The Role of Heat Shock Proteins in the Pathogenesis of Polycystic Ovarian Syndrome: A Review of the Literature
Abstract
1. Introduction to Polycystic Ovarian Syndrome
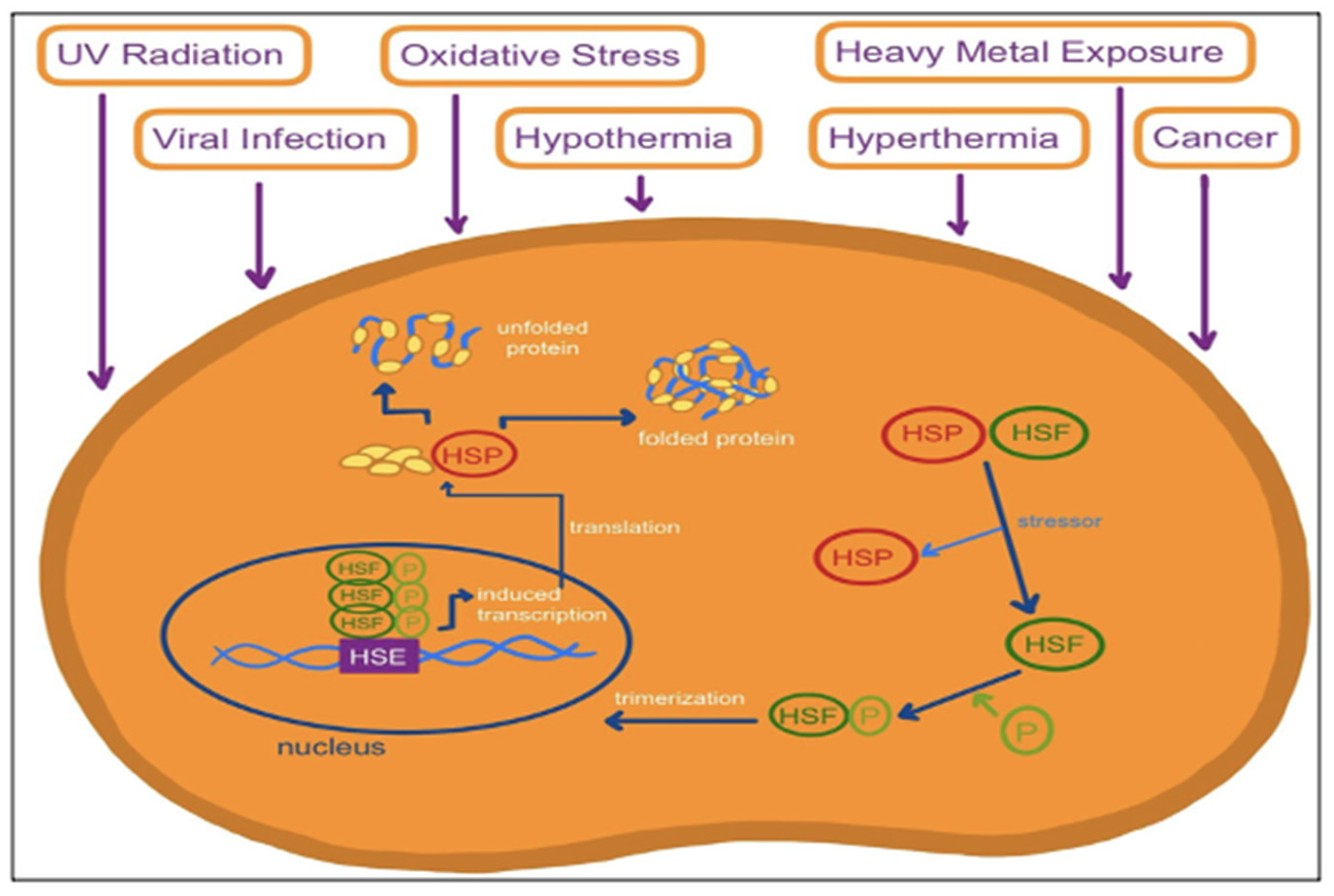
2. Biology of Heat Shock Proteins
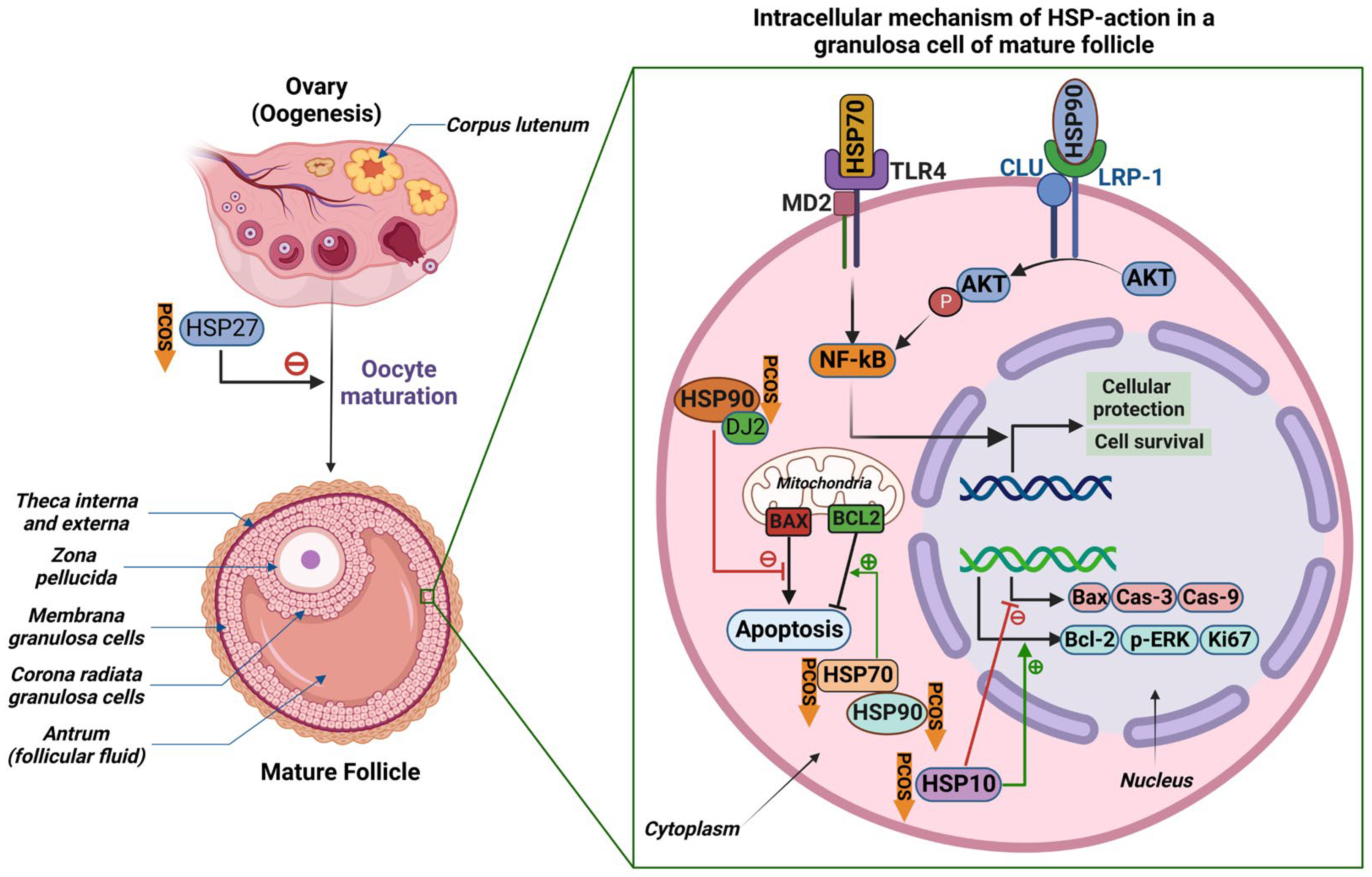
3. The Effects of Heat Shock Proteins on the Pathogenesis of Polycystic Ovarian Syndrome
4. The Role of Heat Shock Proteins in Type 2 Diabetes
5. The Role of Heat Shock Proteins in Obesity
6. Therapeutic Interventions
6.1. Induction of Heat Shock Proteins through Exercise
6.2. Heat Shock Therapy
6.3. Induction of HSPs to Treat Insulin Resistance
6.4. Repression of Heat Shock Proteins through miRNAs
7. Future Directions
- Studies to elucidate the detailed molecular mechanisms underlying the function of HSPs in human ovarian cells, as modulating the activity of HSPs may lead to novel strategies for treating PCOS.
- Further studies focusing upon on the expression profiles and mutations of the different HSPs in ovarian cell function, as current information is limited.
- Studies need to be conducted on the therapeutic and prognostic relevance of expression profiles of HSPs. There is limited research on the involvement of HSPs in the pathogenesis of diabetes for example using PCOS as a model.
- Studies of the effects of HSPs on PCOS pathophysiology using human samples, as a key limitation is the dearth of information in this area and studies on mouse oocyte models in vitro may not simulate PCOS oocyte development.
- Adequately powered studies are needed to address the influence of HSPs on lean, overweight, and obese cohorts with and without PCOS.
- Studies in PCOS populations of differing ethnicity are needed to determine the generalizability of findings across ethnic groups.
- Studies employing targeted manipulation of HSPs to determine their potential clinical utility for treating PCOS and its related conditions.
