (CNS) diseases [50,51,52]. However, cerebrospinal fluid (CSF) collection for selective isolation of neural EVs is quite challenging because of the invasive nature of the procedure and the small size of the obtained samples [53]. For instance, studies performed in animals have revealed that astrocytic EVs are enriched in miR-873a-5p, which inhibits ERK phosphorylation and the NF-κB signaling pathway, thus promoting the release of anti-inflammatory factors from microglia [54]. Similarly, microglial EVs-derived miR-124 promotes the activation of microglia in traumatic brain injured tissue during the acute stage, thus reducing the neuroinflammatory response and restoring neural function [55].
Depending on the parental cell type and activation status, immune cell-derived EVs can induce either immune-stimulatory or immune-suppressing responses and therefore can play dual roles in physiological and pathological processes. Regarding their immune-activating properties, both CD8+ T cell-derived EVs and dendritic cell-derived EVs are being used as antigen delivery tools to hamper tumor growth in metastatic melanoma and non-small cell lung cancer patients [56,57,58]. On the other hand, the immunosuppressive features of immune cell-derived EVs make them also suitable as therapeutic agents in transplantation tolerance and autoimmune diseases. As an example, immature dendritic cells-derived EVs reduce the expression of Major Histocompatibility Complex (MHC) molecules, which helps to prevent graft rejection in models of cardiac and intestinal transplantations [59,60,61]. Similarly, regulatory T lymphocyte-derived EVs have immunomodulatory effects that improve transplantation tolerance [62].
2.3. Body Fluids Versus Cell Cultures as Sources of EVs
The therapeutical potential of EVs is highly related to their uptake by target cells to achieve the desired outcomes. EVs obtained from different cell sources and donors may have different biological cargos, thus exerting different responses on recipient cells [63]. It has been demonstrated that EVs’ internal cargo has a similar pattern to the donor cell, suggesting a selective packaging of the cellular components into EVs [64]. More specially, superficial cargos play a role in EVs uptake. Indeed, specific molecules on the EVs surface might drive the cell-specific receptors for targeted EVs uptake [65]. Hence, the selection of an appropriate cell source could improve EVs targeted uptake.
The isolation of EVs directly from body fluids compared to cell culture media is more time-efficient, cost-efficient, and has relatively higher yields [66]. The choice of the EV isolation method significantly affects EV yield from blood samples, together with lipoprotein and protein contaminants [67]. Moreover, it has been reported that an increased yield is coupled with a short processing time of the sample [68]. Further, obtaining EVs from body fluids eliminates the need for exogenous cell culture media reagents that are not suitable for clinical-grade manufacturing. Indeed, cell culture media are prone to contamination and thus, contaminant removal must be addressed with an extra purification step [69]. On the opposite, in vitro cell culture facilitates EVs engineering to use them as drug delivery carriers. Lastly, the isolation of EVs from body fluids has some disadvantages, including a more heterogenous population and containing high levels of contaminants [27].
3. Modifications for Enhanced EVs Production and EVs’ Cargo Modulation
EVs large-scale production is a critical point for the translation from the bench to the bedside application of EV-based therapies. The “massivEVs” workshop announced the minimum requirements that must be reached towards EVs production in compliance with good manufacturing practices (GMPs), product validation, and regulatory issues [70].
For instance, using a 3D bioreactor was generally accepted as the most efficient method for the large-scale production of EVs from MSCs [71].
Different conditions can also be applied to promote EVs production [72]. For example, stimulation by shear stress [73], high-temperature [74], low oxygen tension [75], or ethanol [76] might increase EV production and shedding. However, the function of these EVs on recipient cells might be different. It has been reported that MSC culture in low oxygen conditions (1–5% O2) increases the number of EVs released and their cargo composition (growth factors, antioxidant enzymes, and miRNAs), thus enhancing their pro-angiogenic, immunomodulatory, anti-senescence, and antioxidant effects [77,78,79].
On top of that, therapeutic agents can be incorporated into EVs through the exogenous loading of drugs [80,81].
Exogenous loading of naive EVs isolated from body fluids or cultured cell media can be conducted by a variety of methods. Among these procedures, the most used are as follows:
- -
- Co-incubation with a drug at different temperatures [82,83]: EVs are incubated with a saturated solution of the desired drug which will diffuse inside EVs to equalize the concentrations. This method is relatively simple to scale up and enables to work of highly fragile therapeutic molecules, although it has a low loading efficiency.
- -
- Sonication of EVs with a drug mixture [84,85,86]: Treatment with ultrasounds produces transient pores in EV membranes that allow drug diffusion inside the vesicles. This method has a higher loading efficiency but might result in the destruction and inactivation of some therapeutic molecules.
- -
- Electroporation of EVs [87,88]: This technique is used to introduce siRNA and miRNA within EVs, but also anticancer drugs such as Paclitaxel, or antibiotics such as Doxycycline. Electroporation may result in the aggregation of RNA particles that will not be incorporated into EVs [89]. Therefore, the last step to ensure an efficient purification of loaded EVs from free RNA aggregates should be performed to obtain reliable and reproducible therapeutic effects.
- -
- Transient permeabilization of EVs membranes with saponin [90]: EVs are incubated with saponin to selectively remove membrane-bound cholesterol, creating transient pores in the EV membrane, thus promoting drug loading. This method is used for enzymes and other protein molecules.
Engineered EVs can be mass-produced by the fusion of the surface of EVs with lipid-based materials (DOTAP, POPC, DPPC, and POPG) using an extrusion technique to form a hybrid lipid membrane structure. Interestingly, this modification increased the number of released EVs from 6- to 43-fold, and their uptake by target cells (lung cancer cells A549) was 14-fold higher compared to non-engineered EVs [91]. Similarly, a recent study reported that both small and large EVs have very low uptake and fusion with target cells (fusogenic activity) that can be ameliorated using a fusogenic protein such as the glycoprotein G of the vesicular stomatitis virus (VSV-G) [92].
On the other hand, exogenous loading of parent cells is mainly performed by two methods: the loading of small therapeutic cargo into cultured parent cells, and the transfection of parent cells with therapeutics encoding DNA plasmids.
- -
- The first method consists of incubating the parent cells with small therapeutic agents, which will be incorporated by the cells and then included in the cargo of the released EVs. This method has been used for loading pancreatic adenocarcinoma cells in vitro with curcumin, which has known anticancer and anti-inflammatory properties [93]. The pros of this approach are that it allows the manufacturing of large batches of EV-based formulations. The cons include high amounts of the drug required for the loading of the parent cells, which is partially metabolized by the cells, thus, lowering the amount of drug loaded onto EVs, which may lead to reduced therapeutical effects.
- -
- The second method is the transfection of parent cells with plasmidic DNA encoding the desired therapeutic molecule, followed by the isolation of loaded EVs from the media. This method has been reported to load macrophages with plasmidic DNA encoding catalase. Loaded EVs collected from cell media displayed encapsulated plasmidic DNA, mRNA, and the encoded therapeutic active catalase [94].
In general, the production of exogenous drug loaded-EV formulations through parental cell modifications seems to be more reproducible than endogenous loading of naive EVs.
4. Methods to Prepare Clinical-Grade EVs
When facing EVs as a therapeutic agent, several key aspects must be addressed, such as their isolation methods, storage conditions, and administration route toward their clinical translation.
4.1. Isolation Methods
Many techniques have been developed for EVs isolation and have been extensively reviewed elsewhere [95,96,97,98]. These techniques are mostly based on EVs biophysical and/or biochemical characteristics, such as size, density, shape, or specific surface markers. It has been described that each isolation technique has the potential to affect the structural integrity and functional activity of EVs [99]. Therefore, the election of the most appropriate method to isolate EVs is of utmost importance for a deep understanding of their biological function before their use in clinics.
Isolation techniques are constantly being improved and combined to provide a strategy with high purity, high yield, and structural and functional integrity. Currently, the most frequently used EVs isolation techniques are briefly described below:
- Ultracentrifugation (UC): Differential ultracentrifugation, with or without density gradients, is the most used and was traditionally considered the “gold standard” technique for EVs isolation [99,100], which combines different densities and gradients with different centrifugal speeds and forces. Differential ultracentrifugation has some disadvantages, such as being not scalable, time-consuming, and resulting in impure EV preparations, which leads to EV aggregation. However, sequential ultracentrifugation has the great advantage that it can be used for large-scale production of clinical-grade MSC-EVs [101].
- Size Exclusion Chromatography (SEC): This method uses a chromatographic column with a porous stationary phase made of polymers. EVs are separated according to their size, which defines the time to elute from the column [102]. SEC has several advantages; among them, the highest purity is GMP-compliant and is a scalable system [103].
- Precipitation: Hydrophilic polymers, such as polyethylene glycol (PEG), are usually used as highly hydrophilic polymers that interact with surroundings to create a hydrophobic microenvironment, thus enabling the precipitation of the EVs [100,102,104,105]. Although the precipitation method has a lower purity and higher contamination, it has many advantages: low price, rapid EV extraction, and high yield, especially for large-scale applications.
- Ultrafiltration (UF): UF uses membranes with molecular weight cut-offs ranging from 10–100 kDa, which enables the separation of EVs according to their size. Ultrafiltration has a great advantage as it reduces isolation times and costs compared with other techniques [106,107]. However, UF has lower yields and purities due to the interaction between vesicles and the filtration membranes that creates aggregates that ultimately block the pores.
