NopAA and NopD Signaling Association-Related Gene GmNAC27 Promotes Nodulation in Soybean (Glycine max)
Abstract
:1. Introduction
2. Results
2.1. HH103ΩNopAA&D Inhibits Nodule Formation
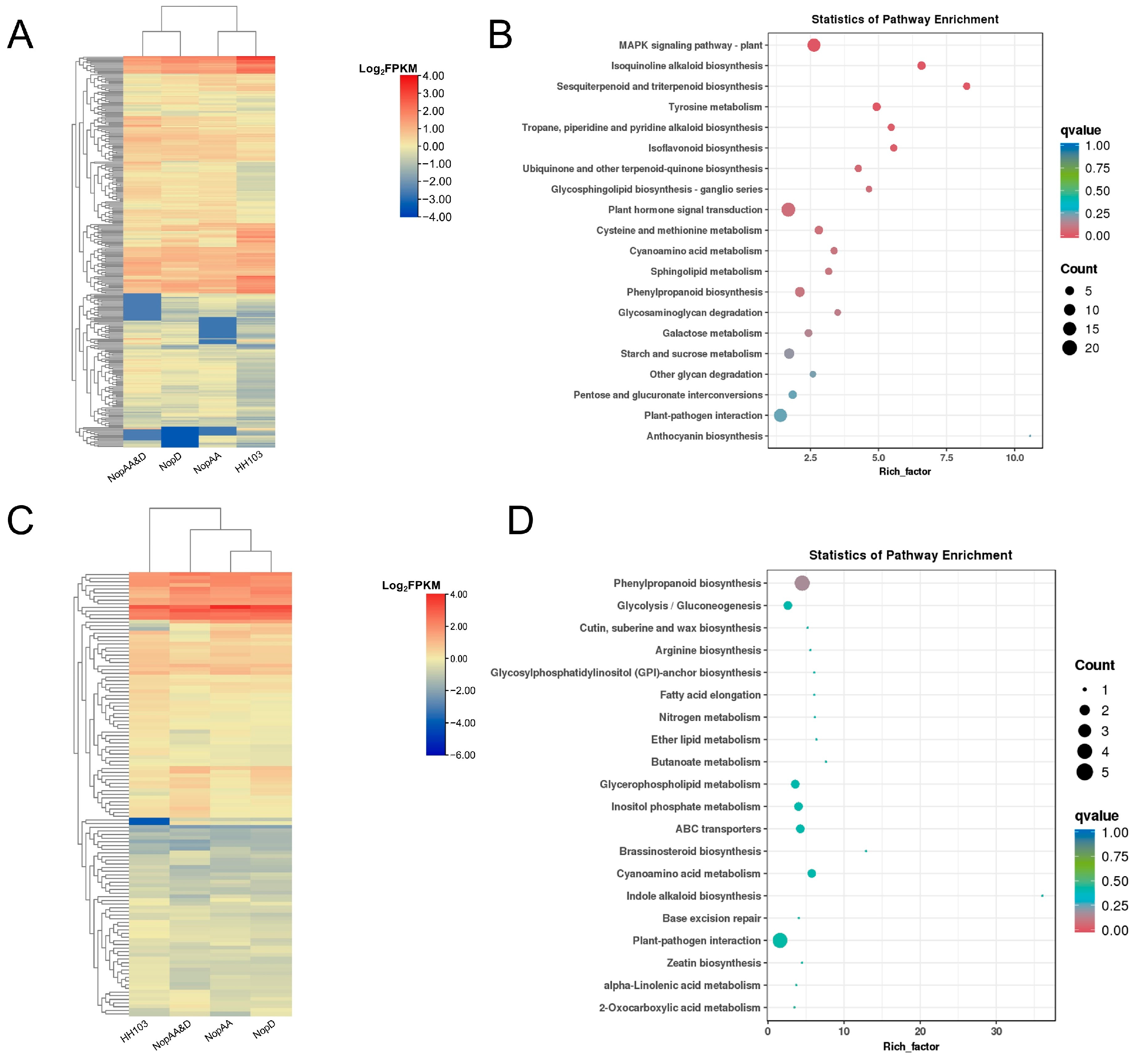
2.2. Similar Patterns of Differential Gene Expression Are Evident in Soybean Roots following HH103ΩNopAA and HH103ΩNopD Inoculation
2.3. NopAA and NopD Co-Mutation Primarily Impacts the Plant–Pathogen Interaction Pathway
2.4. NopAA and NopD Have Similar Effects on the Plant–Pathogen Interaction Pathway
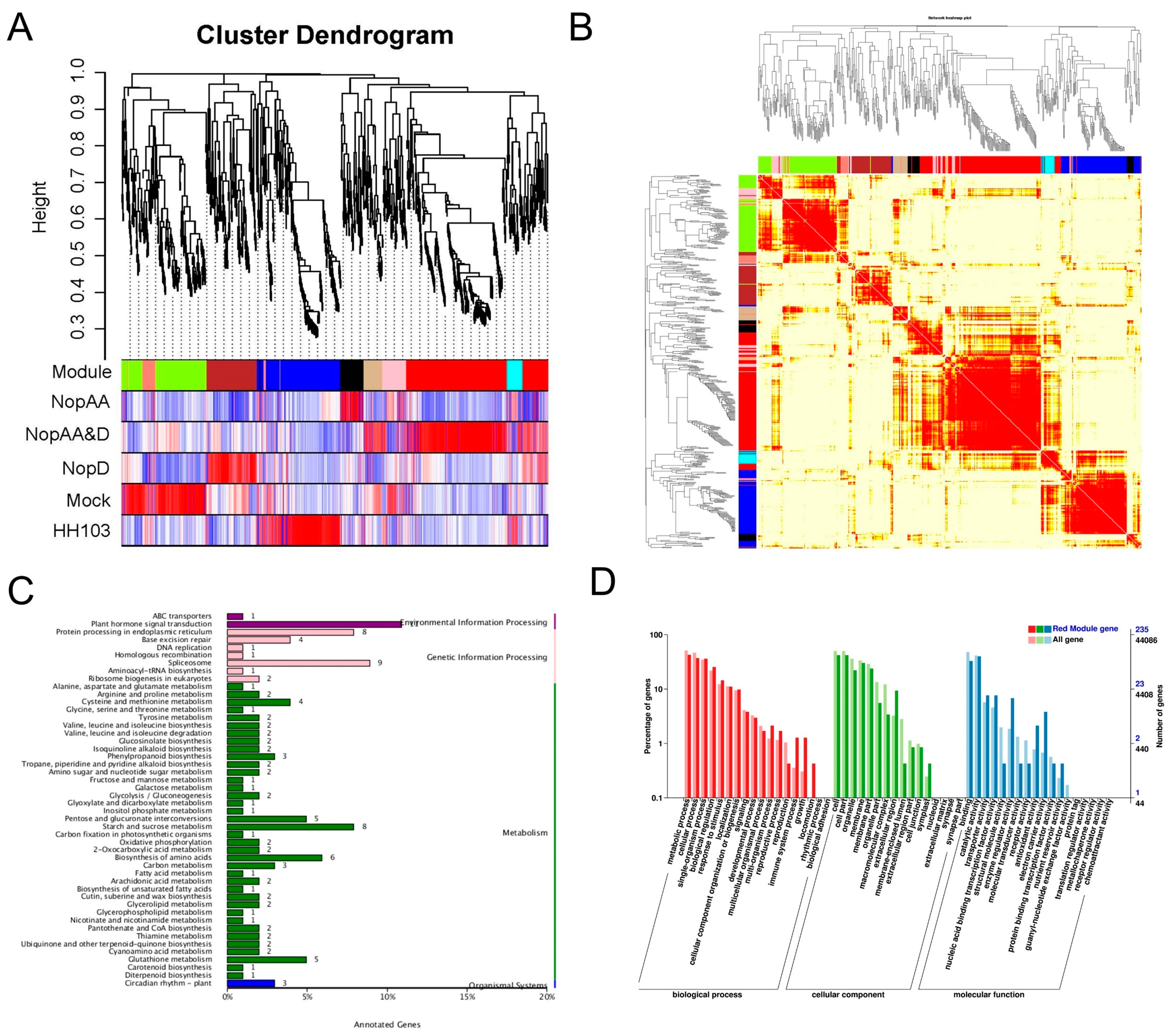
2.5. WGCNA Analyses Reveal Candidate NopAA and NopD-Associated Genes
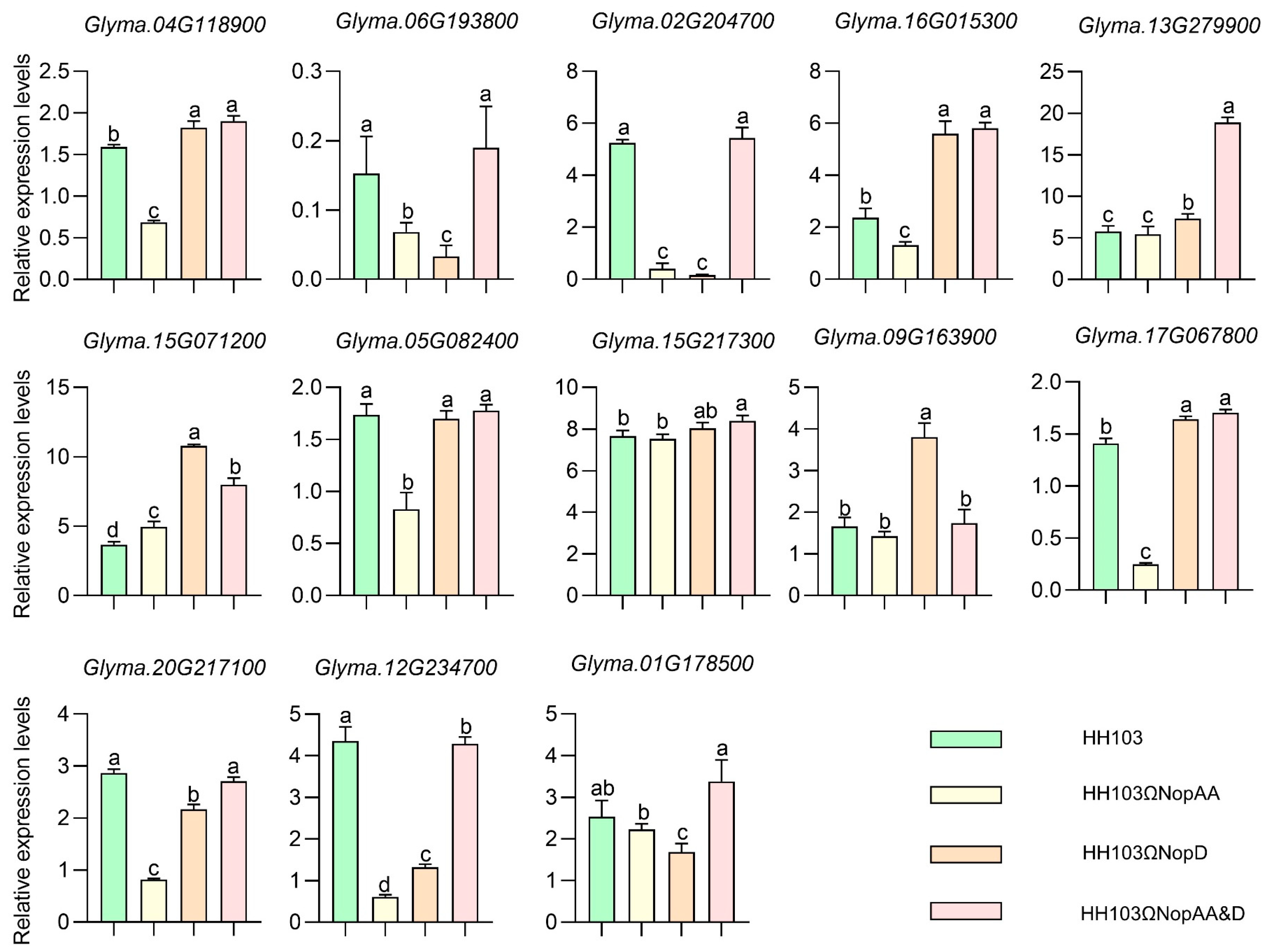
2.6. qRT-PCR Verification of Candidate Genes
2.7. Glyma.13G279900 Is a NAC Family Transcription Factor Located on the Cell Nucleus
2.8. Analyses of the Impact of GmNAC27 RNA-Interference and Overexpression on Soybean Nodulation
3. Discussion
4. Materials and Methods
4.1. Strains, Vectors and Primers
4.2. HH103ΩNopAA&NopD Mutant Strain Construction
4.3. Infection Event Analyses
4.4. Nodulation Test
4.5. qRT-PCR
4.6. RNA-Seq
4.7. Subcellular Localization Analyses
4.8. Soybean Hairy Root Transformation
4.9. Phylogenetic Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Lan, L.; Jin, Y.; Yu, N.; Wang, D.; Wang, E. Mechanisms underlying legume-rhizobium symbioses. J. Integr. Plant Biol. 2022, 64, 244–267. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.K.; Mohammed, M.; Ibny, F.Y.I.; Dakora, F.D. Rhizobia as a Source of Plant Growth-Promoting Molecules: Potential Applications and Possible Operational Mechanisms. Front. Sustain. Food Syst. 2021, 4, 619676. [Google Scholar] [CrossRef]
- Sedivy, E.J.; Wu, F.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Phytol. 2017, 214, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Adalibieke, W.; Cui, X.; Cai, H.; You, L.; Zhou, F. Global crop-specific nitrogen fertilization dataset in 1961–2020. Sci. Data 2023, 10, 617. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.; Brodie, E.L.; Riley, W.; Kaeppler, S.; Lynch, J. Impacts of Agricultural Nitrogen on the Environment and Strategies to Reduce these Impacts. Procedia Environ. Sci. 2015, 29, 303. [Google Scholar] [CrossRef]
