Rice OsANN9 Enhances Drought Tolerance through Modulating ROS Scavenging Systems
Abstract
:1. Introduction
2. Results
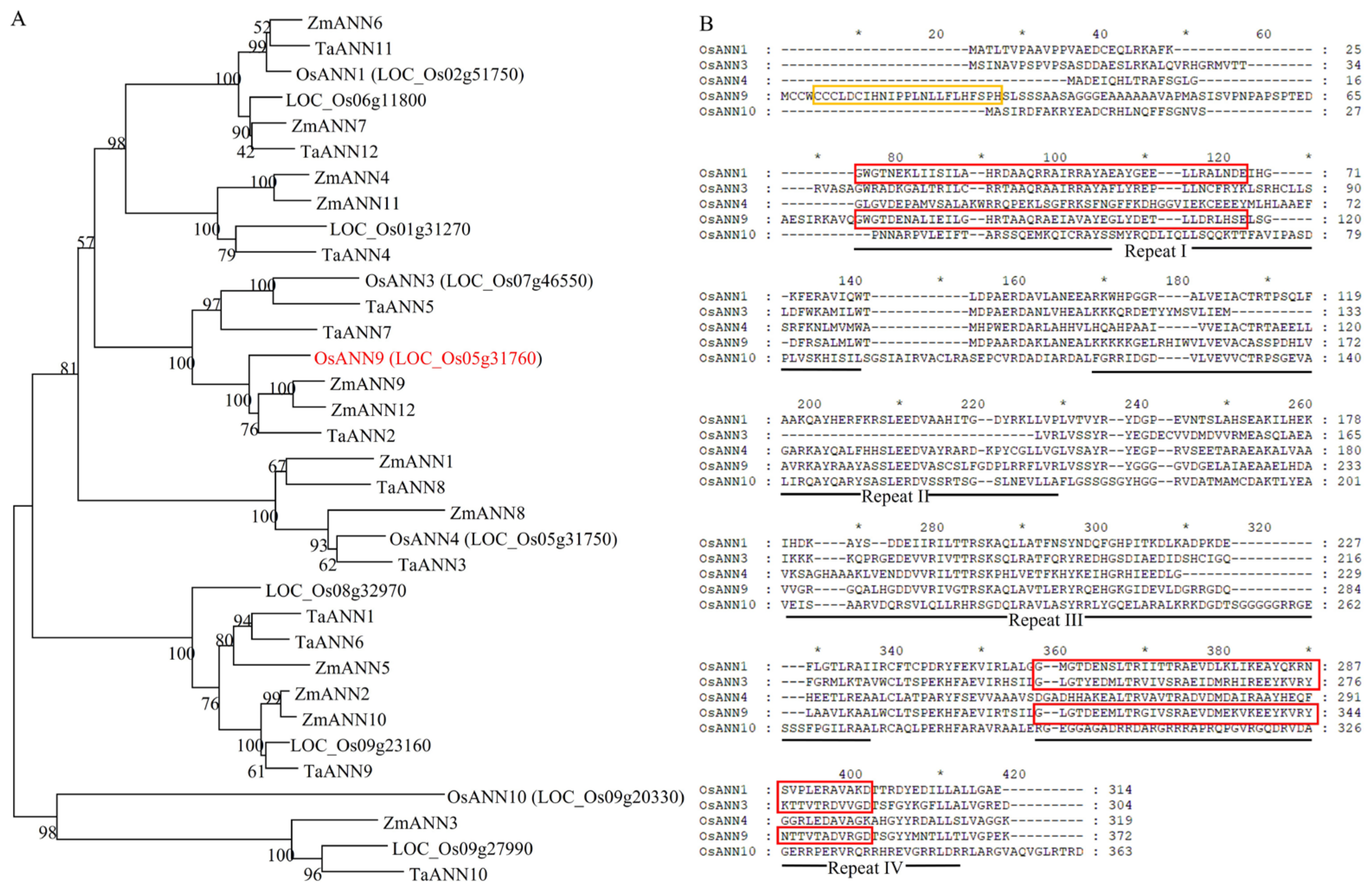
2.1. OsANN9 Encodes an Annexin Family Member
2.2. OsANN9 Expression Is Induced by Osmotic and Drought Stress
2.3. Construction of OsANN9-OE, OsANN9-Ri, and osann9 Mutants
2.4. Overexpression of OsANN9 Activates the ROS Scavenging System, Thereby Enhancing Tolerance to Osmotic and Drought Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Construction of OsANN9 Expression Vectors
4.3. Subcellular Localization of OsANN9
4.4. Recombinant OsANN9-His Protein Purification and Ca2+-Binding Activity
4.5. GUS Staining and GUS Activity
4.6. Antioxidant Enzyme Activity
4.7. Detection of O2− and H2O2 In Situ
4.8. Measurement of MDA and Electrolyte Leakage
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, J.K. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef]
- He, X.Q.; Batáry, P.; Zou, Y.; Zhou, W.W.; Wang, G.H.; Liu, Z.Y.; Bai, Y.Y.; Gong, S.X.; Zhu, Z.R.; Settele, J.; et al. Agricultural diversification promotes sustainable and resilient global rice production. Nat. Food 2023, 4, 788–796. [Google Scholar] [CrossRef]
- Rezvi, H.U.A.; Tahjib-Ul-Arif, M.; Azim, M.A.; Tumpa, T.A.; Tipu, M.M.H.; Najnine, F.; Dawood, M.F.A.; Skalicky, M.; Brestič, M. Rice and food security: Climate change implications and the future prospects for nutritional security. Food Energy Secur. 2022, 12, e430. [Google Scholar] [CrossRef]
- He, F.; Wang, H.L.; Li, H.G.; Su, Y.Y.; Li, S.; Yang, Y.L.; Feng, C.H.; Yin, W.L.; Xia, X.L. PeCHYR1, a ubiquitin E3 ligase from Populus euphratica, enhances drought tolerance via ABA-induced stomatal closure by ROS production in Populus. Plant Biotechnol. J. 2018, 16, 1514–1528. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.L.; Wang, Y.; Xie, Z.Z.; Guo, D.Y.; Chen, C.W.; Fan, Q.J.; Deng, X.D.; Liu, J.H. Enhanced ROS scavenging and sugar accumulation contribute to drought tolerance of naturally occurring autotetraploids in Poncirus trifoliata. Plant Biotechnol. J. 2019, 17, 1394–1407. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Mahmood, A.; Awan, M.I.; Maqbool, R.; Aamer, M.; Alhaithloul, H.A.S.; Huang, G.Q.; Skalicky, M.; Brestic, M.; Pandey, S.; et al. Melatonin-induced protection against plant abiotic stress: Mechanisms and prospects. Front. Plant Sci. 2022, 13, 902694. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Clark, G.B.; Roux, S.J. Annexins of plant cells. Plant Physiol. 1995, 109, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, J.C.; Laohavisit, A.; Macpherson, N.; Webb, A.; Brownlee, C.; Battey, N.H.; Davies, J.M. Annexins: Multifunctional components of growth and adaptation. J. Exp. Bot. 2008, 59, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Laohavisit, A.; Davies, J.M. Annexins. New Phytol. 2011, 189, 40–53. [Google Scholar] [CrossRef]
