Identification and Functional Prediction of CircRNAs in Leaves of F1 Hybrid Poplars with Different Growth Potential and Their Parents
Abstract
1. Introduction
2. Results
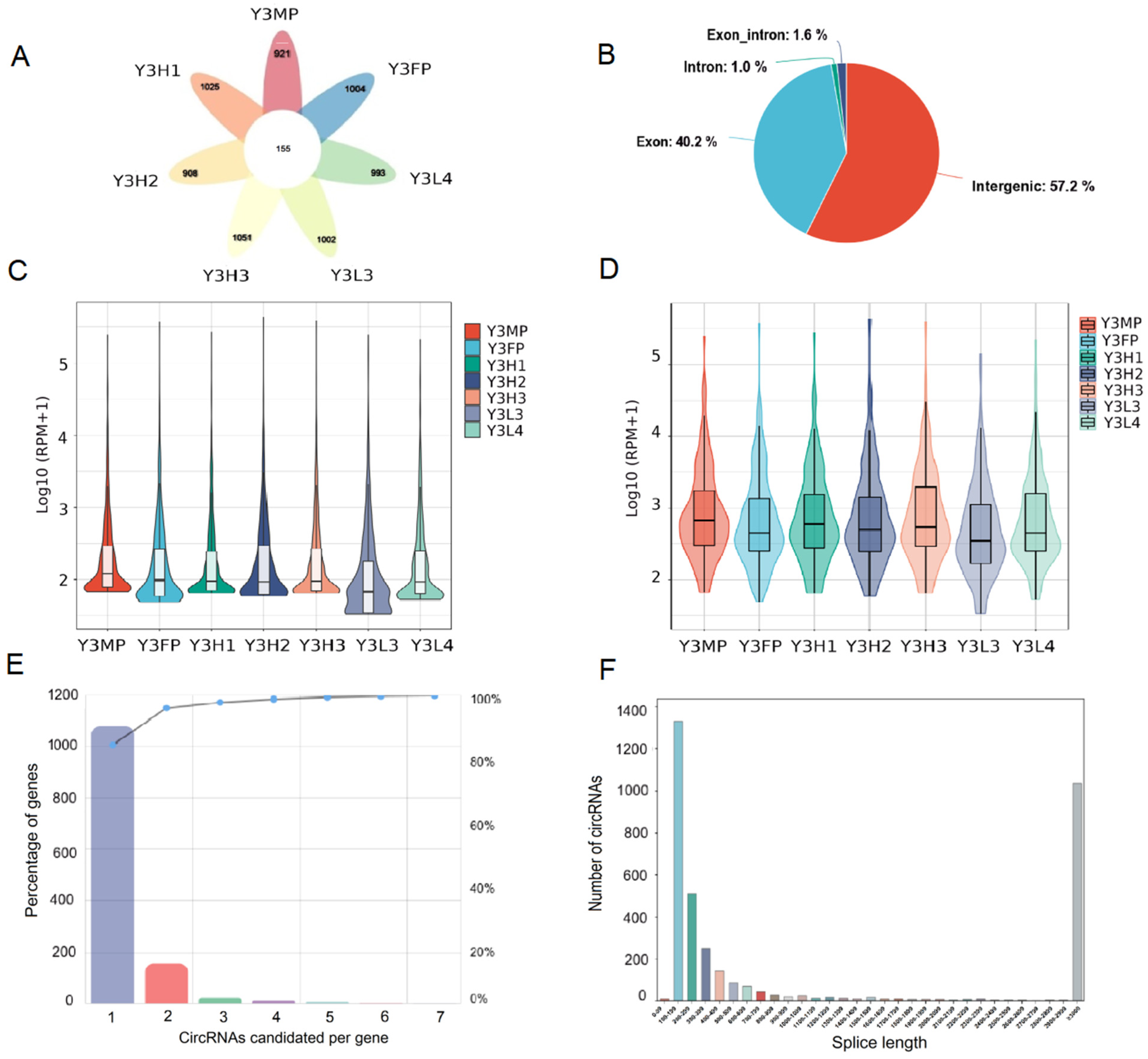
2.1. Identification and Characterization of CircRNA in Poplar’s Leaves
2.2. Differential Expression of CircRNAs in Parents and F1 Hybrids with Different Growth Potential
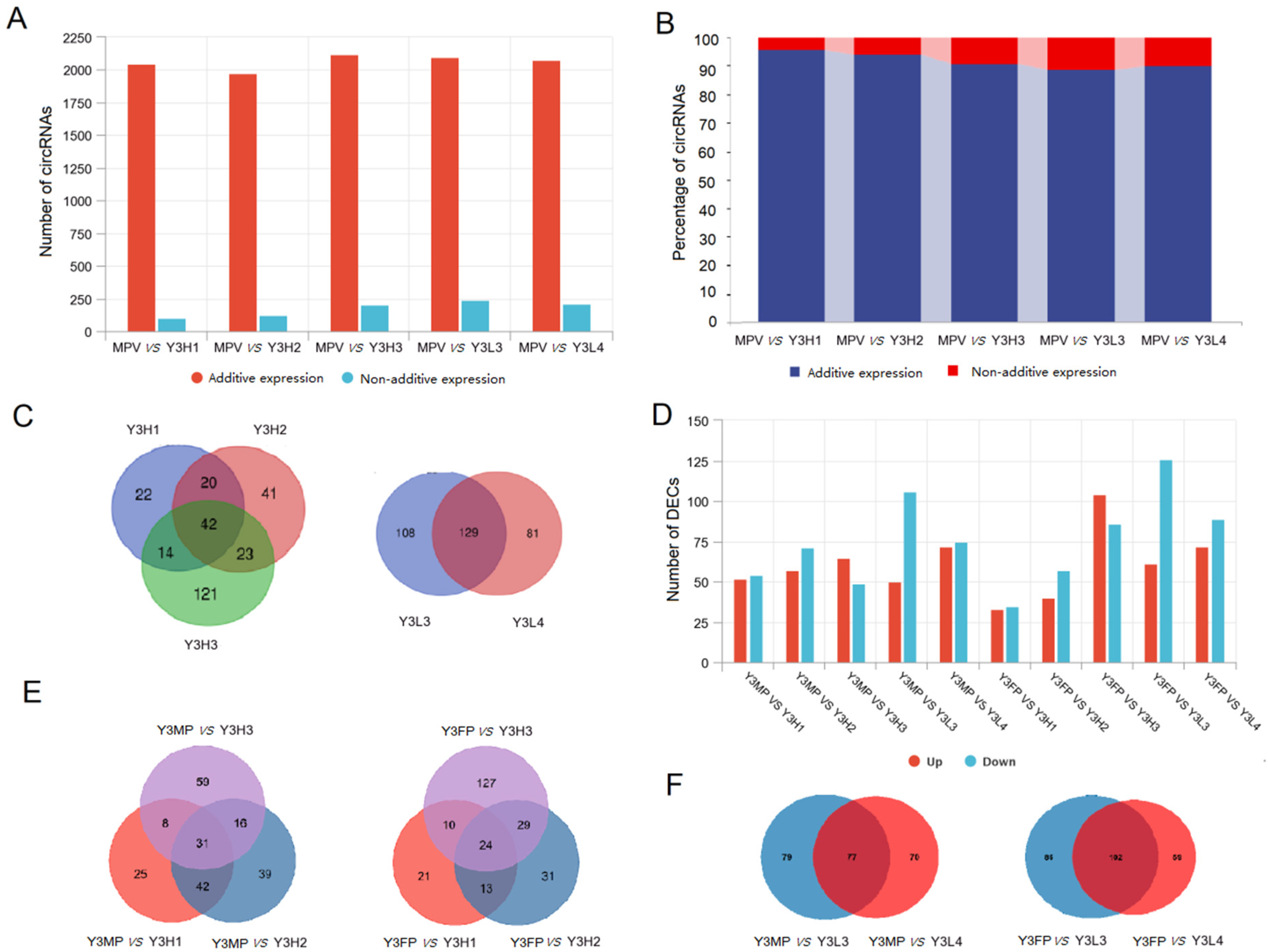
2.2.1. Expression Differences for Additively Expressed CircRNAs and Non-Additively Expressed CircRNAs between Parents and F1 Hybrids
2.2.2. Differential Expression of DECs in the Parents and the F1 Hybrids Groups
2.3. Different Expression of SPE, CoPE and SFE CircRNAs between Parents and the F1 Hybrids
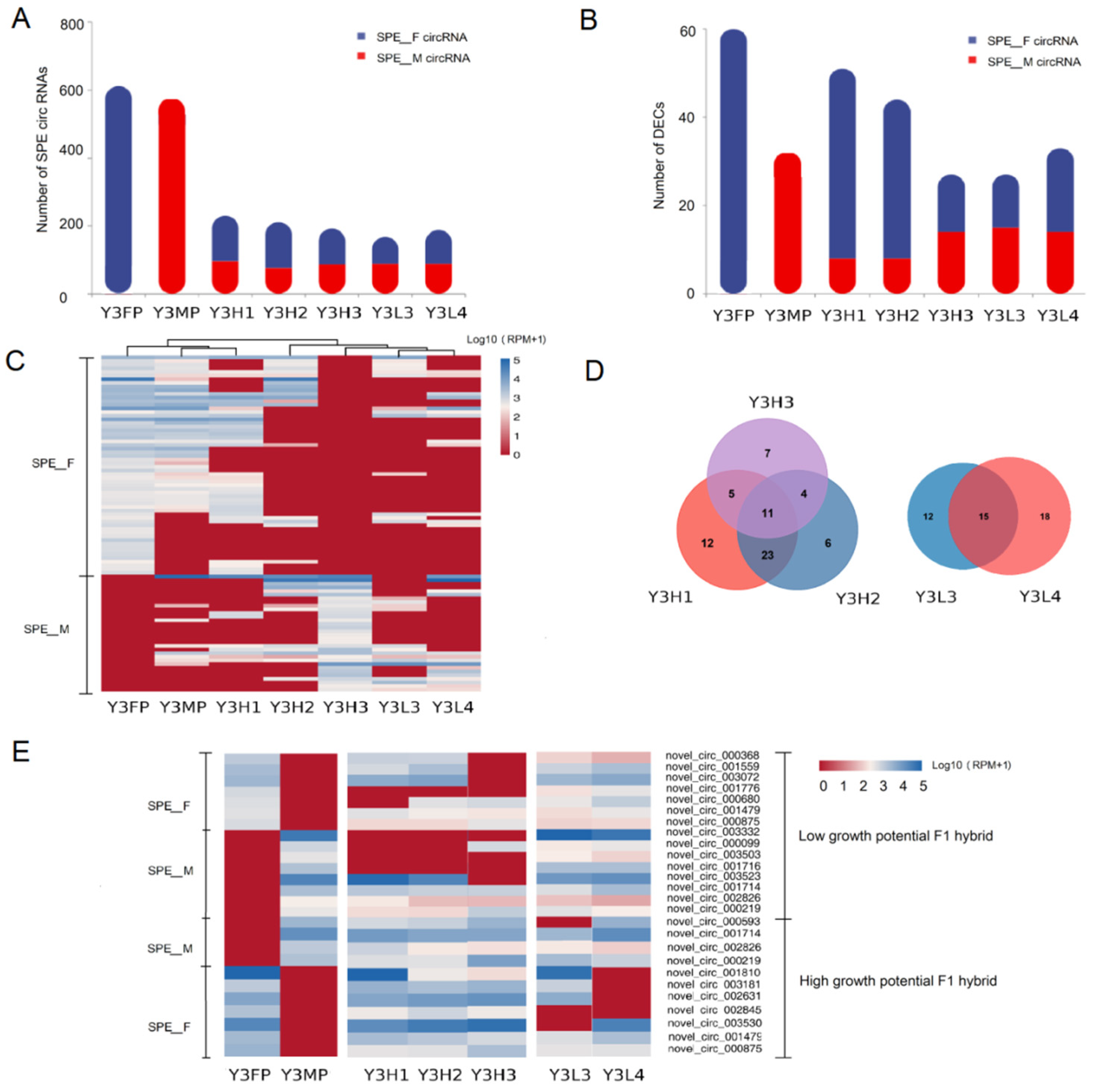
2.3.1. Expression Differences of SPE CircRNAs in the F1 Hybrids and Their Parents
2.3.2. Expression Differences of Co-Expressed CircRNAs in the Parents and the F1 Hybrids
2.3.3. Expression Differences of F1 Hybrid-Specifically Expressed of CircRNAs in F1 Hybrids
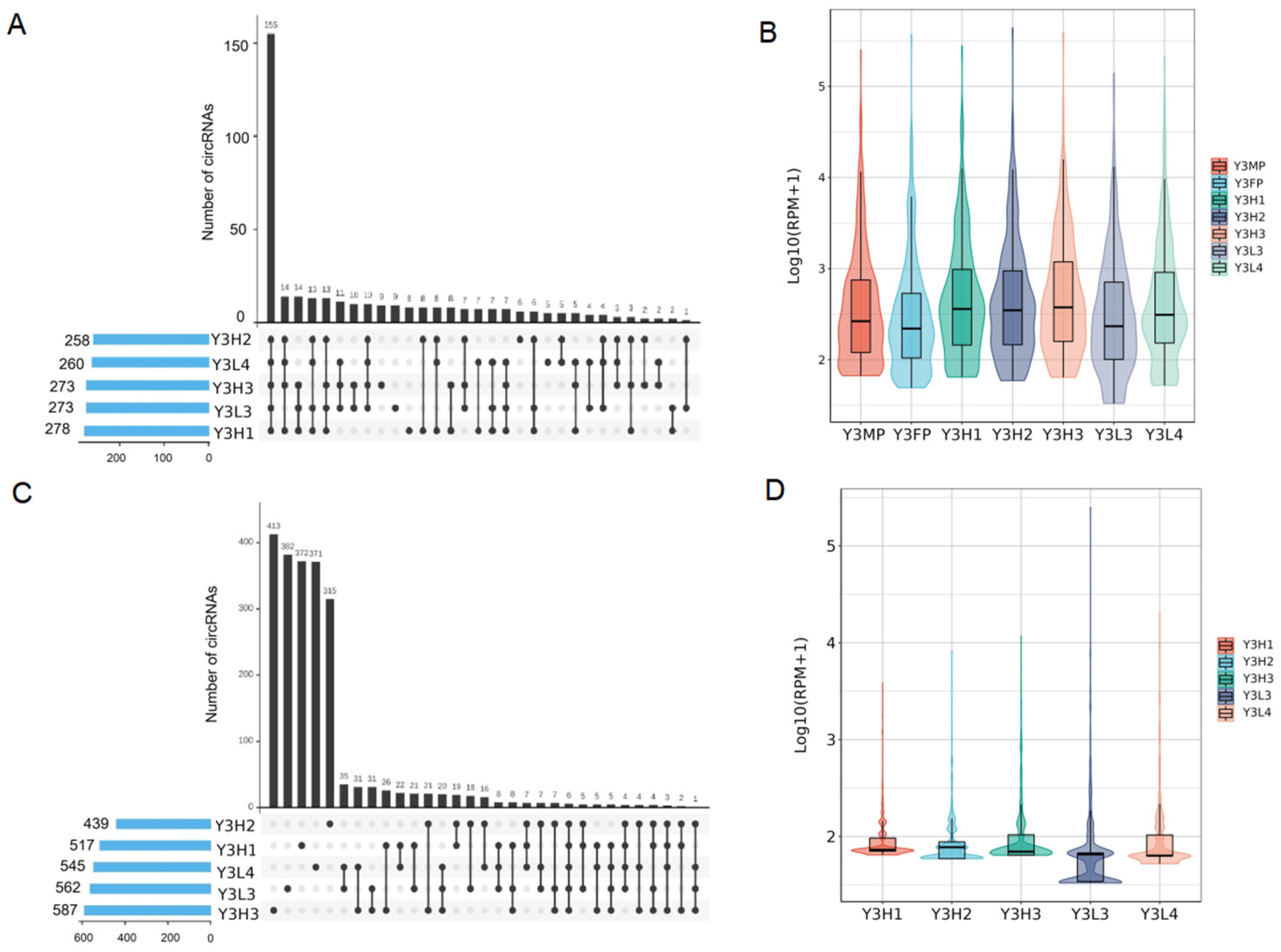
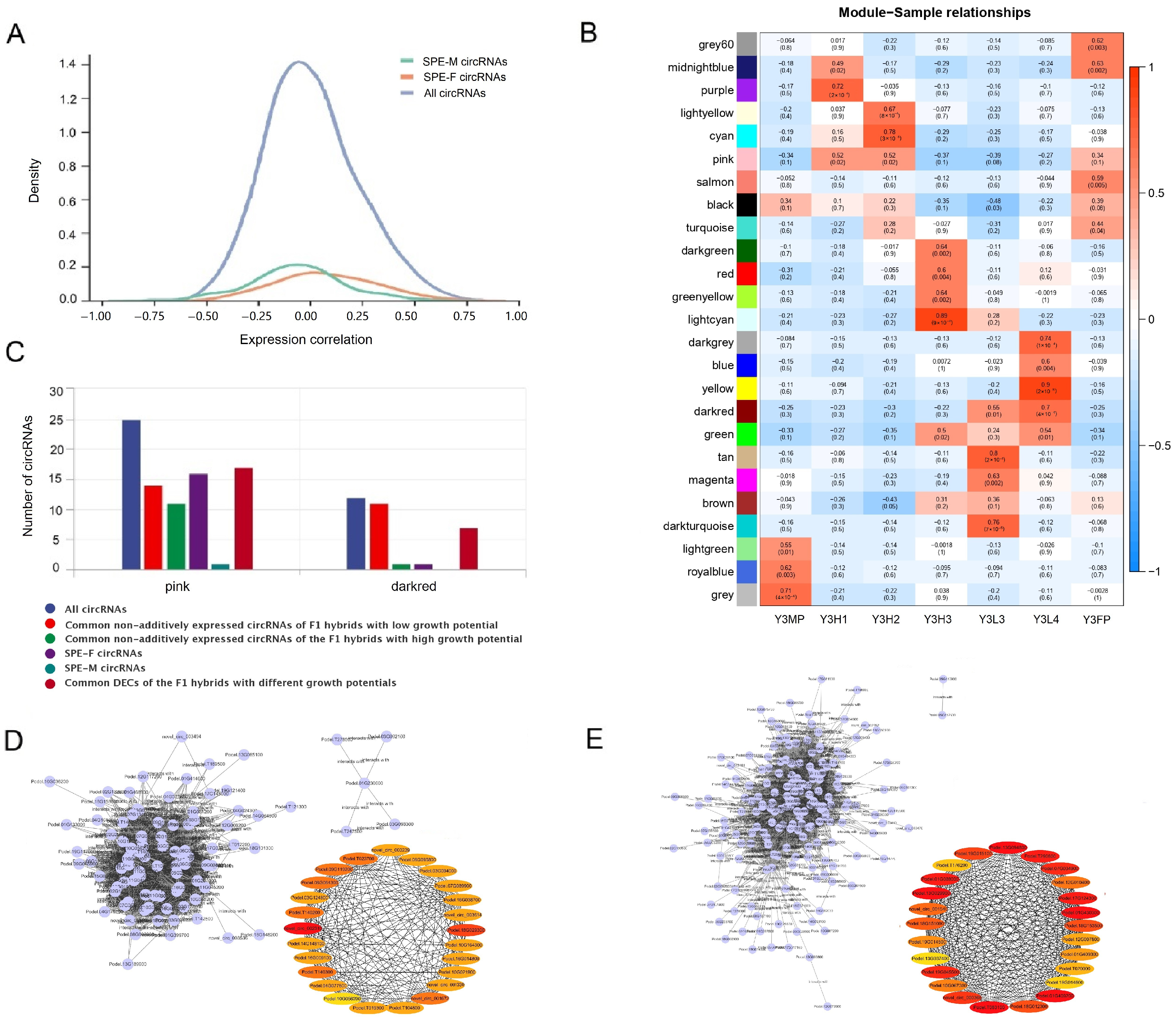
2.4. Expression Differences of CircRNAs among the F1 Hybrids with Different Growth Potentials
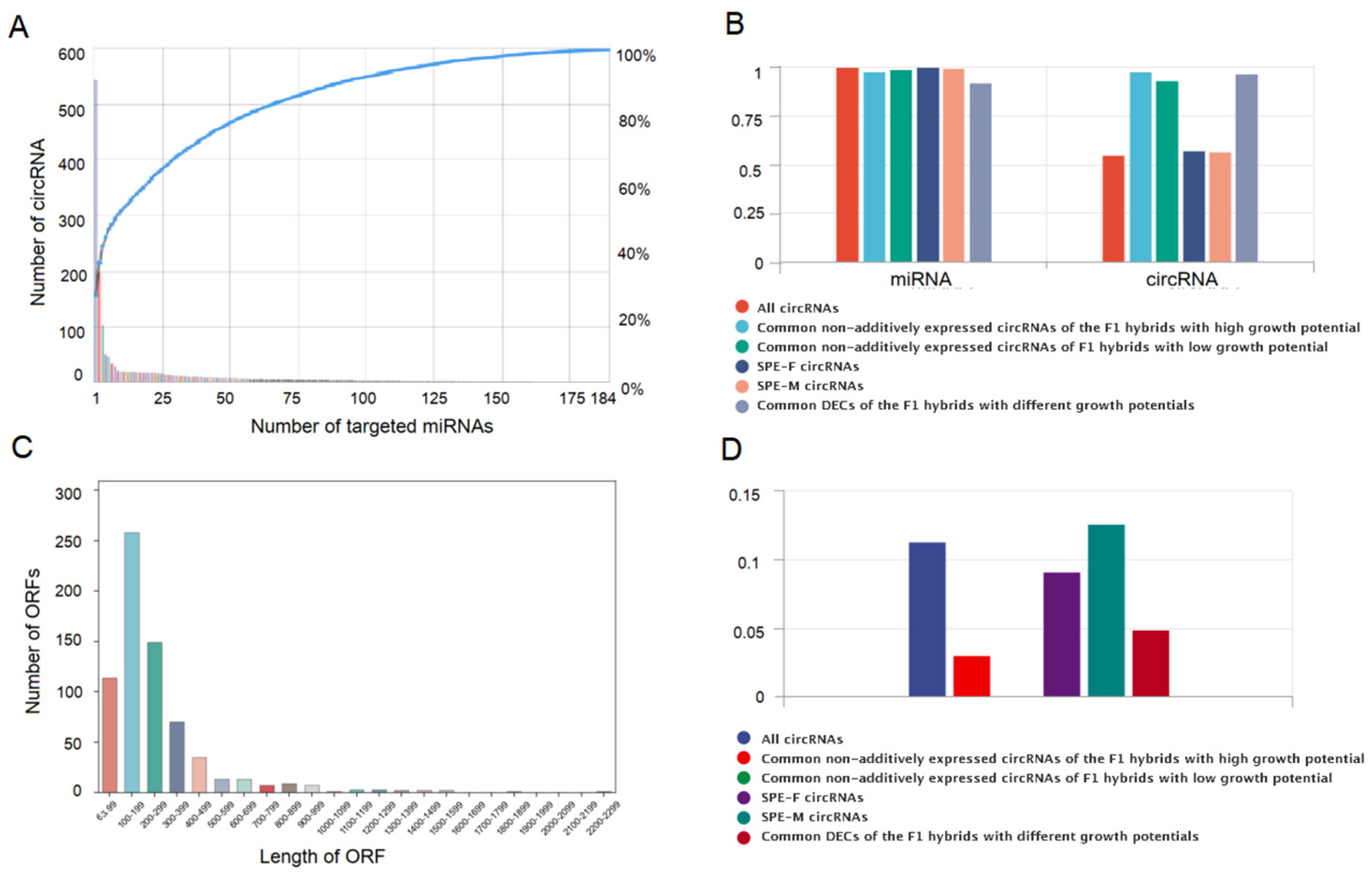
2.5. CircRNAs Regulate the Formation of Different Growth Potentials of Poplars by Acting as miRNA Sponges
2.6. Prediction of the Protein Coding Potential of CircRNA in Poplar Leaves
2.7. Correlation between CircRNA Expression and Their Parental Gene Expression
2.8. Co-Expression of Protein Coding Genes and CircRNAs
3. Discussion
3.1. Expression Level and Source of CircRNAs in Leaves of F1 Poplar Hybrids with Different Growth Potentials and Their Parents
3.2. Differential Expression of CircRNAs in Leaves of F1 Hybrid Poplars with Different Growth Potentials and Their Parents
3.3. Functional Prediction of CircRNAs Related to the Formation of Poplar Heterosis
4. Materials and Methods
4.1. Material Source and Field Test Design
4.2. CircRNA Sequencing and Identification
4.3. Identification of Non-Additively Expressed CircRNAs, SPE CircRNAs, and DECs
4.4. Correlation between the Expression Levels of CircRNAs and Their Parental Genes
4.5. Co-Expression of Protein Coding Genes and CircRNAs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef]