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franks, S.; Hardy, K. What causes anovulation in polycystic ovary syndrome? Curr. Opin. Endocr. Metab. Res. 2020, 12, 59–65. [Google Scholar] [CrossRef]
- Szeliga, A.; Rudnicka, E.; Maciejewska-Jeske, M.; Kucharski, M.; Kostrzak, A.; Hajbos, M.; Niwczyk, O.; Smolarczyk, R.; Meczekalski, B. Neuroendocrine Determinants of Polycystic Ovary Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 3089. [Google Scholar] [CrossRef] [PubMed]
- Rani, R.; Hajam, Y.A.; Kumar, R.; Bhat, R.A.; Rai, S.; Rather, M.A. A landscape analysis of the potential role of polyphenols for the treatment of Polycystic Ovarian Syndrome (PCOS). Phytomed. Plus 2022, 2, 100161. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Cho, L.W.; Jayagopal, V.; Kilpatrick, E.S.; Holding, S.; Atkin, S.L. The LH/FSH ratio has little use in diagnosing polycystic ovarian syndrome. Ann. Clin. Biochem. 2006, 43, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, J.K.; Waqar, A.; Jain, A.; Joseph, C.; Srivastava, K.; Ochuba, O.; Alkayyali, T.; Ruo, S.W.; Poudel, S. Oxidative Stress in Polycystic Ovarian Syndrome and the Effect of Antioxidant N-Acetylcysteine on Ovulation and Pregnancy Rate. Cureus 2021, 13, e17887. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Di, J.; Pan, J.; Yu, R.; Teng, Y.; Cai, Z.; Deng, X. The Association Between Prolactin and Metabolic Parameters in PCOS Women: A Retrospective Analysis. Front. Endocrinol. 2020, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Guo, Y.; Zhang, X.; Liang, X. ENDOMETRIAL ENDOPLASMIC RETICULUM STRESS IN OBESE PCOS PATIENTS. Fertil. Steril. 2021, 116, E313. [Google Scholar] [CrossRef]
- Luan, Y.Y.; Zhang, L.; Peng, Y.Q.; Li, Y.Y.; Liu, R.X.; Yin, C.H. Immune regulation in polycystic ovary syndrome. Clin. Chim. Acta 2022, 531, 265–272. [Google Scholar] [CrossRef]
- Li, L.; Mo, H.; Zhang, J.; Zhou, Y.; Peng, X.; Luo, X. The Role of Heat Shock Protein 90B1 in Patients with Polycystic Ovary Syndrome. PLoS ONE 2016, 11, e0152837. [Google Scholar] [CrossRef] [PubMed]
- Kregel, K.C. Heat shock proteins: Modifying factors in physiological stress responses and acquired thermotolerance. J. Appl. Physiol. (1985) 2002, 92, 2177–2186. [Google Scholar] [CrossRef]
- Tsan, M.F.; Gao, B. Heat shock protein and innate immunity. Cell Mol. Immunol. 2004, 1, 274–279. [Google Scholar] [PubMed]
- Gao, H.; Meng, J.; Xu, M.; Zhang, S.; Ghose, B.; Liu, J.; Yao, P.; Yan, H.; Wang, D.; Liu, L. Serum Heat Shock Protein 70 Concentration in Relation to Polycystic Ovary Syndrome in a Non-Obese Chinese Population. PLoS ONE 2013, 8, e67727. [Google Scholar] [CrossRef] [PubMed]
- Vince, R.V.; Kirk, R.J.; Aye, M.M.; Atkin, S.L.; Madden, L.A. Impaired heat shock protein 72 expression in women with polycystic ovary syndrome following a supervised exercise programme. Cell Stress Chaperones 2020, 25, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zininga, T.; Ramatsui, L.; Shonhai, A. Heat Shock Proteins as Immunomodulants. Molecules 2018, 23, 2846. [Google Scholar] [CrossRef]
- Su, K.H.; Dai, C. Metabolic control of the proteotoxic stress response: Implications in diabetes mellitus and neurodegenerative disorders. Cell Mol. Life Sci. 2016, 73, 4231–4248. [Google Scholar] [CrossRef]
- Butler, A.E.; Abouseif, A.; Dargham, S.R.; Sathyapalan, T.; Atkin, S.L. Metabolic comparison of polycystic ovarian syndrome and control women in Middle Eastern and UK Caucasian populations. Sci. Rep. 2020, 10, 18895. [Google Scholar] [CrossRef]
- Gomez-Pastor, R.; Burchfiel, E.T.; Thiele, D.J. Regulation of heat shock transcription factors and their roles in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 4–19. [Google Scholar] [CrossRef]
- Richter, K.; Haslbeck, M.; Buchner, J. The heat shock response: Life on the verge of death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- Asea, A. Initiation of the Immune Response by Extracellular Hsp72: Chaperokine Activity of Hsp72. Curr. Immunol. Rev. 2006, 2, 209–215. [Google Scholar] [CrossRef]