- Clark, G.B.; Morgan, R.O.; Fernandez, M.P.; Roux, S.J. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol. 2012, 196, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.W.; Han, L.J.; Xia, S.Y.; Zhu, R.J.; Kang, E.; Shang, Z.L. ATANN3 is involved in extracellular ATP-regulated auxin distribution in Arabidopsis thaliana seedlings. Plants 2023, 12, 330. [Google Scholar] [CrossRef] [PubMed]
- Harbaoui, M.; Ben Romdhane, W.; Ben Hsouna, A.; Brini, F.; Ben Saad, R. The durum wheat annexin, TdAnn6, improves salt and osmotic stress tolerance in Arabidopsis via modulation of antioxidant machinery. Protoplasma 2021, 258, 1047–1059. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, J.; Clark, G.; Roux, S.J. ANN1 and ANN2 function in post-phloem sugar transport in root tips to affect primary root growth. Plant Physiol. 2018, 178, 390–401. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Gao, C.H.; Guo, G.Y.; Liu, J.; Gao, Y.; Pan, R.H.; Guan, Y.J.; Hu, J. Maize annexin genes ZmANN33 and ZmANN35 encode proteins that function in cell membrane recovery during seed germination. J. Exp. Bot. 2019, 70, 1183–1195. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.M.; Noh, E.K.; Kim, H.G.; Jeon, B.W.; Bae, K.; Hu, H.C.; Kwak, J.M.; Park, O.K. Arabidopsis annexins AnnAt1 and AnnAt4 interact with each other and regulate drought and salt stress responses. Plant Cell Physiol. 2010, 51, 1499–1514. [Google Scholar] [CrossRef]
- Lee, S.; Lee, E.J.; Yang, E.J.; Lee, J.E.; Park, A.R.; Song, W.H.; Park, O.K. Proteomic identification of annexins, calcium-dependent membrane binding proteins that mediate osmotic stress and abscisic acid signal transduction in Arabidopsis. Plant Cell 2004, 16, 1378–1391. [Google Scholar] [CrossRef]
- Richards, S.L.; Laohavisit, A.; Mortimer, J.C.; Shabala, L.; Swarbreck, S.M.; Shabala, S.; Davies, J.M. Annexin 1 regulates the H2O2-induced calcium signature in Arabidopsis thaliana roots. Plant J. 2013, 77, 136–145. [Google Scholar] [CrossRef]
- Liu, Q.B.; Ding, Y.L.; Shi, Y.T.; Ma, L.; Wang, Y.; Song, C.P.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 2021, 40, e104559. [Google Scholar] [CrossRef]
- Lichocka, M.; Krzymowska, M.; Górecka, M.; Hennig, J. Arabidopsis annexin 5 is involved in maintenance of pollen membrane integrity and permeability. J. Exp. Bot. 2022, 73, 94–109. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Xu, Y.J.; Lei, Y.; Li, Q.; Zhao, J.Q.; Li, Y.; Fan, J.; Xiao, S.; Wang, W.M. ANNEXIN 8 negatively regulates RPW8.1-mediated cell death and disease resistance in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Jami, S.K.; Clark, G.B.; Ayele, B.T.; Roux, S.J.; Kirti, P.B. Identification and characterization of annexin gene family in rice. Plant Cell Rep. 2011, 31, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Que, Z.Q.; Lu, Q.N.; Li, Q.X.; Shen, C.X. The rice annexin gene OsAnn5 is involved in cold stress tolerance at the seedling stage. Plant Direct 2023, 7, e539. [Google Scholar] [CrossRef]
