Cadmium-Induced Proteinuria: Mechanistic Insights from Dose–Effect Analyses
Abstract
1. Introduction
2. Results
2.1. Descriptive Characteristics of Study Subjects According to eGFR
2.2. Predictors of Protein Excretion
2.3. Predictors of eGFR Deterioration
2.4. Prevalence Odds Ratios for Proteinuria and Low eGFR in Relation to Cadmium Exposure
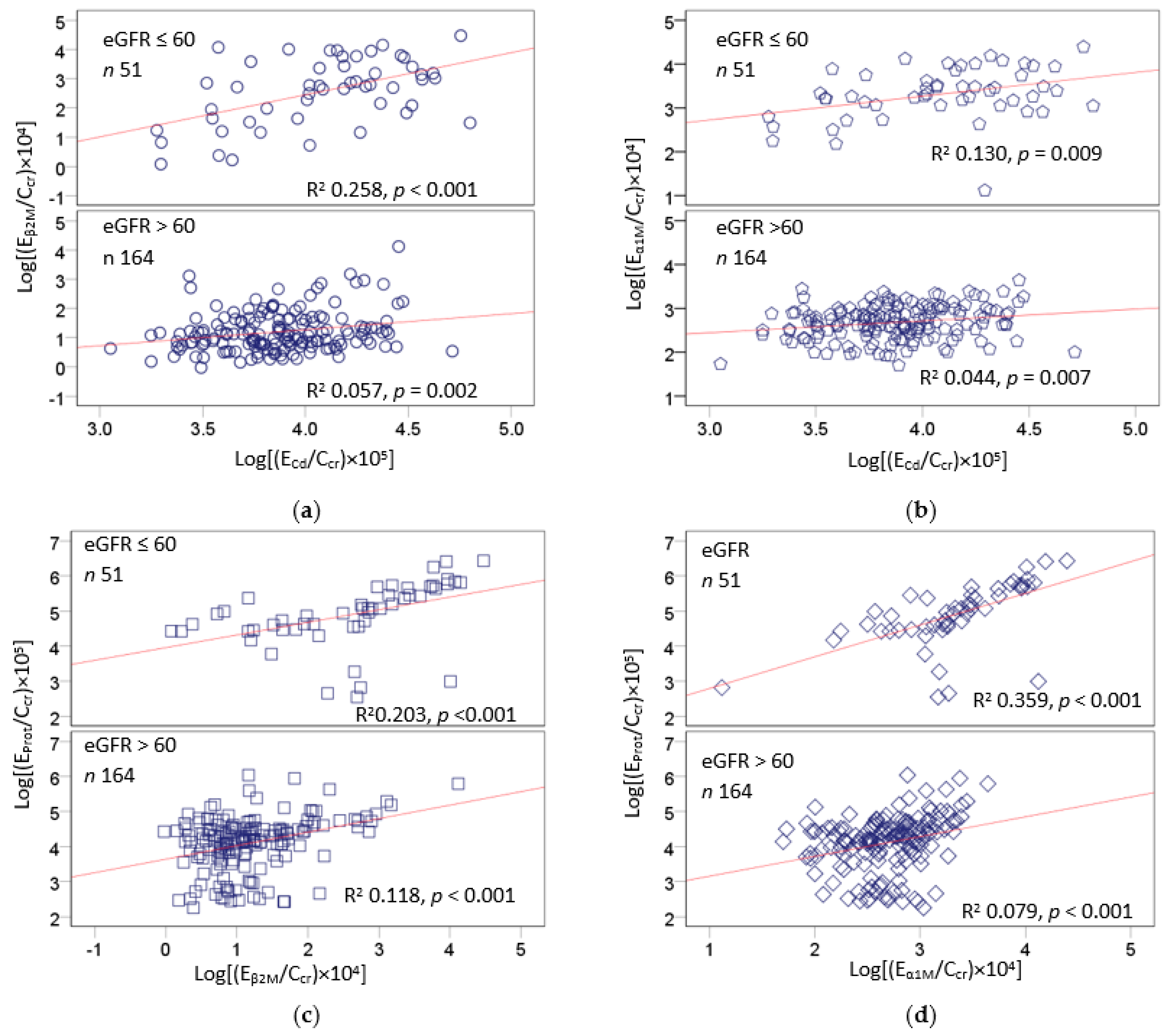
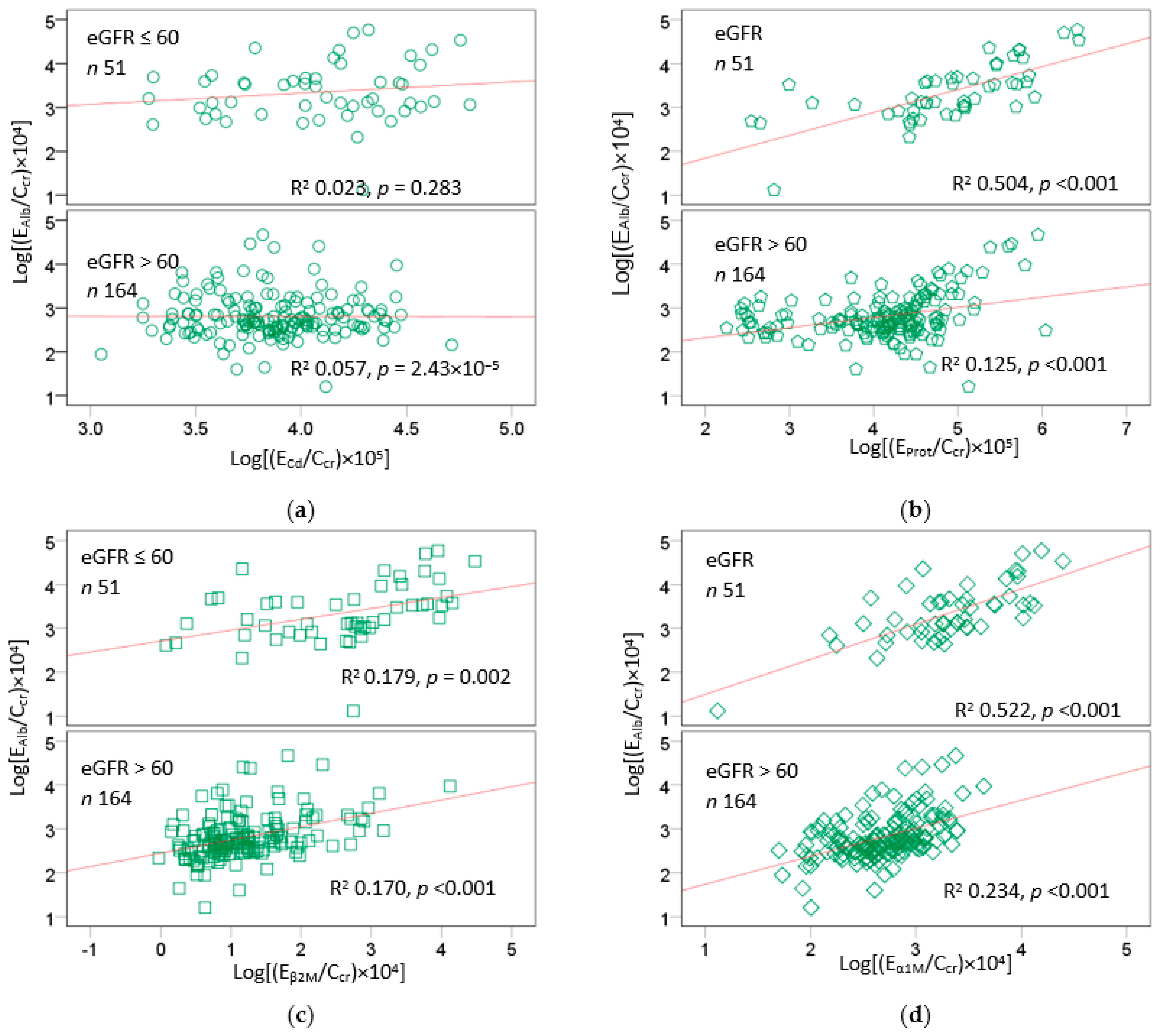
2.5. Excretion Rates of Various Proteins and Cadmum in the High-Exposure Group
2.6. Correlation Analysis of Age, BMI, and Chemical Constiuents of Urine
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Collection and Analysis of Blood and Urine Samples
4.3. Estimated Glomerular Filtration Rates and CKD Stratified by the KDIGO Categories
4.4. Normalization of ECd to Ecr and Ccr
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Satarug, S.; Gobe, G.C.; Vesey, D.A. Multiple targets of toxicity in environmental exposure to low-dose cadmium. Toxics 2022, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Vesey, D.A.; Gobe, G.C.; Phelps, K.R. Estimation of health risks associated with dietary cadmium exposure. Arch. Toxicol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Browar, A.W.; Leavitt, L.L.; Prozialeck, W.C.; Edwards, J.R. Levels of cadmium in human mandibular bone. Toxics 2019, 7, 31. [Google Scholar] [CrossRef]
- Branca, J.J.V.; Fiorillo, C.; Carrino, D.; Paternostro, F.; Taddei, N.; Gulisano, M.; Pacini, A.; Becatti, M. Cadmium-induced oxidative stress: Focus on the central nervous system. Antioxidants 2020, 9, 492. [Google Scholar] [CrossRef]
- Satarug, S. Long-term exposure to cadmium in food and cigarette smoke, liver effects and hepatocellular carcinoma. Curr. Drug Metab. 2012, 13, 257–271. [Google Scholar] [CrossRef]
- Skipper, A.; Sims, J.N.; Yedjou, C.G.; Tchounwou, P.B. Cadmium chloride induces DNA damage and apoptosis of human liver carcinoma cells via oxidative stress. Int. J. Environ. Res. Public Health 2016, 13, 88. [Google Scholar] [CrossRef]
- Demchenkov, E.L.; Nagdalian, A.A.; Budkevich, R.O.; Oboturova, N.P.; Okolelova, A.I. Usage of atomic force microscopy for detection of the damaging effect of CdCl2 on red blood cells membrane. Ecotoxicol. Environ. Saf. 2021, 208, 111683. [Google Scholar] [CrossRef]
- Molitoris, B.A.; Sandoval, R.M.; Yadav, S.P.S.; Wagner, M.C. Albumin uptake and processing by the proximal tubule: Physiological, pathological, and therapeutic implications. Physiol. Rev. 2022, 102, 1625–1667. [Google Scholar] [CrossRef]
