Genome-Wide Identification and Expression Analysis of UBiA Family Genes Associated with Abiotic Stress in Sunflowers (Helianthus annuus L.)
Abstract
1. Introduction
2. Results
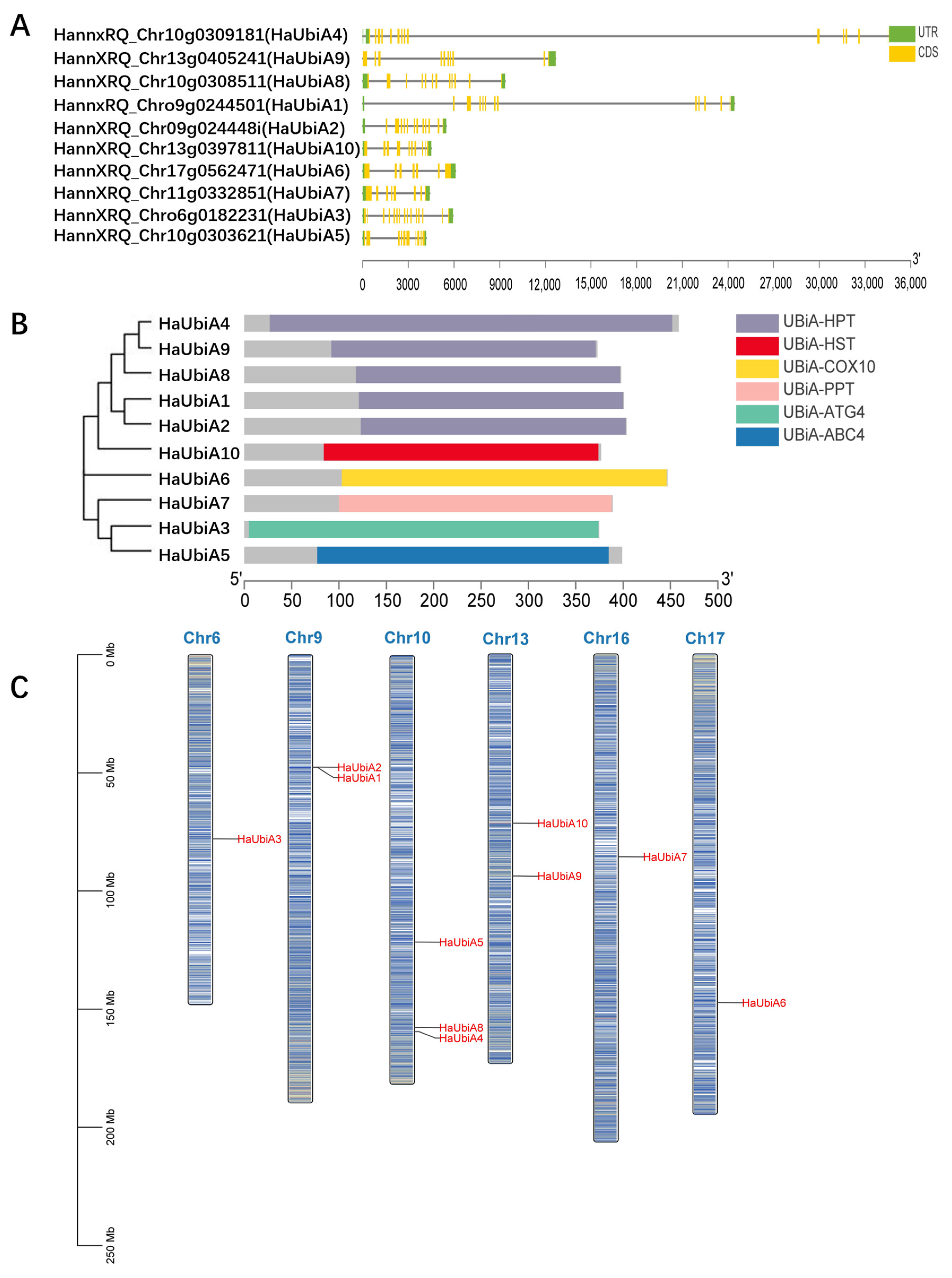
2.1. Identification and Chromosomal Location of UBiA Genes and in Helianthus annuus
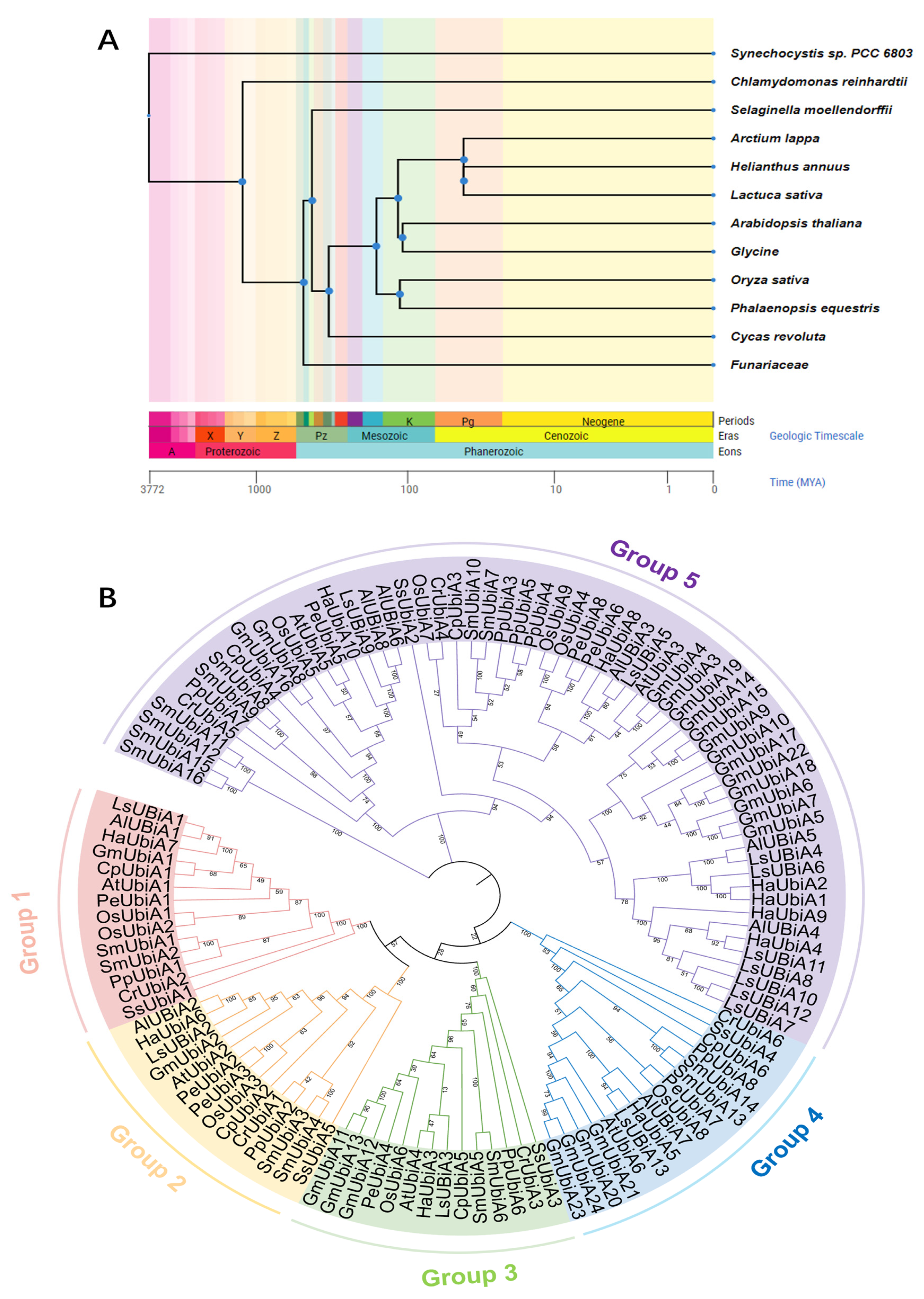
2.2. Identification and Phylogenetic Analysis of UBiA Families in 12 Species
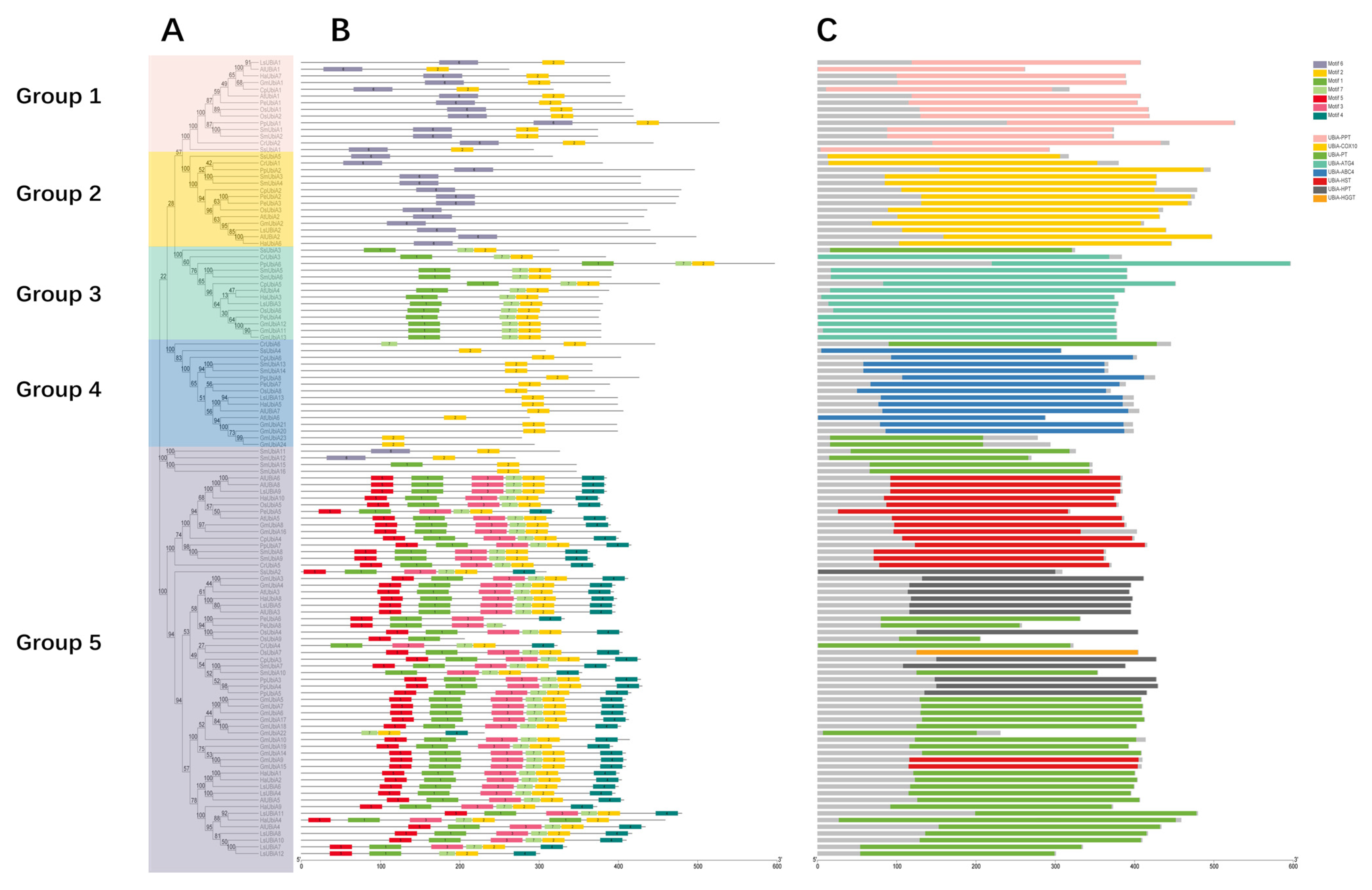
2.3. Structure Analysis of the UBiA Family Members
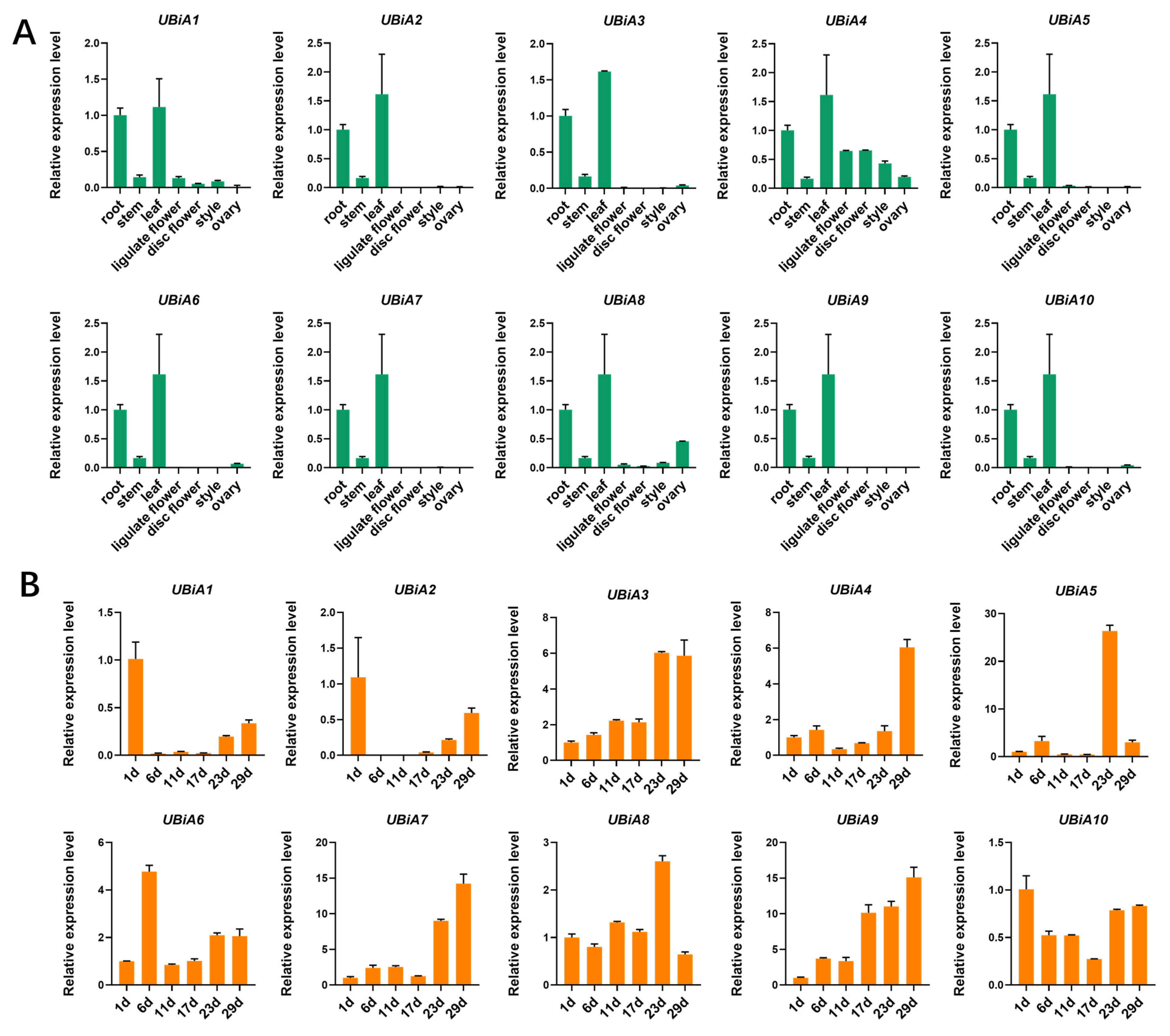
2.4. Expression Profile of UBiA Genes in Helianthus annuus
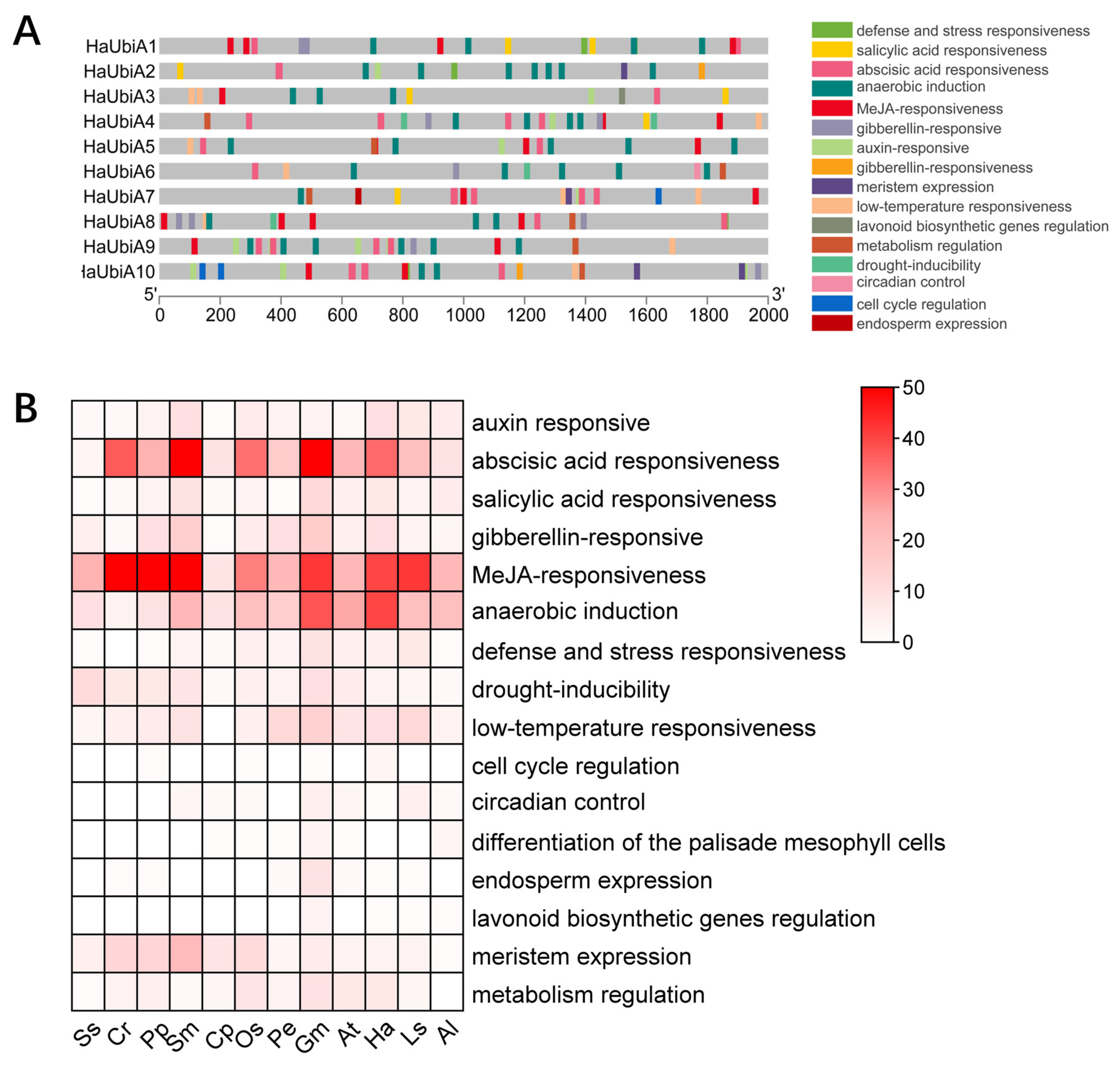
2.5. Cis-Regulatory Elements Analysis of HaUBiA Promoters
2.6. Expression Patterns of HaUBiA Genes under the Treatments of Different Hormones
2.7. Expression Analysis of HaUBiA under Different Stresses
3. Discussion
4. Materials and Methods
4.1. Identification of the UBiA Members in Helianthus annuus L.
4.2. Phylogenetic Tree Analysis of UBiA Proteins
4.3. Chromosomal Location Density, Collinearity, and Ka/Ks Analysis of UBiA Genes
4.4. Gene Structure, Conserved Motifs in Promoter and Three-Dimensional Structure
4.5. Cis-Regulatory Elements Analysis in UBiA Promoters
4.6. Plant Materials and Stress Treatments
4.7. RNA Extraction and Quantitative/Real-Time-PCR (RT-qPCR) Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowicka, B.; Kruk, J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim. Biophys. Acta (BBA) Bioenerg. 2010, 1797, 1587–1605. [Google Scholar] [CrossRef]
- Bonitz, T.; Alva, V.; Saleh, O.; Lupas, A.N.; Heide, L. Evolutionary Relationships of Microbial Aromatic Prenyltransferases. PLoS ONE 2011, 6, e27336. [Google Scholar] [CrossRef]
- Heide, L. Prenyl transfer to aromatic substrates: Genetics and enzymology. Curr. Opin. Chem. Biol. 2009, 13, 171–179. [Google Scholar] [CrossRef]
- Young, I.G.; Leppik, R.A.; Hamilton, J.A.; Gibson, F. Biochemical and Genetic Studies on Ubiquinone Biosynthesis in Escherichia coli K-12: 4-Hydroxybenzoate Octaprenyltransferase. J. Bacteriol. 1972, 110, 18–25. [Google Scholar] [CrossRef]
- Cheng, W.; Li, W. Structural Insights into Ubiquinone Biosynthesis in Membranes. Science 2014, 343, 878–881. [Google Scholar] [CrossRef]
- Grünler, J.; Ericsson, J.; Dallner, G. Branch-point reactions in the biosynthesis of cholesterol, dolichol, ubiquinone and prenylated proteins. Biochim. Biophys. Acta (BBA) Lipids Lipid Metab. 1994, 1212, 259–277. [Google Scholar] [CrossRef]
- Okada, K.; Ohara, K.; Yazaki, K.; Nozaki, K.; Uchida, N.; Kawamukai, M.; Nojiri, H.; Yamane, H. The AtPPT1 gene encoding 4-hydroxybenzoate polyprenyl diphosphate transferase in ubiquinone biosynthesis is required for embryo development in Arabidopsis thaliana. Plant Mol. Biol. 2004, 55, 567–577. [Google Scholar] [CrossRef]