- Tangential Flow Filtration (TFF): TFF couples permeable membrane filtration and flow to obtain EVs from a colloid matrix. TFF enables the concentration of EVs from large volumes of samples in short periods (1 hour). Compared to UC, TFF has a higher yield, fewer aggregates, and better batch-to-batch consistency [108].
- Immunoaffinity capture: This technology is based on EVs membrane surface protein markers such as CD9, CD63, CD81, CD82, and other cell adhesion molecules [109]. Thus, this technique enables the separation of specific EVs subtypes. The main limitation of this method is that it can only detect surface markers, and the antibodies are expensive.
- Microfluidics: Microfluidic devices combine EVs isolation (size and affinity-based) and detection in miniaturized chips with the benefits of reduced time, high sensitivity, specificity, and high production [110,111]. They have some disadvantages such as highly complicated devices, expensive prices, and the need for specialized equipment.
It has been suggested that homogeneous populations of EVs would be safer [112]. Therefore, the isolation of a monodisperse EV population with a smaller size may improve EV uptake by recipient cells and its subsequent therapeutic effects. In addition, EV preparations may include different co-isolates (such as aggregates and precipitates) and exogenous contaminants related to the starting sample or the manufacturing process. Indeed, it has been highlighted that EV purity may not correlate with EV functionality because the co-isolates can contribute to the stability and biological activity of EVs [113,114]. Further, the presence of contaminants could affect EV uptake. Therefore, highly purified EVs appear to have preferential uptake by cells [115].
Concerning clinical applications of EVs, the major challenge is to isolate EVs with high yield and high purity, while preserving their intact structure and biological activity. Preferably, the isolation method should be scalable, cost-effective, and with a high-throughput production process [97]. As mentioned above, all the currently used isolation techniques differ in yield and purity [116]. These methods should be improved to obtain highly purified EVs for preferential uptake in the therapeutic context. In this regard, a combination of several extraction and purification methods should be considered.
4.2. Storage Methods
The storage of EVs is critical to preserve their stability and biological activity. There is still no consent regarding the best method to store EVs, although it is generally accepted that temperature, fluid characteristics, and freeze–thawing cycles deeply influence their conservation [117].
Some studies have reported that it is not practical to directly isolate EVs from a patient sample immediately after biofluid collection. Therefore, the biofluid storage method has also been addressed. Seminal plasma can be stored at -80 °C for short periods without significantly impacting EV yield or bioactivity [118,119]. Moreover, seminal EVs morphology, concentration, and size after short (2 years) and long-term freezing period (30 years) of semen at −80 °C was not affected [120]. Urine stored at −80 °C guaranteed effective preservation of urinal EVs, whereas storage at −20 °C resulted in a significant loss of EVs compared to fresh urine [121]. The storage of plasma at 4 °C, −20 °C, or −80 °C did not result in significant degradation of EV cargo [122]. Interestingly, it has been reported that storage of plasma at room temperature for over 42 h or at −80 °C for 12 years was not accompanied by a degradation of EVs RNA content [123]. BALF storage conditions can also destabilize the morphological features of EVs. The protein content is altered due to the dissociation of membrane-integrated proteins, rather than the loss of internal EV proteins [124].
Regarding EVs produce from in vitro cell cultures, culture media can be stored without altering EV concentration, which remained stable after one week of storage at 4 °C, −20 °C, and −80 °C. However, EV miRNA levels decreased during this period, especially when the media were stored at 4 °C and −20 °C. After 30 days of storage, the EV miRNA content dropped to below 50% compared to the initial amount [125,126].
On the other hand, other studies have addressed the impact of different storage conditions on isolated EVs. In general terms, storage at −80 °C is encouraged. It is accepted that storage at 4 °C and −20 °C can affect EV size and number, and cause EV aggregation [127]. The possibility to freeze-dry isolated EVs for long-term storage at room temperature has been addressed. Freeze-drying might help to preserve EV characteristics and function, and thus might offer a cost-effective storage strategy because it reduces transport costs [128]. In addition, EVs resuspension buffer is critical. Phosphate-buffered saline is the most used for EV resuspension. However, EV aggregation is an inherent consequence of storage at −80 °C which might alter their structure and biological function [39]. A colloidal solution with polymers could prevent the aggregation of EVs [40] and lead to the preservation of biological activity after their uptake [96]. Other possibilities include the addition of disaccharide stabilizers to the buffer to improve EV preservation, such as trehalose [129], mannitol [130], or dimethyl sulfoxide (DMSO) [126]. All three have proven to maintain EV integrity and function.
Taken together, for clinical applications, EVs should be suspended in sterile 0.9% NaCl and stored at −80 °C. EV products should be formulated for single-use because it has been observed that their number decreases and their morphology and content are altered after two cycles of freezing and thawing, which in turn affects their uptake by recipient cells [131]. Finally, independently of the storage formulation and conditions, batch stability will have to be carefully examined and monitored during storage [132].
4.3. Delivery Methods
The selection of the EV administration route is determined by the type of disease. For example, intra-articular injection is recommended for the treatment of osteoarthritic joints and periocular injection is preferred for retinal diseases. Nevertheless, EVs have the intrinsic capacity to cross the blood-brain barrier and can therefore be administered upon intravenous (IV), intraperitoneal (IP), subcutaneous (SC), or intranasal (IN) delivery to treat a broad range of CNS pathologies [133]. Many studies have reported that EVs can be successfully administered both systemically and locally and exert their effect at the desired place.
Systemically administered EVs have proven to successfully reach metastatic cells all over the organism [134], and their uptake was positively correlated with their dosage [135]. However, this route of administration has the inconvenience that EVs can also be uptaken by macrophages in the reticuloendothelial system, thereby promoting EVs clearance [136,137]. Therefore, EVs clearance by macrophages should be decreased to maximize their uptake by target cells and to ensure a high therapeutic effect. Systemic administration of EVs includes intravenous (IV) injection, subcutaneous (SC) injection, intranasal (IN) administration, and oral administration.
SC injection of EVs is the preferred route for wound healing applications. Indeed, SC injection of EVs effectively restored epidermal barrier function [138] and accelerated skin wound repair [139] in animal models.
IN administration of EVs has been used in animal models to treat epilepsy [140], Parkinson’s disease [141], and Alzheimer’s disease [142], among other neurological disorders. Recently, aerosol inhalation administration of EVs has been documented in various clinical trials as a treatment for COVID−19 disease [143,144,145] and other respiratory pathologies [146].
Milk-derived EVs, plant-derived EVS, and bacterial-derived EVs have the potential to cross the gastrointestinal tract barrier, which makes them promising delivery vehicles for orally administered drugs [147]. For instance, milk-EVs can remain unaltered in the gastric acidic environment thanks to their unique lipid composition and membrane rigidity [148,149]. In addition, this lipid bilayer of EVs protects the cargo from enzymatic degradation [150,151]. EVs are mainly absorbed through a transcellular pathway, but they can also be internalized through phagocytosis, pinocytosis, and endocytosis [152,153]. Once absorbed, they are transported by blood and/or lymph throughout the whole organism. Interestingly, orally delivered EVs also influence the immune cells in the digestive tract, which in turn induces local effects [154]. For instance, milk-EVs effects have only been tested in animal models [155].
Compared to systemic administration, local administration provides higher concentrations of EVs to the site of injury and increases EVs uptake by target cells, such as in the knee joint space. However, even faster clearance of EVs was observed and, therefore, repeated administration was needed to ensure the therapeutic effect in a rat model of osteochondral defects [156].
Taken together, it is of utmost importance to select the best administration route for each specific disease to ensure a successful treatment. Regarding clinical application, efficiency, ease of use, safety, and cost must be taken into consideration when choosing the route of administration.
5. Current Use of EVs in Clinical Trials and Human Diseases
For this review, we have analyzed all the clinical trials registered in clinicaltrials.gov in which the primary intervention is the treatment of a concrete disease or condition with EVs. In all described trials, EVs come from a specific cell type, tissue, or biological fluid, whether these cells or tissues have been modified or are being used as drug carriers. Thus, we have excluded trials that include the use of synthetic particles or liposomes that carry a specific drug or component. As this review focuses on the therapeutic properties of EVs, we have also excluded trials dedicated to the basic analysis of EVs or that use EVs as biomarkers [157].
Using a search that includes the words “extracellular vesicles”, “exosomes”, “microvesicles”, “ectosomes” and “microparticles”, we found a total of 383 registered clinical trials. We then identified 60 unique clinical trials that use therapy with EVs as the primary intervention (Table 1). Therefore, although the use of EVs as a therapeutic tool is rising, the number of studies that analyze EVs, use EVs as biomarkers or have them as a secondary measurement is much higher.

Table 1.
Registered clinical trials (clinicaltrials.gov) related to therapy with extracellular vesicles (extracellular vesicles/exosomes/microvesicles search) as the main intervention. ADSCs: Adipose-derived stem cells; ARDS: acute respiratory distress syndrome; ASO: anti-sense oligonucleotide; BMSCs: Bone marrow-derived stem cells.
The COVID-19 pandemic has shaped the way EVs therapy has developed in clinical trials, as 51 of these 60 trials have been registered in the last 4 years. This has biased the use of EVs as a treatment of COVID-19-related syndromes or lung pathologies, in part due to the absence of standard treatment for COVID-19 at the beginning of the pandemic. Easier recruitment of patients with lung pathologies, as well as hampered recruitment for other diseases, have made COVID-19 the predominant disease to treat in EVs human trials (Table 1). This is likely to change in the future, as the COVID-19 pandemic resolves and the application of EVs broadens to other conditions and organs.