- Eshbach, M.L.; Weisz, O.A. Receptor-mediated endocytosis in the proximal tubule. Annu. Rev. Physiol. 2017, 79, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Bökenkamp, A. Proteinuria-take a closer look! Pediatr. Nephrol. 2020, 35, 533–541. [Google Scholar] [CrossRef]
- Comper, W.D.; Vuchkova, J.; McCarthy, K.J. New insights into proteinuria/albuminuria. Front. Physiol. 2022, 13, 991756. [Google Scholar] [CrossRef] [PubMed]
- Gburek, J.; Konopska, B.; Gołąb, K. Renal handling of albumin-from early findings to current concepts. Int. J. Mol. Sci. 2021, 22, 5809. [Google Scholar] [CrossRef]
- Benzing, T.; Salant, D. Insights into glomerular filtration and albuminuria. N. Engl. J. Med. 2021, 384, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.; Long, K.R.; Baty, C.J.; Shipman, K.E.; Weisz, O.A. Modelling normal and nephrotic axial uptake of albumin and other filtered proteins along the proximal tubule. J. Physiol. 2022, 600, 1933–1952. [Google Scholar] [CrossRef]
- Langelueddecke, C.; Roussa, E.; Fenton, R.A.; Wolff, N.A.; Lee, W.K.; Thévenod, F. Lipocalin-2 (24p3/neutrophil gelatinase-associated lipocalin (NGAL) receptor is expressed in distal nephron and mediates protein endocytosis. J. Biol. Chem. 2012, 287, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Dizi, E.; Hasler, U.; Nlandu-Khodo, S.; Fila, M.; Roth, I.; Ernandez, T.; Doucet, A.; Martin, P.Y.; Feraille, E.; de Seigneux, S. Albuminuria induces a proinflammatory and profibrotic response in cortical collecting ducts via the 24p3 receptor. Am. J. Physiol. Renal Physiol. 2013, 305, F1053–F1063. [Google Scholar] [CrossRef] [PubMed]
- Castrop, H.; Schießl, I.M. Novel routes of albumin passage across the glomerular filtration barrier. Acta Physiol. 2017, 219, 544–553. [Google Scholar] [CrossRef]
- Soveri, I.; Berg, U.B.; Björk, J.; Elinder, C.G.; Grubb, A.; Mejare, I.; Sterner, G.; Bäck, S.E.; SBU GFR Review Group. Measuring GFR: A systematic review. Am. J. Kidney Dis. 2014, 64, 411–424. [Google Scholar] [CrossRef]
- Murton, M.; Goff-Leggett, D.; Bobrowska, A.; Garcia Sanchez, J.J.; James, G.; Wittbrodt, E.; Nolan, S.; Sörstadius, E.; Pecoits-Filho, R.; Tuttle, K. Burden of chronic kidney disease by KDIGO categories of glomerular filtration rate and albuminuria: A Systematic review. Adv. Ther. 2021, 38, 180–200. [Google Scholar] [CrossRef]
- Levey, A.S.; Becker, C.; Inker, L.A. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: A systematic review. JAMA 2015, 313, 837–846. [Google Scholar] [CrossRef]
