Surfactin and Capric Acid Affect the Posaconazole Susceptibility of Candida albicans Strains with Altered Sterols and Sphingolipids Biosynthesis
Abstract
:1. Introduction
2. Results
2.1. Susceptibility of Candida albicans Strains with Alterations in Ergosterol and Sphingolipids Biosynthesis to Posaconazol in Combination with Surfactin or Capric Acid
2.1.1. Susceptibility of Candida albicans Strains to Posaconazole in Combination with Surfactin or Capric Acid
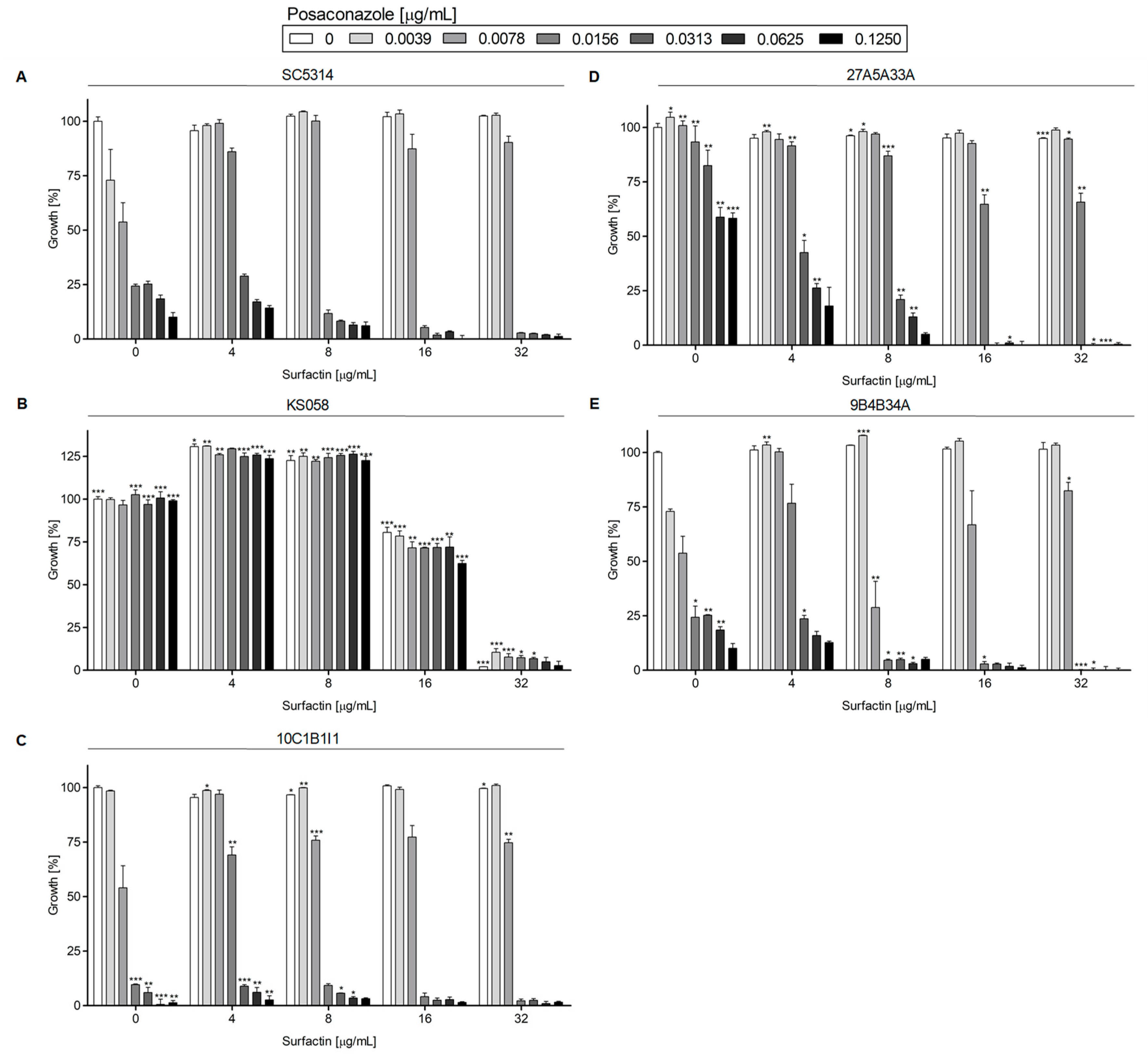
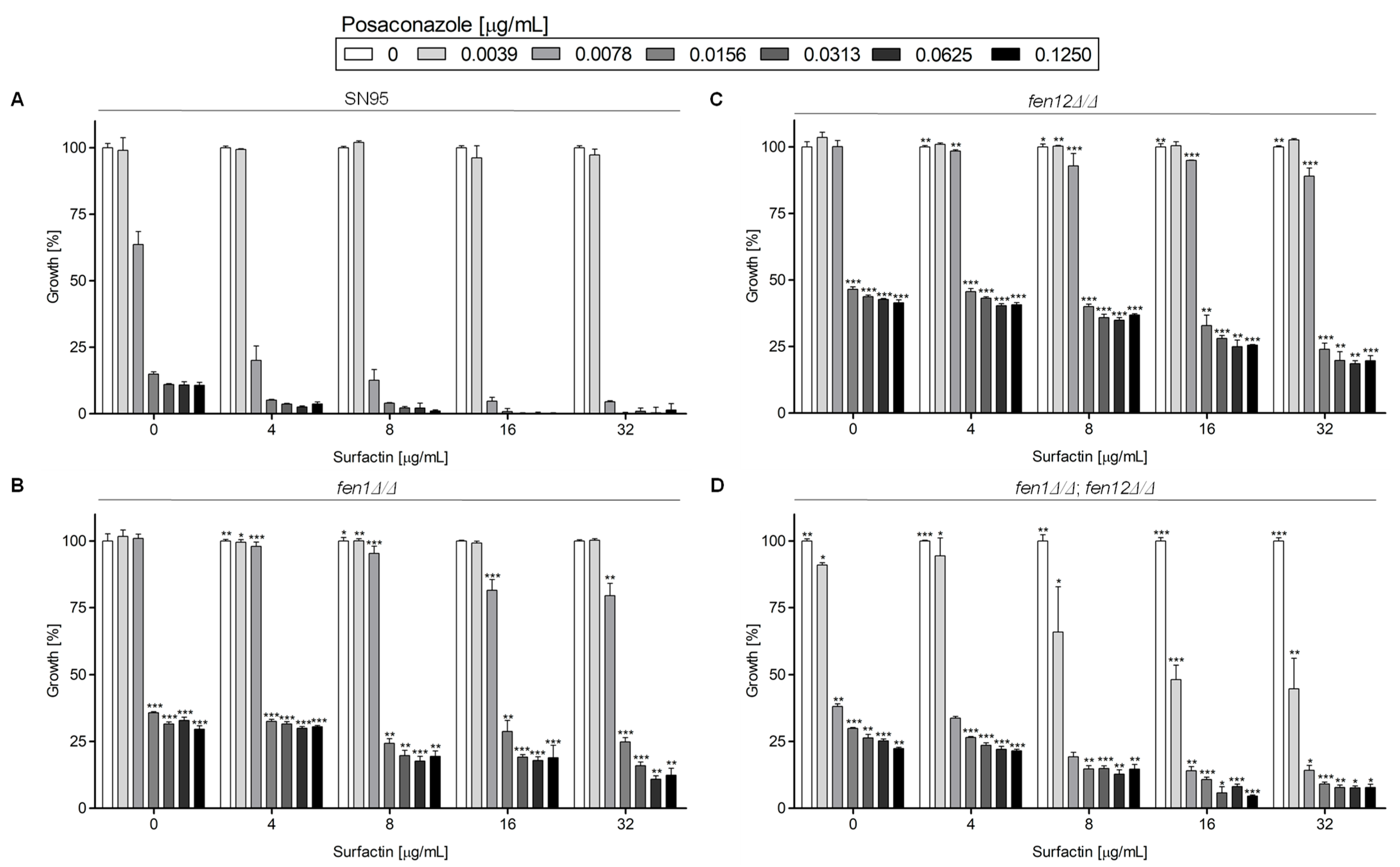
Posaconazole in Combination with Surfactin
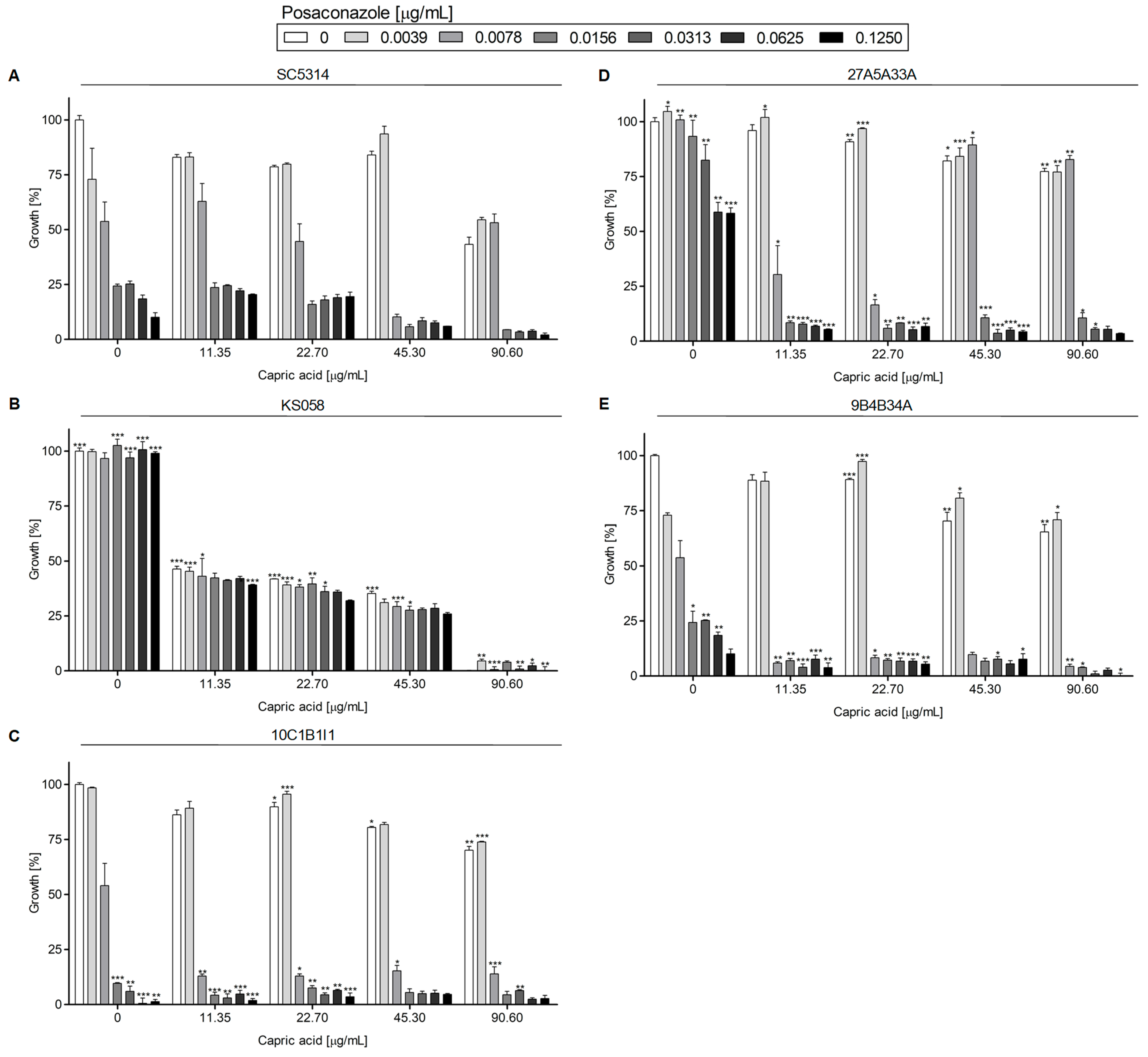
Posaconazole in Combination with Capric Acid
