NGF and Its Role in Immunoendocrine Communication during Metabolic Syndrome
Abstract
1. Introduction
| Condition | NGF | Reference |
|---|---|---|
| Control F | Plasma levels of NGF vary throughout the hormonal cycle Early follicular phase—low levels Mid luteal phase—high levels | Martocchia et al., 2002 [19] |
| MS (F and M) | Plasma levels of NGF decrease in MS | Chaldakov, Fiore, et al., 2001 [20] |
| MS (F and M) | Plasma levels of NGF decrease in MS | Chaldakov et al., 2004 [12] |
| MS (F and M) | Plasma levels of NGF increase in early stages of MS Plasma levels of NGF decrease in late stage of MS | M. Hristova and Aloe, 2006 [13] |
| MS (F) | Plasma levels of NGF and subcutaneous adipose tissue expression of NGF increase in MS | Atanassova et al., 2014 [11] |
| MS (F) | Plasma levels of NGF increase in early stages of MS Plasma levels of NGF decrease in late stage of MS In MS patients with metformin treatment, plasma levels of NGF decrease In MS patients with metformin/aspirin/Diclac treatment, plasma levels of NGF increase | M. G. Hristova, 2011 [14] |
| MS (F) | Plasma levels of NGF increase in MS patients with overweight, obesity, or morbid obesity | Bulló et al., 2007 [21] |
| T2DM | Plasma levels of NGF decrease in T2DM Plasma levels of NGF decrease in T2DM + diabetic neuropathy | Sun et al., 2018 [22] |
| Obesity (F) (BMI > 30; hyperinsulinemia) | Plasma levels of NGF increase in obesity | Molnár, 2020 [23] |
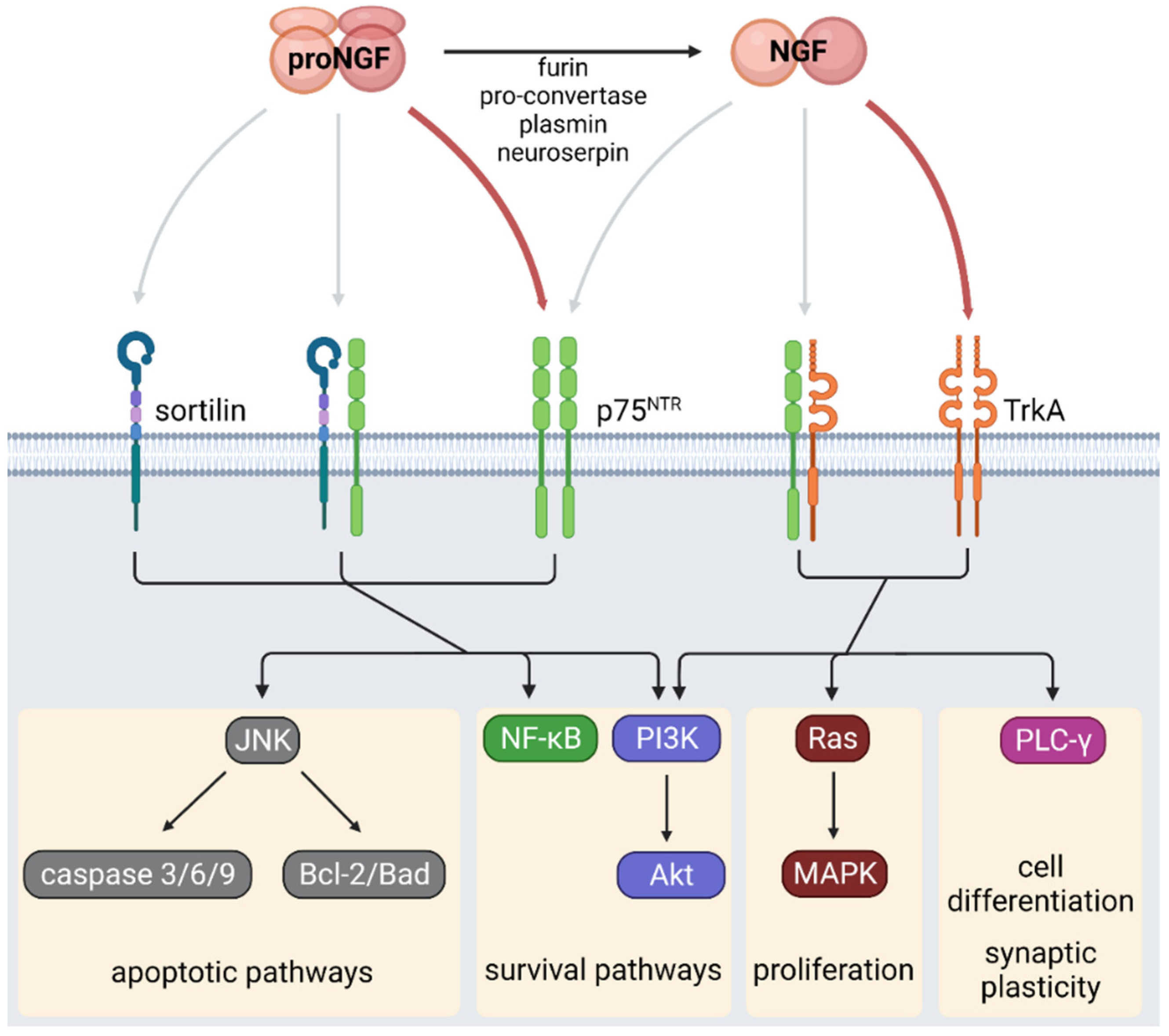
2. NGF Signaling
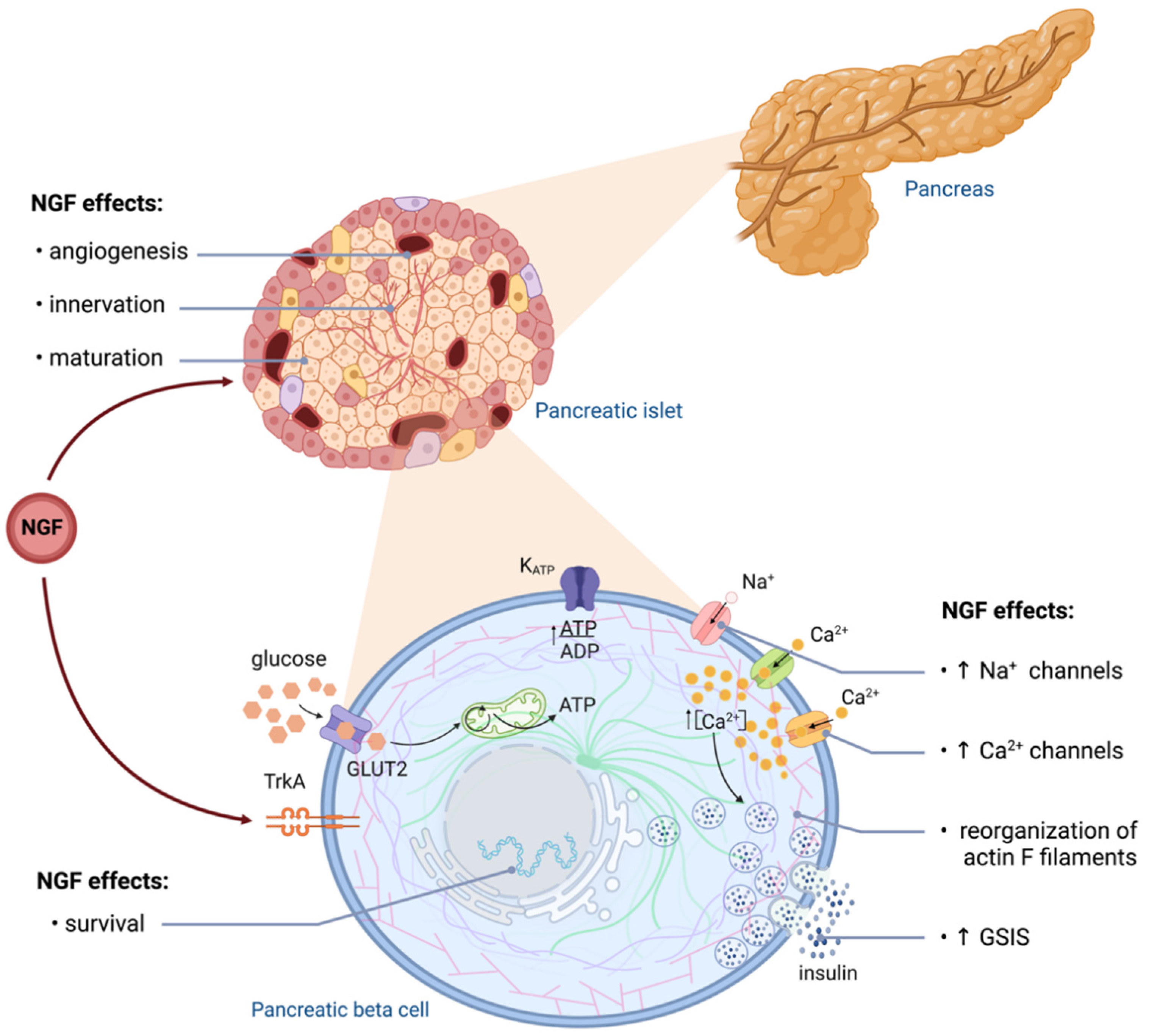
3. NGF in Pancreatic Beta Cells
| Cell Type | NGF | Receptor | Effects Possibly Related to the Development of MS |
|---|---|---|---|
| Pancreatic vascular cells | NGF expression and secretion [50] | NGF deletion impairs glucose tolerance and attenuates GSIS [50] | |
| Pancreatic beta cells | NGF expression and secretion [17,56] | TrkA and p75NTR [49,53] | Low NGF levels induce apoptosis [47,57] |
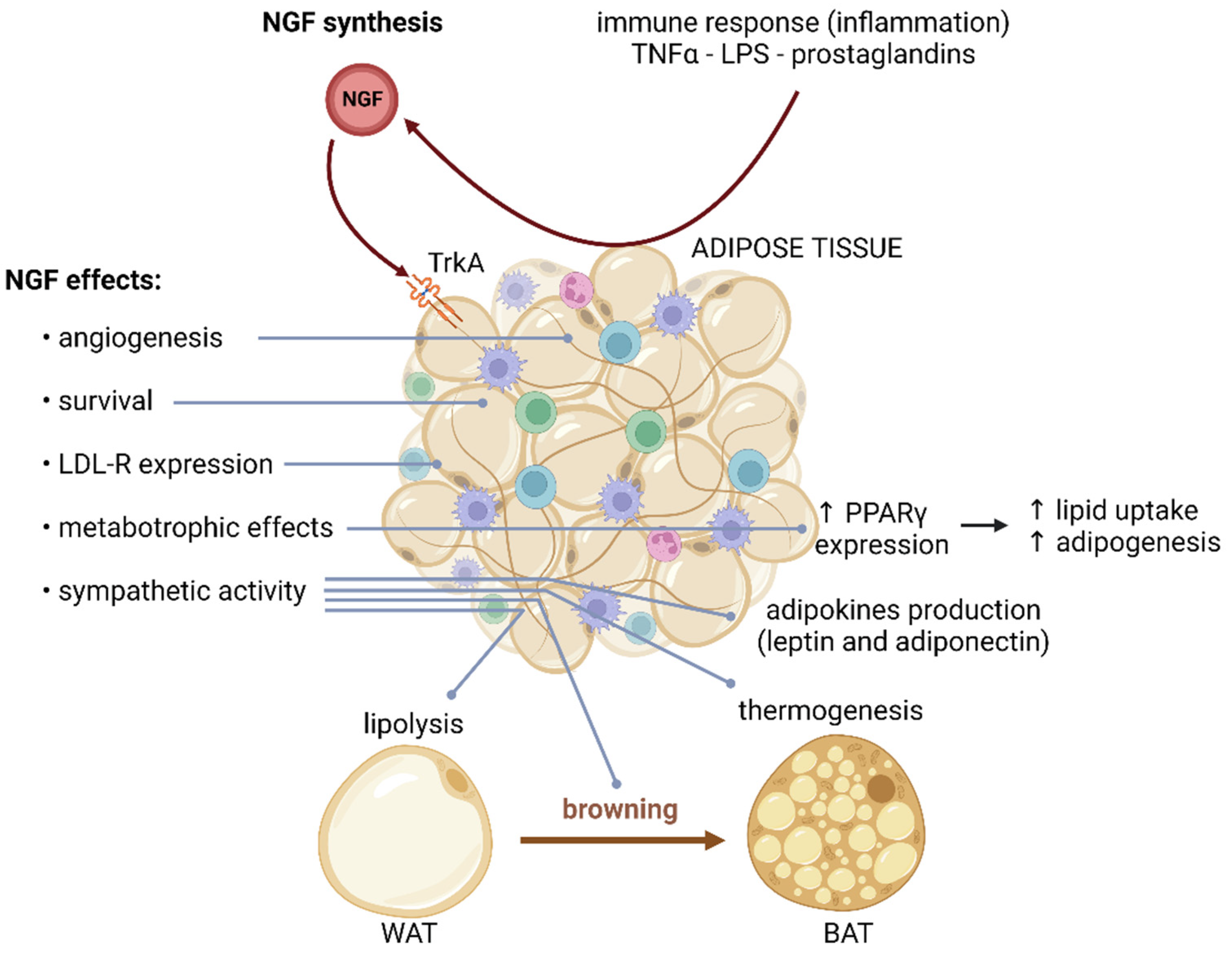
| WAT | NGF expression and secretion [8,58,59] | Stimulates lipolysis Induces browning Controls adipokine production Induces inflammatory response Upregulates PPARγ expression Regulates LDL receptor signaling [45,60] | |
| BAT | NGF expression and secretion NGF [60,61,62] | Induces thermogenesis [7,31] | |
| Adipocytes | NGF expression and secretion NGF | p75NTR [8,58,59] | Impair GLUT4 translocation Decrease lipolysis Induce resistance to catecholamines [45,46] |
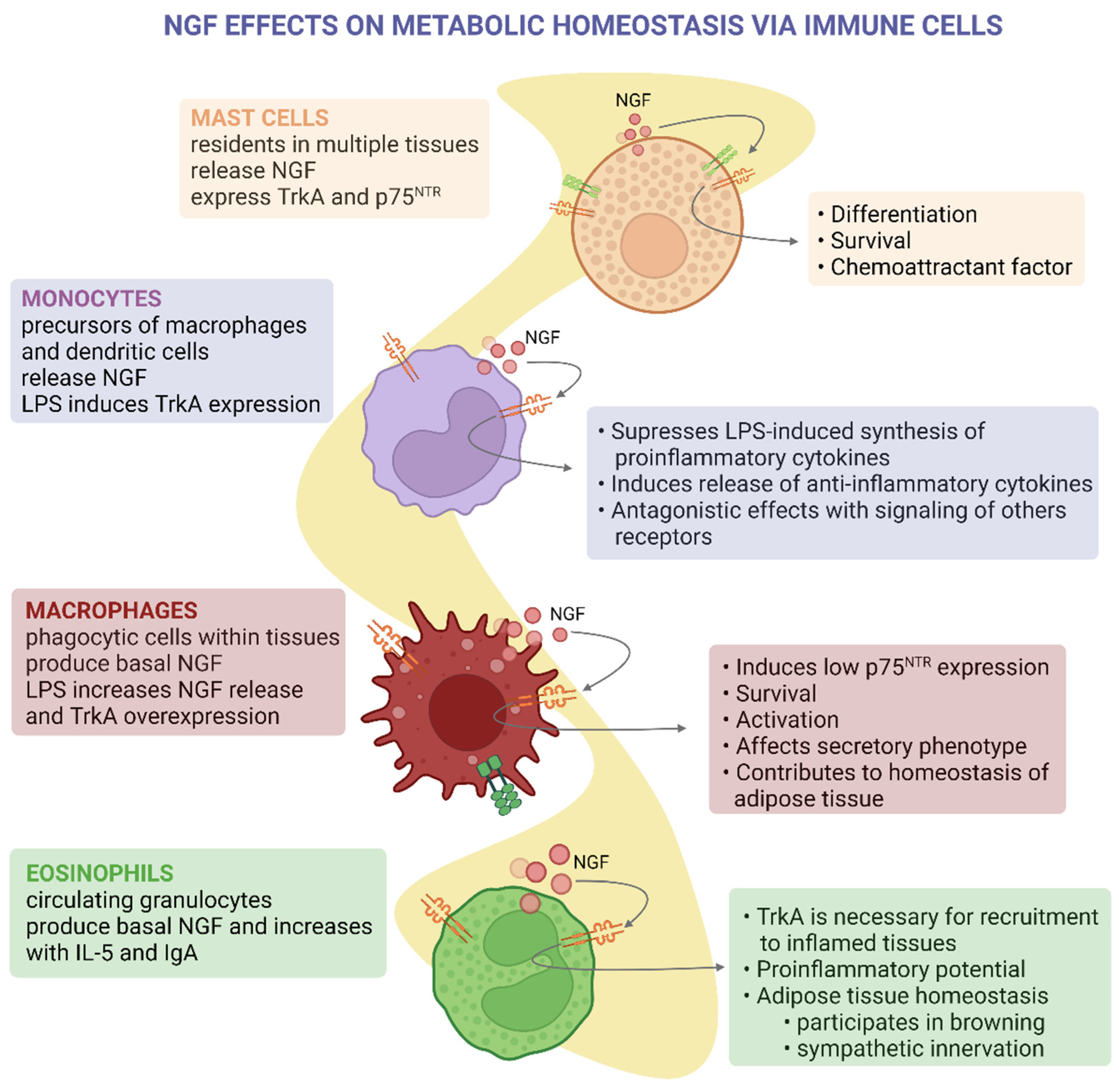
| Mast cells | NGF expression and secretion [63] of proteases involved in NGF maturation [64] | TrkA and p75NTR [64] | Mast cells residing in atherosclerotic lesions express high levels of P75NTR and low levels of NGF [65] |
| Monocytes | TrkA [66] | Modulate the secretion of LPS-induced cytokines [66,67] | |
| Macrophages | NGF expression and secretion NGF [68] | TrkA and P75NTR [68,69] | Resident in adipose tissue and modulate proinflammatory cytokines [70] |
| Eosinophils | NGF expression and secretion NGF [71] | TrkA [72,73] | During cold exposure, NGF secreted by WAT-residing eosinophils induces intra-adipose axon growth and adipocyte browning [74] |
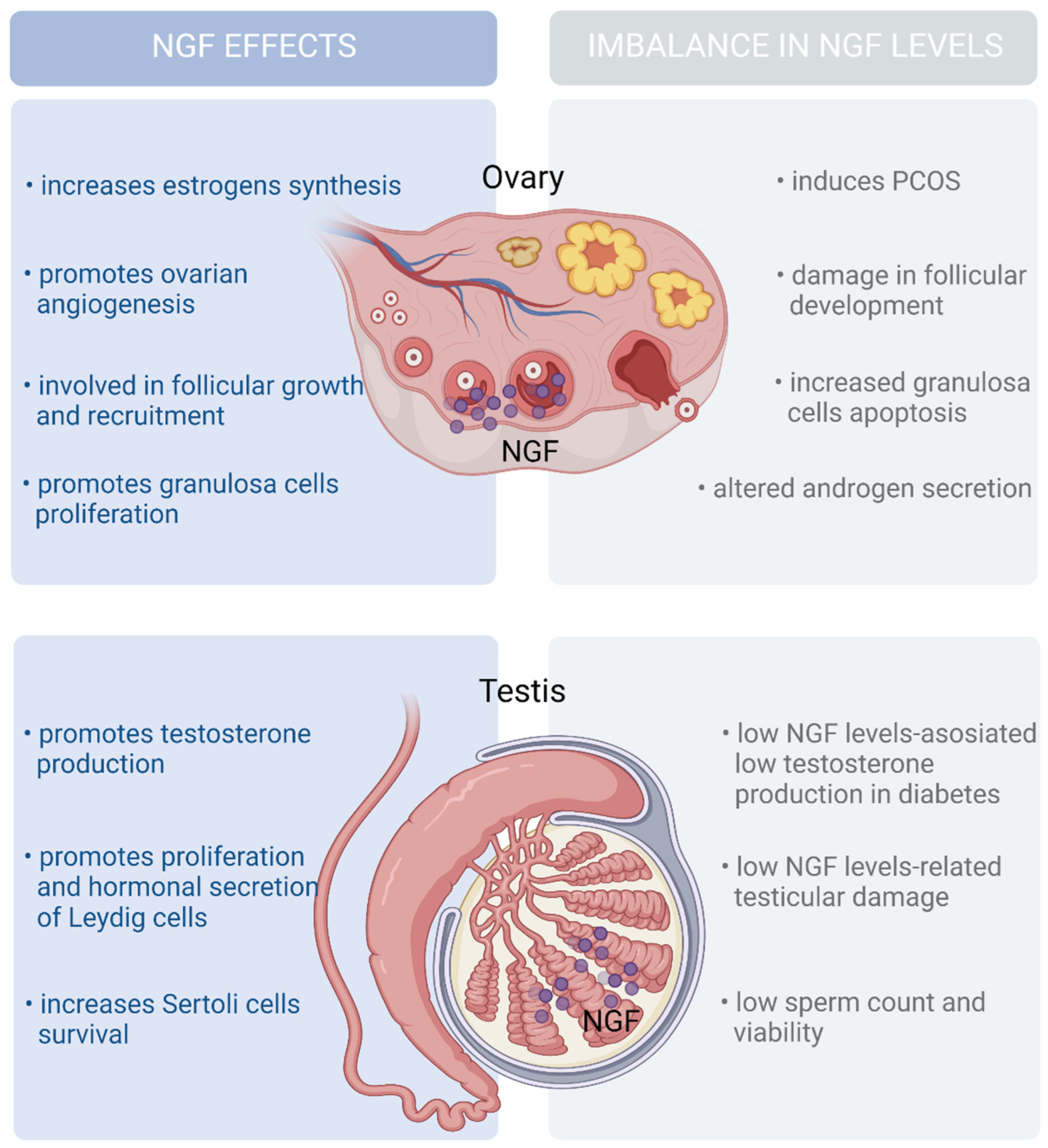
| Somatic/interstitial cells (ovary) | TrkA and P75NTR [75,76] | NGF induces follicular growth and androgen secretion by theca cells [76,77] | |
| Granulosa cells (ovary) | NGF expression and secretion, and it accumulates in the follicular fluid [75,76] | Hyperandrogenism has been linked to insulin resistance in the pathogenesis of PCOS [78,79] | |
| Somatic and meiotic germ cells (testis) | NGF expression and secretion [80,81,82] | P75NTR [80,81,82] | NGF induces steroid hormone synthesis [80,81,82] Low androgen production has been related to increased risk of developing T2DM [82] |
4. NGF in Adipose Tissue
5. Immunomodulatory Effects of NGF and Its Metabolic Implications
6. NGF in the Secretion of Sex Steroid Hormones
7. Discussion
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hiriart, M.; Velasco, M.; Larqué, C.; Diaz-Garcia, C.M. Chapter Four—Metabolic Syndrome and Ionic Channels in Pancreatic Beta Cells. In Vitamins & Hormones; Litwack, G., Ed.; The Pancreatic Beta Cell; Academic Press: Cambridge, MA, USA, 2014; Volume 95, pp. 87–114. [Google Scholar] [CrossRef]