- Argyropoulos, C.P.; Chen, S.S.; Ng, Y.H.; Roumelioti, M.E.; Shaffi, K.; Singh, P.P.; Tzamaloukas, A.H. Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front. Med. 2017, 4, 73. [Google Scholar] [CrossRef] [PubMed]
- Portman, R.J.; Kissane, J.M.; Robson, A.M. Use of B2-microglobulin to diagnose tubulo-interstitial renal lesions in children. Kidney Int. 1986, 30, 91–98. [Google Scholar] [CrossRef]
- Gauthier, C.; Nguyen-Simonnet, H.; Vincent, C.; Revillard, J.-P.; Pellet, M.V. Renal tubular absorption of beta 2 micro-globulin. Kidney Int. 1984, 26, 170–175. [Google Scholar] [CrossRef]
- Satarug, S.; Vesey, D.A.; Nishijo, M.; Ruangyuttikarn, W.; Gobe, G.C. The inverse association of glomerular function and urinary β2-MG excretion and its implications for cadmium health risk assessment. Environ. Res. 2019, 173, 40–47. [Google Scholar] [CrossRef]
- Phelps, K.R.; Gosmanova, E.O. A generic method for analysis of plasma concentrations. Clin. Nephrol. 2020, 94, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Santoyo-Sánchez, M.P.; Pedraza-Chaverri, J.; Molina-Jijón, E.; Arreola-Mendoza, L.; Rodríguez-Muñoz, R.; Barbier, O.C. Impaired endocytosis in proximal tubule from subchronic exposure to cadmium involves angiotensin II type 1 and cubilin receptors. BMC Nephrol. 2013, 14, 211. [Google Scholar] [CrossRef]
- Gena, P.; Calamita, G.; Guggino, W.B. Cadmium impairs albumin reabsorption by down-regulating megalin and ClC5 channels in renal proximal tubule cells. Environ. Health Perspect. 2010, 118, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Dong, F.; Xu, D.; Du, L.; Yan, S.; Hu, H.; Lobe, C.G.; Yi, F.; Kapron, C.M.; Liu, J. Short-term, low-dose cadmium exposure induces hyperpermeability in human renal glomerular endothelial cells. J. Appl. Toxicol. 2016, 36, 257–265. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, L.; Tao, T.; Su, W.; Guo, Y.; Yu, H.; Qin, J. Assessment of cadmium-induced nephrotoxicity using a kidney-on-a-chip device. Toxicol. Res. 2017, 6, 372–380. [Google Scholar] [CrossRef]
- Nichols, G.A.; Déruaz-Luyet, A.; Brodovicz, K.G.; Kimes, T.M.; Rosales, A.G.; Hauske, S.J. Kidney disease progression and all-cause mortality across estimated glomerular filtration rate and albuminuria categories among patients with vs. without type 2 diabetes. BMC Nephrol. 2020, 21, 167. [Google Scholar]