- Chen, L. The biogenesis and emerging roles of circular RNAs. Nat. Rev. Mol. Cell Biol. 2016, 17, 205–211. [Google Scholar] [CrossRef]
- Zhao, W.; Chu, S.; Jiao, Y. Present scenario of circular RNAs (circRNAs) in plants. Front. Plant Sci. 2019, 10, 379. [Google Scholar] [CrossRef]
- Wang, X.; Qin, T.; Peng, Z.; Zhang, Y.; Yang, Q.; Geng, X.; Salihc, H.; Sun, J.; He, S.; Wang, Q.; et al. Genome-wide profiling of circular RNAs in the hybridization of two elite inbred lines of Gossypium hirsutum. Ind. Crops Prod. 2021, 170, 113754. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, L.; Jia, C.; Shi, W.; Deng, S.; Luo, Z. Identification and Functional Prediction of Poplar Root circRNAs Involved in Treatment with Different Forms of Nitrogen. Front. Plant Sci. 2022, 13, 941380. [Google Scholar] [CrossRef]
- Ye, C.; Chen, L.; Liu, C.; Zhu, Q.; Fan, L. Widespread noncoding circular RNA s in plants. New Phytol. 2015, 208, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, L.; Ding, J.; Wang, Y.; Wang, J.; Zhang, X.; Che, Y.; Liu, Z.; Zhang, X.; Ye, J.; et al. Integrative analysis of Arabidopsis thaliana transcriptomics reveals intuitive splicing mechanism for circular RNA. FEBS Lett. 2016, 590, 3510–3516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Fan, Y.; Sun, X.; Chen, L.; Terzaghi, W.; Bucher, E.; Li, L.; Dai, M. A large-scale circular RNA profiling reveals universal molecular mechanisms responsive to drought stress in maize and Arabidopsis. Plant J. 2019, 98, 697–713. [Google Scholar] [CrossRef]
- Lu, T.; Cui, L.; Zhou, Y.; Zhu, C.; Fan, D.; Gong, H.; Zhao, Q.; Zhou, C.; Zhao, Y.; Lu, D.; et al. Transcriptome-wide investigation of circular RNAs in rice. RNA 2015, 21, 2076–2087. [Google Scholar] [CrossRef]
- Luo, Z.; Qian, J.; Chen, S.; Li, L. Dynamic patterns of circular and linear RNAs in maize hybrid and parental lines. Theo. Appl. Genet. 2020, 133, 593–604. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Zhang, H.; Wang, H.; Liu, X.; Xu, X.; Zhang, Z.; Kohnen, M.V.; Hu, K.; Wang, H.; et al. Genome-wide profiling of circular RNAs in the rapidly growing shoots of moso bamboo (Phyllostachys edulis). Plant Cell Physiol. 2019, 60, 1354–1373. [Google Scholar] [CrossRef]
- Wang, J.; Lin, J.; Wang, H.; Li, X.; Yang, Q.; Li, H.; Chang, Y. Identification and characterization of circRNAs in Pyrus betulifolia Bunge under drought stress. PLoS ONE 2018, 13, e0200692. [Google Scholar] [CrossRef]