- Qiao, B.; Zhang, Q.; Liu, D.L.; Wang, H.Q.; Yin, J.Y.; Wang, R.; He, M.L.; Cui, M.; Shang, Z.L.; Wang, D.K.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Q.; Liu, R.; Zhou, Q.Z.; Ye, J.; Meng, F.W.; Liu, J.; Yang, C. Calcium-binding protein OsANN1 regulates rice blast disease resistance by inactivating jasmonic acid signaling. Plant Physiol. 2023, 192, 1621–1637. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, Q.; Yang, X.; Han, J.B.; Zhu, Z.G. OsANN3, a calcium-dependent lipid binding annexin is a positive regulator of ABA-dependent stress tolerance in rice. Plant Sci. 2019, 284, 212–220. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, T.; Guan, C.; Gao, Y.J.; Ma, J.C.; Gu, X.Y.; Qi, Z.G.; Wang, X.J.; Zhu, Z.G. OsANN4 modulates ROS production and mediates Ca2+ influx in response to ABA. BMC Plant Biol. 2021, 21, 474. [Google Scholar] [CrossRef]
- Gao, S.X.; Song, T.; Han, J.B.; He, M.L.; Zhang, Q.; Zhu, Y.; Zhu, Z.G. A calcium-dependent lipid binding protein, OsANN10, is a negative regulator of osmotic stress tolerance in rice. Plant Sci. 2020, 293, 110420. [Google Scholar] [CrossRef]
- Foyer, C.H.; Hanke, G. ROS production and signalling in chloroplasts: Cornerstones and evolving concepts. Plant J. 2022, 111, 642–661. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef]
- Shi, Y.; Chang, Y.L.; Wu, H.T.; Shalmani, A.; Liu, W.T.; Li, W.Q.; Xu, J.W.; Chen, K.M. OsRbohB-mediated ROS production plays a crucial role in drought stress tolerance of rice. Plant Cell Rep. 2020, 39, 1767–1784. [Google Scholar] [CrossRef]
- Santosh Kumar, V.V.; Yadav, S.K.; Verma, R.K.; Shrivastava, S.; Ghimire, O.; Pushkar, S.; Rao, M.V.; Senthil Kumar, T.; Chinnusamy, V. The abscisic acid receptor OsPYL6 confers drought tolerance to indica rice through dehydration avoidance and tolerance mechanisms. J. Exp. Bot. 2021, 72, 1411–1431. [Google Scholar] [CrossRef]
- Long, Q.; Qiu, S.C.; Man, J.M.; Ren, D.H.; Xu, N.; Luo, R. OsAAI1 increases rice yield and drought tolerance dependent on ABA-mediated regulatory and ROS scavenging pathway. Rice 2023, 16, 35. [Google Scholar] [CrossRef]
- Ben, S.R.; Harbaoui, M.; Ben, R.W.; Zouari, N.; Giang, K.N.; Ben, H.A.; Brini, F. Overexpression of Triticum durum TdAnn12 gene confers stress tolerance through scavenging reactive oxygen species in transgenic tobacco. Funct. Plant Biol. 2019, 46, 885–895. [Google Scholar]
- Niu, S.K.; Gu, X.Y.; Zhang, Q.; Tian, X.M.; Chen, Z.; Liu, J.R.; Wei, X.J.; Yan, C.X.; Liu, Z.W.; Wang, X.J.; et al. Grapevine bZIP transcription factor bZIP45 regulates VvANN1 and confers drought tolerance in Arabidopsis. Front. Plant Sci. 2023, 14, 1128002. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Hu, C.; Elias, E.; Nawrocki, W.J.; Croce, R. Drought affects both photosystems in Arabidopsis thaliana. New Phytol. 2023, 240, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Qin, F. Genetic dissection of maize drought tolerance for trait improvement. Mol. Breed. 2021, 41, 8. [Google Scholar] [CrossRef]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X.M. Insights into drought stress signaling in plants and the molecular genetic basis of cotton drought tolerance. Cells 2020, 9, 105. [Google Scholar] [CrossRef]