- Ohara, K.; Yamamoto, K.; Hamamoto, M.; Sasaki, K.; Yazaki, K. Functional Characterization of OsPPT1, Which Encodes p-Hydroxybenzoate Polyprenyltransferase Involved in Ubiquinone Biosynthesis in Oryza sativa. Plant Cell Physiol. 2006, 47, 581–590. [Google Scholar] [CrossRef]
- Ohara, K.; Kokado, Y.; Yamamoto, H.; Sato, F.; Yazaki, K. Engineering of ubiquinone biosynthesis using the yeast coq2 gene confers oxidative stress tolerance in transgenic tobacco: Engineering of ubiquinone biosynthesis in tobacco. Plant J. 2004, 40, 734–743. [Google Scholar] [CrossRef]
- Tian, L.; DellaPenna, D.; Dixon, R.A. The pds2 mutation is a lesion in the Arabidopsis homogentisate solanesyltransferase gene involved in plastoquinone biosynthesis. Planta 2007, 226, 1067–1073. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, A.; Sedlářová, M.; Ksas, B.; Havaux, M.; Pospíšil, P. Interplay between antioxidants in response to photooxidative stress in Arabidopsis. Free Radic. Biol. Med. 2020, 160, 894–907. [Google Scholar] [CrossRef]
- Yang, W.; Cahoon, R.E.; Hunter, S.C.; Zhang, C.; Han, J.; Borgschulte, T.; Cahoon, E.B. Vitamin E biosynthesis: Functional characterization of the monocot homogentisate geranylgeranyl transferase: Vitamin E biosynthesis: Monocot HGGT. Plant J. 2011, 65, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Han, N.; Liu, C.; Buerte, B.; Zhou, C.; Chen, J.; Wang, M.; Zhang, Y.; Tang, Y.; Zhu, M.; et al. Functional dissection of HGGT and HPT in barley vitamin E biosynthesis via CRISPR/Cas9-enabled genome editing. Ann. Bot. 2020, 126, 929–942. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Zhu, X.; Wu, Y.; Zhang, S.; Chen, H.; Ling, J.; Wang, Y.; Fang, X. Rice tocopherol deficiency 1 encodes a homogentisate phytyltransferase essential for tocopherol biosynthesis and plant development in rice. Plant Cell Rep. 2018, 37, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, A.; San Martín, A.; López-Climent, M.; Ruiz-Lara, S.; Gómez-Cadenas, A.; Casaretto, J.A. Engineered drought-induced biosynthesis of α-tocopherol alleviates stress-induced leaf damage in tobacco. J. Plant Physiol. 2013, 170, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Botta, B.; Monache, G.D.; Menendez, P.; Boffi, A. Novel prenyltransferase enzymes as a tool for flavonoid prenylation. Trends Pharmacol. Sci. 2005, 26, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Eckhardt, U.; Grimm, B.; Hortensteiner, S. Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol. Biol. 2004, 56, 1–14. [Google Scholar] [CrossRef]
- Shimada, H.; Ohno, R.; Shibata, M.; Ikegami, I.; Onai, K.; Ohto, M.; Takamiya, K. Inactivation and deficiency of core proteins of photosystems I and II caused by genetical phylloquinone and plastoquinone deficiency but retained lamellar structure in a T-DNA mutant of Arabidopsis: Phylloquinone-deficient mutant in Arabidopsis. Plant J. 2005, 41, 627–637. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Oilseed crop sunflower (Helianthus annuus) as a source of food: Nutritional and health benefits. Food Sci. Nutr. 2020, 8, 4666–4684. [Google Scholar] [CrossRef]