- Sreedhar, A.S.; Kalmár, E.; Csermely, P.; Shen, Y.F. Hsp90 isoforms: Functions, expression and clinical importance. FEBS Lett. 2004, 562, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Walsh, P.; Bursać, D.; Law, Y.C.; Cyr, D.; Lithgow, T. The J-protein family: Modulating protein assembly, disassembly and translocation. EMBO Rep. 2004, 5, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Esser, C.; Alberti, S.; Höhfeld, J. Cooperation of molecular chaperones with the ubiquitin/proteasome system. Biochim. Biophys. Acta 2004, 1695, 171–188. [Google Scholar] [CrossRef]
- Park, S.H.; Bolender, N.; Eisele, F.; Kostova, Z.; Takeuchi, J.; Coffino, P.; Wolf, D.H. The cytoplasmic Hsp70 chaperone machinery subjects misfolded and endoplasmic reticulum import-incompetent proteins to degradation via the ubiquitin-proteasome system. Mol. Biol. Cell 2007, 18, 153–165. [Google Scholar] [CrossRef]
- Dokladny, K.; Myers, O.B.; Moseley, P.L. Heat shock response and autophagy--cooperation and control. Autophagy 2015, 11, 200–213. [Google Scholar] [CrossRef]
- Dokladny, K.; Zuhl, M.N.; Mandell, M.; Bhattacharya, D.; Schneider, S.; Deretic, V.; Moseley, P.L. Regulatory coordination between two major intracellular homeostatic systems: Heat shock response and autophagy. J. Biol. Chem. 2013, 288, 14959–14972. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Ciocca, D.R. Heat shock proteins: Stress proteins with Janus-like properties in cancer. Int. J. Hyperth. 2008, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiao, S.; Chen, J.; Wang, M.; Zheng, Z.; Song, S.; Zhang, L. Heat shock protein 90 mediates the apoptosis and autophage in nicotinic-mycoepoxydiene-treated HeLa cells. Acta Biochim. Biophys. Sin. 2015, 47, 451–458. [Google Scholar] [CrossRef]
- Xu, Q. Role of heat shock proteins in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xia, J.; Yang, Z.; Wu, G.; Yang, J. The abnormal level of HSP70 is related to Treg/Th17 imbalance in PCOS patients. J. Ovarian Res. 2021, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Hu, X.; Ding, J.; Yang, J. Abnormal expression of HSP70 may contribute to PCOS pathology. J. Ovarian Res. 2019, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.; Linka, R.M.; Reinke, H. HSP90 affects the stability of BMAL1 and circadian gene expression. J. Biol. Rhythm. 2014, 29, 87–96. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Audouard, C.; Le Masson, F.; Charry, C.; Li, Z.; Christians, E.S. Oocyte-targeted deletion reveals that hsp90b1 is needed for the completion of first mitosis in mouse zygotes. PLoS ONE 2011, 6, e17109. [Google Scholar] [CrossRef]
- Das, M.; Djahanbakhch, O.; Hacihanefioglu, B.; Saridogan, E.; Ikram, M.; Ghali, L.; Raveendran, M.; Storey, A. Granulosa cell survival and proliferation are altered in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 881–887. [Google Scholar] [CrossRef]
- Velázquez, M.M.; Salvetti, N.R.; Amweg, A.N.; Díaz, P.U.; Matiller, V.; Ortega, H.H. Changes in the expression of Heat Shock Proteins in ovaries from bovines with cystic ovarian disease induced by ACTH. Res. Vet. Sci. 2013, 95, 1059–1067. [Google Scholar] [CrossRef]
- Gotoh, T.; Terada, K.; Oyadomari, S.; Mori, M. hsp70-DnaJ chaperone pair prevents nitric oxide- and CHOP-induced apoptosis by inhibiting translocation of Bax to mitochondria. Cell Death Differ. 2004, 11, 390–402. [Google Scholar] [CrossRef]
- Ma, X.; Fan, L.; Meng, Y.; Hou, Z.; Mao, Y.D.; Wang, W.; Ding, W.; Liu, J.Y. Proteomic analysis of human ovaries from normal and polycystic ovarian syndrome. Mol. Hum. Reprod. 2007, 13, 527–535. [Google Scholar] [CrossRef]
- Zhao, K.K.; Cui, Y.G.; Jiang, Y.Q.; Wang, J.; Li, M.; Zhang, Y.; Ma, X.; Diao, F.Y.; Liu, J.Y. Effect of HSP10 on apoptosis induced by testosterone in cultured mouse ovarian granulosa cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 171, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Isobe, N.; Yoshimura, Y. Deficient proliferation and apoptosis in the granulosa and theca interna cells of the bovine cystic follicle. J. Reprod. Dev. 2007, 53, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Ma, X.; Cai, L.B.; Cui, Y.G.; Liu, J.Y. Downregulation of both gene expression and activity of Hsp27 improved maturation of mouse oocyte in vitro. Reprod. Biol. Endocrinol. 2010, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ma, X.; Liu, S.; Liu, J.; Wang, W.; Cui, Y.; Ding, W.; Mao, Y.; Chen, H.; Huang, J.; et al. Effects of upregulation of Hsp27 expression on oocyte development and maturation derived from polycystic ovary syndrome. PLoS ONE 2013, 8, e83402. [Google Scholar] [CrossRef] [PubMed]