- Liu, S.; Wu, L.; Qi, H.; Xu, M. LncRNA/circRNA–miRNA–mRNA networks regulate the development of root and shoot meristems of Populus. Ind. Crops Prod. 2019, 133, 333–347. [Google Scholar] [CrossRef]
- Zhan, N.; Wang, Z.; Xie, Y.; Shang, X.; Liu, G.; Wu, Z. Expression Patterns and Regulation of Non-Coding RNAs during Synthesis of Cellulose in Eucalyptus grandis Hill. Forests 2021, 12, 1565. [Google Scholar] [CrossRef]
- Tan, J.; Zhou, Z.; Niu, Y.; Sun, X.; Deng, Z. Identification and functional characterization of tomato circRNAs derived from genes involved in fruit pigment accumulation. Sci. Rep. 2017, 7, 8594. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qin, S.; Bao, L.; Guo, Z.; Zhao, L. Identification and functional prediction of circRNAs in Populus euphratica Oliv. heteromorphic leaves. Genomics 2020, 112, 92–98. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, Y.; Chen, B.; Wei, M.; Wang, X.; Du, L. Identification and Characterization of circRNAs in the Developing Stem Cambium of Poplar Seedlings. Mol. Biol. 2020, 54, 708–718. [Google Scholar] [CrossRef]
- Xu, H.; Chen, B.; Zhao, Y.; Guo, Y.; Liu, G.; Li, R.; Zeisler-Diehl, V.V.; Chen, Y.; He, X.; Schreiber, L.; et al. Non-Coding RNA analyses of seasonal cambium activity in Populus tomentosa. Cells 2022, 11, 640. [Google Scholar] [CrossRef]
- Li, G.; Niu, Z.; Zheng, Z.; Lv, J.; Chen, Q.; Liu, J.; Wan, D. Contrasting origins, expression patterns and functions of circRNAs between salt-sensitive and salt-tolerant poplars. Environ. Exp. Bot. 2021, 185, 104403. [Google Scholar] [CrossRef]
- Chen, Z. Genomic and epigenetic insights into the molecular bases of heterosis. Nat. Rev. Genet. 2013, 14, 471–482. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Zhang, Y.; Gu, R. Advances in research on the mechanism of heterosis in plants. Front. Plant Sci. 2021, 12, 2124. [Google Scholar] [CrossRef]
- Stobrawa, K. Poplars (Populus spp.): Ecological role, applications and scientific perspectives in the 21st century. Balt. For 2014, 20, 204–213. [Google Scholar]
- Birchler, J.A.; Auger, D.L.; Riddle, N.C. In search of the molecular basis of heterosis. Plant J. 2003, 15, 2236–2239. [Google Scholar] [CrossRef]