- Lu, H.; Wang, Z.; Xu, C.; Li, L.; Yang, C. Multiomics analysis provides insights into alkali stress tolerance of sunflower (Helianthus annuus L.). Plant Physiol. Biochem. 2021, 166, 66–77. [Google Scholar] [CrossRef]
- Wang, J.; Chu, S.; Zhu, Y.; Cheng, H.; Yu, D. Positive selection drives neofunctionalization of the UbiA prenyltransferase gene family. Plant Mol. Biol. 2015, 87, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014, 217–218, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Fraudentali, I.; Pedalino, C.; Tavladoraki, P.; Angelini, R.; Cona, A. A New Player in Jasmonate-Mediated Stomatal Closure: The Arabidopsis thaliana Copper Amine Oxidase β. Cells 2021, 10, 3399. [Google Scholar] [CrossRef]
- Nakashima, K.; Yamaguchi-Shinozaki, K. ABA signaling in stress-response and seed development. Plant Cell Rep. 2013, 32, 959–970. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, L.; Zhang, L.; Wang, Y.; Cui, L.; Tang, Y.; Sun, X.; Tang, K. Molecular analysis of a homogentisate phytyltransferase gene from Lactuca sativa L. Mol. Biol. Rep. 2011, 38, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H.K.; Niyogi, K.K. Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ. 2005, 28, 1037–1045. [Google Scholar] [CrossRef]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef]
- Kruk, J.; Szymańska, R.; Cela, J.; Munne-Bosch, S. Plastochromanol-8: Fifty years of research. Phytochemistry 2014, 108, 9–16. [Google Scholar] [CrossRef]
- Collakova, E.; DellaPenna, D. Homogentisate Phytyltransferase Activity Is Limiting for Tocopherol Biosynthesis in Arabidopsis. Plant Physiol. 2003, 131, 632–642. [Google Scholar] [CrossRef]
- Diao, P.; Chen, C.; Zhang, Y.; Meng, Q.; Lv, W.; Ma, N. The role of NAC transcription factor in plant cold response. Plant Signal. Behav. 2020, 15, 1785668. [Google Scholar] [CrossRef]
- Antonicka, H. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum. Mol. Genet. 2003, 12, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Pitceathly, R.D.S. COX10 Mutations Resulting in Complex Multisystem Mitochondrial Disease That Remains Stable Into Adulthood. JAMA Neurol. 2013, 70, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Vitali, M.; Venturelli, E.; Galimberti, D.; Benerini Gatta, L.; Scarpini, E.; Finazzi, D. Analysis of the genes coding for subunit 10 and 15 of cytochrome c oxidase in Alzheimer’s disease. J. Neural Transm. 2009, 116, 1635–1641. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, B.S.; van den Heuvel, L.P.; Smeets, R.J.P.; de Vries, M.C.; Hien, S.; Schaible, T.; Smeitink, J.A.M.; Wevers, R.A.; Wortmann, S.B.; Rodenburg, R.J.T. A novel mutation in COQ2 leading to fatal infantile multisystem disease. J. Neurol. Sci. 2013, 326, 24–28. [Google Scholar] [CrossRef]