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; Zito, M.C.; et al. From Metabolic Syndrome to Neurological Diseases: Role of Autophagy. Front Cell Dev. Biol. 2021, 9, 651021. [Google Scholar] [CrossRef] [PubMed]
- Pánico, P.; Velasco, M.; Salazar, A.M.; Picones, A.; Ortiz-Huidobro, R.I.; Guerrero-Palomo, G.; Salgado-Bernabé, M.E.; Ostrosky-Wegman, P.; Hiriart, M. Is Arsenic Exposure a Risk Factor for Metabolic Syndrome? A Review of the Potential Mechanisms. Front. Endocrinol. 2022, 13, 860. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic Syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Ortiz-Huidobro, R.I.; Larqué, C.; Sánchez-Zamora, Y.I.; Romo-Yáñez, J.; Hiriart, M. Sexual Dimorphism in Insulin Resistance in a Metabolic Syndrome Rat Model. Endocr. Connect. 2020, 9, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Larqué, C.; Velasco, M.; Navarro-Tableros, V.; Duhne, M.; Aguirre, J.; Gutiérrez-Reyes, G.; Moreno, J.; Robles-Diaz, G.; Hong, E.; Hiriart, M. Early Endocrine and Molecular Changes in Metabolic Syndrome Models. IUBMB Life 2011, 63, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Bulló, M.; Peeraully, M.R.; Trayhurn, P. Stimulation of NGF Expression and Secretion in 3T3-L1 Adipocytes by Prostaglandins PGD2, PGJ2, and Delta12-PGJ2. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E62–E67. [Google Scholar] [CrossRef] [PubMed]
- Chaldakov, G.N.; Tonchev, A.B.; Aloe, L. NGF and BDNF: From Nerves to Adipose Tissue, from Neurokines to Metabokines. Riv. Psichiatr. 2009, 44, 79–87. [Google Scholar]
- Grimble, R.F. Inflammatory status and insulin resistance. Curr. Opin. Clin. Nutr. Metab. Care. 2002, 5, 551–559. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and Metabolic Disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Atanassova, P.; Hrischev, P.; Orbetzova, M.; Nikolov, P.; Nikolova, J.; Georgieva, E. Expression of Leptin, NGF and Adiponectin in Metabolic Syndrome. Folia Biol. (Krakow) 2014, 62, 301–306. [Google Scholar] [CrossRef]
- Chaldakov, G.N.; Fiore, M.; Stankulov, I.S.; Manni, L.; Hristova, M.G.; Antonelli, A.; Ghenev, P.I.; Aloe, L. Neurotrophin Presence in Human Coronary Atherosclerosis and Metabolic Syndrome: A Role for NGF and BDNF in Cardiovascular Disease? In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2004; Volume 146, pp. 279–289. [Google Scholar] [CrossRef]
- Hristova, M.; Aloe, L. Metabolic Syndrome—Neurotrophic Hypothesis. Med. Hypotheses 2006, 66, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Hristova, M.G. Metabolic Syndrome and Neurotrophins: Effects of Metformin and Non-Steroidal Antiinflammatory Drug Treatment. Eurasian J. Med. 2011, 43, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Pingitore, A.; Caroleo, M.C.; Cione, E.; Castañera Gonzalez, R.; Huang, G.C.; Persaud, S.J. Fine Tuning of Insulin Secretion by Release of Nerve Growth Factor from Mouse and Human Islet β-Cells. Mol. Cell Endocrinol. 2016, 436, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Rocco, M.L.; Soligo, M.; Manni, L.; Aloe, L. Nerve Growth Factor: Early Studies and Recent Clinical Trials. Curr. Neuropharmacol. 2018, 16, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Vidaltamayo, R.; Sánchez-Soto, M.C.; Zentella, A.; Hiriart, M. Pancreatic Beta Cells Synthesize and Secrete Nerve Growth Factor. Proc. Natl. Acad. Sci. USA 1998, 95, 7784–7788. [Google Scholar] [CrossRef]
- Skaper, S.D. Nerve Growth Factor: A Neurokine Orchestrating Neuroimmune-Endocrine Functions. Mol. Neurobiol. 2001, 24, 183–199. [Google Scholar] [CrossRef]
- Martocchia, A.; Sigala, S.; Proietti, A.; D’Urso, R.; Spano, P.F.; Missale, C.; Falaschi, P. Sex-Related Variations in Serum Nerve Growth Factor Concentration in Humans. Neuropeptides 2002, 36, 391–395. [Google Scholar] [CrossRef]
- Chaldakov, G.N.; Fiore, M.; Stankulov, I.S.; Hristova, M.; Antonelli, A.; Manni, L.; Ghenev, P.I.; Angelucci, F.; Aloe, L. NGF, BDNF, Leptin, and Mast Cells in Human Coronary Atherosclerosis and Metabolic Syndrome. Arch. Physiol. Biochem. 2001, 109, 357–360. [Google Scholar] [CrossRef]