- Gao, M.; Huang, Q.; Chu, Y.; Ding, C.; Zhang, B.; Su, X. Analysis of the leaf methylomes of parents and their hybrids provides new insight into hybrid vigor in Populus deltoides. BMC Genet. 2014, 15, S8. [Google Scholar] [CrossRef]
- Zhuang, Y.; Adams, K.L. Extensive allelic variation in gene expression in Populus F1 hybrids. Genetics 2007, 177, 1987–1996. [Google Scholar] [CrossRef]
- Zhao, P.; Ding, D.; Zhang, F.; Zhao, X.; Xue, Y.; Li, W.; Fu, Z.; Li, H.; Tang, J. Investigating the molecular genetic basis of heterosis for internode expansion in maize by microRNA transcriptomic deep sequencing. Funct. Integr. Genomics 2015, 15, 261–270. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, S.; Hua, S.; Shen, E.; Ye, C.; Cai, D.; Timko, M.P.; Zhu, Q.; Fan, L. Analysis of transcriptional and epigenetic changes in hybrid vigor of allopolyploid Brassica napus uncovers key roles for small RNAs. Plant J. 2017, 91, 874–893. [Google Scholar] [CrossRef]
- Seifert, F.; Thiemann, A.; Grant-Downton, R.; Edelmann, S.; Rybka, R.; Schrag, T.A.; Frisch, M.; Dickinson, H.G.; Melchinger, A.E.; Scholten, S. Parental expression variation of small RNAs is negatively correlated with grain yield heterosis in a maize breeding population. Front. Plant Sci. 2018, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Tao, X.; Feng, S.; Wang, L.; Hong, H.; Ma, W.; Shang, G.; Guo, S.; He, Y.; Zhou, B.; et al. LncRNAs in polyploid cotton interspecific hybrids are derived from transposon neofunctionalization. Genome Biol. 2018, 19, 195. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, X.; Zhang, Q. Understanding the genetic and molecular constitutions of heterosis for developing hybrid rice. J. Genet. Genomics 2022, 49, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Messing, J. Gene expression of a gene family in maize based on noncollinear haplotypes. Proc. Natl. Acad. Sci. USA 2003, 100, 9055–9060. [Google Scholar] [CrossRef]
- Swanson-Wagner, R.A.; Jia, Y.; DeCook, R.; Lisa, A.; Borsuk, L.A.; Nettleton, D.; Schnable, S.P. All possible modes of gene action are observed in a global comparison of gene expression in a maize F1 hybrid and its inbred parents. Proc. Natl. Acad. Sci. USA 2006, 103, 6805–6810. [Google Scholar] [CrossRef]
- Auger, D.L.; Gray, A.D.; Ream, T.S.; Kato, A.; Coe, E.H.; Birchler, J.A. Non-additive gene expression in diploid and triploid hybrids of maize. Genetics 2005, 169, 389–397. [Google Scholar] [CrossRef]