5.1. Trial Status
Regarding trial status (Figure 2A), only nine trials have been completed; of these, three of them have had their results published in peer-reviewed journals. Two of the published results are safety studies of several doses of nebulized EVs. In the first one, the authors demonstrate the efficacy of inhaled EVs from human ADSCs in a preclinical model of lung injury and test the safety of one dose of 2 × 108 to 16 × 108 particles of EVs from hADSCs in healthy adults, which was well-tolerated in all volunteers [146]. In the second study, authors studied the safety of daily doses of 2 × 108 inhaled particles of EVs from hADSCs for 5 days in seven COVID-19 patients, with no evidence of adverse events [144]. The third published study is a randomized controlled clinical trial with 11 patients per group, that shows some efficacy of autologous platelet and EV-enriched plasma in the treatment of chronic temporal bone inflammation after surgery when compared to standard treatment [158]. Other completed clinical trials, with unpublished results, include two more studies on COVID-19, an ex vivo study of blood coagulation, a trial on the safety of intra-discal injections of plasma enriched with platelets and EVs for low back pain, an oral treatment with curcumin-loaded EVs from the ginger plant on inflammatory bowel disease, and a safety study of a topic ointment that includes EVs from MSCs. On the other hand, most of the studies are still recruiting patients or have not started the recruitment, and five of them have been suspended, mainly due to recruitment problems or lack of funding; it is important to highlight that none of the studies have found safety-related issues.
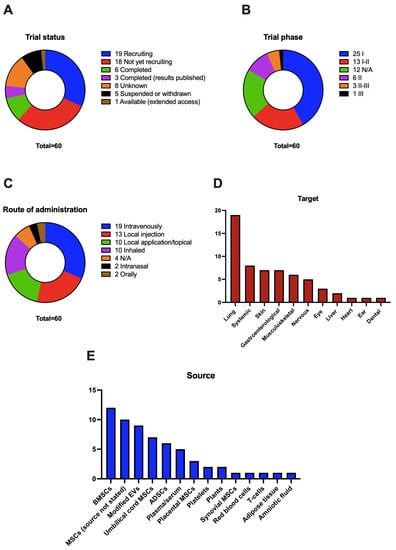
Figure 2.
Graphical representation of the analysis of Table 1 results regarding ongoing and finalized clinical trials that use EVs therapy as the main intervention. (A): Trial status defined by clinicaltrials.gov. (B): Trial phase defined by clinicaltrials.gov, when the phase is not available or is not applicable N/A is shown. (C): Route of administration of the EV preparation, when not stated N/A is shown. (D): Organs or systems related to the primary outcome measured in the trial. (E): Source of the EVs used in the trial.
5.2. Trial Phase
When analyzing the trial phase (Figure 2B), it becomes evident that we are still in the initial stage of EVs therapeutics, as 60% of the studies are phase I or I-IIa and include a small number of individuals, mostly related to the safety of the treatments, and just some of them use a small comparator group. There is only one study in phase III (NCT05354141), approved in April 2022, that will involve 400 patients with COVID-19-related ARDS; patients will be treated intravenously with 1,2 × 109 EVs from bone marrow MSCs. This study is based on two earlier trials that have been carried out by the same company. Three other trials have some characteristics of a phase III study, that will focus on the treatment of moderate COVID-19 (NCT05216562), retinitis pigmentosa (NCT05413148), and chronic middle ear infections (NCT04761562).
5.3. Route of Administration
In terms of the route of administration (Figure 2C), intravenously is the preferred one, with 30% of the studies using it. This approach has some advantages, it is widely used in preclinical models of diseases, and, as we are yet to determine the mechanism of action and delivery to target organs of EVs, IV injection secures a systemic distribution of EVs [159]. Still, the use of IV injections in humans is tightly regulated compared to other topical routes. In the second place comes the use of local injections in the desired target, such as intra-articular or epineural injections. The main advantage of this proposal is that it secures the arrival of EVs to the intended site and minimizes the secondary effects on other tissues. However, some of the organs or tissues, such as the brain, are difficult to reach, and these injections may in part miss the beneficial systemic effects of EVs. Local or topical application to an accessible tissue such as the skin is another interesting approach, mainly for skin regeneration and immune-mediated diseases, which can maximize the effect on the skin while reducing systemic absorption. We still need more studies on the degree of penetration of EVs in wounds and intact skin and how this affects its therapeutic effects [160,161]. The COVID-19 pandemic has increased the number of studies that use the inhaled route for EVs administration. This route seems to be ideal for lung diseases, as EVs can be included in aerosol liquid and delivered to the bronchi and alveolus, to exert their therapeutic effect [162,163]. It is of interest to note the oral administration of EVs; there are only two trials that use this route, but this may be an appealing approach to treat gastroenterological diseases, such as inflammatory bowel disease [164]. Indeed, some studies have highlighted the potential of oral-administered extracellular vesicles to treat systemic diseases, as these vesicles can reach the circulation and distant organs such as the liver or spleen [154,158].
5.4. Targeted Tissues and Diseases
Many different diseases are being studied in clinical trials using EVs therapeutics; we have divided trials into the organs or tissues intended to treat (Figure 2D). Currently, lung diseases are the most common in trials, as we have stated before. The COVID-19 pandemic has boosted trials in this field, being the most studied disease. Other lung diseases studied include ARDS and non-COVID-19 pulmonary infections. We have included systemic affections as the second most studied ones, referring to conditions such as solid organ transplantation, metastatic cancer, familial hypercholesterolemia, or intensively ill children. Skin is the third most studied tissue in these trials. The main application is for wound healing and regeneration, appearing from different origins, such as burn wounds or venous trophic lesions, and immune-mediated diseases such as psoriasis. Along with the skin, gastroenterological diseases are the third most studied condition, with trials mainly focusing on inflammatory bowel disease, including Crohn’s and ulcerative colitis, and its complications, mainly perianal fistula. The clinical research on musculoskeletal conditions includes osteoarthritis, bone defects, or meniscal injury. Regarding the nervous system, clinical trials include diverse conditions, such as Alzheimer’s, depression, neuralgia, or stroke. To conclude, other least studied tissues and organs include disorders ranging from macular holes and retinitis pigmentosa in the eye to myocardial infarction or middle ear infections.
5.5. Source of EVs
The source of choice to obtain the EVs for most of the studies are MSCs from different tissues, mainly bone marrow, umbilical cord, and adipose tissue (Figure 2E). The experience in the clinical research environment with these cells—leading to the development of clinical grade, ready-to-use cells—along with their regenerative and immunomodulatory properties, with a lower chance of immune rejection, has shifted the use of whole cells to concrete factors released by these cells such as EVs. EVs from MSCs have shown great potential in preclinical trials, mainly improving the regenerative potential of the tissues, and serving as anti-inflammatory and immunomodulatory factors. Plasma or serum, and other biological fluids, are also being used in clinical trials as a source of EVs, however, the use of these fluids makes it more challenging to obtain a pure preparation of EVs. Of particular interest is the use of modified EVs and whether these modifications affect the cells of origin or the vesicles themselves; these modifications aim to improve the therapeutic power of the EV preparation. The most used modification is loading the vesicles with some drugs or factors; in the first registered study, researchers tried to use EVs from erythrocytes loaded with methotrexate to treat malignant ascites (NCT03230708). Another study with preclinical evidence [101,165] is using EVs from BMSCs loaded with a siRNA directed to G12D mutated KRAS to treat metastatic pancreatic cancer (NCT0308631). EVs from MSCs loaded with miR-124 are being studied for the treatment of ischemic stroke [166] (NCT03384433). Three trials using EVs from cells that overexpress the CD24 receptor to treat COVID-19-related conditions (NCT04747574, NCT04902183, NCT04969172). Researchers will use EVs from BMSCs loaded with LDL receptor mRNA to treat familial hypercholesterolemia [167] (NCT05043181). Ultimately, a recent study will study the effect of EVs from HEK-293 cells loaded with an antisense oligonucleotide directed to STAT6 in advanced hepatocellular carcinoma and metastasis from gastric and colorectal cancer [168] (NCT05375604).
6. Conclusions
The field of EVs has experienced an unprecedented rise in the last decade [169]. Originally seen as merely cellular waste products, these information carriers are being exhaustively studied in cell biology and intercellular communication [170]. We are still learning the basic cellular processes that give place to EVs [7], however, their application in many diseases is rapidly growing—even faster than our knowledge of the basic biology of EVs. Their particular mechanism of action, carrying molecules from the original cells that can be detected in systemic fluids such as plasma, has led to the study of EVs as diagnostic tools and biomarkers for many diseases, including the development of liquid biopsies based on EVs analysis [171].
Due to their innate ability to reach different tissues and cells, these vesicles are excellent drug carriers that can deliver these drugs, for example, to cancerous cells [172]. However, EVs can have a therapeutic effect themselves, especially those coming from the stem or progenitor cells [48]. This has led to the development of multiple vesicle-based therapies targeting diseases affecting humans. Results in preclinical models of these diseases have been generally encouraging, especially in fields such as regenerative medicine and immune diseases, as these vesicles appear to have pro-regenerative and immunomodulatory properties [173], as well as in cancer [174], given the vesicles’ tropism for cancer tissues [175]. There are currently multiple companies developing EVs for clinical use, and there are already several clinical trials testing the safety and efficacy of EVs in various conditions (Table 1 and Figure 2).