- Bulló, M.; Peeraully, M.R.; Trayhurn, P.; Folch, J.; Salas-Salvadó, J. Circulating Nerve Growth Factor Levels in Relation to Obesity and the Metabolic Syndrome in Women. Eur. J. Endocrinol. 2007, 157, 303–310. [Google Scholar] [CrossRef]
- Sun, Q.; Tang, D.-D.; Yin, E.-G.; Wei, L.-L.; Chen, P.; Deng, S.-P.; Tu, L.-L. Diagnostic Significance of Serum Levels of Nerve Growth Factor and Brain Derived Neurotrophic Factor in Diabetic Peripheral Neuropathy. Med. Sci. Monit 2018, 24, 5943–5950. [Google Scholar] [CrossRef]
- Molnár, I. Interactions among Thyroid Hormone (FT4), Chemokine (MCP-1) and Neurotrophin (NGF-β) Levels Studied in Hungarian Postmenopausal and Obese Women. Cytokine 2020, 127, 154948. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.A.; Cuello, A.C. Activity-Dependent Release of Precursor Nerve Growth Factor, Conversion to Mature Nerve Growth Factor, and Its Degradation by a Protease Cascade. Proc. Natl. Acad. Sci. USA 2006, 103, 6735–6740. [Google Scholar] [CrossRef] [PubMed]
- Seidah, N.G.; Benjannet, S.; Pareek, S.; Savaria, D.; Hamelin, J.; Goulet, B.; Laliberte, J.; Lazure, C.; Chrétien, M.; Murphy, R.A. Cellular Processing of the Nerve Growth Factor Precursor by the Mammalian Pro-Protein Convertases. Biochem. J. 1996, 314 Pt 3, 951–960. [Google Scholar] [CrossRef]
- Al-Shawi, R.; Hafner, A.; Olsen, J.; Olson, J.; Chun, S.; Raza, S.; Thrasivoulou, C.; Lovestone, S.; Killick, R.; Simons, P.; et al. Neurotoxic and Neurotrophic Roles of ProNGF and the Receptor Sortilin in the Adult and Ageing Nervous System. Eur. J. Neurosci. 2008, 27, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, S.; Kannel, B.; Cuello, A.C. The Nerve Growth Factor Metabolic Pathway Dysregulation as Cause of Alzheimer’s Cholinergic Atrophy. Cells 2021, 11, 16. [Google Scholar] [CrossRef]
- Mysona, B.A.; Matragoon, S.; Stephens, M.; Mohamed, I.N.; Farooq, A.; Bartasis, M.L.; Fouda, A.Y.; Shanab, A.Y.; Espinosa-Heidmann, D.G.; El-Remessy, A.B. Imbalance of the Nerve Growth Factor and Its Precursor as a Potential Biomarker for Diabetic Retinopathy. Biomed. Res. Int. 2015, 2015, 571456. [Google Scholar] [CrossRef]
- Fahnestock, M.; Yu, G.; Coughlin, M.D. ProNGF: A Neurotrophic or an Apoptotic Molecule? In Progress in Brain Research; NGF and Related Molecules in Health and Disease; Elsevier: Amsterdam, The Netherlands, 2004; Volume 146, pp. 101–110. [Google Scholar] [CrossRef]
- Lobos, E.; Gebhardt, C.; Kluge, A.; Spanel-Borowski, K. Expression of Nerve Growth Factor (NGF) Isoforms in the Rat Uterus during Pregnancy: Accumulation of Precursor ProNGF. Endocrinology 2005, 146, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Skaper, S.D.; Pollock, M.; Facci, L. Mast Cells Differentially Express and Release Active High Molecular Weight Neurotrophins. Mol. Brain Res. 2001, 97, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Deinhardt, K.; Chao, M.V. Trk Receptors. Handb Exp. Pharm. 2014, 220, 103–119. [Google Scholar] [CrossRef]
- Chao, M.V. Neurotrophins and Their Receptors: A Convergence Point for Many Signalling Pathways. Nat. Rev. Neurosci. 2003, 4, 299–309. [Google Scholar] [CrossRef]
- Settanni, G.; Cattaneo, A.; Carloni, P. Molecular Dynamics Simulations of the NGF-TrkA Domain 5 Complex and Comparison with Biological Data. Biophys. J. 2003, 84, 2282–2292. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.M.; Massa, S.M. Small-Molecule Modulation of Neurotrophin Receptors: A Strategy for the Treatment of Neurological Disease. Nat. Rev. Drug Discov. 2013, 12, 507–525. [Google Scholar] [CrossRef]
- Bucci, C.; Alifano, P.; Cogli, L. The Role of Rab Proteins in Neuronal Cells and in the Trafficking of Neurotrophin Receptors. Membranes 2014, 4, 642–677. [Google Scholar] [CrossRef] [PubMed]
- Colardo, M.; Martella, N.; Pensabene, D.; Siteni, S.; Di Bartolomeo, S.; Pallottini, V.; Segatto, M. Neurotrophins as Key Regulators of Cell Metabolism: Implications for Cholesterol Homeostasis. Int. J. Mol. Sci. 2021, 22, 5692. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D.R.; Miller, F.D. Neurotrophin Signal Transduction in the Nervous System. Curr. Opin. Neurobiol. 2000, 10, 381–391. [Google Scholar] [CrossRef]