- George, C.; Mogueo, A.; Okpechi, I.; Echouffo-Tcheugui, J.B.; Kengne, A.P. Chronic kidney disease in low-income to middle-income countries: Case Increased Screening. BMJ Glob Health. 2017, 2, e000256. [Google Scholar] [CrossRef]
- George, C.; Echouffo-Tcheugui, J.B.; Jaar, B.G.; Okpechi, I.G.; Kengne, A.P. The need for screening, early diagnosis, and prediction of chronic kidney disease in people with diabetes in low- and middle-income countries-a review of the current literature. BMC Med. 2022, 20, 247. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.M.; Costanzi, S.; Naticchia, A.; Sturniolo, A.; Gambaro, G. Low level exposure to cadmium increases the risk of chronic kidney disease: Analysis of the NHANES 1999–2006. BMC Publ. Health 2010, 10, 304. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Tellez-Plaza, M.; Guallar, E.; Muntner, P.; Silbergeld, E.; Jaar, B.; Weaver, V. Blood cadmium and lead and chronic kidney disease in US adults: A joint analysis. Am. J. Epidemiol. 2009, 170, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Ho, W.C.; Caffrey, J.L.; Sonawane, B. Low serum zinc is associated with elevated risk of cadmium nephrotoxicity. Environ. Res. 2014, 134, 33–38. [Google Scholar]
- Madrigal, J.M.; Ricardo, A.C.; Persky, V.; Turyk, M. Associations between blood cadmium concentration and kidney function in the U.S. population: Impact of sex, diabetes and hypertension. Environ. Res. 2018, 169, 180–188. [Google Scholar] [CrossRef]
- Zhu, X.J.; Wang, J.J.; Mao, J.H.; Shu, Q.; Du, L.Z. Relationships of cadmium, lead, and mercury levels with albuminuria in US adults: Results from the National Health and Nutrition Examination Survey Database, 2009–2012. Am. J. Epidemiol. 2019, 188, 1281–1287. [Google Scholar] [CrossRef]
- Grau-Perez, M.; Pichler, G.; Galan-Chilet, I.; Briongos-Figuero, L.S.; Rentero-Garrido, P.; Lopez-Izquierdo, R.; Navas-Acien, A.; Weaver, V.; García-Barrera, T.; Gomez-Ariza, J.L.; et al. Urine cadmium levels and albuminuria in a general population from Spain: A gene-environment interaction analysis. Environ. Int. 2017, 106, 27–36. [Google Scholar] [CrossRef]
- Feng, X.; Zhou, R.; Jiang, Q.; Wang, Y.; Chen, C. Analysis of cadmium accumulation in community adults and its correlation with low-grade albuminuria. Sci. Total Environ. 2022, 834, 155210. [Google Scholar] [CrossRef]
- Jalili, C.; Kazemi, M.; Cheng, H.; Mohammadi, H.; Babaei, A.; Taheri, E.; Moradi, S. Associations between exposure to heavy metals and the risk of chronic kidney disease: A systematic review and meta-analysis. Crit. Rev. Toxicol. 2021, 51, 165–182. [Google Scholar] [CrossRef]