- Baldauf, J.A.; Marcon, C.; Lithio, A.; Vedder, L.; Altrogge, L.; Piepho, P.; Schoof, H.; Nettleton, D.; Hochholdinger, F. Single-parent expression is a general mechanism driving extensive complementation of non-syntenic genes in maize hybrids. Curr. Biol. 2018, 28, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Henry, I.M.; Zinkgraf, M.S.; Groover, A.T.; Zinkgraf, M.S.; Groover, A.T.; Comai, L. A system for dosage-based functional genomics in poplar. Plant Cell 2015, 27, 2370–2383. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Min, Y.; Dong, C.; Wang, Y.; Cheng, S.; Kang, X. MicroRNA expression changes following synthesis of three full-sib Populus triploid populations with different heterozygosities. Plant Mol. Biol. 2017, 95, 215–225. [Google Scholar] [CrossRef]
- Zhang, G.; Diao, S.; Zhang, T.; Chen, D.; He, C.; Zhang, J. Identification and characterization of circular RNAs during the sea buckthorn fruit development. RNA Biol. 2019, 16, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Gandikota, M.; Birkenbihl, R.P.; Höhmann, S.; Cardon, G.H.; Saedler, H.; Huijser, P. The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J. 2007, 49, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Lian, C.; Han, S.; Huang, M.; Shen, C.; Li, Q.; Niu, M.; Yu, X.; Yin, W.; Xia, X. PtmiR169o plays a positive role in regulating drought tolerance and growth by targeting the PtNF-YA6 gene in poplar. Environ. Exp. Bot. 2021, 189, 104549. [Google Scholar] [CrossRef]
- Li, H.; Luo, Y.; Ma, B.; Hu, J.; Lv, Z.; Wei, W.; Hao, H.; Yuan, J.; He, N. Hierarchical Action of Mulberry miR156 in the Vegetative Phase Transition. Int. J. Mol. Sci. 2021, 22, 5550. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, D.; Li, L.; Zhang, Q.; Wang, S.; Huang, H. Identification of circular RNAs in kiwifruit and their species-specific response to bacterial canker pathogen invasion. Front. Plant Sci. 2017, 8, 413. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Zhang, Q.; Wei, X.; Huang, X. Exploring the molecular basis of heterosis for plant breeding. J. Integr. Plant Biol. 2020, 62, 287–298. [Google Scholar] [CrossRef]
- Chen, G.; Cui, J.; Wang, L.; Zhu, Y.; Lu, Z.; Jin, B. Genome-wide identification of circular RNAs in Arabidopsis thaliana. Front. Plant Sci. 2017, 8, 1678. [Google Scholar] [CrossRef]