- Ren, N.; Wang, Y.; Ye, Y.; Zhao, Y.; Huang, Y.; Fu, W.; Chu, X. Effects of Continuous Nitrogen Fertilizer Application on the Diversity and Composition of Rhizosphere Soil Bacteria. Front. Microbiol. 2020, 11, 1948. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, J.; Ahmad, S.; Malik, M. Nitrogenous fertilizers: Impact on environment sustainability, mitigation strategies, and challenges. Int. J. Environ. Sci. Technol. 2022, 19, 11649–11672. [Google Scholar] [CrossRef]
- Lopez-Baena, F.J.; Monreal, J.A.; Perez-Montano, F.; Guasch-Vidal, B.; Bellogin, R.A.; Vinardell, J.M.; Ollero, F.J. The absence of Nops secretion in Sinorhizobium fredii HH103 increases GmPR1 expression in Williams soybean. Mol. Plant Microbe Interact. 2009, 22, 1445–1454. [Google Scholar] [CrossRef]
- Ge, Y.Y.; Xiang, Q.W.; Wagner, C.; Zhang, D.; Xie, Z.P.; Staehelin, C. The type 3 effector NopL of Sinorhizobium sp. strain NGR234 is a mitogen-activated protein kinase substrate. J. Exp. Bot. 2016, 67, 2483–2494. [Google Scholar] [CrossRef]
- Canonne, J.; Pichereaux, C.; Marino, D.; Roby, D.; Rossignol, M.; Rivas, S. Identification of the protein sequence of the type III effector XopD from the B100 strain of Xanthomonas campestris pv campestris. Plant Signal Behav. 2012, 7, 184–187. [Google Scholar] [CrossRef]
- Busset, N.; Gully, D.; Teulet, A.; Fardoux, J.; Camuel, A.; Cornu, D.; Severac, D.; Giraud, E.; Mergaert, P. The Type III Effectome of the Symbiotic Bradyrhizobium vignae Strain ORS3257. Biomolecules 2021, 11, 1592. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Murray, J.D. The Role of Flavonoids in Nodulation Host-Range Specificity: An Update. Plants 2016, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Lei, N.; Chen, L.; Kiba, A.; Hikichi, Y.; Zhang, Y.; Ohnishi, K. Super-Multiple Deletion Analysis of Type III Effectors in Ralstonia solanacearum OE1-1 for Full Virulence Toward Host Plants. Front. Microbiol. 2020, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Bolzan de Campos, S.; Deakin, W.J.; Broughton, W.J.; Passaglia, L.M.P. Roles of flavonoids and the transcriptional regulator TtsI in the activation of the type III secretion system of Bradyrhizobium elkanii SEMIA587. Microbiology 2011, 157 Pt 3, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The Role of Nutrient Efficient Plants in Improving Crop Yields in the Twenty First Century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- Hidalgo-Castellanos, J.; Marín-Peña, A.J.; Herrera-Cervera, J.A.; López-Gómez, M. Polyamines: Key elements in the rhizobia-legume symbiosis? Phytochem. Rev. 2021, 21, 127–140. [Google Scholar] [CrossRef]