- Thijssen, S.; Lambrichts, I.; Maringwa, J.; Van Kerkhove, E. Changes in expression of fibrotic markers and histopathological alterations in kidneys of mice chronically exposed to low and high Cd doses. Toxicology 2007, 238, 200–210. [Google Scholar] [CrossRef]
- Liang, L.; Huang, K.; Yuan, W.; Liu, L.; Zou, F.; Wang, G. Dysregulations of miR-503-5p and Wnt/β-catenin pathway coordinate in mediating cadmium-induced kidney fibrosis. Ecotoxicol. Environ. Saf. 2021, 224, 112667. [Google Scholar] [CrossRef] [PubMed]
- Gobe, G.C.; Mott, S.A.; de Jonge, M.; Hoy, W.E. Heavy metal imaging in fibrotic human kidney tissue using the synchrotron X-ray fluorescence microprobe. Transl. Androl. Urol. 2019, 8, S184–S191. [Google Scholar] [CrossRef]
- Barregard, L.; Sallsten, G.; Lundh, T.; Mölne, J. Low-level exposure to lead, cadmium and mercury, and histopathological findings in kidney biopsies. Environ. Res. 2022, 211, 113119. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Nogawa, K.; Suwazono, Y.; Kido, T.; Sakurai, M.; Nakagawa, H. Lifetime cadmium exposure and mortality for renal diseases in residents of the cadmium-polluted Kakehashi River Basin in Japan. Toxics 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Satarug, S.; Swaddiwudhipong, W.; Ruangyuttikarn, W.; Nishijo, M.; Ruiz, P. Modeling cadmium exposures in low- and high-exposure areas in Thailand. Environ. Health Perspect. 2013, 121, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.W.; Reed, L.D. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 1990, 5, 46–51. [Google Scholar] [CrossRef]
- White, C.A.; Allen, C.M.; Akbari, A.; Collier, C.P.; Holland, D.C.; Day, A.G.; Knoll, G.A. Comparison of the new and traditional CKD-EPI GFR estimation equations with urinary inulin clearance: A study of equation performance. Clin. Chim. Acta 2019, 488, 189–195. [Google Scholar] [CrossRef] [PubMed]
| Parameters | All Subjects n = 405 | eGFR a, mL/min/1.73 m2 | p | ||
|---|---|---|---|---|---|
| >90, n = 207 | 61–90, n = 147 | ≤60, n = 51 | |||
| Low-exposure controls (%) | 46.9 | 84.1 | 10.9 | 0 | <0.001 |
| Females (%) | 51.4 | 48.3 | 56.5 | 49.0 | 0.299 |
| Smoking (%) | 45.9 | 32.9 | 56.5 | 68.6 | <0.001 |
| Diabetes (%) | 2.7 | 0 | 4.8 | 7.8 | 0.001 |
| Hypertension (%) | 13.8 | 2.9 | 19.7 | 41.2 | <0.001 |
| Age, years | 44.6 ± 16.2 | 33.2 ± 9.9 | 53.2 ± 11.5 | 65.6 ± 10.6 | <0.001 |