- Zhao, T.; Wang, L.; Li, S.; Xu, M.; Guan, X.; Zhou, B. Characterization of conserved circular RNA in polyploid Gossypium species and their ancestors. FEBS Lett. 2017, 591, 3660–3669. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Yu, K.; Lü, H.; Zhang, X.; Liu, X.; Sun, C.; Xu, H.; Zhang, J.; He, X.; Zhang, D. Transcriptome-wide identification of novel circular RNAs in soybean in response to low-phosphorus stress. PLoS ONE 2020, 15, e0227243. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Gao, L.; Zhu, B.; Luo, Y.; Deng, Z.; Zuo, Z. Integrative analysis of circRNAs acting as ceRNAs involved in ethylene pathway in tomato. Physiol. Plant. 2017, 161, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Cheng, Y.; Zhang, C.; You, Q.; Shen, X.; Guo, W.; Jiao, Y. Genome-wide identification and characterization of circular RNAs by high throughput sequencing in soybean. Sci. Rep. 2017, 7, 5636. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Chen, X.; Wu, S.; Chen, X. Recent advances in understanding plant heterosis. Agric. Sci. 2015, 6, 1033. [Google Scholar] [CrossRef]
- Jones, D.F. Dominance of linked factors as a means of accounting for heterosis. Proc. Natl. Acad. Sci. USA 1917, 3, 310–312. [Google Scholar] [CrossRef]
- Baldauf, J.A.; Liu, M.; Vedder, L.; Yu, P.; Piepho, H.P.; Schoof, H.; Nettleton, D.; Hochholdinger, F. Single-parent expression complementation contributes to phenotypic heterosis in maize hybrids. Plant Physiol. 2022, 189, 1625–1638. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, S.; Xing, C.; Fan, S.; Song, M.; Ye, W. Differential gene expression between hybrids and their parents during the four crucial stages of cotton growth and development. Agric. Sci. China 2009, 8, 144–153. [Google Scholar] [CrossRef]
- Ekchaweng, K.; Khunjan, U.; Churngchow, N. Molecular cloning and characterization of three novel subtilisin-like serine protease genes from Hevea brasiliensis. Physiol. Mol. Plant Pathol. 2017, 97, 79–95. [Google Scholar] [CrossRef]
- Jia, X.; Wang, W.; Ren, L.; Chen, Q.; Mendu, V.; Willcut, B.; Dinkins, R.; Tang, X.; Tang, G. Differential and dynamic regulation of miR398 in response to ABA and salt stress in Populus tremula and Arabidopsis thaliana. Plant Mol. Biol. 2009, 71, 51–59. [Google Scholar] [CrossRef]