Nonetheless, it must be said that we are still at a very early stage, as most of the studies are in early phases, and there is not a single completed phase III study. For the moment, the disease that is the target of the largest number of trials is COVID-19, partly due to the ease of testing new therapies for this disease, and the greater funding it has received in recent years. It is to be expected that in the coming years, the number of studies in this regard will decrease, increasing the number of studies related to other diseases.
As for the preferred route of administration, at the moment it is intravenously, closely followed by the local administration at the site of interest, either by injection or topical treatment. Much research remains to be done on the optimal methods for isolating, storing, and delivery of the vesicles, as well as the preferred dose. Due to the effort needed for isolating EVs, few studies perform a comprehensive dose-response study, and even fewer in humans, so these studies need to be developed to find out the optimal methods and doses to obtain clinical-grade EVs and achieve success in clinical trials to make EVs the medicine of tomorrow.
Author Contributions
Conceptualization, figure preparation, and writing—original draft preparation, J.S.-R. and C.M.-B.; writing—review and editing, C.B.; visualization and supervision, N.R.-G., J.H.-A. and M.D.; supervision, C.B.; funding acquisition, C.M.-B. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the following grants: Grant PID2020–113839RB-I00 funded by MCIN/AEI/10.13039/501100011033, PCIN-2017–117 of the Ministry of Economy and Competitiveness, and the EU Joint Programming Initiative “A Healthy Diet for a Healthy Life” (JPI HDHL INTIMIC-085) to C.B., and CIGE/2021/134 from Conselleria de Innovación, Universidades, Ciencia y Sociedad Digital to C.M.-B. Part of the equipment employed in this work has been funded by Generalitat Valenciana and co-financed with ERDF funds (OP ERDF of Comunitat Valenciana 2014–2020).
Acknowledgments
Figures and graphs were created using BioRender and GraphPad Prism 9.0.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Hristov, M.; Erl, W.; Linder, S.; Weber, P.C. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 2004, 104, 2761–2766. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Ostrowski, M.; Segura, E. Membrane vesicles as conveyors of immune responses. Nat. Rev. Immunol. 2009, 9, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Zöller, M. Tetraspanins: Push and pull in suppressing and promoting metastasis. Nat. Rev. Cancer 2009, 9, 40–55. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell-cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; Del Portillo, H.A.; et al. Applying extracellular vesicles based therapeutics in clinical trials—An ISEV position paper. J. Extracell Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Reiner, A.T.; Witwer, K.W.; van Balkom, B.W.M.; de Beer, J.; Brodie, C.; Corteling, R.L.; Gabrielsson, S.; Gimona, M.; Ibrahim, A.G.; de Kleijn, D.; et al. Concise Review: Developing Best-Practice Models for the Therapeutic Use of Extracellular Vesicles. Stem Cells Transl. Med. 2017, 6, 1730–1739. [Google Scholar] [CrossRef]
- Witwer, K.W.; Van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; De Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef]
- Claridge, B.; Lozano, J.; Poh, Q.H.; Greening, D.W. Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front. Cell Dev. Biol. 2021, 9, 734720. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Caro, H.; Dragovic, R.; Shen, M.; Dombi, E.; Mounce, G.; Field, K.; Meadows, J.; Turner, K.; Lunn, D.; Child, T.; et al. In vitro decidualisation of human endometrial stromal cells is enhanced by seminal fluid extracellular vesicles. J. Extracell Vesicles 2019, 8, 1565262. [Google Scholar] [CrossRef] [PubMed]
- Sun, I.O.; Lerman, L.O. Urinary Extracellular Vesicles as Biomarkers of Kidney Disease: From Diagnostics to Therapeutics. Diagnostics 2020, 10, 311. [Google Scholar] [CrossRef]
- Erdbrugger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borras, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcon-Perez, J.M.; et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Morrow, A.L.; Newburg, D.S. Human Milk Extracellular Vesicles: A Biological System with Clinical Implications. Cells 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Arslanoglu, S.; Boquien, C.Y.; King, C.; Lamireau, D.; Tonetto, P.; Barnett, D.; Bertino, E.; Gaya, A.; Gebauer, C.; Grovslien, A.; et al. Fortification of Human Milk for Preterm Infants: Update and Recommendations of the European Milk Bank Association (EMBA) Working Group on Human Milk Fortification. Front. Pediatr. 2019, 7, 76. [Google Scholar] [CrossRef]
- Parker, M.G.; Stellwagen, L.M.; Noble, L.; Kim, J.H.; Poindexter, B.B.; Puopolo, K.M.; Section on Breastfeeding, Committee on Nutrition, Committee on Fetus and Newborn. Promoting Human Milk and Breastfeeding for the Very Low Birth Weight Infant. Pediatrics 2021, 148, 54272. [Google Scholar] [CrossRef]
- Guo, M.M.; Zhang, K.; Zhang, J.H. Human Breast Milk-Derived Exosomal miR-148a-3p Protects Against Necrotizing Enterocolitis by Regulating p53 and Sirtuin 1. Inflammation 2022, 45, 1254–1268. [Google Scholar] [CrossRef]
- Yenuganti, V.R.; Afroz, S.; Khan, R.A.; Bharadwaj, C.; Nabariya, D.K.; Nayak, N.; Subbiah, M.; Chintala, K.; Banerjee, S.; Reddanna, P.; et al. Milk exosomes elicit a potent anti-viral activity against dengue virus. J. Nanobiotechnol. 2022, 20, 317. [Google Scholar] [CrossRef]
- Reif, S.; Elbaum Shiff, Y.; Golan-Gerstl, R. Milk-derived exosomes (MDEs) have a different biological effect on normal fetal colon epithelial cells compared to colon tumor cells in a miRNA-dependent manner. J. Transl. Med. 2019, 17, 325. [Google Scholar] [CrossRef]
- Zareba, L.; Szymanski, J.; Homoncik, Z.; Czystowska-Kuzmicz, M. EVs from BALF-Mediators of Inflammation and Potential Biomarkers in Lung Diseases. Int. J. Mol. Sci. 2021, 22, 3651. [Google Scholar] [CrossRef] [PubMed]
- Qazi, K.R.; Torregrosa Paredes, P.; Dahlberg, B.; Grunewald, J.; Eklund, A.; Gabrielsson, S. Proinflammatory exosomes in bronchoalveolar lavage fluid of patients with sarcoidosis. Thorax 2010, 65, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Wahlund, C.J.E.; Gucluler Akpinar, G.; Steiner, L.; Ibrahim, A.; Bandeira, E.; Lepzien, R.; Lukic, A.; Smed-Sorensen, A.; Kullberg, S.; Eklund, A.; et al. Sarcoidosis exosomes stimulate monocytes to produce pro-inflammatory cytokines and CCL2. Sci. Rep. 2020, 10, 15328. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhang, S.; Du, X.; Zhang, W.; Jing, R.; Wang, X.; Pan, L. RhoA inhibitor suppresses the production of microvesicles and rescues high ventilation induced lung injury. Int. Immunopharmacol. 2019, 72, 74–81. [Google Scholar] [CrossRef]
- Paredes, P.T.; Esser, J.; Admyre, C.; Nord, M.; Rahman, Q.K.; Lukic, A.; Radmark, O.; Gronneberg, R.; Grunewald, J.; Eklund, A.; et al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy 2012, 67, 911–919. [Google Scholar] [CrossRef]
- Heim, M.U.; Meyer, B.; Hellstern, P. Recommendations for the use of therapeutic plasma. Curr. Vasc. Pharm. 2009, 7, 110–119. [Google Scholar] [CrossRef]
- Iannotta, D.; Yang, M.; Celia, C.; Di Marzio, L.; Wolfram, J. Extracellular vesicle therapeutics from plasma and adipose tissue. Nano Today 2021, 39, 101195. [Google Scholar] [CrossRef]
- Vicencio, J.M.; Yellon, D.M.; Sivaraman, V.; Das, D.; Boi-Doku, C.; Arjun, S.; Zheng, Y.; Riquelme, J.A.; Kearney, J.; Sharma, V.; et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 2015, 65, 1525–1536. [Google Scholar] [CrossRef]
- Shi, G.; Wang, Y.; Wang, Z.; Thoreson, A.R.; Jacobson, D.S.; Amadio, P.C.; Behfar, A.; Moran, S.L.; Zhao, C. A novel engineered purified exosome product patch for tendon healing: An explant in an ex vivo model. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2021, 39, 1825–1837. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Roelofs, A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharm. 2018, 40, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Vonk, L.A.; van Dooremalen, S.F.J.; Liv, N.; Klumperman, J.; Coffer, P.J.; Saris, D.B.F.; Lorenowicz, M.J. Mesenchymal Stromal/stem Cell-derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics 2018, 8, 906–920. [Google Scholar] [CrossRef]