| eGFR, mL/min/1.73 m2 | 87.3 ± 23.3 | 106.0 ± 10.1 | 75.5 ± 8.2 | 45.4 ± 11.3 | <0.001 |
| Plasma creatinine, mg/dL | 0.98 ± 0.29 | 0.84 ± 0.1392 | 0.98 ± 0.14 | 1.50 ± 0.44 | <0.001 |
| Urine creatinine, mg/dL | 106 ± 68 | 92 ± 68 | 116 ± 62 | 135 ± 69 | <0.001 |
| Plasma total protein, g/dL | 8.04 ± 0.45 | 8.05 ± 0.45 | 7.93 ± 0.47 | − | 0.337 |
| Plasma albumin, g/dL | 4.97 ± 0.30 | 4.97± 0.31 | 4.98 ± 0.28 | − | 0.861 |
| Urine protein, mg/L | 47.18 ± 150.6 | 5.42 ± 10.33 | 50.83 ± 137.3 | 206.2 ± 307.7 | <0.001 |
| Urine protein ≥ 20 mg/L (%) | 28.6 | 3.9 | 45.6 | 80.4 | <0.001 |
| Urine Cd, µg/L | 6.54 ± 10.59 | 2.24 ± 8.38 | 9.46 ± 8.22 | 15.6 ± 15.3 | <0.001 |
| Normalized to Ecr as Ex/Ecr b | |||||
| EProt/Ecr, mg/g creatinine | 43.76 ± 132.57 | 6.52 ± 11.58 | 52.36 ± 141.78 | 170 ± 246 | <0.001 |
| EProt/Ecr ≥ 100 mg/g creatinine (%) | 8.6 | 0.5 | 8.8 | 41.2 | <0.001 |
| ECd/Ecr, µg/g creatinine | 5.81 ± 7.64 | 2.14 ± 5.43 | 8.95 ± 7.06 | 11.69 ± 9.20 | <0.001 |
| Normalized to Ccr as Ex/Ccr c | |||||
| (EProt/Ccr) × 100, mg/L filtrate | 60.2 ± 236.5 | 5.32 ± 9.00 | 51 ± 134 | 310 ± 568 | <0.001 |
| (EProt/Ccr) × 100 ≥ 20 mg/L (%) | 30.6 | 3.9 | 49 | 86.3 | <0.001 |
| (ECd/Ccr) × 100, µg/L filtrate | 6.21 ± 9.00 | 1.70 ± 4.55 | 8.67 ± 6.85 | 17.44 ± 14.17 | <0.001 |
| Independent Variables/ Factors | Urinary Excretion of Protein a | |||||
|---|---|---|---|---|---|---|
| All subjects, n = 405 | Males, n = 197 | Females, n = 208 | ||||
| β b | p | β | p | β | p | |
| Age, years | 0.263 | <0.001 | 0.222 | 0.028 | 0.260 | 0.011 |
| Log [(ECd/Ccr) × 105], µg/L filtrate | 0.252 | <0.001 | 0.376 | <0.001 | 0.179 | 0.050 |
| Diabetes | −0.039 | 0.353 | 0.012 | 0.831 | −0.097 | 0.114 |
| Sex | 0.078 | 0.107 | − | − | − | − |
| Hypertension | −0.065 | 0.152 | −0.116 | 0.069 | −0.002 | 0.974 |
| Smoking | −0.075 | 0.150 | 0.007 | 0.911 | −0.152 | 0.040 |
| Adjusted R2 | 0.306 | <0.001 | 0.371 | <0.001 | 0.259 | <0.001 |
| Independent Variables/ Factors | eGFR, mL/min/1.73 m2 a | |||||
|---|---|---|---|---|---|---|
| All Subjects, n = 405 | Males, n = 197 | Females, n = 208 | ||||
| β b | p | β | p | β | p | |
| Age, years | −0.558 | <0.001 | −0.603 | <0.001 | −0.504 | <0.001 |
| Log [(ECd/Ccr) × 105], µg/L filtrate | −0.266 | <0.001 | −0.178 | 0.012 | −0.334 | <0.001 |
| Diabetes | 0.034 | 0.256 | 0.048 | 0.246 | 0.033 | 0.441 |
| Sex | −0.049 | 0.155 | − | − | − | − |
| Hypertension | 0.084 | 0.010 | 0.158 | 0.001 | 0.012 | 0.790 |
| Smoking | −0.043 | 0.247 | −0.061 | 0.168 | 0.016 | 0.750 |