2.1.2. Growth of Candida albicans Strains in the Presence of Posaconazole in Combination with Surfactin or Capric Acid
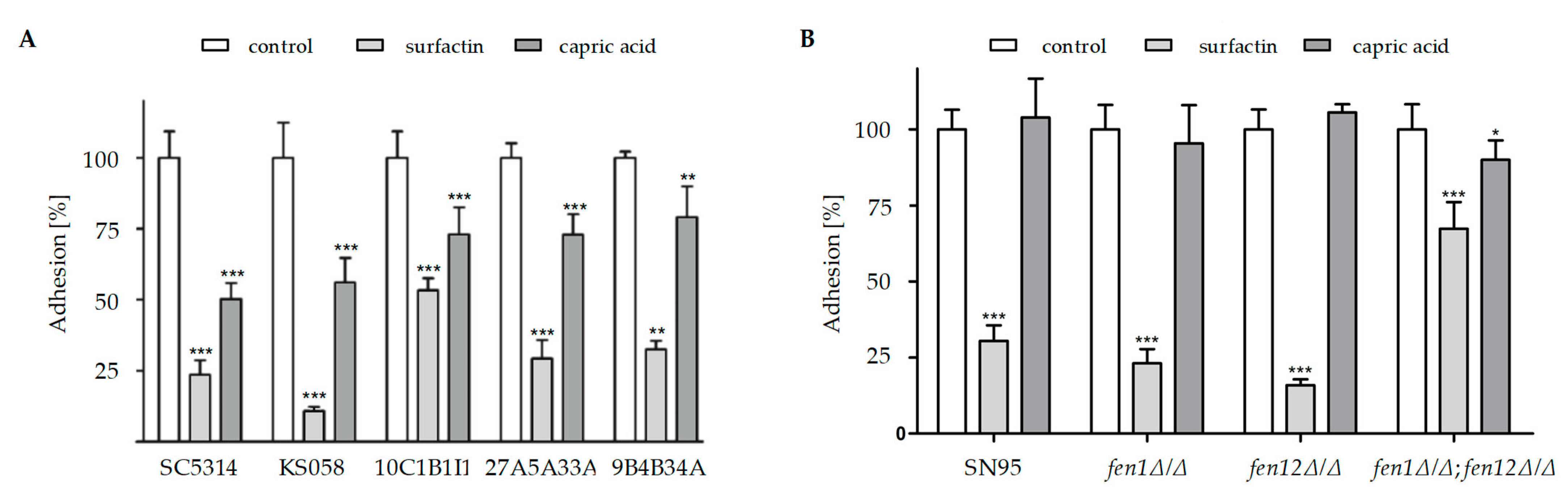
2.2. Candida albicans Strains with Altered