- Buteva-Hristova, I.; Lazarov, V.; Lozanov, V.; Gateva, A.; Bechev, B.; Kavaldzieva, K.; Mladenov, N.; Trifonova, N.; Dimitrova-Dikanarova, D.; Kamenov, Z. Serum anti-α-crystallin antibodies in women with endocrine disorders. Biotechnol. Biotechnol. Equip. 2017, 31, 574–580. [Google Scholar] [CrossRef]
- Salvetti, N.R.; Panzani, C.G.; Gimeno, E.J.; Neme, L.G.; Alfaro, N.S.; Ortega, H.H. An imbalance between apoptosis and proliferation contributes to follicular persistence in polycystic ovaries in rats. Reprod. Biol. Endocrinol. 2009, 7, 68. [Google Scholar] [CrossRef]
- Isobe, N.; Yoshimura, Y. Immunocytochemical study of cell proliferation in the cystic ovarian follicles in cows. Theriogenology 2000, 54, 1159–1169. [Google Scholar] [CrossRef]
- Bas, D.; Abramovich, D.; Hernandez, F.; Tesone, M. Altered expression of Bcl-2 and Bax in follicles within dehydroepiandrosterone-induced polycystic ovaries in rats. Cell Biol. Int. 2011, 35, 423–429. [Google Scholar] [CrossRef]
- Li, Y.; Kong, L.; Deng, M.; Lian, Z.; Han, Y.; Sun, B.; Guo, Y.; Liu, G.; Liu, D. Heat Stress-Responsive Transcriptome Analysis in the Liver Tissue of Hu Sheep. Genes 2019, 10, 395. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, J.; Zhang, Z.; Shang, X.; Chu, Z.; Fu, Y.; Chu, M. Identification and analysis of differentially expressed long non-coding RNAs of Chinese Holstein cattle responses to heat stress. Anim. Biotechnol. 2020, 31, 9–16. [Google Scholar] [CrossRef]
- Shamovsky, I.; Ivannikov, M.; Kandel, E.S.; Gershon, D.; Nudler, E. RNA-mediated response to heat shock in mammalian cells. Nature 2006, 440, 556–560. [Google Scholar] [CrossRef]
- Yang, Z.; Li, W.; Su, X.; Ge, P.; Zhou, Y.; Hao, Y.; Shu, H.; Gao, C.; Cheng, S.; Zhu, G.; et al. Early Response of Radish to Heat Stress by Strand-Specific Transcriptome and miRNA Analysis. Int. J. Mol. Sci. 2019, 20, 3321. [Google Scholar] [CrossRef]
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef]
- Lellahi, S.M.; Rosenlund, I.A.; Hedberg, A.; Kiær, L.T.; Mikkola, I.; Knutsen, E.; Perander, M. The long noncoding RNA NEAT1 and nuclear paraspeckles are up-regulated by the transcription factor HSF1 in the heat shock response. J. Biol. Chem. 2018, 293, 18965–18976. [Google Scholar] [CrossRef]
- Bernabò, P.; Viero, G.; Lencioni, V. A long noncoding RNA acts as a post-transcriptional regulator of heat shock protein (HSP70) synthesis in the cold hardy Diamesa tonsa under heat shock. PLoS ONE 2020, 15, e0227172. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, Y.; Pu, S.; Zhang, X.; Li, Z.; Chen, J. Third-generation sequencing found LncRNA associated with heat shock protein response to heat stress in Populus qiongdaoensis seedlings. BMC Genom. 2020, 21, 572. [Google Scholar] [CrossRef]
- Dou, J.; Schenkel, F.; Hu, L.; Khan, A.; Khan, M.Z.; Yu, Y.; Wang, Y.; Wang, Y. Genome-wide identification and functional prediction of long non-coding RNAs in Sprague-Dawley rats during heat stress. BMC Genom. 2021, 22, 122. [Google Scholar] [CrossRef]
- Murri, M.; Insenser, M.; Fernández-Durán, E.; San-Millán, J.L.; Escobar-Morreale, H.F. Effects of polycystic ovary syndrome (PCOS), sex hormones, and obesity on circulating miRNA-21, miRNA-27b, miRNA-103, and miRNA-155 expression. J. Clin. Endocrinol. Metab. 2013, 98, E1835–E1844. [Google Scholar] [CrossRef]
- Naji, M.; Aleyasin, A.; Nekoonam, S.; Arefian, E.; Mahdian, R.; Amidi, F. Differential Expression of miR-93 and miR-21 in Granulosa Cells and Follicular Fluid of Polycystic Ovary Syndrome Associating with Different Phenotypes. Sci. Rep. 2017, 7, 14671. [Google Scholar] [CrossRef]
- Tu, M.; Wu, Y.; Mu, L.; Zhang, D. Long non-coding RNAs: Novel players in the pathogenesis of polycystic ovary syndrome. Ann. Transl. Med. 2021, 9, 173. [Google Scholar] [CrossRef]