- Wang, T.; Balla, B.; Kovacs, S.; Kereszt, A. Varietas Delectat: Exploring Natural Variations in Nitrogen-Fixing Symbiosis Research. Front. Plant Sci. 2022, 13, 856187. [Google Scholar] [CrossRef]
- Redžepović, S.; Sikora, S.; Čolo, J.; Blažinkov, M. Influence of plant growth regulator and rhizobial inoculation on nodulation and soybean nitrogen contant. Cereal Res. Commun. 2007, 35, 993–996. [Google Scholar] [CrossRef]
- Shea, Z.; Singer, W.M.; Zhang, B. Soybean Production, Versatility, and Improvement. In Legume Crops—Prospects, Production and Uses; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Timilsina, S.; Potnis, N.; Newberry, E.A.; Liyanapathiranage, P.; Iruegas-Bocardo, F.; White, F.F.; Goss, E.M.; Jones, J.B. Xanthomonas diversity, virulence and plant–pathogen interactions. Nat. Rev. Microbiol. 2020, 18, 415–427. [Google Scholar] [CrossRef]
- Ma, C.; Liu, C.; Yu, Y.; Ma, S.; Pan, S.; Feng, H.; Chen, Q.; Xin, D.; Wu, X.; Wang, J. GmTNRP1, associated with rhizobial type-III effector NopT, regulates nitrogenase activity in the nodules of soybean (Glycine max). Food Energy Secur. 2023, 12, e466. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Sun, Y.; Zhao, P.; Liu, C.; Qing, K.; Hu, X.; Zhong, Z.; Cheng, J.; Wang, H.; et al. Glycine max NNL1 restricts symbiotic compatibility with widely distributed bradyrhizobia via root hair infection. Nat. Plants 2021, 7, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.W.; Liao, S.; Xie, Z.P.; Hann, D.R.; Steinle, L.; Boller, T.; Staehelin, C. Functional analysis of NopM, a novel E3 ubiquitin ligase (NEL) domain effector of Rhizobium sp. strain NGR234. PLoS Pathog. 2012, 8, e1002707. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, C.; Ma, S.; Zheng, H.; Feng, H.; Wang, Y.; Wang, J.; Liu, C.; Xin, D.; Chen, Q.; et al. GmARP is Related to the Type III Effector NopAA to Promote Nodulation in Soybean (Glycine max). Front. Genet. 2022, 13, 889795. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Marshall, N.C.; Rowland, J.L.; McCoy, J.M.; Worrall, L.J.; Santos, A.S.; Strynadka, N.C.J.; Finlay, B.B. Assembly, structure, function and regulation of type III secretion systems. Nat. Rev. Microbiol. 2017, 15, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Teulet, A.; Camuel, A.; Perret, X.; Giraud, E. The Versatile Roles of Type III Secretion Systems in Rhizobium-Legume Symbioses. Annu. Rev. Microbiol. 2022, 76, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Guerrero, I.; Perez-Montano, F.; Medina, C.; Ollero, F.J.; Lopez-Baena, F.J. The Sinorhizobium (Ensifer) fredii HH103 Nodulation Outer Protein NopI Is a Determinant for Efficient Nodulation of Soybean and Cowpea Plants. Appl. Environ. Microbiol. 2017, 83, e02770-16. [Google Scholar] [CrossRef]
- Ratu, S.T.N.; Teulet, A.; Miwa, H.; Masuda, S.; Nguyen, H.P.; Yasuda, M.; Sato, S.; Kaneko, T.; Hayashi, M.; Giraud, E.; et al. Rhizobia use a pathogenic-like effector to hijack leguminous nodulation signalling. Sci. Rep. 2021, 11, 2034. [Google Scholar] [CrossRef]
- Piromyou, P.; Nguyen, H.P.; Songwattana, P.; Boonchuen, P.; Teamtisong, K.; Tittabutr, P.; Boonkerd, N.; Alisha Tantasawat, P.; Gottfert, M.; Okazaki, S.; et al. The Bradyrhizobium diazoefficiens type III effector NopE modulates the regulation of plant hormones towards nodulation in Vigna radiata. Sci. Rep. 2021, 11, 16604. [Google Scholar] [CrossRef]
- Songwattana, P.; Chaintreuil, C.; Wongdee, J.; Teulet, A.; Mbaye, M.; Piromyou, P.; Gully, D.; Fardoux, J.; Zoumman, A.M.A.; Camuel, A.; et al. Identification of type III effectors modulating the symbiotic properties of Bradyrhizobium vignae strain ORS3257 with various Vigna species. Sci. Rep. 2021, 11, 4874. [Google Scholar] [CrossRef]
- Teulet, A.; Gully, D.; Rouy, Z.; Camuel, A.; Koebnik, R.; Giraud, E.; Lassalle, F. Phylogenetic distribution and evolutionary dynamics of nod and T3SS genes in the genus Bradyrhizobium. Microb. Genom. 2020, 6, mgen000407. [Google Scholar] [CrossRef]
- Jimenez-Guerrero, I.; Perez-Montano, F.; Medina, C.; Ollero, F.J.; Lopez-Baena, F.J. NopC Is a Rhizobium-Specific Type 3 Secretion System Effector Secreted by Sinorhizobium (Ensifer) fredii HH103. PLoS ONE 2015, 10, e0142866. [Google Scholar] [CrossRef] [PubMed]