- Geetha, T.; Zheng, C.; McGregor, W.C.; White, B.D.; Diaz-Meco, M.T.; Moscat, J.; Babu, J.R. TRAF6 and P62 Inhibit Amyloid β-Induced Neuronal Death through P75 Neurotrophin Receptor. Neurochem. Int. 2012, 61, 1289–1293. [Google Scholar] [CrossRef]
- Kisiswa, L.; Fernández-Suárez, D.; Sergaki, M.C.; Ibáñez, C.F. RIP2 Gates TRAF6 Interaction with Death Receptor P75NTR to Regulate Cerebellar Granule Neuron Survival. Cell Rep. 2018, 24, 1013–1024. [Google Scholar] [CrossRef]
- Skaper, S.D. Neurotrophic Factors: An Overview. Methods Mol. Biol. 2018, 1727, 1–17. [Google Scholar] [CrossRef]
- Gonçalves, N.P.; Yan, Y.; Ulrichsen, M.; Venø, M.T.; Poulsen, E.T.; Enghild, J.J.; Kjems, J.; Vægter, C.B. Modulation of Small RNA Signatures in Schwann-Cell-Derived Extracellular Vesicles by the P75 Neurotrophin Receptor and Sortilin. Biomedicines 2020, 8, 450. [Google Scholar] [CrossRef]
- Fahnestock, M.; Shekari, A. ProNGF and Neurodegeneration in Alzheimer’s Disease. Front Neurosci. 2019, 13, 129. [Google Scholar] [CrossRef]
- Niederhauser, O.; Mangold, M.; Schubenel, R.; Kusznir, E.A.; Schmidt, D.; Hertel, C. NGF Ligand Alters NGF Signaling via P75(NTR) and TrkA. J. Neurosci. Res. 2000, 61, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Baeza-Raja, B.; Li, P.; Le Moan, N.; Sachs, B.D.; Schachtrup, C.; Davalos, D.; Vagena, E.; Bridges, D.; Kim, C.; Saltiel, A.R.; et al. P75 Neurotrophin Receptor Regulates Glucose Homeostasis and Insulin Sensitivity. Proc. Natl. Acad. Sci. USA 2012, 109, 5838–5843. [Google Scholar] [CrossRef]
- Baeza-Raja, B.; Sachs, B.D.; Li, P.; Christian, F.; Vagena, E.; Davalos, D.; Le Moan, N.; Ryu, J.K.; Sikorski, S.L.; Chan, J.P.; et al. P75 Neurotrophin Receptor Regulates Energy Balance in Obesity. Cell Rep. 2016, 14, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Gezginci-Oktayoglu, S.; Bolkent, S. Ras Signaling in NGF Reduction and TNF-α-Related Pancreatic β Cell Apoptosis in Hyperglycemic Rats. Apoptosis 2012, 17, 14–24. [Google Scholar] [CrossRef]
- Almaça, J.; Caicedo, A.; Landsman, L. Beta Cell Dysfunction in Diabetes: The Islet Microenvironment as an Unusual Suspect. Diabetologia 2020, 63, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Sakata, N.; Yoshimatsu, G.; Tsuchiya, H.; Fukase, M.; Ishida, M.; Aoki, T.; Katayose, Y.; Egawa, S.; Unno, M. Nerve Growth Factor Improves Survival and Function of Transplanted Islets Via TrkA-Mediated β Cell Proliferation and Revascularization. Transplantation 2015, 99, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Houtz, J.; Borden, P.; Ceasrine, A.; Minichiello, L.; Kuruvilla, R. Neurotrophin Signaling Is Required for Glucose-Induced Insulin Secretion. Dev. Cell 2016, 39, 329–345. [Google Scholar] [CrossRef]
- Aguayo-Mazzucato, C.; Sanchez-Soto, C.; Godinez-Puig, V.; Gutiérrez-Ospina, G.; Hiriart, M. Restructuring of Pancreatic Islets and Insulin Secretion in a Postnatal Critical Window. PLoS ONE 2006, 1, e35. [Google Scholar] [CrossRef]
- Navarro-Tableros, V.; Fiordelisio, T.; Hernandez-Cruz, A.; Hiriart, M. Nerve Growth Factor Promotes Development of Glucose-Induced Insulin Secretion in Rat Neonate Pancreatic Beta Cells by Modulating Calcium Channels. Channels (Austin) 2007, 1, 408–416. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Sánchez-Soto, M.C.; Hiriart, M. Nerve Growth Factor Increases Insulin Secretion and Barium Current in Pancreatic Beta-Cells. Diabetes 2001, 50, 1755–1762. [Google Scholar] [CrossRef]
- Rosenbaum, T.; Castañares, D.T.; López-Valdés, H.E.; Hiriart, M. Nerve Growth Factor Increases L-Type Calcium Current in Pancreatic Beta Cells in Culture. J. Membr. Biol. 2002, 186, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Vidaltamayo, R.; Sánchez-Soto, M.C.; Hiriart, M. Nerve Growth Factor Increases Sodium Channel Expression in Pancreatic Beta Cells: Implications for Insulin Secretion. FASEB J. 2002, 16, 891–892. [Google Scholar] [CrossRef] [PubMed]
- Vidaltamayo, R.; Mery, C.M.; Angeles-Angeles, A.; Robles-Díaz, G.; Hiriart, M. Expression of Nerve Growth Factor in Human Pancreatic Beta Cells. Growth Factors 2003, 21, 103–107. [Google Scholar] [CrossRef]
- Gezginci-Oktayoglu, S.; Karatug, A.; Bolkent, S. The Relation among NGF, EGF and Insulin Is Important for Triggering Pancreatic β Cell Apoptosis. Diabetes Metab. Res. Rev. 2012, 28, 654–662. [Google Scholar] [CrossRef]