| Adjusted R2 | 0.650 | <0.001 | 0.681 | <0.001 | 0.635 | <0.001 |
| Parameters | Number of Subjects | Proteinuria a | Low eGFR b | ||
|---|---|---|---|---|---|
| POR (95% CI) | p | POR (95% CI) | p | ||
| Age, years | 405 | 0.923 (0.897, 0.949) | <0.001 | 0.888 (0.854, 0.924) | <0.001 |
| Diabetes | 11 | 0.726 (0.181, 2.916) | 0.652 | 0.582 (0.119, 2.861) | 0.506 |
| Sex (females) | 208 | 1.030 (0.539, 1.971) | 0.928 | 0.775 (0.336, 1.787) | 0.550 |
| Hypertension | 56 | 0.498 (0.244, 1.017) | 0.055 | 0.363 (0.159, 0.826) | 0.016 |
| Smoking | 186 | 0.778 (0.398, 1.520) | 0.462 | 1.271 (0.523, 3.092) | 0.597 |
| ECd/Ccr × 100, µg/L filtrate | |||||
| 0.04–2.71 | 203 | Referent | |||
| 2.72–8.28 | 102 | 1.252 (0.670, 2.341) | 0.482 | 4.579 (1.116, 18.79) | 0.035 |
| 8.29–63 | 100 | 4.575 (1.880, 11.13) | 0.001 | 5.109 (2.093, 12.47) | <0.001 |
| Parameters | All Subjects n = 215 | eGFR a, mL/min/1.73 m2 | p | ||
|---|---|---|---|---|---|
| >90, n = 33 | 61–90, n = 131 | ≤60, n = 51 | |||
| Age, years | 57.0 ± 11.1 | 49.4 ± 9.4 | 55.6 ± 9.6 | 65.6 ± 10.6 | <0.001 |
| BMI, kg/m2 | 21.4 ± 3.6 | 21.2 ± 3.2 | 21.3 ± 3.5 | 21.7 ± 4.3 | 0.822 |
| eGFR, mL/min/1.73 m2 | 71.6 ± 19.4 | 100.4 ± 8.3 | 74.6 ± 8.2 | 45.4 ± 11.3 | <0.001 |
| Plasma creatinine, mg/dL | 1.07 ± 0.35 | 0.79 ± 0.13 | 0.98 ± 0.14 | 1.50 ± 0.44 | <0.001 |
| Urine creatinine, mg/dL | 118.4 ± 62.2 | 99.1 ± 53.1 | 116.8 ± 60.2 | 135.2 ± 69.4 | 0.054 |
| Plasma to urine creatinine ratio | 0.0125 ± 0.0096 | 0.0116 ± 0.0097 | 0.0118 ± 0.0094 | 0.0148 ± 0.0098 | 0.034 |
| Urine Cd, µg/L | 11.85 ± 12.28 | 11.18 ± 18.70 | 10.56 ± 8.05 | 15.61 ± 15.31 | 0.079 |
| Urine β2M, mg/L | 4.92 ± 17.43 | 0.20 ± 0.36 | 1.18 ± 4.02 | 17.57 ± 32.31 | <0.001 |
| Urine α1M, mg/L | 13.09 ± 18.68 | 5.66 ± 6.17 | 8.37 ± 7.91 | 30.04 ± 30.31 | <0.001 |
| Urine albumin, mg/L | 25.57 ± 70.59 | 7.62 ± 7.29 | 22.64 ± 76.57 | 44.72 ± 73.74 | <0.001 |
| Urine protein, mg/L | 85.4 ± 199.1 | 14.9 ± 22.6 | 56.2 ± 144.6 | 206.2 ± 307.7 | <0.001 |
| Normalized to Ecr as Ex/Ecr b | |||||
| ECd/Ecr, µg/g creatinine | 10.43 ± 8.02 | 10.26 ± 10.35 | 9.98 ± 6.79 | 11.69 ± 9.20 | 0.641 |
| Eβ2M/Ecr, mg/g creatinine | 4.87 ± 16.55 | 0.23 ± 0.37 | 1.66 ± 9.72 | 16.13 ± 27.49 | <0.001 |
| Eα1M/Ecr, mg/g creatinine | 11.34 ± 15.00 | 5.78 ± 4.95 | 7.53 ± 6.30 | 24.72 ± 24.57 | <0.001 |
| EAlb/Ecr, mg/g creatinine | 23.21 ± 55.07 | 10.47 ± 15.68 | 20.71 ± 59.50 | 37.88 ± 57.23 | <0.001 |