- Kambara, K.; Ardissone, S.; Kobayashi, H.; Saad, M.M.; Schumpp, O.; Broughton, W.J.; Deakin, W.J. Rhizobia utilize pathogen-like effector proteins during symbiosis. Mol. Microbiol. 2009, 71, 92–106. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, S.; Higasitani, N.; Kaneko, T.; Yasuda, M.; Miwa, H.; Okazaki, S.; Saeki, K.; Higashitani, A.; Sato, S. Lotus Accessions Possess Multiple Checkpoints Triggered by Different Type III Secretion System Effectors of the Wide-Host-Range Symbiont Bradyrhizobium elkanii USDA61. Microbes Environ. 2020, 35, ME19141. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Ratu, S.T.N.; Yasuda, M.; Gottfert, M.; Okazaki, S. InnB, a Novel Type III Effector of Bradyrhizobium elkanii USDA61, Controls Symbiosis With Vigna Species. Front. Microbiol. 2018, 9, 3155. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.J.; Zeng, Y.; Xie, Z.P.; Staehelin, C. Symbiosis-promoting and deleterious effects of NopT, a novel type 3 effector of Rhizobium sp. strain NGR234. J. Bacteriol. 2008, 190, 5101–5110. [Google Scholar] [CrossRef] [PubMed]
- Skorpil, P.; Saad, M.M.; Boukli, N.M.; Kobayashi, H.; Ares-Orpel, F.; Broughton, W.J.; Deakin, W.J. NopP, a phosphorylated effector of Rhizobium sp. strain NGR234, is a major determinant of nodulation of the tropical legumes Flemingia congesta and Tephrosia vogelii. Mol. Microbiol. 2005, 57, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Teulet, A.; Busset, N.; Fardoux, J.; Gully, D.; Chaintreuil, C.; Cartieaux, F.; Jauneau, A.; Comorge, V.; Okazaki, S.; Kaneko, T.; et al. The rhizobial type III effector ErnA confers the ability to form nodules in legumes. Proc. Natl. Acad. Sci. USA 2019, 116, 21758–21768. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, Z.; Deng, X.; Zou, J.; Ma, C.; Li, C.; Yin, T.; Liu, C.; Wang, J.; Chen, Q.; et al. GmPBS1, a Hub Gene Interacting with Rhizobial Type-III Effectors NopT and NopP, Regulates Soybean Nodulation. Agronomy 2023, 13, 1242. [Google Scholar] [CrossRef]
- Ni, H.; Peng, Y.; Wang, J.; Wang, J.; Yuan, Y.; Fu, T.; Zhu, Z.; Zhang, J.; Pan, X.; Cui, Z.; et al. Mapping of Quantitative Trait Loci Underlying Nodule Traits in Soybean (Glycine max (L.) Merr.) and Identification of Genes Whose Expression Is Affected by the Sinorhizobium fredii HH103 Effector Proteins NopL and NopT. Agronomy 2022, 12, 946. [Google Scholar] [CrossRef]