- Colitti, M.; Loor, J.J.; Stefanon, B. Expression of NGF, BDNF and Their Receptors in Subcutaneous Adipose Tissue of Lactating Cows. Res. Vet. Sci. 2015, 102, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Peeraully, M.R.; Jenkins, J.R.; Trayhurn, P. NGF Gene Expression and Secretion in White Adipose Tissue: Regulation in 3T3-L1 Adipocytes by Hormones and Inflammatory Cytokines. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E331–E339. [Google Scholar] [CrossRef]
- Frohlich, J.; Chaldakov, G.N.; Vinciguerra, M. Cardio- and Neurometabolic Adipobiology: Consequences and Implications for Therapy. Int. J. Mol. Sci. 2021, 22, 4137. [Google Scholar] [CrossRef]
- Camerino, C.; Conte, E.; Caloiero, R.; Fonzino, A.; Carratù, M.; Lograno, M.D.; Tricarico, D. Evaluation of Short and Long Term Cold Stress Challenge of Nerve Grow Factor, Brain-Derived Neurotrophic Factor, Osteocalcin and Oxytocin MRNA Expression in BAT, Brain, Bone and Reproductive Tissue of Male Mice Using Real-Time PCR and Linear Correlation Analysis. Front. Physiol. 2017, 8, 1101. [Google Scholar] [CrossRef]
- Münzberg, H.; Floyd, E.; Chang, J.S. Sympathetic Innervation of White Adipose Tissue: To Beige or Not to Beige? Physiology 2021, 36, 246–255. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, H.; Zeng, W. Whole-Tissue 3D Imaging Reveals Intra-Adipose Sympathetic Plasticity Regulated by NGF-TrkA Signal in Cold-Induced Beiging. Protein. Cell 2018, 9, 527–539. [Google Scholar] [CrossRef]
- Spinnler, K.; Fröhlich, T.; Arnold, G.J.; Kunz, L.; Mayerhofer, A. Human Tryptase Cleaves Pro-Nerve Growth Factor (Pro-NGF): HINTS OF LOCAL, MAST CELL-DEPENDENT REGULATION OF NGF/PRO-NGF ACTION *. J. Biol. Chem. 2011, 286, 31707–31713. [Google Scholar] [CrossRef]
- Chaldakov, G.N.; Stankulov, I.S.; Fiore, M.; Ghenev, P.I.; Aloe, L. Nerve Growth Factor Levels and Mast Cell Distribution in Human Coronary Atherosclerosis. Atherosclerosis 2001, 159, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Prencipe, G.; Minnone, G.; Strippoli, R.; De Pasquale, L.; Petrini, S.; Caiello, I.; Manni, L.; De Benedetti, F.; Bracci-Laudiero, L. Nerve Growth Factor Downregulates Inflammatory Response in Human Monocytes through TrkA. J. Immunol. 2014, 192, 3345–3354. [Google Scholar] [CrossRef]
- Datta-Mitra, A.; Kundu-Raychaudhuri, S.; Mitra, A.; Raychaudhuri, S.P. Cross Talk between Neuroregulatory Molecule and Monocyte: Nerve Growth Factor Activates the Inflammasome. PLoS ONE 2015, 10, e0121626. [Google Scholar] [CrossRef]
- Caroleo, M.C.; Costa, N.; Bracci-Laudiero, L.; Aloe, L. Human Monocyte/Macrophages Activate by Exposure to LPS Overexpress NGF and NGF Receptors. J. Neuroimmunol. 2001, 113, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.S.; Killebrew, D.A.; Clary, G.P.; Seawell, J.A.; Meeker, R.B. Differential Regulation of Macrophage Phenotype by Mature and Pro-Nerve Growth Factor. J. Neuroimmunol. 2015, 285, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What We Talk about When We Talk about Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Kobayashi, H.; Gleich, G.J.; Butterfield, J.H.; Kita, H. Human Eosinophils Produce Neurotrophins and Secrete Nerve Growth Factor on Immunologic Stimuli. Blood 2002, 99, 2214–2220. [Google Scholar] [CrossRef]
- Dileepan, M.; Ge, X.N.; Bastan, I.; Greenberg, Y.G.; Liang, Y.; Sriramarao, P.; Rao, S.P. Regulation of Eosinophil Recruitment and Allergic Airway Inflammation by Tropomyosin Receptor Kinase A. J. Immunol. 2020, 204, 682–693. [Google Scholar] [CrossRef]
- Ziogas, A.; Maekawa, T.; Wiessner, J.R.; Le, T.T.; Sprott, D.; Troullinaki, M.; Neuwirth, A.; Anastasopoulou, V.; Grossklaus, S.; Chung, K.-J.; et al. DHEA Inhibits Leukocyte Recruitment through Regulation of the Integrin Antagonist DEL-1. J. Immunol. 2020, 204, 1214–1224. [Google Scholar] [CrossRef]
- Meng, X.; Qian, X.; Ding, X.; Wang, W.; Yin, X.; Zhuang, G.; Zeng, W. Eosinophils Regulate Intra-Adipose Axonal Plasticity. Proc. Natl. Acad. Sci. USA 2022, 119, e2112281119. [Google Scholar] [CrossRef]