- Shi, Z.; Wang, Q.; Jiang, D. Extracellular vesicles from bone marrow-derived multipotent mesenchymal stromal cells regulate inflammation and enhance tendon healing. J. Transl. Med. 2019, 17, 211. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Fan, H.; Shou, Z.; Xu, M.; Chen, Q.; Ai, C.; Dong, Y.; Liu, Y.; Nan, Z.; Wang, Y.; et al. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int. Immunopharmacol. 2019, 68, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, L.; Zhou, D.; Li, L.; Zhang, W.; Zhen, Y.; Wang, T.; Su, J.; Chen, D.; Mao, C.; et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J. Cell. Mol. Med. 2019, 23, 2822–2835. [Google Scholar] [CrossRef]
- Joerger-Messerli, M.S.; Oppliger, B.; Spinelli, M.; Thomi, G.; di Salvo, I.; Schneider, P.; Schoeberlein, A. Extracellular Vesicles Derived from Wharton’s Jelly Mesenchymal Stem Cells Prevent and Resolve Programmed Cell Death Mediated by Perinatal Hypoxia-Ischemia in Neuronal Cells. Cell Transplant. 2018, 27, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Thomi, G.; Surbek, D.; Haesler, V.; Joerger-Messerli, M.; Schoeberlein, A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res. Ther. 2019, 10, 105. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, L.; Zou, S.; Feng, Y.; Miao, X.; Huang, L.; Wu, Y. Human Umbilical Cord Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying MicroRNA-181c-5p Promote BMP2-Induced Repair of Cartilage Injury through Inhibition of SMAD7 Expression. Stem. Cells Int. 2022, 2022, 1157498. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, T.; Zhou, C.; Cai, J.; Zhang, X.; Liang, J.; Sui, X.; Chen, X.; Chen, L.; Sun, Y.; et al. Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Protect Liver Ischemia/Reperfusion Injury by Reducing CD154 Expression on CD4+ T Cells via CCT2. Adv. Sci. (Weinh) 2020, 7, 1903746. [Google Scholar] [CrossRef]
- Jiang, W.; Tan, Y.; Cai, M.; Zhao, T.; Mao, F.; Zhang, X.; Xu, W.; Yan, Z.; Qian, H.; Yan, Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl(4)-Induced Liver Injury through Antioxidant Effect. Stem. Cells Int. 2018, 2018, 6079642. [Google Scholar] [CrossRef]
- Nassar, W.; El-Ansary, M.; Sabry, D.; Mostafa, M.A.; Fayad, T.; Kotb, E.; Temraz, M.; Saad, A.N.; Essa, W.; Adel, H. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomater. Res. 2016, 20, 21. [Google Scholar] [CrossRef]
- Hong, P.; Yang, H.; Wu, Y.; Li, K.; Tang, Z. The functions and clinical application potential of exosomes derived from adipose mesenchymal stem cells: A comprehensive review. Stem Cell Res. Ther. 2019, 10, 242. [Google Scholar] [CrossRef]
- Tofino-Vian, M.; Guillen, M.I.; Perez Del Caz, M.D.; Castejon, M.A.; Alcaraz, M.J. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxidative Med. Cell. Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef] [PubMed]
- de Castro, L.L.; Xisto, D.G.; Kitoko, J.Z.; Cruz, F.F.; Olsen, P.C.; Redondo, P.A.G.; Ferreira, T.P.T.; Weiss, D.J.; Martins, M.A.; Morales, M.M.; et al. Human adipose tissue mesenchymal stromal cells and their extracellular vesicles act differentially on lung mechanics and inflammation in experimental allergic asthma. Stem Cell Res. Ther. 2017, 8, 151. [Google Scholar] [CrossRef]
- Cho, B.S.; Kim, J.O.; Ha, D.H.; Yi, Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wang, J.; Zhou, X.; Xiong, Z.; Zhao, J.; Yu, R.; Huang, F.; Zhang, H.; Chen, L. Exosomes derived from human adipose mensenchymal stem cells accelerates cutaneous wound healing via optimizing the characteristics of fibroblasts. Sci. Rep. 2016, 6, 32993. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Zhou, X.; Xiong, Z.; Zhang, C.; Shehada, H.M.A.; Hu, B.; Song, J.; Chen, L. Exosomes secreted by human adipose mesenchymal stem cells promote scarless cutaneous repair by regulating extracellular matrix remodelling. Sci. Rep. 2017, 7, 13321. [Google Scholar] [CrossRef]
- Lee, M.; Ban, J.J.; Kim, K.Y.; Jeon, G.S.; Im, W.; Sung, J.J.; Kim, M. Adipose-derived stem cell exosomes alleviate pathology of amyotrophic lateral sclerosis in vitro. Biochem. Biophys. Res. Commun. 2016, 479, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, T.; Oki, K.; Ochiya, T. Potential application of extracellular vesicles of human adipose tissue-derived mesenchymal stem cells in Alzheimer’s disease therapeutics. Methods Mol. Biol. 2015, 1212, 171–181. [Google Scholar]
- Sanz-Ros, J.; Romero-Garcia, N.; Mas-Bargues, C.; Monleon, D.; Gordevicius, J.; Brooke, R.T.; Dromant, M.; Diaz, A.; Derevyanko, A.; Guio-Carrion, A.; et al. Small extracellular vesicles from young adipose-derived stem cells prevent frailty, improve health span, and decrease epigenetic age in old mice. Sci. Adv. 2022, 8, eabq2226. [Google Scholar] [CrossRef]
- Sharma, P.; Schiapparelli, L.; Cline, H.T. Exosomes function in cell-cell communication during brain circuit development. Curr. Opin. Neurobiol. 2013, 23, 997–1004. [Google Scholar] [CrossRef]
- Sharma, P.; Mesci, P.; Carromeu, C.; McClatchy, D.R.; Schiapparelli, L.; Yates, J.R., 3rd; Muotri, A.R.; Cline, H.T. Exosomes regulate neurogenesis and circuit assembly. Proc. Natl. Acad. Sci. USA 2019, 116, 16086–16094. [Google Scholar] [CrossRef] [PubMed]
- Watson, L.S.; Hamlett, E.D.; Stone, T.D.; Sims-Robinson, C. Neuronally derived extracellular vesicles: An emerging tool for understanding Alzheimer’s disease. Mol. Neurodegener 2019, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Akers, J.C.; Ramakrishnan, V.; Yang, I.; Hua, W.; Mao, Y.; Carter, B.S.; Chen, C.C. Optimizing preservation of extracellular vesicular miRNAs derived from clinical cerebrospinal fluid. Cancer Biomark 2016, 17, 125–132. [Google Scholar] [CrossRef]
- Long, X.; Yao, X.; Jiang, Q.; Yang, Y.; He, X.; Tian, W.; Zhao, K.; Zhang, H. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J. Neuroinflamm. 2020, 17, 89. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.; Kong, C.; Su, X.; Zhang, X.; Bai, W.; He, X. MiR-124 Enriched Exosomes Promoted the M2 Polarization of Microglia and Enhanced Hippocampus Neurogenesis After Traumatic Brain Injury by Inhibiting TLR4 Pathway. Neurochem. Res. 2019, 44, 811–828. [Google Scholar] [CrossRef]
- Seo, N.; Shirakura, Y.; Tahara, Y.; Momose, F.; Harada, N.; Ikeda, H.; Akiyoshi, K.; Shiku, H. Activated CD8(+) T cell extracellular vesicles prevent tumour progression by targeting of lesional mesenchymal cells. Nat. Commun. 2018, 9, 435. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; Andre, F.; Caby, M.P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
- Morse, M.A.; Garst, J.; Osada, T.; Khan, S.; Hobeika, A.; Clay, T.M.; Valente, N.; Shreeniwas, R.; Sutton, M.A.; Delcayre, A.; et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 2005, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Yang, J.Y.; Song, W.J.; Ding, R.; Zhang, Z.C.; Ji, H.C.; Zhang, X.; Wang, J.L.; Yang, X.S.; Tao, K.S.; et al. Combining Exosomes Derived from Immature DCs with Donor Antigen-Specific Treg Cells Induces Tolerance in a Rat Liver Allograft Model. Sci. Rep. 2016, 6, 32971. [Google Scholar] [CrossRef]
- Li, X.; Li, J.J.; Yang, J.Y.; Wang, D.S.; Zhao, W.; Song, W.J.; Li, W.M.; Wang, J.F.; Han, W.; Zhang, Z.C.; et al. Tolerance induction by exosomes from immature dendritic cells and rapamycin in a mouse cardiac allograft model. PLoS ONE 2012, 7, e44045. [Google Scholar] [CrossRef]
- Yang, X.; Meng, S.; Jiang, H.; Zhu, C.; Wu, W. Exosomes derived from immature bone marrow dendritic cells induce tolerogenicity of intestinal transplantation in rats. J. Surg. Res. 2011, 171, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Aiello, S.; Rocchetta, F.; Longaretti, L.; Faravelli, S.; Todeschini, M.; Cassis, L.; Pezzuto, F.; Tomasoni, S.; Azzollini, N.; Mister, M.; et al. Extracellular vesicles derived from T regulatory cells suppress T cell proliferation and prolong allograft survival. Sci. Rep. 2017, 7, 11518. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.P.; Kim, E.Y.; Badr, C.E.; Weissleder, R.; Mempel, T.R.; Tannous, B.A.; Breakefield, X.O. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun. 2015, 6, 7029. [Google Scholar] [CrossRef] [PubMed]