- Wang, J.; Park, M.; Wang, L.; Koo, Y.; Chen, X.; Weigel, D.; Poethig, R.S. miRNA control of vegetative phase change in trees. PLos Genet. 2011, 7, e1002012. [Google Scholar] [CrossRef]
- Cui, J.; Lu, W.; Lu, Z.; Ren, S.; Zhao, B.; Wang, L.; Teng, N.; Jin, B. Identification and analysis of microRNAs in the SAM and leaves of Populus tomentosa. Forests 2019, 10, 130. [Google Scholar] [CrossRef]
- Lawrence, E.H.; Leichty, A.R.; Doody, E.E.; Ma, C.; Strauss, S.H.; Poethig, R.S. Vegetative phase change in Populus tremula× alba. New Phytol. 2021, 231, 351–364. [Google Scholar] [CrossRef]
- Legnini, I.; Di Timoteo, G.F.; Morlando, M.; Briganti, F.; Sthandier, O.; Fatica, A.; Santini, T.; Andronache, A.; Wade, M.; Laneve, P.; et al. Circ-ZNF609 Is a Circular RNA that Can Be Translated and Functions in Myogenesis. Mol. Cell 2017, 66, 22–37. [Google Scholar] [CrossRef]
- Wang, X.; Jian, W.; Luo, Q.; Fang, L. CircSEMA4B inhibits the progression of breast cancer by encoding a novel protein SEMA4B-211aa and regulating AKT phosphorylation. Cell Death Dis. 2022, 13, 794. [Google Scholar] [CrossRef]
- Marquez-Molins, J.; Navarro, J.A.; Seco, L.C.; Pallas, V.; Gomez, G. Might exogenous circular RNAs act as protein-coding transcripts in plants? RNA Biol. 2021, 18, 98–107. [Google Scholar] [CrossRef]
- Liao, X.; Li, X.; Zheng, G.; Chang, F.; Fang, L.; Yu, H.; Huang, J.; Zhang, Y. Mitochondrion-encoded circular RNAs are widespread and translatable in plants. Plant Physiol. 2022, 189, 1482–1500. [Google Scholar] [CrossRef]
- Wang, X.; Chang, X.; Jing, Y.; Zhao, J.; Fang, Q.; Sun, M.; Zhang, Y.; Li, W.; Li, Y. Identification and functional prediction of soybean CircRNAs involved in low-temperature responses. J. Plant Physiol. 2020, 250, 153188. [Google Scholar] [CrossRef]
- Li, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Groszmann, M.; Gonzalez-Bayon, R.; Lyons, R.L.; Greaves, I.K.; Kazan, K.; Peacock, W.J.; Dennis, E.S. Hormone-regulated defense and stress response networks contribute to heterosis in Arabidopsis F1 hybrids. Proc. Natl. Acad. Sci. USA 2015, 112, E6397–E6406. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, W.; Gao, M.; Huang, Q.; Chu, Y.; Su, X. Analysis of Transcriptome Differences among Populus deltoides with Different Growth Potentials. Sci. Silvae Sin. 2016, 52, 47–58. [Google Scholar]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. Gigascience 2018, 7, gix120. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Zhao, F. CIRI: An efficient and unbiased algorithm for de novo circular RNA identification. Genome Biol. 2015, 16, 4. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Wu, H.; Ma, Y.; Chen, T.; Wang, M.; Wang, X. PsRobot: A web-based plant small RNA meta-analysis toolbox. Nucleic Acids Res. 2012, 40, W22–W28. [Google Scholar] [CrossRef]
- Fahlgren, N.; Carrington, J.C. miRNA Target Prediction in Plants; Humana Press: Clifton, America, 2010; pp. 51–57. [Google Scholar]
- Zhao, J.; Wu, J.; Xu, T.; Yang, Q.; He, J.; Song, X. IRESfinder: Identifying RNA internal ribosome entry site in eukaryotic cell using framed k-mer features. J. Genet. Genomics 2018, 45, 403–406. [Google Scholar] [CrossRef]
- Stupar, R.M.; Gardiner, J.M.; Oldre, A.G.; Haun, W.J.; Chandler, V.L.; Springer, N.M. Gene expression analyses in maize inbreds and hybrids with varying levels of heterosis. BMC Plant Biol. 2008, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinf. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Yuan, Z.; Zhang, J.; Su, X.; Huang, Q.; Liu, Q.; Ding, C. Identification and Functional Prediction of CircRNAs in Leaves of F1 Hybrid Poplars with Different Growth Potential and Their Parents. Int. J. Mol. Sci. 2023, 24, 2284. https://doi.org/10.3390/ijms24032284
Zhang W, Yuan Z, Zhang J, Su X, Huang Q, Liu Q, Ding C. Identification and Functional Prediction of CircRNAs in Leaves of F1 Hybrid Poplars with Different Growth Potential and Their Parents. International Journal of Molecular Sciences. 2023; 24(3):2284. https://doi.org/10.3390/ijms24032284
Chicago/Turabian StyleZhang, Weixi, Zhengsai Yuan, Jing Zhang, Xiaohua Su, Qinjun Huang, Qi Liu, and Changjun Ding. 2023. "Identification and Functional Prediction of CircRNAs in Leaves of F1 Hybrid Poplars with Different Growth Potential and Their Parents" International Journal of Molecular Sciences 24, no. 3: 2284. https://doi.org/10.3390/ijms24032284
APA StyleZhang, W., Yuan, Z., Zhang, J., Su, X., Huang, Q., Liu, Q., & Ding, C. (2023). Identification and Functional Prediction of CircRNAs in Leaves of F1 Hybrid Poplars with Different Growth Potential and Their Parents. International Journal of Molecular Sciences, 24(3), 2284. https://doi.org/10.3390/ijms24032284