- Lu, W.; Zheng, Z.; Kang, Q.; Liu, H.; Jia, H.; Yu, F.; Zhang, Y.; Han, D.; Zhang, X.; Yan, X.; et al. Detection of type III effector-induced transcription factors that regulate phytohormone content during symbiosis establishment in soybean. Physiol. Plant 2023, 175, e13872. [Google Scholar] [CrossRef]
- Zou, J.-n.; Zhang, Z.-G.; Kang, Q.-L.; Yu, S.-Y.; Wang, J.-Q.; Chen, L.; Liu, Y.-R.; Ma, C.; Zhu, R.-S.; Zhu, Y.-X.; et al. Characterization of chromosome segment substitution lines reveals candidate genes associated with the nodule number in soybean. J. Integr. Agric. 2022, 21, 2197–2210. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, Z.; Wen, Y.; Yu, G.; Zou, J.; Huang, S.; Wang, J.; Zhu, J.; Wang, J.; Chen, L.; et al. RNA Sequencing-Associated Study Identifies GmDRR1 as Positively Regulating the Establishment of Symbiosis in Soybean. Mol. Plant Microbe Interact. 2020, 33, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, J.; Ma, C.; Zhou, Z.; Yang, D.; Zheng, J.; Wang, Q.; Li, H.; Zhou, H.; Sun, Z.; et al. QTL Mapping and Data Mining to Identify Genes Associated with the Sinorhizobium fredii HH103 T3SS Effector NopD in Soybean. Front. Plant Sci. 2020, 11, 453. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.W.; Bai, J.; Cai, J.; Huang, Q.Y.; Wang, Y.; Liang, Y.; Zhong, Z.; Wagner, C.; Xie, Z.P.; Staehelin, C. NopD of Bradyrhizobium sp. XS1150 Possesses SUMO Protease Activity. Front. Microbiol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Canonne, J.; Marino, D.; Jauneau, A.; Pouzet, C.; Briere, C.; Roby, D.; Rivas, S. The Xanthomonas type III effector XopD targets the Arabidopsis transcription factor MYB30 to suppress plant defense. Plant Cell 2011, 23, 3498–3511. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, S.; Wang, J.; Li, Q.; Xin, X.F.; Zhou, S.; Wang, Y.; Li, D.; Xu, J.; Luo, Z.Q.; et al. A bacterial kinase phosphorylates OSK1 to suppress stomatal immunity in rice. Nat. Commun. 2021, 12, 5479. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, C.; Oldroyd, G.E. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Zribi, I.; Ghorbel, M.; Brini, F. Pathogenesis Related Proteins (PRs): From Cellular Mechanisms to Plant Defense. Curr. Protein Pept. Sci. 2021, 22, 396–412. [Google Scholar] [CrossRef]