- Abir, R.; Fisch, B.; Jin, S.; Barnnet, M.; Ben-Haroush, A.; Felz, C.; Kessler-Icekson, G.; Feldberg, D.; Nitke, S.; Ao, A. Presence of NGF and Its Receptors in Ovaries from Human Fetuses and Adults. Mol. Hum. Reprod. 2005, 11, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Dissen, G.A.; Romero, C.; Hirshfield, A.N.; Ojeda, S.R. Nerve Growth Factor Is Required for Early Follicular Development in the Mammalian Ovary. Endocrinology 2001, 142, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.; Julio-Pieper, M.; Valladares, M.; Pommer, R.; Vega, M.; Mastronardi, C.; Kerr, B.; Ojeda, S.R.; Lara, H.E.; Romero, C. Nerve Growth Factor-Dependent Activation of TrkA Receptors in the Human Ovary Results in Synthesis of Follicle-Stimulating Hormone Receptors and Estrogen Secretion. J. Clin. Endocrinol. Metab. 2006, 91, 2396–2403. [Google Scholar] [CrossRef] [PubMed]
- Dissen, G.A.; Garcia-Rudaz, C.; Paredes, A.; Mayer, C.; Mayerhofer, A.; Ojeda, S.R. Excessive Ovarian Production of Nerve Growth Factor Facilitates Development of Cystic Ovarian Morphology in Mice and Is a Feature of Polycystic Ovarian Syndrome in Humans. Endocrinology 2009, 150, 2906–2914. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.L.; Chen, W.; Dissen, G.A.; Ojeda, S.R.; Cowley, M.A.; Garcia-Rudaz, C.; Enriori, P.J. Excess of Nerve Growth Factor in the Ovary Causes a Polycystic Ovary-like Syndrome in Mice, Which Closely Resembles Both Reproductive and Metabolic Aspects of the Human Syndrome. Endocrinology 2014, 155, 4494–4506. [Google Scholar] [CrossRef] [PubMed]
- Cupp, A.S.; Kim, G.H.; Skinner, M.K. Expression and Action of Neurotropin-3 and Nerve Growth Factor in Embryonic and Early Postnatal Rat Testis Development. Biol. Reprod. 2000, 63, 1617–1628. [Google Scholar] [CrossRef]
- Li, C.; Watanabe, G.; Weng, Q.; Jin, W.; Furuta, C.; Suzuki, A.K.; Kawaguchi, M.; Taya, K. Expression of Nerve Growth Factor (NGF), and Its Receptors TrkA and P75 in the Reproductive Organs of the Adult Male Rats. Zool. Sci. 2005, 22, 933–937. [Google Scholar] [CrossRef]
- Müller, D.; Davidoff, M.S.; Bargheer, O.; Paust, H.-J.; Pusch, W.; Koeva, Y.; Jezek, D.; Holstein, A.F.; Middendorff, R. The Expression of Neurotrophins and Their Receptors in the Prenatal and Adult Human Testis: Evidence for Functions in Leydig Cells. Histochem. Cell Biol. 2006, 126, 199–211. [Google Scholar] [CrossRef]
- Alejandro, E.U.; Gregg, B.; Blandino-Rosano, M.; Cras-Méneur, C.; Bernal-Mizrachi, E. Natural History of β-Cell Adaptation and Failure in Type 2 Diabetes. Mol. Asp. Med. 2015, 42, 19–41. [Google Scholar] [CrossRef]
- Wysham, C.; Shubrook, J. Beta-Cell Failure in Type 2 Diabetes: Mechanisms, Markers, and Clinical Implications. Postgrad. Med. 2020, 132, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Pierucci, D.; Cicconi, S.; Bonini, P.; Ferrelli, F.; Pastore, D.; Matteucci, C.; Marselli, L.; Marchetti, P.; Ris, F.; Halban, P.; et al. NGF-Withdrawal Induces Apoptosis in Pancreatic Beta Cells in Vitro. Diabetologia 2001, 44, 1281–1295. [Google Scholar] [CrossRef]
- Cheng, H.T.; Dauch, J.R.; Hayes, J.M.; Hong, Y.; Feldman, E.L. Nerve Growth Factor Mediates Mechanical Allodynia in a Mouse Model of Type 2 Diabetes. J. Neuropathol. Exp. Neurol. 2009, 68, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Larrieta, M.E.; Vital, P.; Mendoza-Rodríguez, A.; Cerbón, M.; Hiriart, M. Nerve Growth Factor Increases in Pancreatic Beta Cells after Streptozotocin-Induced Damage in Rats. Exp. Biol. Med. (Maywood) 2006, 231, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Sposato, V.; Manni, L.; Chaldakov, G.N.; Aloe, L. Streptozotocin-Induced Diabetes Is Associated with Changes in NGF Levels in Pancreas and Brain. Arch. Ital. Biol. 2007, 145, 87–97. [Google Scholar]
- Steckiewicz, K.P.; Barcińska, E.; Woźniak, M. Nerve Growth Factor as an Important Possible Component of Novel Therapy for Cancer, Diabetes and Cardiovascular Diseases. Cell. Mol. Biol. (Noisy-Le-Grand) 2018, 64, 16–23. [Google Scholar] [CrossRef]
- Fasshauer, M.; Blüher, M. Adipokines in Health and Disease. Trends. Pharm. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Scheja, L.; Heeren, J. The Endocrine Function of Adipose Tissues in Health and Cardiometabolic Disease. Nat. Rev. Endocrinol. 2019, 15, 507–524. [Google Scholar] [CrossRef]