- Diomedi-Camassei, F.; Di Giandomenico, S.; Santorelli, F.M.; Caridi, G.; Piemonte, F.; Montini, G.; Ghiggeri, G.M.; Murer, L.; Barisoni, L.; Pastore, A.; et al. COQ2 Nephropathy: A Newly Described Inherited Mitochondriopathy with Primary Renal Involvement. J. Am. Soc. Nephrol. 2007, 18, 2773–2780. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef]
- Tian, D.; Wang, Q.; Zhang, P.; Araki, H.; Yang, S.; Kreitman, M.; Nagylaki, T.; Hudson, R.; Bergelson, J.; Chen, J.-Q. Single-nucleotide mutation rate increases close to insertions/deletions in eukaryotes. Nature 2008, 455, 105–108. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef]
- The perversion of knowledge: The true story of Soviet science. Choice Rev. Online 2002, 39, 39–4541. [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- The UniProt Consortium; Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; et al. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Lescot, M. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]


| Species | I | II | III | IV | V | Total No. |
|---|---|---|---|---|---|---|
| Synechocystis | 1 | 1 | 1 | 1 | 1 | 5 |
| Chlamydomonas reinhardtii | 1 | 1 | 1 | 1 | 2 | 6 |
| Physcomitrium patens | 1 | 1 | 1 | 1 | 4 | 8 |
| Selaginella moellendorffii | 4 | 4 | 2 | 2 | 4 | 16 |
| Cycas revoluta | 1 | 1 | 1 | 1 | 2 | 6 |
| Oryza sativa | 2 | 1 | 1 | 1 | 4 | 9 |
| Phalaenopsis equestris | 1 | 1 | 1 | 2 | 3 | 8 |
| Glycine max | 1 | 3 | 4 | 1 | 15 | 24 |
| Arabidopsis thaliana | 1 | 1 | 1 | 1 | 2 | 6 |
| Helianthus annuus | 1 | 1 | 1 | 1 | 6 | 10 |
| Lactuca sativa | 1 | 1 | 2 | 1 | 8 | 13 |
| Arctium lappa | 1 | 0 | 1 | 1 | 5 | 8 |
| Total No. | 16 | 16 | 17 | 14 | 56 | 119 |
| ID | Rename | Number of Amino Acids | MW (Da) | PI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | LOCATION |
|---|---|---|---|---|---|---|---|---|
| XM_006602661.3 | GmUBiA1 | 389 | 42,818.65 | 9.51 | 39.86 | 97.56 | 0.192 | Plasma Membrane |
| XM_003556504.5 | GmUBiA2 | 411 | 44,599.15 | 9.53 | 46.35 | 86.33 | 0.098 | Plasma Membrane |
| NM_001251443.2 | GmUBiA3 | 411 | 46,172.21 | 9.46 | 41.75 | 106.28 | 0.477 | Plasma Membrane |
| NM_001254567.1 | GmUBiA4 | 395 | 44,364.22 | 9.37 | 43.28 | 109.82 | 0.532 | Plasma Membrane |
| XM_003541408.5 | GmUBiA5 | 408 | 46,233.75 | 9.65 | 46.56 | 106.59 | 0.42 | Plasma Membrane |
| XM_006588774.4 | GmUBiA6 | 409 | 46,464.65 | 9.45 | 43.87 | 104.43 | 0.414 | Plasma Membrane |
| XM_003541409.4 | GmUBiA7 | 410 | 46,311.92 | 9.66 | 49.08 | 109.61 | 0.438 | Plasma Membrane |
| NM_001250971.1 | GmUBiA8 | 389 | 43,362.87 | 9.77 | 38.49 | 107.89 | 0.491 | Plasma Membrane |
| NM_001249061.2 | GmUBiA9 | 409 | 46,011.81 | 9.36 | 38.15 | 96.36 | 0.308 | Plasma Membrane |
| XM_041014270.1 | GmUBiA10 | 413 | 47,079.83 | 9.56 | 53.68 | 112.4 | 0.443 | Plasma Membrane |
| NM_001252704.2 | GmUBiA11 | 377 | 40,923.53 | 7.67 | 29.12 | 107.67 | 0.336 | Plasma Membrane |
| XM_003552213.4 | GmUBiA12 | 377 | 40,981.45 | 6.9 | 33.9 | 104.8 | 0.3 | Plasma Membrane |