- Kimura, K.; Iwatsuki, M.; Nagai, T.; Matsumoto, A.; Takahashi, Y.; Shiomi, K.; Omura, S.; Abe, A. A small-molecule inhibitor of the bacterial type III secretion system protects against in vivo infection with Citrobacter rodentium. J. Antibiot. (Tokyo) 2011, 64, 197–203. [Google Scholar] [CrossRef]
- Zhang, R.; Zheng, F.; Wei, S.; Zhang, S.; Li, G.; Cao, P.; Zhao, S. Evolution of Disease Defense Genes and Their Regulators in Plants. Int. J. Mol. Sci. 2019, 20, 335. [Google Scholar] [CrossRef]
- Gourion, B.; Berrabah, F.; Ratet, P.; Stacey, G. Rhizobium-legume symbioses: The crucial role of plant immunity. Cell Press 2015, 20, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Sun, J.; Li, X.; Guan, Y.; Xie, F. Asymmetric redundancy of soybean Nodule Inception (NIN) genes in root nodule symbiosis. Plant Physiol. 2022, 188, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Katinakis, P.; Hendriks, P.; Smolders, A.; de Vries, F.; Spee, J.; van Kammen, A.; Bisseling, T.; Franssen, H. Characterization of GmENOD40, a gene showing novel patterns of cell-specific expression during soybean nodule development. Plant J. 1993, 3, 573–585. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Gao, H.; Wang, H.; Guo, Y.; He, M.; Peng, Y.; Wang, X. GSK3-mediated stress signaling inhibits legume-rhizobium symbiosis by phosphorylating GmNSP1 in soybean. Mol. Plant 2021, 14, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Takahashi, S.; Umehara, Y.; Iwano, H.; Tsurumaru, H.; Odake, H.; Suzuki, Y.; Kondo, H.; Konno, Y.; Yamakawa, T.; et al. Variation in bradyrhizobial NopP effector determines symbiotic incompatibility with Rj2-soybeans via effector-triggered immunity. Nat. Commun. 2018, 9, 3139. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Chen, L.; Fu, Y.; Li, C.; Qi, Z.; Zou, J.; Zhu, R.; Li, S.; Wei, W.; et al. Mining for genes encoding proteins associated with NopL of Sinorhizobium fredii HH103 using quantitative trait loci in soybean (Glycine max Merr.) recombinant inbred lines. Plant Soil. 2018, 431, 245–255. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Liu, C.; Ma, C.; Li, C.; Zhang, Y.; Qi, Z.; Zhu, R.; Shi, Y.; Zou, J.; et al. Identification of Soybean Genes Whose Expression is Affected by the Ensifer fredii HH103 Effector Protein NopP. Int. J. Mol. Sci. 2018, 19, 3438. [Google Scholar] [CrossRef]