- Chiou, N.T.; Kageyama, R.; Ansel, K.M. Selective Export into Extracellular Vesicles and Function of tRNA Fragments during T Cell Activation. Cell Rep. 2018, 25, 3356–3370.e4. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, W.; Freeman, M.L.; Lederman, M.M.; Vasilieva, E.; Romero, R.; Margolis, L. A System of Cytokines Encapsulated in ExtraCellular Vesicles. Sci. Rep. 2018, 8, 8973. [Google Scholar] [CrossRef]
- Tian, M.; Ticer, T.; Wang, Q.; Walker, S.; Pham, A.; Suh, A.; Busatto, S.; Davidovich, I.; Al-Kharboosh, R.; Lewis-Tuffin, L.; et al. Adipose-Derived Biogenic Nanoparticles for Suppression of Inflammation. Small 2020, 16, e1904064. [Google Scholar] [CrossRef]
- Brennan, K.; Martin, K.; FitzGerald, S.P.; O’Sullivan, J.; Wu, Y.; Blanco, A.; Richardson, C.; Mc Gee, M.M. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020, 10, 1039. [Google Scholar] [CrossRef]
- Lobb, R.J.; Becker, M.; Wen, S.W.; Wong, C.S.; Wiegmans, A.P.; Leimgruber, A.; Moller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Staubach, S.; Bauer, F.N.; Tertel, T.; Borger, V.; Stambouli, O.; Salzig, D.; Giebel, B. Scaled preparation of extracellular vesicles from conditioned media. Adv. Drug Deliv. Rev. 2021, 177, 113940. [Google Scholar] [CrossRef]
- Paolini, L.; Monguió-Tortajada, M.; Costa, M.; Antenucci, F.; Barilani, M.; Clos-Sansalvador, M.; Andrade, A.C.; Driedonks, T.A.P.; Giancaterino, S.; Kronstadt, S.M.; et al. Large-scale production of extracellular vesicles: Report on the “massivEVs” ISEV workshop. J. Extracell. Biol. 2022, 1, e63. [Google Scholar] [CrossRef]
- Bellani, C.F.; Ajeian, J.; Duffy, L.; Miotto, M.; Groenewegen, L.; Connon, C.J. Scale-Up Technologies for the Manufacture of Adherent Cells. Front. Nutr. 2020, 7, 575146. [Google Scholar] [CrossRef] [PubMed]
- Syromiatnikova, V.; Prokopeva, A.; Gomzikova, M. Methods of the Large-Scale Production of Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 10522. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.B.; Santoro, M.; Born, L.J.; Fisher, J.P.; Jay, S.M. Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: Impact of the bioproduction microenvironment. Biotechnol Adv. 2018, 36, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Yamamoto, Y.; Ochiya, T. Uncovering temperature-dependent extracellular vesicle secretion in breast cancer. J. Extracell Vesicles 2020, 10, e12049. [Google Scholar] [CrossRef]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef]
- Patel, D.B.; Luthers, C.R.; Lerman, M.J.; Fisher, J.P.; Jay, S.M. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta. Biomater 2019, 95, 236–244. [Google Scholar] [CrossRef]
- Cui, G.H.; Wu, J.; Mou, F.F.; Xie, W.H.; Wang, F.B.; Wang, Q.L.; Fang, J.; Xu, Y.W.; Dong, Y.R.; Liu, J.R.; et al. Exosomes derived from hypoxia-preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 654–668. [Google Scholar] [CrossRef]
- Xue, C.; Shen, Y.; Li, X.; Li, B.; Zhao, S.; Gu, J.; Chen, Y.; Ma, B.; Wei, J.; Han, Q.; et al. Exosomes Derived from Hypoxia-Treated Human Adipose Mesenchymal Stem Cells Enhance Angiogenesis Through the PKA Signaling Pathway. Stem Cells Dev. 2018, 27, 456–465. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Sanz-Ros, J.; Roman-Dominguez, A.; Gimeno-Mallench, L.; Ingles, M.; Vina, J.; Borras, C. Extracellular Vesicles from Healthy Cells Improves Cell Function and Stemness in Premature Senescent Stem Cells by miR-302b and HIF-1alpha Activation. Biomolecules 2020, 10, 957. [Google Scholar] [CrossRef]
- Xi, X.M.; Xia, S.J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. Pharmazie 2021, 76, 61–67. [Google Scholar]
- Klyachko, N.L.; Arzt, C.J.; Li, S.M.; Gololobova, O.A.; Batrakova, E.V. Extracellular Vesicle-Based Therapeutics: Preclinical and Clinical Investigations. Pharmaceutics 2020, 12, 1171. [Google Scholar] [CrossRef] [PubMed]
- O’Loughlin, A.J.; Mager, I.; de Jong, O.G.; Varela, M.A.; Schiffelers, R.M.; El Andaloussi, S.; Wood, M.J.A.; Vader, P. Functional Delivery of Lipid-Conjugated siRNA by Extracellular Vesicles. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Meng, Y.; Wang, B.; Wang, C.X.; Hou, C.X.; Zhu, Q.H.; Tang, Y.T.; Ye, J.H. In vitro experimental study on the formation of microRNA-34a loaded exosomes and their inhibitory effect in oral squamous cell carcinoma. Cell Cycle 2022, 21, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Wu, J.Y.; Wang, J.M.; Hu, X.B.; Cai, J.X.; Xiang, D.X. Gemcitabine loaded autologous exosomes for effective and safe chemotherapy of pancreatic cancer. Acta Biomater. 2020, 101, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Yuan, D.; Deygen, I.; Klyachko, N.L.; Kabanov, A.V.; Batrakova, E.V. Engineering macrophage-derived exosomes for targeted paclitaxel delivery to pulmonary metastases: In vitro and in vivo evaluations. Nanomedicine 2018, 14, 195–204. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Jeyaram, A.; Patel, D.B.; Parajuli, B.; Livingston, N.K.; Arumugasaamy, N.; Schardt, J.S.; Jay, S.M. Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cell Mol. Bioeng. 2016, 9, 315–324. [Google Scholar] [CrossRef]
- Pomatto, M.A.C.; Bussolati, B.; D’Antico, S.; Ghiotto, S.; Tetta, C.; Brizzi, M.F.; Camussi, G. Improved Loading of Plasma-Derived Extracellular Vesicles to Encapsulate Antitumor miRNAs. Mol. Methods Clin. Dev. 2019, 13, 133–144. [Google Scholar] [CrossRef]
- Wan, Z.; Gan, X.; Mei, R.; Du, J.; Fan, W.; Wei, M.; Yang, G.; Qin, W.; Zhu, Z.; Liu, L. ROS triggered local delivery of stealth exosomes to tumors for enhanced chemo/photodynamic therapy. J. Nanobiotechnol. 2022, 20, 385. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; de Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release Off. J. Control. Release Soc. 2013, 172, 229–238. [Google Scholar] [CrossRef]
- Thakur, A.; Sidu, R.K.; Zou, H.; Alam, M.K.; Yang, M.; Lee, Y. Inhibition of Glioma Cells’ Proliferation by Doxorubicin-Loaded Exosomes via Microfluidics. Int. J. Nanomed. 2020, 15, 8331–8343. [Google Scholar] [CrossRef]
- Jhan, Y.Y.; Prasca-Chamorro, D.; Palou Zuniga, G.; Moore, D.M.; Arun Kumar, S.; Gaharwar, A.K.; Bishop, C.J. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int. J. Pharm. 2020, 573, 118802. [Google Scholar] [CrossRef]
- Hirose, H.; Hirai, Y.; Sasaki, M.; Sawa, H.; Futaki, S. Quantitative Analysis of Extracellular Vesicle Uptake and Fusion with Recipient Cells. Bioconjug Chem 2022, 33, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Osterman, C.J.; Lynch, J.C.; Leaf, P.; Gonda, A.; Ferguson Bennit, H.R.; Griffiths, D.; Wall, N.R. Curcumin Modulates Pancreatic Adenocarcinoma Cell-Derived Exosomal Function. PLoS ONE 2015, 10, e0132845. [Google Scholar] [CrossRef]
- Haney, M.J.; Zhao, Y.; Harrison, E.B.; Mahajan, V.; Ahmed, S.; He, Z.; Suresh, P.; Hingtgen, S.D.; Klyachko, N.L.; Mosley, R.L.; et al. Specific transfection of inflamed brain by macrophages: A new therapeutic strategy for neurodegenerative diseases. PLoS ONE 2013, 8, e61852. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Alini, M.; Baghaban Eslaminejad, M.; Hosseini, S. Engineering strategies for customizing extracellular vesicle uptake in a therapeutic context. Stem Cell Res. Ther. 2022, 13, 129. [Google Scholar] [CrossRef]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noel, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng Biotechnol 2020, 8, 997. [Google Scholar] [CrossRef] [PubMed]
- Mas-Bargues, C.; Borrás, C. Importance of stem cell culture conditions for their derived extracellular vesicles therapeutic effect. Free. Radic. Biol. Med. 2021, 168, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of small extracellular vesicles isolated from plasma by ultracentrifugation or size-exclusion chromatography: Yield, purity and functional potential. J. Extracell Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef]
- Coughlan, C.; Bruce, K.D.; Burgy, O.; Boyd, T.D.; Michel, C.R.; Garcia-Perez, J.E.; Adame, V.; Anton, P.; Bettcher, B.M.; Chial, H.J.; et al. Exosome Isolation by Ultracentrifugation and Precipitation and Techniques for Downstream Analyses. Curr. Protoc. Cell Biol. 2020, 88, e110. [Google Scholar] [CrossRef]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. JCI Insight 2018, 3, 99263. [Google Scholar] [CrossRef] [PubMed]