| XM_014768972.3 | GmUBiA13 | 377 | 40,959.48 | 8.31 | 30.94 | 105.86 | 0.308 | Plasma Membrane |
| NM_001366992.1 | GmUBiA14 | 408 | 45,830.1 | 9.27 | 51.83 | 114.24 | 0.526 | Plasma Membrane |
| XM_041017800.1 | GmUBiA15 | 408 | 45,834.76 | 9.49 | 41.57 | 102.99 | 0.332 | Plasma Membrane |
| XM_014761623.3 | GmUBiA16 | 402 | 44,363.28 | 9.94 | 36.09 | 109.93 | 0.496 | Plasma Membrane |
| XM_041005679.1 | GmUBiA17 | 412 | 46,579.91 | 9.55 | 54.66 | 106.67 | 0.397 | Plasma Membrane |
| NM_001366991.1 | GmUBiA18 | 402 | 45,250.3 | 9.61 | 48.84 | 105.97 | 0.446 | Plasma Membrane |
| NM_001348662.1 | GmUBiA19 | 392 | 43,502.4 | 9.3 | 35.8 | 114.67 | 0.589 | Plasma Membrane |
| XM_003532557.5 | GmUBiA20 | 398 | 44,124.58 | 8.73 | 35 | 101.91 | 0.417 | Plasma Membrane |
| XM_003543880.5 | GmUBiA21 | 397 | 44,097.67 | 8.98 | 36.77 | 102.64 | 0.369 | Plasma Membrane |
| XM_041006158.1 | GmUBiA22 | 230 | 25,921.12 | 9.84 | 36.66 | 124.96 | 0.861 | Plasma Membrane |
| XM_026126145.2 | GmUBiA23 | 277 | 30,997.81 | 9 | 35.8 | 110.47 | 0.627 | Plasma Membrane |
| XM_041006200.1 | GmUBiA24 | 293 | 32,271.04 | 8.8 | 41.52 | 103.45 | 0.566 | Plasma Membrane |
| XM_020738038.1 | PeUBiA1 | 403 | 44,033.25 | 9.13 | 50.67 | 105.56 | 0.309 | Plasma Membrane |
| XM_020718723.1 | PeUBiA2 | 475 | 51,887.23 | 9.19 | 46.28 | 93.75 | 0.315 | Plasma Membrane |
| XM_020732720.1 | PeUBiA3 | 471 | 51,393.55 | 8.93 | 50.25 | 93.27 | 0.293 | Plasma Membrane |
| XM_020722467.1 | PeUBiA4 | 374 | 40,536.16 | 8.79 | 31.78 | 109.09 | 0.305 | Plasma Membrane |
| XM_020722914.1 | PeUBiA5 | 318 | 35,194.82 | 9.15 | 29.75 | 128.87 | 0.874 | Plasma Membrane |
| XM_020729489.1 | PeUBiA6 | 331 | 37,301.06 | 9.47 | 38.54 | 109.94 | 0.569 | Plasma Membrane |
| XM_020726705.1 | PeUBiA7 | 388 | 42,121.47 | 8.98 | 36.94 | 96.29 | 0.465 | Plasma Membrane |
| XM_020738474.1 | PeUBiA8 | 257 | 29,003.29 | 9.88 | 39.1 | 102.88 | 0.594 | Plasma Membrane |
| WP_010874074.1 | SsUBiA1 | 292 | 31,645.74 | 6.54 | 34.17 | 130.27 | 0.899 | Plasma Membrane |
| WP_010872404.1 | SsUBiA2 | 308 | 34,410.07 | 9.02 | 28.08 | 134.87 | 0.853 | Plasma Membrane |
| WP_010873507.1 | SsUBiA3 | 324 | 35,208.44 | 6.05 | 19.93 | 116.23 | 0.546 | Plasma Membrane |
| WP_010872655.1 | SsUBiA4 | 307 | 33,246.9 | 7.08 | 29.88 | 116.91 | 0.639 | Plasma Membrane |
| WP_010872804.1 | SsUBiA5 | 316 | 34,857.68 | 9.13 | 27.37 | 128.73 | 0.703 | Plasma Membrane |
| XM_043068307.1 | CrUBiA1 | 379 | 40,389.72 | 8.97 | 37.32 | 98.05 | 0.186 | Plasma Membrane |
| XM_001695355.2 | CrUBiA2 | 443 | 45,902.39 | 7.7 | 54.22 | 87.43 | 0.197 | Plasma Membrane |
| XM_001701536.2 | CrUBiA3 | 383 | 41,448.11 | 8.49 | 28.62 | 103.26 | 0.298 | Plasma Membrane |
| XM_043066342.1 | CrUBiA4 | 322 | 32,758.75 | 9.01 | 24.07 | 127.39 | 0.987 | Plasma Membrane |
| XM_001695289.2 | CrUBiA5 | 370 | 39,347.15 | 9.37 | 41.36 | 111.41 | 0.595 | Plasma Membrane |
| XM_043061866.1 | CrUBiA6 | 445 | 43,763.78 | 9.17 | 41.98 | 100.54 | 0.659 | Plasma Membrane |