- Schmidt, R.; Mieulet, D.; Hubberten, H.M.; Obata, T.; Hoefgen, R.; Fernie, A.R.; Fisahn, J.; San Segundo, B.; Guiderdoni, E.; Schippers, J.H.M.; et al. Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell 2013, 25, 2115–2131. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.H.; Rizwan, M.; Fahad, S.; Xu, Z.H.; Hu, L.Y. Seed priming with melatonin coping drought stress in rapeseed by regulating reactive oxygen species detoxification: Antioxidant defense system, osmotic adjustment, stomatal traits and chloroplast ultrastructure perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Villadangos, S. Cheap, cost-effective, and quick stress biomarkers for drought stress detection and monitoring in plants. Trends Plant Sci. 2023, 28, 527–536. [Google Scholar] [CrossRef]
- Terletskaya, N.V.; Shcherban, A.B.; Nesterov, M.A.; Perfil‘ev, R.N.; Salina, E.A.; Altayeva, N.A.; Blavachinskaya, I.V. Drought stress tolerance and photosynthetic activity of alloplasmic lines T. dicoccum x T. aestivum. Int. J. Mol. Sci. 2020, 21, 3356. [Google Scholar] [CrossRef]
- Smith, E.N.; van Aalst, M.; Tosens, T.; Niinemets, Ü.; Stich, B.; Morosinotto, T.; Alboresi, A.; Erb, T.J.; Gómez-Coronado, P.A.; Tolleter, D.; et al. Improving photosynthetic efficiency toward food security: Strategies, advances, and perspectives. Mol. Plant 2023, 16, 1547–1563. [Google Scholar] [CrossRef] [PubMed]
- Dodd, A.N.; Kudla, J.; Sanders, D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010, 61, 593–620. [Google Scholar] [CrossRef] [PubMed]
- Laohavisit, A.; Richards, S.L.; Shabala, L.; Chen, C.; Colaço, R.D.D.R.; Swarbreck, S.M.; Shaw, E.; Dark, A.; Shabala, S.; Shang, Z.L.; et al. Salinity-induced calcium signaling and root adaptation in Arabidopsis require the calcium regulatory protein Annexin1. Plant Physiol. 2013, 163, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Boyidi, P.; Trishla, V.S.; Botta, H.K.; Yadav, D.; Kirti, P.B. Heterologous expression of rice annexin OsANN5 potentiates abiotic stress tolerance in transgenic tobacco through ROS amelioration. Plant Stress 2021, 2, 100022. [Google Scholar] [CrossRef]
- Ma, L.; Ye, J.M.; Yang, Y.Q.; Lin, H.X.; Yue, L.L.; Luo, J.; Long, Y.; Fu, H.Q.; Liu, X.N.; Zhang, Y.L.; et al. The SOS2-SCaBP8 complex generates and fine-tunes an AtANN4-dependent calcium signature under salt stress. Dev. Cell 2019, 48, 697–709.e5. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, J.Y.; Chong, K.; Harter, K.; Lee, Y.; Leung, J.; Martinoia, E.; Matsuoka, M.; Offringa, R.; Qu, L.J.; et al. Toward a molecular understanding of plant hormone actions. Mol. Plant 2016, 9, 1–3. [Google Scholar] [CrossRef]