- Vargas-Castillo, A.; Torres, N.; Tovar, A.R. Chapter 12—Endocrine Regulation of Brown and Beige Adipose Tissue. In Cellular Endocrinology in Health and Disease, 2nd ed.; Ulloa-Aguirre, A., Tao, Y.-X., Eds.; Academic Press: Boston, FL, USA, 2021; pp. 247–259. [Google Scholar] [CrossRef]
- Emont, M.P.; Jacobs, C.; Essene, A.L.; Pant, D.; Tenen, D.; Colleluori, G.; Di Vincenzo, A.; Jørgensen, A.M.; Dashti, H.; Stefek, A.; et al. A Single-Cell Atlas of Human and Mouse White Adipose Tissue. Nature 2022, 603, 926–933. [Google Scholar] [CrossRef]
- Romo-Yañez, J.; Velasco, M.; Larqué, C.; Chávez-Maldonado, J.P.; Hiriart, M. Differential Expression of NGF and BDNF in Rat Adipose Depots during Early Development and Adulthood. Adipobiology 2013, 5, 39. [Google Scholar] [CrossRef]
- Sornelli, F.; Fiore, M.; Chaldakov, G.N.; Aloe, L. Adipose Tissue-Derived Nerve Growth Factor and Brain-Derived Neurotrophic Factor: Results from Experimental Stress and Diabetes. Gen. Physiol. Biophys. 2009, 28, 179–183. [Google Scholar] [PubMed]
- Harrington, A.W.; Ginty, D.D. Long-Distance Retrograde Neurotrophic Factor Signalling in Neurons. Nat. Rev. Neurosci. 2013, 14, 177–187. [Google Scholar] [CrossRef]
- Nisoli, E.; Tonello, C.; Benarese, M.; Liberini, P.; Carruba, M.O. Expression of Nerve Growth Factor in Brown Adipose Tissue: Implications for Thermogenesis and Obesity. Endocrinology 1996, 137, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Estève, D.; Boulet, N.; Belles, C.; Zakaroff-Girard, A.; Decaunes, P.; Briot, A.; Veeranagouda, Y.; Didier, M.; Remaury, A.; Guillemot, J.C.; et al. Lobular Architecture of Human Adipose Tissue Defines the Niche and Fate of Progenitor Cells. Nat. Commun. 2019, 10, 2549. [Google Scholar] [CrossRef] [PubMed]
- Del Rey, A.; Besedovsky, H.O. Immune-Neuro-Endocrine Reflexes, Circuits, and Networks: Physiologic and Evolutionary Implications. Front. Horm. Res. 2017, 48, 1–18. [Google Scholar] [CrossRef]
- Morimoto, K.; Nakajima, K. Role of the Immune System in the Development of the Central Nervous System. Front. Neurosci. 2019, 13, 916. [Google Scholar] [CrossRef]
- Ryder, E.; Diez-Ewald, M.; Mosquera, J.; Fernández, E.; Pedreañez, A.; Vargas, R.; Peña, C.; Fernández, N. Association of Obesity with Leukocyte Count in Obese Individuals without Metabolic Syndrome. Diabetes Metab. Syndr. Clin. Res. Rev. 2014, 8, 197–204. [Google Scholar] [CrossRef]
- Andersen, C.J.; Murphy, K.E.; Fernandez, M.L. Impact of Obesity and Metabolic Syndrome on Immunity. Adv. Nutr. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Cao, Z.; Zheng, X.; Yang, H.; Li, S.; Xu, F.; Yang, X.; Wang, Y. Association of Obesity Status and Metabolic Syndrome with Site-Specific Cancers: A Population-Based Cohort Study. Br. J. Cancer 2020, 123, 1336–1344. [Google Scholar] [CrossRef]
- Ferlita, S.; Yegiazaryan, A.; Noori, N.; Lal, G.; Nguyen, T.; To, K.; Venketaraman, V. Type 2 Diabetes Mellitus and Altered Immune System Leading to Susceptibility to Pathogens, Especially Mycobacterium Tuberculosis. J. Clin. Med. 2019, 8, 2219. [Google Scholar] [CrossRef] [PubMed]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.; Buriani, A.; Dal Toso, R.; Fabris, M.; Romanello, S.; Aloe, L.; Levi-Montalcini, R. Mast Cells Synthesize, Store, and Release Nerve Growth Factor. Proc. Natl. Acad. Sci. USA 1994, 91, 3739–3743. [Google Scholar] [CrossRef] [PubMed]
- Bracci-Laudiero, L.; Aloe, L.; Caroleo, M.C.; Buanne, P.; Costa, N.; Starace, G.; Lundeberg, T. Endogenous NGF Regulates CGRP Expression in Human Monocytes, and Affects HLA-DR and CD86 Expression and IL-10 Production. Blood 2005, 106, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Sawada, J.; Itakura, A.; Tanaka, A.; Furusaka, T.; Matsuda, H. Nerve Growth Factor Functions as a Chemoattractant for Mast Cells through Both Mitogen-Activated Protein Kinase and Phosphatidylinositol 3-Kinase Signaling Pathways. Blood 2000, 95, 2052–2058. [Google Scholar] [CrossRef] [PubMed]
- Neuropeptides Activate Human Mast Cell Degranulation and Chemokine Production—Kulka—2008—Immunology—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2567.2007.02705.x (accessed on 4 July 2022).