| NM_001341624.1 | AtUBiA1 | 407 | 44,603.53 | 9.23 | 35.01 | 96.34 | 0.137 | Plasma Membrane |
| NM_130015.4 | AtUBiA2 | 431 | 46,475.03 | 9.87 | 45.51 | 90.93 | 0.27 | Plasma Membrane |
| NM_179653.4 | AtUBiA3 | 393 | 43,908.96 | 9.74 | 41.9 | 113.36 | 0.56 | Plasma Membrane |
| NM_115041.4 | AtUBiA4 | 387 | 41,881.44 | 8.52 | 27.04 | 104.81 | 0.267 | Plasma Membrane |
| NM_001084669.2 | AtUBiA5 | 386 | 42,840.52 | 10.15 | 30.82 | 115.75 | 0.542 | Plasma Membrane |
| NM_104743.3 | AtUBiA6 | 287 | 31,054.75 | 9.18 | 30.63 | 123.66 | 0.706 | Plasma Membrane |
| XM_023879341.2 | LsUBiA1 | 407 | 45,328.19 | 9.19 | 42.95 | 94.91 | 0.008 | Plasma Membrane |
| XM_023874978.2 | LsUBiA2 | 439 | 47,603.05 | 9.46 | 34.16 | 92.51 | 0.171 | Plasma Membrane |
| XM_023904443.2 | LsUBiA3 | 379 | 41,111.8 | 8.73 | 29.3 | 102.51 | 0.267 | Plasma Membrane |
| XM_023909767.2 | LsUBiA4 | 395 | 44,741.91 | 9.62 | 44.83 | 107.85 | 0.443 | Plasma Membrane |
| XM_023901890.2 | LsUBiA5 | 395 | 44,761.73 | 9.58 | 47.37 | 111.77 | 0.637 | Plasma Membrane |
| XM_023909580.2 | LsUBiA6 | 399 | 45,145.59 | 9.46 | 42.71 | 102.68 | 0.418 | Plasma Membrane |
| XM_023913555.2 | LsUBiA7 | 334 | 37,050.93 | 9.57 | 33 | 123.17 | 0.505 | Plasma Membrane |
| XM_023901886.2 | LsUBiA8 | 416 | 46,609.98 | 9.66 | 34.11 | 117.88 | 0.42 | Plasma Membrane |
| XM_023901043.2 | LsUBiA9 | 384 | 42,777.3 | 9.67 | 34.2 | 107.71 | 0.52 | Plasma Membrane |
| XM_023893904.2 | LsUBiA10 | 409 | 45,553.65 | 9.65 | 33.88 | 116.31 | 0.36 | Plasma Membrane |
| XM_042902057.1 | LsUBiA11 | 479 | 53,606.59 | 8.9 | 41.44 | 110.33 | 0.252 | Plasma Membrane |
| XM_023908970.2 | LsUBiA12 | 300 | 33,379.45 | 9.38 | 30.33 | 118.93 | 0.467 | Plasma Membrane |
| XM_023909766.2 | LsUBiA13 | 398 | 43,239.93 | 9.37 | 32.4 | 111.73 | 0.442 | Plasma Membrane |
| JAKOEK|L6452_mrna25818 | AlUBiA1 | 261 | 28,191.91 | 9.11 | 31.89 | 107.24 | 0.448 | Plasma Membrane |
| JAKOEK|L6452_mrna20277 | AlUBiA2 | 497 | 53,962.3 | 9.68 | 40.86 | 90.78 | 0.059 | Plasma Membrane |
| JAKOEK|L6452_mrna39700 | AlUBiA3 | 395 | 43,986.85 | 9.64 | 45.87 | 115.47 | 0.631 | Plasma Membrane |
| JAKOEK|L6452_mrna22236 | AlUBiA4 | 433 | 48,739.19 | 9.51 | 35.29 | 110.81 | 0.37 | Plasma Membrane |
| JAKOEK|L6452_mrna22240 | AlUBiA5 | 406 | 45,958.41 | 9.29 | 40.73 | 108.97 | 0.55 | Plasma Membrane |
| JAKOEK|L6452_mrna24308 | AlUBiA6 | 384 | 42,580.01 | 9.69 | 37.47 | 107.94 | 0.563 | Plasma Membrane |
| JAKOEK|L6452_mrna22828 | AlUBiA7 | 405 | 43,960.54 | 9.58 | 36.37 | 112.2 | 0.404 | Plasma Membrane |
| JAKOEK|L6452_mrna24308 | AlUBiA8 | 384 | 42,580.01 | 9.69 | 37.47 | 107.94 | 0.563 | Plasma Membrane |
| XM_002978823.2 | SmUBiA1 | 373 | 40,376.75 | 9.6 | 48.87 | 102.01 | 0.186 | Plasma Membrane |
| XM_002988434.2 | SmUBiA2 | 373 | 40,295.69 | 9.42 | 43.85 | 102.28 | 0.245 | Plasma Membrane |
| XM_024669487.1 | SmUBiA3 | 427 | 45,928.2 | 9.12 | 35.26 | 89.77 | 0.181 | Plasma Membrane |