- Gambineri, A.; Patton, L.; Altieri, P.; Pagotto, U.; Pizzi, C.; Manzoli, L.; Pasquali, R. Polycystic ovary syndrome is a risk factor for type 2 diabetes: Results from a long-term prospective study. Diabetes 2012, 61, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. International Diabetes Federation: A consensus on Type 2 diabetes prevention. Diabet. Med. 2007, 24, 451–463. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Screening for type 2 diabetes. Diabetes Care 2003, 26 (Suppl. 1), S21–S24. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.; Morteza, A.; Khajeali, L.; Esteghamati, A.; Khalilzadeh, O.; Asgarani, F.; Outeiro, T.F. Increased serum HSP70 levels are associated with the duration of diabetes. Cell Stress Chaperones 2010, 15, 959–964. [Google Scholar] [CrossRef]
- Nakhjavani, M.; Morteza, A.; Asgarani, F.; Khalilzadeh, O.; Ghazizadeh, Z.; Bathaie, S.Z.; Esteghamati, A. The dual behavior of heat shock protein 70 and asymmetric dimethylarginine in relation to serum CRP levels in type 2 diabetes. Gene 2012, 498, 107–111. [Google Scholar] [CrossRef]
- Kurucz, I.; Morva, A.; Vaag, A.; Eriksson, K.F.; Huang, X.; Groop, L.; Koranyi, L. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes 2002, 51, 1102–1109. [Google Scholar] [CrossRef]
- Chung, J.; Nguyen, A.K.; Henstridge, D.C.; Holmes, A.G.; Chan, M.H.; Mesa, J.L.; Lancaster, G.I.; Southgate, R.J.; Bruce, C.R.; Duffy, S.J.; et al. HSP72 protects against obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 2008, 105, 1739–1744. [Google Scholar] [CrossRef]
- Abubaker, J.; Tiss, A.; Abu-Farha, M.; Al-Ghimlas, F.; Al-Khairi, I.; Baturcam, E.; Cherian, P.; Elkum, N.; Hammad, M.; John, J.; et al. DNAJB3/HSP-40 cochaperone is downregulated in obese humans and is restored by physical exercise. PLoS ONE 2013, 8, e69217. [Google Scholar] [CrossRef]
- Kleinridders, A.; Lauritzen, H.P.; Ussar, S.; Christensen, J.H.; Mori, M.A.; Bross, P.; Kahn, C.R. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J. Clin. Investig. 2013, 123, 4667–4680. [Google Scholar] [CrossRef]
- Purwana, I.; Liu, J.J.; Portha, B.; Buteau, J. HSF1 acetylation decreases its transcriptional activity and enhances glucolipotoxicity-induced apoptosis in rat and human beta cells. Diabetologia 2017, 60, 1432–1441. [Google Scholar] [CrossRef]
- Bruce, C.R.; Carey, A.L.; Hawley, J.A.; Febbraio, M.A. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: Evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes 2003, 52, 2338–2345. [Google Scholar] [CrossRef]
- Kitano, S.; Kondo, T.; Matsuyama, R.; Ono, K.; Goto, R.; Takaki, Y.; Hanatani, S.; Sakaguchi, M.; Igata, M.; Kawashima, J.; et al. Impact of hepatic HSP72 on insulin signaling. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E305–E318. [Google Scholar] [CrossRef] [PubMed]
- Drew, B.G.; Ribas, V.; Le, J.A.; Henstridge, D.C.; Phun, J.; Zhou, Z.; Soleymani, T.; Daraei, P.; Sitz, D.; Vergnes, L.; et al. HSP72 is a mitochondrial stress sensor critical for Parkin action, oxidative metabolism, and insulin sensitivity in skeletal muscle. Diabetes 2014, 63, 1488–1505. [Google Scholar] [CrossRef] [PubMed]
- Henstridge, D.C.; Forbes, J.M.; Penfold, S.A.; Formosa, M.F.; Dougherty, S.; Gasser, A.; de Courten, M.P.; Cooper, M.E.; Kingwell, B.A.; de Courten, B. The relationship between heat shock protein 72 expression in skeletal muscle and insulin sensitivity is dependent on adiposity. Metabolism 2010, 59, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Pettit-Mee, R.J.; Power, G.; Cabral-Amador, F.J.; Ramirez-Perez, F.I.; Soares, R.N.; Sharma, N.; Liu, Y.; Christou, D.D.; Kanaley, J.A.; Martinez-Lemus, L.A.; et al. Endothelial HSP72 is not reduced in type 2 diabetes nor is it a key determinant of endothelial insulin sensitivity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R43–R58. [Google Scholar] [CrossRef]
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod. Health 2019, 13, 1179558119874042. [Google Scholar] [CrossRef]
- Sabbah, N.A.; Rezk, N.A.; Saad, M.S.S. Relationship Between Heat Shock Protein Expression and Obesity With and Without Metabolic Syndrome. Genet. Test. Mol. Biomark. 2019, 23, 737–743. [Google Scholar] [CrossRef]
- González, F.; Considine, R.V.; Abdelhadi, O.A.; Xue, J.; Acton, A.J. Saturated fat ingestion stimulates proatherogenic inflammation in polycystic ovary syndrome. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E689–E701. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Sagili, H. Metabolic syndrome in women with polycystic ovary syndrome. Obstet. Gynaecol. 2018, 20, 245–252. [Google Scholar] [CrossRef]