- Gamez-Valero, A.; Monguio-Tortajada, M.; Carreras-Planella, L.; Franquesa, M.; Beyer, K.; Borras, F.E. Size-Exclusion Chromatography-based isolation minimally alters Extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016, 6, 33641. [Google Scholar] [CrossRef] [PubMed]
- Paganini, C.; Capasso Palmiero, U.; Pocsfalvi, G.; Touzet, N.; Bongiovanni, A.; Arosio, P. Scalable Production and Isolation of Extracellular Vesicles: Available Sources and Lessons from Current Industrial Bioprocesses. Biotechnol. J. 2019, 14, e1800528. [Google Scholar] [CrossRef]
- Weng, Y.; Sui, Z.; Shan, Y.; Hu, Y.; Chen, Y.; Zhang, L.; Zhang, Y. Effective isolation of exosomes with polyethylene glycol from cell culture supernatant for in-depth proteome profiling. Analyst 2016, 141, 4640–4646. [Google Scholar] [CrossRef]
- Martins, T.S.; Catita, J.; Rosa, I.M.; da Cruz e SilvaSilva, O.A.B.; Henriques, A.G. Exosome isolation from distinct biofluids using precipitation and column-based approaches. PLoS ONE 2018, 13, e0198820. [Google Scholar] [CrossRef]
- Saxena, A.; Tripathi, B.P.; Kumar, M.; Shahi, V.K. Membrane-based techniques for the separation and purification of proteins: An overview. Adv. Colloid Interface Sci. 2009, 145, 1–22. [Google Scholar] [CrossRef]
- Bari, E.; Ferrarotti, I.; Torre, M.L.; Corsico, A.G.; Perteghella, S. Mesenchymal stem/stromal cell secretome for lung regeneration: The long way through "pharmaceuticalization" for the best formulation. J. Control. Release Off. J. Control. Release Soc. 2019, 309, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef]
- Fortunato, D.; Giannoukakos, S.; Gimenez-Capitan, A.; Hackenberg, M.; Molina-Vila, M.A.; Zarovni, N. Selective isolation of extracellular vesicles from minimally processed human plasma as a translational strategy for liquid biopsies. Biomark Res. 2022, 10, 57. [Google Scholar] [CrossRef]
- Wang, J.; Ma, P.; Kim, D.H.; Liu, B.F.; Demirci, U. Towards Microfluidic-Based Exosome Isolation and Detection for Tumor Therapy. Nano. Today 2021, 37, 101066. [Google Scholar] [CrossRef]
- Cheng, S.; Li, Y.; Yan, H.; Wen, Y.; Zhou, X.; Friedman, L.; Zeng, Y. Advances in microfluidic extracellular vesicle analysis for cancer diagnostics. Lab Chip 2021, 21, 3219–3243. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.G.; Andrade, A.C.; Wolf, M.; Hochmann, S.; Krisch, L.; Maeding, N.; Regl, C.; Poupardin, R.; Ebner-Peking, P.; Huber, C.G.; et al. Synergy of Human Platelet-Derived Extracellular Vesicles with Secretome Proteins Promotes Regenerative Functions. Biomedicines 2022, 10, 238. [Google Scholar] [CrossRef]
- Wolf, M.; Poupardin, R.W.; Ebner-Peking, P.; Andrade, A.C.; Blochl, C.; Obermayer, A.; Gomes, F.G.; Vari, B.; Maeding, N.; Eminger, E.; et al. A functional corona around extracellular vesicles enhances angiogenesis, skin regeneration and immunomodulation. J. Extracell Vesicles 2022, 11, e12207. [Google Scholar] [CrossRef] [PubMed]
- Zwi-Dantsis, L.; Winter, C.W.; Kauscher, U.; Ferrini, A.; Wang, B.; Whittaker, T.E.; Hood, S.R.; Terracciano, C.M.; Stevens, M.M. Highly purified extracellular vesicles from human cardiomyocytes demonstrate preferential uptake by human endothelial cells. Nanoscale 2020, 12, 19844–19854. [Google Scholar] [CrossRef]
- Tian, Y.; Gong, M.; Hu, Y.; Liu, H.; Zhang, W.; Zhang, M.; Hu, X.; Aubert, D.; Zhu, S.; Wu, L.; et al. Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J. Extracell Vesicles 2020, 9, 1697028. [Google Scholar] [CrossRef]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2017, 20, 1. [Google Scholar] [CrossRef]
- Madison, M.N.; Roller, R.J.; Okeoma, C.M. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 2014, 11, 102. [Google Scholar] [CrossRef]
- Madison, M.N.; Jones, P.H.; Okeoma, C.M. Exosomes in human semen restrict HIV-1 transmission by vaginal cells and block intravaginal replication of LP-BM5 murine AIDS virus complex. Virology 2015, 482, 189–201. [Google Scholar] [CrossRef]
- Welch, J.L.; Madison, M.N.; Margolick, J.B.; Galvin, S.; Gupta, P.; Martinez-Maza, O.; Dash, C.; Okeoma, C.M. Effect of prolonged freezing of semen on exosome recovery and biologic activity. Sci. Rep. 2017, 7, 45034. [Google Scholar] [CrossRef]
- Zhou, H.; Yuen, P.S.; Pisitkun, T.; Gonzales, P.A.; Yasuda, H.; Dear, J.W.; Gross, P.; Knepper, M.A.; Star, R.A. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006, 69, 1471–1476. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. miRNA in plasma exosome is stable under different storage conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef]
- Du, K.; Sun, X.; Tang, X.; Xu, H.; Duan, P.; Xu, Q.; Sang, M. Effects of storage temperature and time on quality of plasma exosomes extracted by ExoQuick(TM) and Umibio kits. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2020, 36, 330–336. [Google Scholar]
- Maroto, R.; Zhao, Y.; Jamaluddin, M.; Popov, V.L.; Wang, H.; Kalubowilage, M.; Zhang, Y.; Luisi, J.; Sun, H.; Culbertson, C.T.; et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell Vesicles 2017, 6, 1359478. [Google Scholar] [CrossRef]
- Wright, A.; Snyder, O.L.; Christenson, L.K.; He, H.; Weiss, M.L. Effect of Pre-Processing Storage Condition of Cell Culture-Conditioned Medium on Extracellular Vesicles Derived from Human Umbilical Cord-Derived Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2022, 23, 7716. [Google Scholar] [CrossRef]
- Romanov, Y.A.; Volgina, N.E.; Dugina, T.N.; Kabaeva, N.V.; Sukhikh, G.T. Effect of Storage Conditions on the Integrity of Human Umbilical Cord Mesenchymal Stromal Cell-Derived Microvesicles. Bull Exp. Biol. Med. 2019, 167, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, A.M.; Timar, C.I.; Marosvari, K.A.; Veres, D.S.; Otrokocsi, L.; Kittel, A.; Ligeti, E. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J. Extracell Vesicles 2014, 3, 25465. [Google Scholar] [CrossRef] [PubMed]
- Charoenviriyakul, C.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Preservation of exosomes at room temperature using lyophilization. Int. J. Pharm. 2018, 553, 1–7. [Google Scholar] [CrossRef]
- Bosch, S.; de Beaurepaire, L.; Allard, M.; Mosser, M.; Heichette, C.; Chretien, D.; Jegou, D.; Bach, J.M. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 2016, 6, 36162. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Perteghella, S.; Catenacci, L.; Sorlini, M.; Croce, S.; Mantelli, M.; Avanzini, M.A.; Sorrenti, M.; Torre, M.L. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine 2019, 14, 753–765. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Barabadi, M.; Tan, J.L.; Morton, D.A.V.; Frith, J.E.; Lim, R. To Protect and to Preserve: Novel Preservation Strategies for Extracellular Vesicles. Front. Pharm. 2018, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Buzas, E.I.; Bemis, L.T.; Bora, A.; Lasser, C.; Lotvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, M.J.; Vandendriessche, C.; Vandenbroucke, R.E. Special delEVery: Extracellular Vesicles as Promising Delivery Platform to the Brain. Biomedicines 2021, 9, 1734. [Google Scholar] [CrossRef]
- Lun, X.Q.; Jang, J.H.; Tang, N.; Deng, H.; Head, R.; Bell, J.C.; Stojdl, D.F.; Nutt, C.L.; Senger, D.L.; Forsyth, P.A.; et al. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin. Cancer Res. 2009, 15, 2777–2788. [Google Scholar] [CrossRef] [PubMed]
- Wiklander, O.P.; Nordin, J.Z.; O’Loughlin, A.; Gustafsson, Y.; Corso, G.; Mager, I.; Vader, P.; Lee, Y.; Sork, H.; Seow, Y.; et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell Vesicles 2015, 4, 26316. [Google Scholar] [CrossRef]
- Imai, T.; Takahashi, Y.; Nishikawa, M.; Kato, K.; Morishita, M.; Yamashita, T.; Matsumoto, A.; Charoenviriyakul, C.; Takakura, Y. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J. Extracell Vesicles 2015, 4, 26238. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol 2013, 165, 77–84. [Google Scholar] [CrossRef]
- Shin, K.O.; Ha, D.H.; Kim, J.O.; Crumrine, D.A.; Meyer, J.M.; Wakefield, J.S.; Lee, Y.; Kim, B.; Kim, S.; Kim, H.K.; et al. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing de Novo Synthesis of Ceramides in Atopic Dermatitis. Cells 2020, 9, 680. [Google Scholar] [CrossRef]