| EProt/Ecr, mg/g creatinine | 78.25 ± 174.96 | 16.73 ± 24.54 | 57.98 ± 149.26 | 170.13 ± 246.01 | <0.001 |
| Eβ2M/Ecr, µg/g creatinine (%) | |||||
| <100 | 36.7 | 51.5 | 43.5 | 9.8 | <0.001 |
| 100–999 | 37.7 | 42.4 | 42.0 | 23.5 | <0.001 |
| 1000 | 25.6 | 6.1 | 14.5 | 66.7 | <0.001 |
| EAlb/Ecr, mg/g creatinine (%) | |||||
| <30 | 84.2 | 93.9 | 88.5 | 66.7 | <0.001 |
| 30–300 | 15.3 | 0.1 | 10.7 | 33.3 | <0.001 |
| >300 | 0.5 | 0 | 0.8 | 0 | 0.017 |
| Normalized to Ccr as Ex/Ccr c | |||||
| (ECd/Ccr) × 100, µg/L filtrate | 11.27 ± 9.89 | 8.10 ± 9.06 | 9.67 ± 6.60 | 17.44 ± 14.17 | <0.001 |
| (Eβ2M/Ccr) × 100, mg/L filtrate | 7.74 ± 29.06 | 0.18 ± 0.28 | 1.82 ± 11.58 | 27.82 ± 52.20 | <0.001 |
| (Eα1M/Ccr) × 100, mg/L filtrate | 15.00 ± 28.25 | 4.46 ± 3.59 | 7.45 ± 6.63 | 41.20 ± 48.68 | <0.001 |
| (EAlb/Ccr) × 100, mg/L filtrate | 29.06 ± 75.93 | 7.50 ± 9.83 | 20.23 ± 56.82 | 65.68 ± 119.75 | <0.001 |
| (EProt/Ccr) × 100, mg/L filtrate | 109.9 ± 316.8 | 13.0 ± 19.1 | 56.3 ± 141.0 | 310.2 ± 568.2 | <0.001 |
| Variables | Age | BMI | ECd/Ccr | EProt/Ccr | EAlb/Ccr | Eβ2M/Ccr | Eα1M/Ccr |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| BMI | −0.248 *** | ||||||
| ECd/Ccr | 0.778 *** | −0.050 | |||||
| EProt/Ccr | 0.516 *** | −0.133 | 0.507 *** | ||||
| EAlb/Ccr | 0.320 *** | −0.120 | 0.152 * | 0.546 *** | |||
| Eβ2M/Ccr | 0.421 *** | −0.184 ** | 0.430 *** | 0.508 *** | 0.537 *** | ||
| Eα1M/Ccr | 0.368 *** | −0.164 * | 0.364 *** | 0.508 *** | 0.653 *** | 0.825 *** | |
| Creatinine | 0.152 ** | 0.084 | 0.169 ** | 0.217 *** | −0.024 | −0.067 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satarug, S.; Vesey, D.A.; Gobe, G.C. Cadmium-Induced Proteinuria: Mechanistic Insights from Dose–Effect Analyses. Int. J. Mol. Sci. 2023, 24, 1893. https://doi.org/10.3390/ijms24031893
Satarug S, Vesey DA, Gobe GC. Cadmium-Induced Proteinuria: Mechanistic Insights from Dose–Effect Analyses. International Journal of Molecular Sciences. 2023; 24(3):1893. https://doi.org/10.3390/ijms24031893
Chicago/Turabian StyleSatarug, Soisungwan, David A. Vesey, and Glenda C. Gobe. 2023. "Cadmium-Induced Proteinuria: Mechanistic Insights from Dose–Effect Analyses" International Journal of Molecular Sciences 24, no. 3: 1893. https://doi.org/10.3390/ijms24031893
APA StyleSatarug, S., Vesey, D. A., & Gobe, G. C. (2023). Cadmium-Induced Proteinuria: Mechanistic Insights from Dose–Effect Analyses. International Journal of Molecular Sciences, 24(3), 1893. https://doi.org/10.3390/ijms24031893