- Sell, H.; Poitou, C.; Habich, C.; Bouillot, J.L.; Eckel, J.; Clément, K. Heat Shock Protein 60 in Obesity: Effect of Bariatric Surgery and its Relation to Inflammation and Cardiovascular Risk. Obesity 2017, 25, 2108–2114. [Google Scholar] [CrossRef]
- Sharma, H.S.; Westman, J.A.N. 17—The Heat Shock Proteins and Hemeoxygenase Response in Central Nervous System Injuries. In Blood-Spinal Cord and Brain Barriers in Health and Disease; Sharma, H.S., Westman, J., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 329–360. [Google Scholar]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Denis-Larose, C.; Massie, B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell Biol. 1997, 17, 5317–5327. [Google Scholar] [CrossRef]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2017, 11, 2. [Google Scholar] [CrossRef]
- Pericleous, P.; Stephanides, S. Can resistance training improve the symptoms of polycystic ovary syndrome? BMJ Open Sport Exerc. Med. 2018, 4, e000372. [Google Scholar] [CrossRef]
- Ely, B.R.; Francisco, M.A.; Halliwill, J.R.; Bryan, S.D.; Comrada, L.N.; Larson, E.A.; Brunt, V.E.; Minson, C.T. Heat therapy reduces sympathetic activity and improves cardiovascular risk profile in women who are obese with polycystic ovary syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R630–R640. [Google Scholar] [CrossRef]
- Ely, B.R.; Clayton, Z.S.; McCurdy, C.E.; Pfeiffer, J.; Minson, C.T. Meta-inflammation and cardiometabolic disease in obesity: Can heat therapy help? Temperature (Austin) 2018, 5, 9–21. [Google Scholar] [CrossRef]
- Ely, B.R.; Clayton, Z.S.; McCurdy, C.E.; Pfeiffer, J.; Needham, K.W.; Comrada, L.N.; Minson, C.T. Heat therapy improves glucose tolerance and adipose tissue insulin signaling in polycystic ovary syndrome. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E172–E182. [Google Scholar] [CrossRef]
- Rogers, R.S.; Beaudoin, M.S.; Wheatley, J.L.; Wright, D.C.; Geiger, P.C. Heat shock proteins: In vivo heat treatments reveal adipose tissue depot-specific effects. J. Appl. Physiol. (1985) 2015, 118, 98–106. [Google Scholar] [CrossRef]
- Hooper, P.L.; Hooper, P.L. Inflammation, heat shock proteins, and type 2 diabetes. Cell Stress Chaperones 2009, 14, 113–115. [Google Scholar] [CrossRef]
- McCarty, M.F. Induction of heat shock proteins may combat insulin resistance. Med. Hypotheses 2006, 66, 527–534. [Google Scholar] [CrossRef]
- Literáti-Nagy, B.; Kulcsár, E.; Literáti-Nagy, Z.; Buday, B.; Péterfai, E.; Horváth, T.; Tory, K.; Kolonics, A.; Fleming, A.; Mandl, J.; et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: A proof of concept randomized double-blind clinical trial. Horm. Metab. Res. 2009, 41, 374–380. [Google Scholar] [CrossRef]
- Morino, S.; Kondo, T.; Sasaki, K.; Adachi, H.; Suico, M.A.; Sekimoto, E.; Matsuda, T.; Shuto, T.; Araki, E.; Kai, H. Mild electrical stimulation with heat shock ameliorates insulin resistance via enhanced insulin signaling. PLoS ONE 2008, 3, e4068. [Google Scholar] [CrossRef] [PubMed]
- Molina, M.N.; Ferder, L.; Manucha, W. Emerging Role of Nitric Oxide and Heat Shock Proteins in Insulin Resistance. Curr. Hypertens. Rep. 2016, 18, 1. [Google Scholar] [CrossRef]
- Sørensen, A.E.; Wissing, M.L.; Salö, S.; Englund, A.L.; Dalgaard, L.T. MicroRNAs Related to Polycystic Ovary Syndrome (PCOS). Genes 2014, 5, 684–708. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, A.; Tutar, L.; Tutar, Y. Regulation of Heat Shock Proteins by miRNAs in human breast cancer. Microrna 2014, 3, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Qu, B.; Zhao, Q.; Ma, Q.; Yang, T.; Li, X.; Chen, Y.; Yang, J.; Zhang, Y. Overexpression of miR-144-3p alleviates polycystic ovaries syndrome through targeting expression of HSP-70. Gene Ther. 2022, 29, 217–226. [Google Scholar] [CrossRef]
- Okusha, Y.; Guerrero-Gimenez, M.E.; Lang, B.J.; Borges, T.J.; Stevenson, M.A.; Truman, A.W.; Calderwood, S.K. MicroRNA-570 targets the HSP chaperone network, increases proteotoxic stress and inhibits mammary tumor cell migration. Sci. Rep. 2022, 12, 15582. [Google Scholar] [CrossRef]
- Ou-Yang, Y.; Liu, Z.L.; Xu, C.L.; Wu, J.L.; Peng, J.; Peng, Q.H. miR-223 induces retinal ganglion cells apoptosis and inflammation via decreasing HSP-70 in vitro and in vivo. J. Chem. Neuroanat. 2020, 104, 101747. [Google Scholar] [CrossRef]