- Lu, M.; Peng, L.; Ming, X.; Wang, X.; Cui, A.; Li, Y.; Wang, X.; Meng, D.; Sun, N.; Xiang, M.; et al. Enhanced wound healing promotion by immune response-free monkey autologous iPSCs and exosomes vs. their allogeneic counterparts. EBioMedicine 2019, 42, 443–457. [Google Scholar] [CrossRef]
- Long, Q.; Upadhya, D.; Hattiangady, B.; Kim, D.K.; An, S.Y.; Shuai, B.; Prockop, D.J.; Shetty, A.K. Intranasal MSC-derived A1-exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc. Natl. Acad. Sci. USA 2017, 114, E3536–E3545. [Google Scholar] [CrossRef]
- Herman, S.; Djaldetti, R.; Mollenhauer, B.; Offen, D. CSF-derived extracellular vesicles from patients with Parkinson’s disease induce symptoms and pathology. Brain 2022, 146, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Huang, M.; Zheng, M.; Dai, C.; Song, Q.; Zhang, Q.; Li, Q.; Gu, X.; Chen, H.; Jiang, G.; et al. ADSCs-derived extracellular vesicles alleviate neuronal damage, promote neurogenesis and rescue memory loss in mice with Alzheimer’s disease. J. Control. Release Off. J. Control. Release Soc. 2020, 327, 688–702. [Google Scholar] [CrossRef]
- Jiang, L.; Driedonks, T.A.P.; Jong, W.S.P.; Dhakal, S.; van den Berg van Saparoea, H.B.; Sitaras, I.; Zhou, R.; Caputo, C.; Littlefield, K.; Lowman, M.; et al. A bacterial extracellular vesicle-based intranasal vaccine against SARS-CoV-2 protects against disease and elicits neutralizing antibodies to wild-type and Delta variants. Biorxiv 2022, 11, e12192. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Shi, M.M.; Monsel, A.; Dai, C.X.; Dong, X.; Shen, H.; Li, S.K.; Chang, J.; Xu, C.L.; Li, P.; et al. Nebulized exosomes derived from allogenic adipose tissue mesenchymal stromal cells in patients with severe COVID-19: A pilot study. Stem Cell Res. Ther. 2022, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Payares-Herrera, C.; Martinez-Munoz, M.E.; Vallhonrat, I.L.; de Molina, R.M.; Torres, M.P.; Trisan, A.; de Diego, I.S.; Alonso, R.; Zafra, R.; Donaire, T.; et al. Double-blind, randomized, controlled, trial to assess the efficacy of allogenic mesenchymal stromal cells in patients with acute respiratory distress syndrome due to COVID-19 (COVID-AT): A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 9. [Google Scholar]
- Shi, M.M.; Yang, Q.Y.; Monsel, A.; Yan, J.Y.; Dai, C.X.; Zhao, J.Y.; Shi, G.C.; Zhou, M.; Zhu, X.M.; Li, S.K.; et al. Preclinical efficacy and clinical safety of clinical-grade nebulized allogenic adipose mesenchymal stromal cells-derived extracellular vesicles. J. Extracell Vesicles 2021, 10, e12134. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cui, M.; Fang, Z.; Liu, K. Advanced research on extracellular vesicles based oral drug delivery systems. J. Control. Release Off. J. Control. Release Soc. 2022, 351, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, L.; Liu, X.; Bai, Y.; Wu, R.; Li, X.; Mao, Y.; Zhang, L.; Zheng, Y.; Gong, T.; et al. Milk-derived exosomes exhibit versatile effects for improved oral drug delivery. Acta. Pharm Sin. B 2022, 12, 2029–2042. [Google Scholar] [CrossRef]
- Skotland, T.; Hessvik, N.P.; Sandvig, K.; Llorente, A. Exosomal lipid composition and the role of ether lipids and phosphoinositides in exosome biology. J. Lipid Res. 2019, 60, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Xia, B.; Shan, S.; Zheng, A.; Zhang, S.; Chen, J.; Liang, X.J. High-quality milk exosomes as oral drug delivery system. Biomaterials 2021, 277, 121126. [Google Scholar] [CrossRef]
- Reiner, A.T.; Somoza, V. Extracellular Vesicles as Vehicles for the Delivery of Food Bioactives. J. Agric. Food Chem. 2019, 67, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, E.J.; Krachler, A.M. Mechanisms of outer membrane vesicle entry into host cells. Cell Microbiol. 2016, 18, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Wolf, T.; Baier, S.R.; Zempleni, J. The Intestinal Transport of Bovine Milk Exosomes Is Mediated by Endocytosis in Human Colon Carcinoma Caco-2 Cells and Rat Small Intestinal IEC-6 Cells. J. Nutr. 2015, 145, 2201–2206. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, M.; Nazimek, K.; Bryniarski, K. Extracellular Vesicles-Oral Therapeutics of the Future. Int. J. Mol. Sci. 2022, 23, 7554. [Google Scholar] [CrossRef]
- Du, C.; Quan, S.; Nan, X.; Zhao, Y.; Shi, F.; Luo, Q.; Xiong, B. Effects of oral milk extracellular vesicles on the gut microbiome and serum metabolome in mice. Food Funct 2021, 12, 10938–10949. [Google Scholar] [CrossRef]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Vozel, D.; Božič, D.; Jeran, M.; Jan, Z.; Pajnič, M.; Pađen, L.; Steiner, N.; Kralj-Iglič, V.; Battelino, S. Autologous Platelet- and Extracellular Vesicle-Rich Plasma Is an Effective Treatment Modality for Chronic Postoperative Temporal Bone Cavity Inflammation: Randomized Controlled Clinical Trial. Front. Bioeng. Biotechnol. 2021, 9, 677541. [Google Scholar] [CrossRef]
- Kang, M.; Jordan, V.; Blenkiron, C.; Chamley, L.W. Biodistribution of extracellular vesicles following administration into animals: A systematic review. J. Extracell Vesicles 2021, 10, e12085. [Google Scholar] [CrossRef]
- Mitragotri, S. Modeling skin permeability to hydrophilic and hydrophobic solutes based on four permeation pathways. J. Control Release 2003, 86, 69–92. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, L.; Li, F.R.; Li, X.; Wang, Z.; Zou, X.; Zhang, C.; Lv, K.; Zhou, B.; Mitragotri, S.; et al. Topical Application of Exosomes Derived from Human Umbilical Cord Mesenchymal Stem Cells in Combination with Sponge Spicules for Treatment of Photoaging. Int. J. Nanomed. 2020, 15, 2859–2872. [Google Scholar] [CrossRef] [PubMed]
- Dinh, P.C.; Paudel, D.; Brochu, H.; Popowski, K.D.; Gracieux, M.C.; Cores, J.; Huang, K.; Hensley, M.T.; Harrell, E.; Vandergriff, A.C.; et al. Inhalation of lung spheroid cell secretome and exosomes promotes lung repair in pulmonary fibrosis. Nat. Commun. 2020, 11, 1064. [Google Scholar] [CrossRef] [PubMed]
- Popowski, K.D.; López de Juan Abad, B.; George, A.; Silkstone, D.; Belcher, E.; Chung, J.; Ghodsi, A.; Lutz, H.; Davenport, J.; Flanagan, M.; et al. Inhalable exosomes outperform liposomes as mRNA and protein drug carriers to the lung. Extracell Vesicle 2022, 1, 100002. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, G.; Zhou, W.; Miao, C. Exosomes as a New Delivery Vehicle in Inflammatory Bowel Disease. Pharmaceutics 2021, 13, 1644. [Google Scholar] [CrossRef] [PubMed]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.; Chen, X.; Wang, L.; Yang, G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol. Nucleic Acids. 2017, 7, 278–287. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, P.; Zhang, Y.; Wang, J.; Wang, C.; Liu, Y.; Yang, G.; Yuan, L. Exosome-based. Theranostics 2021, 11, 2953–2965. [Google Scholar] [CrossRef]
- Kamerkar, S.; Leng, C.; Burenkova, O.; Jang, S.C.; McCoy, C.; Zhang, K.; Dooley, K.; Kasera, S.; Zi, T.; Sisó, S.; et al. Exosome-mediated genetic reprogramming of tumor-associated macrophages by exoASO-STAT6 leads to potent monotherapy antitumor activity. Sci. Adv. 2022, 8, eabj7002. [Google Scholar] [CrossRef]
- Couch, Y.; Buzas, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lotvall, J.; Raposo, G.; Stahl, P.D.; Thery, C.; et al. A brief history of nearly EV-erything—The rise and rise of extracellular vesicles. J. Extracell Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- Rashed, M.H.; Bayraktar, E.; Helal, G.K.; Abd-Ellah, M.F.; Amero, P.; Chavez-Reyes, A.; Rodriguez-Aguayo, C. Exosomes: From Garbage Bins to Promising Therapeutic Targets. Int. J. Mol. Sci. 2017, 18, 538. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, K.; Zheng, X.; Chen, T.; Wang, J.; Song, Y.; Shao, Y.; Zheng, S. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct. Target 2020, 5, 144. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, L.; Perazzoli, G.; Peña, M.; Cepero, A.; Luque, C.; Melguizo, C.; Prados, J. Cancer therapy based on extracellular vesicles as drug delivery vehicles. J. Control. Release 2020, 327, 296–315. [Google Scholar] [CrossRef] [PubMed]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis 2022, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef]
- Tominaga, N.; Yoshioka, Y.; Ochiya, T. A novel platform for cancer therapy using extracellular vesicles. Adv. Drug Deliv. Rev. 2015, 95, 50–55. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).