- Zhang, J.; Shi, G.-P. Mast Cells and Metabolic Syndrome. Biochim. Biophys. Acta 2012, 1822, 14–20. [Google Scholar] [CrossRef]
- Teh, Y.C.; Ding, J.L.; Ng, L.G.; Chong, S.Z. Capturing the Fantastic Voyage of Monocytes through Time and Space. Front. Immunol. 2019, 10, 834. [Google Scholar] [CrossRef]
- Fain, J.N.; Tichansky, D.S.; Madan, A.K. Most of the Interleukin 1 Receptor Antagonist, Cathepsin S, Macrophage Migration Inhibitory Factor, Nerve Growth Factor, and Interleukin 18 Release by Explants of Human Adipose Tissue Is by the Non-Fat Cells, Not by the Adipocytes. Metabolism 2006, 55, 1113–1121. [Google Scholar] [CrossRef]
- Wen, T.; Rothenberg, M.E. The Regulatory Function of Eosinophils. Microbiol. Spectr. 2016, 4, 4–5. [Google Scholar] [CrossRef]
- Meinel, S.; Blohberger, J.; Berg, D.; Berg, U.; Dissen, G.A.; Ojeda, S.R.; Mayerhofer, A. Pro-Nerve Growth Factor in the Ovary and Human Granulosa Cells. Horm. Mol. Biol. Clin. Investig. 2015, 24, 91–99. [Google Scholar] [CrossRef]
- Azziz, R. Polycystic Ovary Syndrome. Obs. Gynecol. 2018, 132, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Buyuk, E.; Seifer, D.B. Follicular-Fluid Neurotrophin Levels in Women Undergoing Assisted Reproductive Technology for Different Etiologies of Infertility. Fertil. Steril. 2008, 90, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, F.Z.; Naghizadeh, M.M.; Bagheri, M.; Jafarabadi, M. Are CRH & NGF as Psychoneuroimmune Regulators in Women with Polycystic Ovary Syndrome? Gynecol. Endocrinol. 2017, 33, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Moghetti, P.; Tosi, F. Insulin Resistance and PCOS: Chicken or Egg? J. Endocrinol. Investig. 2021, 44, 233–244. [Google Scholar] [CrossRef]
- Skarra, D.V.; Hernández-Carretero, A.; Rivera, A.J.; Anvar, A.R.; Thackray, V.G. Hyperandrogenemia Induced by Letrozole Treatment of Pubertal Female Mice Results in Hyperinsulinemia Prior to Weight Gain and Insulin Resistance. Endocrinology 2017, 158, 2988–3003. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Yang, Y.; Liu, H.; Zhang, Q.; Xiang, Q.; Ge, R.; Su, Z.; Huang, Y. NGF Induces Adult Stem Leydig Cells to Proliferate and Differentiate during Leydig Cell Regeneration. Biochem. Biophys. Res. Commun. 2013, 436, 300–305. [Google Scholar] [CrossRef]
- Lönnerberg, P.; Söder, O.; Parvinen, M.; Martin Ritzén, E.; Persson, H. β-Nerve Growth Factor Influences the Expression of Androgen-Binding Protein Messenger Ribonucleic Acid in the Rat Testis1. Biol. Reprod. 1992, 47, 381–388. [Google Scholar] [CrossRef]
- Metsis, M.; Timmusk, T.; Allikmets, R.; Saarma, M.; Persson, H. Regulatory Elements and Transcriptional Regulation by Testosterone and Retinoic Acid of the Rat Nerve Growth Factor Receptor Promoter. Gene 1992, 121, 247–254. [Google Scholar] [CrossRef]
- Schultz, R.; Metsis, M.; Hökfelt, T.; Parvinen, M.; Pelto-Huikko, M. Expression of Neurotrophin Receptors in Rat Testis. Upregulation of TrkA MRNA with HCG Treatment. Mol. Cell. Endocrinol. 2001, 182, 121–127. [Google Scholar] [CrossRef]
- Persson, H.; Ayer-Le Lievre, C.; Söder, O.; Villar, M.J.; Metsis, M.; Olson, L.; Ritzen, M.; Hökfelt, T. Expression of Beta-Nerve Growth Factor Receptor MRNA in Sertoli Cells Downregulated by Testosterone. Science 1990, 247, 704–707. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, C.; Meng, F.; Que, F.; Xiao, W.; Rao, H.; Wan, Y.; Taylor, H.S.; Lu, L. Nerve Growth Factor Improves the Outcome of Type 2 Diabetes—Induced Hypotestosteronemia and Erectile Dysfunction. Reprod. Sci. 2019, 26, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Seeley, R.J.; Clegg, D.J. Sexual Differences in the Control of Energy Homeostasis. Front. Neuroendocrinol. 2009, 30, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Larqué, C.; Velasco, M.; Barajas-Olmos, F.; García-Delgado, N.; Chávez-Maldonado, J.P.; García-Morales, J.; Orozco, L.; Hiriart, M. Transcriptome Landmarks of the Functional Maturity of Rat Beta-Cells, from Lactation to Adulthood. J. Mol. Endocrinol. 2016, 57, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Velasco, M.; Larqué, C.; Gutiérrez-Reyes, G.; Arredondo, R.; Sanchez-Soto, C.; Hiriart, M. Metabolic Syndrome Induces Changes in KATP-Channels and Calcium Currents in Pancreatic β-Cells. Islets 2012, 4, 302–311. [Google Scholar] [CrossRef]
- Ortiz-Huidobro, R.I.; Velasco, M.; Larqué, C.; Escalona, R.; Hiriart, M. Molecular Insulin Actions Are Sexually Dimorphic in Lipid Metabolism. Front. Endocrinol. 2021, 18, 12. [Google Scholar] [CrossRef]
- Ortiz-Huidobro, R.I.; Larqué, C.; Velasco, M.; Chávez-Maldonado, J.P.; Sabido, J.; Sanchez-Zamora, Y.I.; Hiriart, M. Sexual Dimorphism in the Molecular Mechanisms of Insulin Resistance during a Critical Developmental Window in Wistar Rats. Cell. Commun. Signal. 2022, 20, 154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samario-Román, J.; Larqué, C.; Pánico, P.; Ortiz-Huidobro, R.I.; Velasco, M.; Escalona, R.; Hiriart, M. NGF and Its Role in Immunoendocrine Communication during Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 1957. https://doi.org/10.3390/ijms24031957
Samario-Román J, Larqué C, Pánico P, Ortiz-Huidobro RI, Velasco M, Escalona R, Hiriart M. NGF and Its Role in Immunoendocrine Communication during Metabolic Syndrome. International Journal of Molecular Sciences. 2023; 24(3):1957. https://doi.org/10.3390/ijms24031957
Chicago/Turabian StyleSamario-Román, Jazmín, Carlos Larqué, Pablo Pánico, Rosa Isela Ortiz-Huidobro, Myrian Velasco, Rene Escalona, and Marcia Hiriart. 2023. "NGF and Its Role in Immunoendocrine Communication during Metabolic Syndrome" International Journal of Molecular Sciences 24, no. 3: 1957. https://doi.org/10.3390/ijms24031957
APA StyleSamario-Román, J., Larqué, C., Pánico, P., Ortiz-Huidobro, R. I., Velasco, M., Escalona, R., & Hiriart, M. (2023). NGF and Its Role in Immunoendocrine Communication during Metabolic Syndrome. International Journal of Molecular Sciences, 24(3), 1957. https://doi.org/10.3390/ijms24031957