| XM_024687212.1 | SmUBiA4 | 427 | 45,908.12 | 9.36 | 34.92 | 87.73 | 0.134 | Plasma Membrane |
| XM_024689909.1 | SmUBiA5 | 390 | 42,593.43 | 8.71 | 29.5 | 101.87 | 0.26 | Plasma Membrane |
| XM_024659843.1 | SmUBiA6 | 390 | 42,686.6 | 8.71 | 28.82 | 102.62 | 0.257 | Plasma Membrane |
| XM_002985500.3 | SmUBiA7 | 388 | 42,192.01 | 9.75 | 54.4 | 114.48 | 0.678 | Plasma Membrane |
| XM_002979186.2 | SmUBiA8 | 363 | 40,060.19 | 9.59 | 27.46 | 117.66 | 0.552 | Plasma Membrane |
| XM_024664099.1 | SmUBiA9 | 363 | 40,066.16 | 9.59 | 26.63 | 117.11 | 0.546 | Plasma Membrane |
| XM_024659418.1 | SmUBiA10 | 353 | 39,419.15 | 9.89 | 58.54 | 97.88 | 0.271 | Plasma Membrane |
| XM_024664337.1 | SmUBiA11 | 325 | 34,633.71 | 8.48 | 24.56 | 120.09 | 0.632 | Plasma Membrane |
| XM_024688846.1 | SmUBiA12 | 269 | 28,648.87 | 7.6 | 22.61 | 120.45 | 0.778 | Plasma Membrane |
| XM_002961810.2 | SmUBiA13 | 366 | 39,183.08 | 8.93 | 40.02 | 114.92 | 0.546 | Plasma Membrane |
| XM_002980750.2 | SmUBiA14 | 366 | 39,231.12 | 8.93 | 40.02 | 114.13 | 0.542 | Plasma Membrane |
| XM_024659390.1 | SmUBiA15 | 346 | 37,322.91 | 9.36 | 48.66 | 109.91 | 0.518 | Plasma Membrane |
| XM_024660820.1 | SmUBiA16 | 346 | 37,496.13 | 9.37 | 47.18 | 109.62 | 0.527 | Plasma Membrane |
| XM_015794109.2 | OsUBiA1 | 417 | 45,579.06 | 9.49 | 46.47 | 96.47 | 0.14 | Plasma Membrane |
| XM_015793105.2 | OsUBiA2 | 418 | 45,307.63 | 9.03 | 58.59 | 94.4 | 0.143 | Plasma Membrane |
| XM_015766928.2 | OsUBiA3 | 435 | 46,539.75 | 9.61 | 44.01 | 90.6 | 0.175 | Plasma Membrane |
| XM_015789024.2 | OsUBiA4 | 404 | 44,501.31 | 9.85 | 52.66 | 108.94 | 0.51 | Plasma Membrane |
| XM_015791419.2 | OsUBiA5 | 379 | 41,421.94 | 9.99 | 40.71 | 111.56 | 0.565 | Plasma Membrane |
| XM_015782164.2 | OsUBiA6 | 376 | 40,578.99 | 8.23 | 29.2 | 104.63 | 0.289 | Plasma Membrane |
| XM_015786513.2 | OsUBiA7 | 404 | 44,899.63 | 9.15 | 42.67 | 102.92 | 0.378 | Plasma Membrane |
| XM_015772366.2 | OsUBiA8 | 369 | 39,316.01 | 9.57 | 37.68 | 104.99 | 0.521 | Plasma Membrane |
| XM_026025964.1 | OsUBiA9 | 205 | 22,086.31 | 6.64 | 39.67 | 99.51 | 0.26 | Plasma Membrane |
| CYCAS_007186 | CpUBiA1 | 317 | 34,783.72 | 8.74 | 36.19 | 108.36 | 0.332 | Plasma Membrane |
| CYCAS_028733 | CpUBiA2 | 478 | 51,943.14 | 9.31 | 41.34 | 91.72 | 0.13 | Plasma Membrane |
| CYCAS_026755 | CpUBiA3 | 427 | 47,516.28 | 9.81 | 42.87 | 110.77 | 0.51 | Plasma Membrane |
| CYCAS_009776 | CpUBiA4 | 399 | 44,110.11 | 10.01 | 33.16 | 114.46 | 0.637 | Plasma Membrane |
| CYCAS_028820 | CpUBiA5 | 451 | 49,065.88 | 7.62 | 36.65 | 110.29 | 0.264 | Plasma Membrane |
| CYCAS_029627 | CpUBiA6 | 402 | 43,142.54 | 9.39 | 38.72 | 106.02 | 0.43 | Plasma Membrane |
| XM_035977189.1 | HaUBiA1 | 400 | 44,671 | 9.5 | 36.43 | 103.15 | 0.455 | Plasma Membrane |
| XM_022125636.2 | HaUBiA2 | 403 | 44,765.53 | 9.31 | 39.62 | 96.38 | 0.389 | Plasma Membrane |