- El Bezawy, R.; Percio, S.; Ciniselli, C.M.; De Cesare, M.; Colella, G.; Dugo, M.; Veneroni, S.; Doldi, V.; Martini, S.; Baratti, D.; et al. miR-550a-3p is a prognostic biomarker and exerts tumor-suppressive functions by targeting HSP90AA1 in diffuse malignant peritoneal mesothelioma. Cancer Gene Ther. 2022, 29, 1394–1404. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.E.; Hong, C.Y.; Lin, Y.L.; Chen, R.M. MicroRNA-1 participates in nitric oxide-induced apoptotic insults to MC3T3-E1 cells by targeting heat-shock protein-70. Int. J. Biol. Sci. 2015, 11, 246–255. [Google Scholar] [CrossRef]
- Kalmar, B.; Greensmith, L. Induction of heat shock proteins for protection against oxidative stress. Adv. Drug Deliv. Rev. 2009, 61, 310–318. [Google Scholar] [CrossRef]

| Authors | Year | HSPs | Aim of the Study | Main Findings |
|---|---|---|---|---|
| Li L et al. [11] | 2016 | 90B1 | To analyze protein expression profiles in the ovarian tissues of subjects with PCOS | HSP90B1’s expression was increased by at least 2-fold and associated with the proliferation and survival of ovarian cells |
| Velázquez MML et al. [38] | 2013 | 27 and 60 | To analyze HSP expression changes in bovine ovaries with cystic ovarian disease (COD) (induced by ACTH) | Induced COD caused differences in HSP protein expression |
| Wu G et al. [33] | 2016 | 70 | To study the correlation between HSP70 and hormones and inflammatory factors and investigate the role of HSP70 in the pathogenesis of PCOS | HSP70 showed abnormal expression in PCOS, which correlated with testosterone and inflammatory factors |
| Zhao K-K et al. [41] | 2013 | 10 | To determine the effect of HSP10 on apoptosis induced by testosterone in granulosa cells of mouse ovaries | Testosterone may reduce HSP10 expression in granulosa cells causing reduced Bcl-2 expression and increased Bax expression |
| Liu J-J et al. [43] | 2010 | 27 | To investigate the effects of HSP27 downregulation on oocyte development | Reduction in HSP27 levels improved the maturation of mouse oocytes and increased the early stage of apoptosis in oocytes |
| Cai L et al. [44] | 2013 | 27 | To investigate the effects of upregulation of HSP27 on oocyte development and maturation in PCOS | Upregulation of HSP27 led to inhibition of oocyte maturation in women with PCOS |
| Yang Y et al. [32] | 2021 | 70 | To study the correlation between HSP70 and Treg/Th17 ratio | Abnormal levels of HSP70 were correlated with Treg/Th17 imbalance, indicating that HSP70 plays a role in PCOS immunological pathogenesis |
| MicroRNA | Role in Regulation of HSPs |
|---|---|
| miR-570 [97] | miR-570 was found to affect tumor cell growth and migration by targeting the HSP chaperone network |
| miR-223 [98] | miR-223 can inhibit cell proliferation and increase cell apoptosis by targeting HSP 70 |
| miR-550a-3p [99] | miR-550a-3p can have antiproliferative and proapoptotic effects in prostate and ovarian cancer cells by inhibiting HSP90AA1 |
| miR-1 [100] | miR-1 was found to play a role in nitric oxide-induced apoptosis in osteoblasts by targeting HSP70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niinuma, S.A.; Lubbad, L.; Lubbad, W.; Moin, A.S.M.; Butler, A.E. The Role of Heat Shock Proteins in the Pathogenesis of Polycystic Ovarian Syndrome: A Review of the Literature. Int. J. Mol. Sci. 2023, 24, 1838. https://doi.org/10.3390/ijms24031838
Niinuma SA, Lubbad L, Lubbad W, Moin ASM, Butler AE. The Role of Heat Shock Proteins in the Pathogenesis of Polycystic Ovarian Syndrome: A Review of the Literature. International Journal of Molecular Sciences. 2023; 24(3):1838. https://doi.org/10.3390/ijms24031838
Chicago/Turabian StyleNiinuma, Sara Anjum, Laila Lubbad, Walaa Lubbad, Abu Saleh Md Moin, and Alexandra E. Butler. 2023. "The Role of Heat Shock Proteins in the Pathogenesis of Polycystic Ovarian Syndrome: A Review of the Literature" International Journal of Molecular Sciences 24, no. 3: 1838. https://doi.org/10.3390/ijms24031838
APA StyleNiinuma, S. A., Lubbad, L., Lubbad, W., Moin, A. S. M., & Butler, A. E. (2023). The Role of Heat Shock Proteins in the Pathogenesis of Polycystic Ovarian Syndrome: A Review of the Literature. International Journal of Molecular Sciences, 24(3), 1838. https://doi.org/10.3390/ijms24031838