- Huang, J.-C.; Piater, L.A.; Dubery, I.A. The NAC transcription factor gene ANAC072 is differentially expressed in Arabidopsis thaliana in response to microbe-associated molecular pattern (MAMP) molecules. Physiol. Mol. Plant Pathol. 2012, 80, 19–27. [Google Scholar] [CrossRef]
- Liu, N.; Xu, Y.; Li, Q.; Cao, Y.; Yang, D.; Liu, S.; Wang, X.; Mi, Y.; Liu, Y.; Ding, C.; et al. A lncRNA fine-tunes salicylic acid biosynthesis to balance plant immunity and growth. Cell Host Microbe 2022, 30, 1124–1138.e1128. [Google Scholar] [CrossRef]
- Li, S.; Wang, N.; Ji, D.; Zhang, W.; Wang, Y.; Yu, Y.; Zhao, S.; Lyu, M.; You, J.; Zhang, Y.; et al. A GmSIN1/GmNCED3s/GmRbohBs Feed-Forward Loop Acts as a Signal Amplifier That Regulates Root Growth in Soybean Exposed to Salt Stress. Plant Cell 2019, 31, 2107–2130. [Google Scholar] [CrossRef]
- Krehenbrink, M.; Downie, J.A. Identification of protein secretion systems and novel secreted proteins in Rhizobium leguminosarum bv. viciae. BMC Genom. 2008, 9, 55. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jia, X.; Li, Y.; Ma, S.; Ma, C.; Xin, D.; Wang, J.; Chen, Q.; Liu, C. NopAA and NopD Signaling Association-Related Gene GmNAC27 Promotes Nodulation in Soybean (Glycine max). Int. J. Mol. Sci. 2023, 24, 17498. https://doi.org/10.3390/ijms242417498
Wang Y, Jia X, Li Y, Ma S, Ma C, Xin D, Wang J, Chen Q, Liu C. NopAA and NopD Signaling Association-Related Gene GmNAC27 Promotes Nodulation in Soybean (Glycine max). International Journal of Molecular Sciences. 2023; 24(24):17498. https://doi.org/10.3390/ijms242417498
Chicago/Turabian StyleWang, Yue, Xiaoke Jia, Yansong Li, Shengnan Ma, Chao Ma, Dawei Xin, Jinhui Wang, Qingshan Chen, and Chunyan Liu. 2023. "NopAA and NopD Signaling Association-Related Gene GmNAC27 Promotes Nodulation in Soybean (Glycine max)" International Journal of Molecular Sciences 24, no. 24: 17498. https://doi.org/10.3390/ijms242417498
APA StyleWang, Y., Jia, X., Li, Y., Ma, S., Ma, C., Xin, D., Wang, J., Chen, Q., & Liu, C. (2023). NopAA and NopD Signaling Association-Related Gene GmNAC27 Promotes Nodulation in Soybean (Glycine max). International Journal of Molecular Sciences, 24(24), 17498. https://doi.org/10.3390/ijms242417498