| XM_022115079.2 | HaUBiA3 | 374 | 40,492.14 | 8.87 | 33.66 | 108.05 | 0.294 | Plasma Membrane |
| XM_035978370.1 | HaUBiA4 | 458 | 50,808.91 | 8.07 | 36.01 | 122.42 | 0.572 | Plasma Membrane |
| XM_022133493.2 | HaUBiA5 | 398 | 43,348.1 | 9.43 | 36.1 | 109.05 | 0.456 | Plasma Membrane |
| XM_022170044.2 | HaUBiA6 | 446 | 48,452.02 | 9.84 | 36.17 | 90.81 | 0.13 | Plasma Membrane |
| XM_022137525.2 | HaUBiA7 | 388 | 42,826.65 | 9.46 | 35.46 | 101.11 | 0.211 | Plasma Membrane |
| XM_022133910.2 | HaUBiA8 | 397 | 44,132.01 | 9.64 | 45.68 | 113.93 | 0.642 | Plasma Membrane |
| XM_022148176.2 | HaUBiA9 | 372 | 41,976.64 | 9.5 | 39.1 | 114.52 | 0.39 | Plasma Membrane |
| XM_022144901.2 | HaUBiA10 | 375 | 41,704.12 | 9.81 | 33.01 | 114.21 | 0.601 | Plasma Membrane |
| XM_024545301.1 | PpUBiA1 | 526 | 58,058.76 | 9.09 | 35.24 | 95.48 | −0.025 | Plasma Membrane |
| XM_024539677.1 | PpUBiA2 | 495 | 52,852.45 | 9.91 | 33.75 | 90.3 | 0.206 | Plasma Membrane |
| XM_024546331.1 | PpUBiA3 | 427 | 47,112.91 | 9.52 | 40.99 | 98.45 | 0.319 | Plasma Membrane |
| XM_024523725.1 | PpUBiA4 | 429 | 46,955.35 | 9.85 | 35.81 | 109.6 | 0.484 | Plasma Membrane |
| XM_024533894.1 | PpUBiA5 | 415 | 45,263.45 | 9.94 | 41.24 | 111.93 | 0.537 | Plasma Membrane |
| XM_024536594.1 | PpUBiA6 | 596 | 64,246.01 | 9.36 | 39.27 | 94.85 | 0.057 | Plasma Membrane |
| XM_024541651.1 | PpUBiA7 | 415 | 44,777.38 | 9.73 | 34.17 | 108.92 | 0.471 | Plasma Membrane |
| XM_024541769.1 | PpUBiA8 | 425 | 45,232.4 | 9.31 | 35.04 | 112.92 | 0.502 | Plasma Membrane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Cai, M.; Zeng, Q.; Han, Y.; Zhang, S.; Wang, Y.; Xie, Q.; Chen, Y.; Zeng, Y.; Chen, T. Genome-Wide Identification and Expression Analysis of UBiA Family Genes Associated with Abiotic Stress in Sunflowers (Helianthus annuus L.). Int. J. Mol. Sci. 2023, 24, 1883. https://doi.org/10.3390/ijms24031883
Sun M, Cai M, Zeng Q, Han Y, Zhang S, Wang Y, Xie Q, Chen Y, Zeng Y, Chen T. Genome-Wide Identification and Expression Analysis of UBiA Family Genes Associated with Abiotic Stress in Sunflowers (Helianthus annuus L.). International Journal of Molecular Sciences. 2023; 24(3):1883. https://doi.org/10.3390/ijms24031883
Chicago/Turabian StyleSun, Mingzhe, Maohong Cai, Qinzong Zeng, Yuliang Han, Siqi Zhang, Yingwei Wang, Qinyu Xie, Youheng Chen, Youling Zeng, and Tao Chen. 2023. "Genome-Wide Identification and Expression Analysis of UBiA Family Genes Associated with Abiotic Stress in Sunflowers (Helianthus annuus L.)" International Journal of Molecular Sciences 24, no. 3: 1883. https://doi.org/10.3390/ijms24031883
APA StyleSun, M., Cai, M., Zeng, Q., Han, Y., Zhang, S., Wang, Y., Xie, Q., Chen, Y., Zeng, Y., & Chen, T. (2023). Genome-Wide Identification and Expression Analysis of UBiA Family Genes Associated with Abiotic Stress in Sunflowers (Helianthus annuus L.). International Journal of Molecular Sciences, 24(3), 1883. https://doi.org/10.3390/ijms24031883