- Liao, Z.G.; Zhang, Y.C.; Yu, Q.; Fang, W.C.; Chen, M.Y.; Li, T.F.; Liu, Y.; Liu, Z.C.; Chen, L.; Yu, S.W.; et al. Coordination of growth and drought responses by GA-ABA signaling in rice. New Phytol. 2023, 240, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Z.; Hou, L.Y.; Meng, J.J.; You, H.W.; Li, Z.; Gong, Z.Z.; Yang, S.H.; Shi, Y.T. The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, S.S.; Li, W.Q.; Feng, W.Q.; Li, J.; Wu, Z.D.; Gao, X.W.; Liu, F.Q.; Shao, M. Overexpression of a Harpin-encoding gene hrf1 in rice enhances drought tolerance. J. Exp. Bot. 2011, 62, 4229–4238. [Google Scholar] [CrossRef] [PubMed]
- Li, X.T.; Xie, Y.Y.; Zhu, Q.L.; Liu, Y.G. Targeted genome editing in genes and cis-regulatory regions improves qualitative and quantitative traits in crops. Mol. Plant 2017, 10, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Toki, S.; Hara, N.; Ono, K.; Onodera, H.; Tagiri, A.; Oka, S.; Tanaka, H. Early infection of scutellum tissue with Agrobacterium allows high-speed transformation of rice. Plant J. 2006, 47, 969–976. [Google Scholar] [CrossRef]
- Tang, N.; Ma, S.Q.; Zong, W.; Yang, N.; Lv, Y.; Yan, C.; Guo, Z.L.; Li, J.; Li, X.; Xiang, Y.; et al. MODD mediates deactivation and degradation of OsbZIP46 to negatively regulate ABA signaling and drought resistance in rice. Plant Cell 2016, 28, 2161–2177. [Google Scholar] [CrossRef]
- Jiang, Y.J.; Qiu, Y.P.; Hu, Y.R.; Yu, D.Q. Heterologous expression of AtWRKY57 confers drought tolerance in Oryza sativa. Front. Plant Sci. 2016, 7, 145. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Liu, C.; Tang, D.Y.; Yan, L.; Wang, D.; Yang, Y.Z.; Gui, J.S.; Zhao, X.Y.; Li, L.G.; Tang, X.D.; et al. The receptor-like cytoplasmic kinase STRK1 phosphorylates and activates CatC, thereby regulating H2O2 homeostasis and improving salt tolerance in rice. Plant Cell 2018, 30, 1100–1118. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Gu, X.; Chai, J.; Yao, X.; Cheng, S.; Liu, L.; He, S.; Peng, Y.; Zhang, Q.; Zhu, Z. Rice OsANN9 Enhances Drought Tolerance through Modulating ROS Scavenging Systems. Int. J. Mol. Sci. 2023, 24, 17495. https://doi.org/10.3390/ijms242417495
Jia Y, Gu X, Chai J, Yao X, Cheng S, Liu L, He S, Peng Y, Zhang Q, Zhu Z. Rice OsANN9 Enhances Drought Tolerance through Modulating ROS Scavenging Systems. International Journal of Molecular Sciences. 2023; 24(24):17495. https://doi.org/10.3390/ijms242417495
Chicago/Turabian StyleJia, Yangyang, Xiangyang Gu, Jiaxin Chai, Xiaohong Yao, Shoutao Cheng, Lirui Liu, Saiya He, Yizhuo Peng, Qian Zhang, and Zhengge Zhu. 2023. "Rice OsANN9 Enhances Drought Tolerance through Modulating ROS Scavenging Systems" International Journal of Molecular Sciences 24, no. 24: 17495. https://doi.org/10.3390/ijms242417495
APA StyleJia, Y., Gu, X., Chai, J., Yao, X., Cheng, S., Liu, L., He, S., Peng, Y., Zhang, Q., & Zhu, Z. (2023). Rice OsANN9 Enhances Drought Tolerance through Modulating ROS Scavenging Systems. International Journal of Molecular Sciences, 24(24), 17495. https://doi.org/10.3390/ijms242417495