Ergosterol or Sphingolipids Biosynthesis Exhibit Diminished Adhesion to Abiotic Surfaces in the Presence of Surfactin or Capric Acid
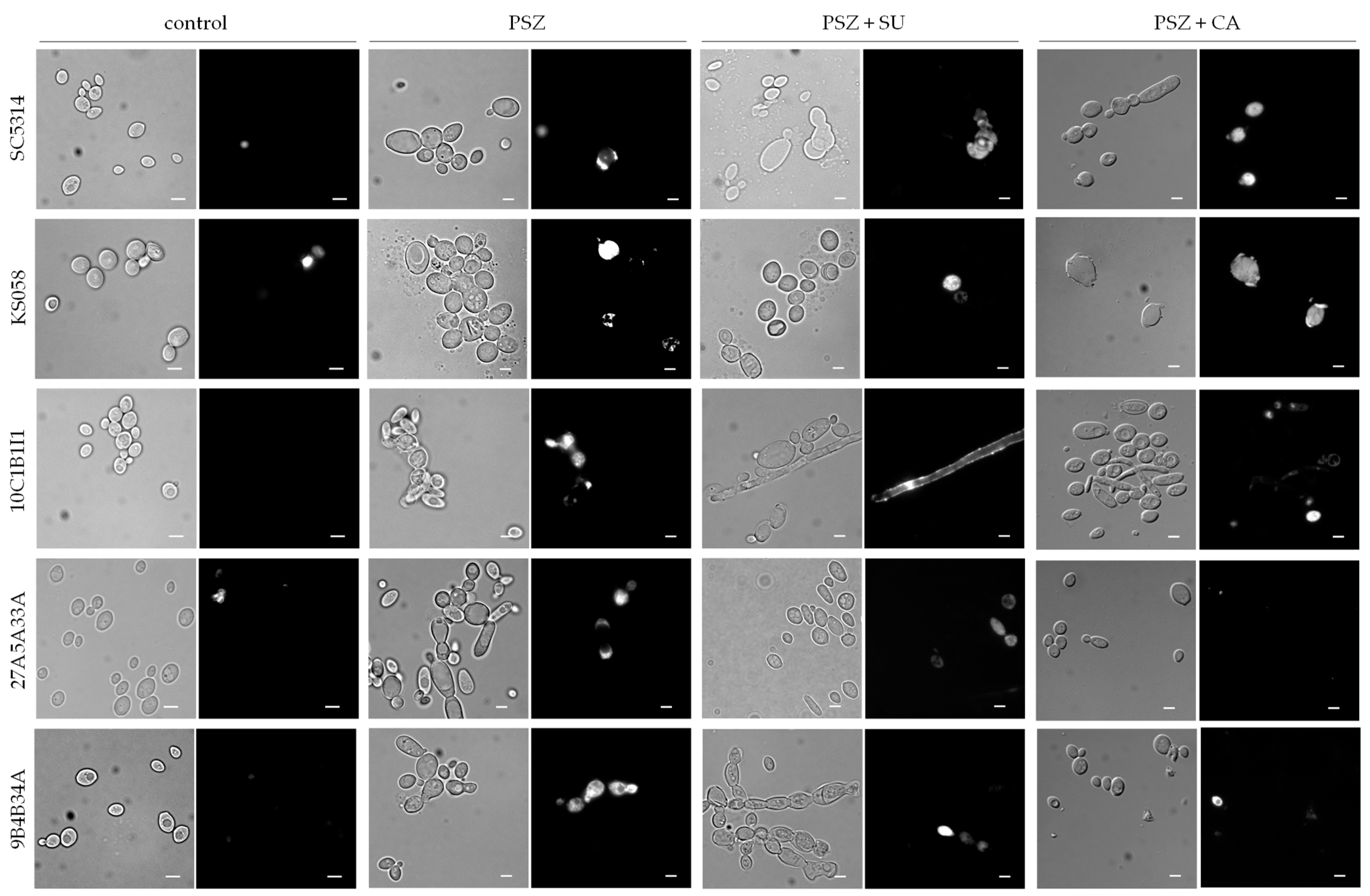
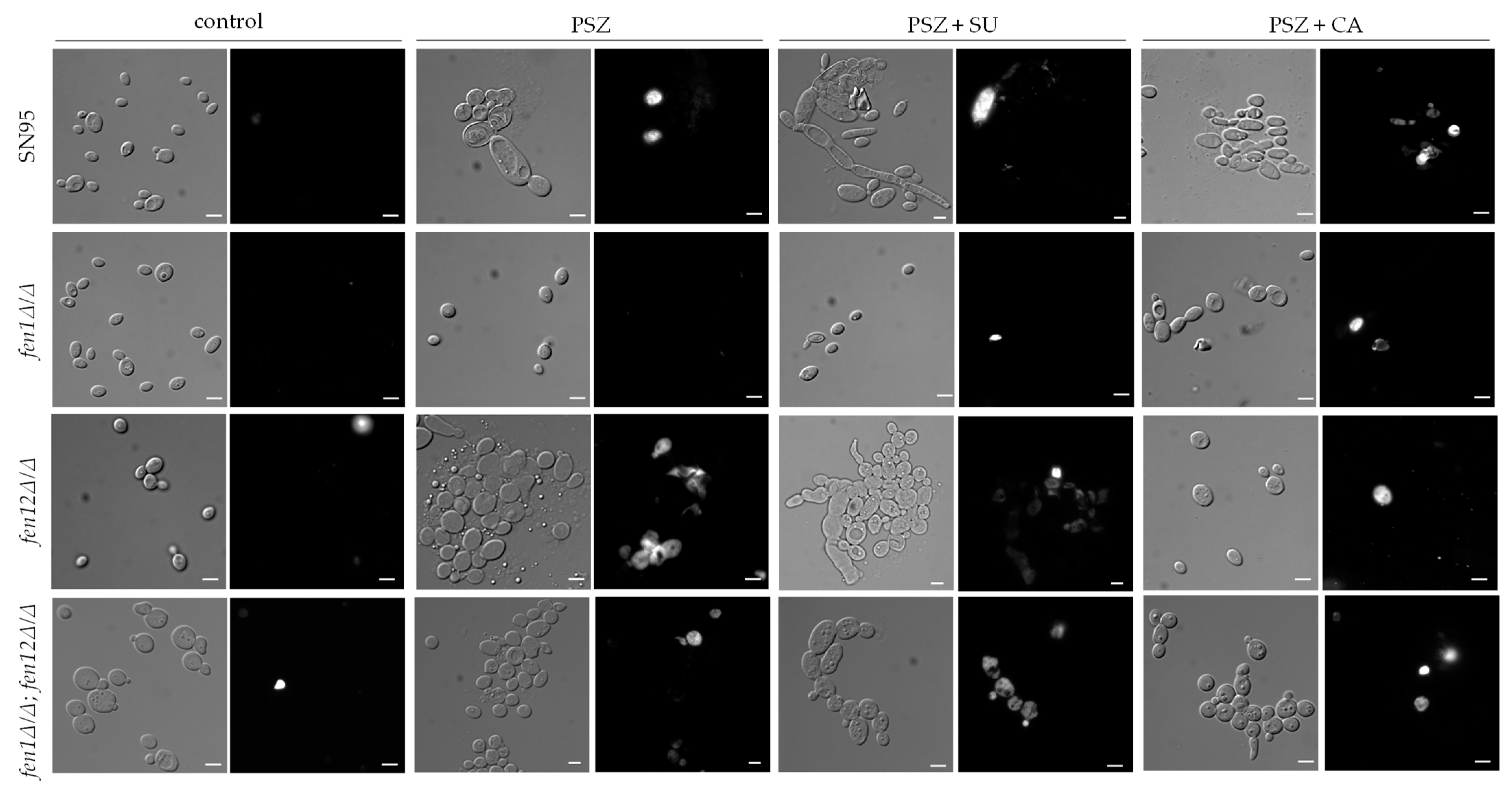
2.3. The Combination of Posaconazole and Surfactin or Capric Acid Results in Plasma Membrane Permeabilization in Candida albicans Strains with Altered Ergosterol or Sphingolipids Biosynthesis
3. Discussion
4. Materials and Methods
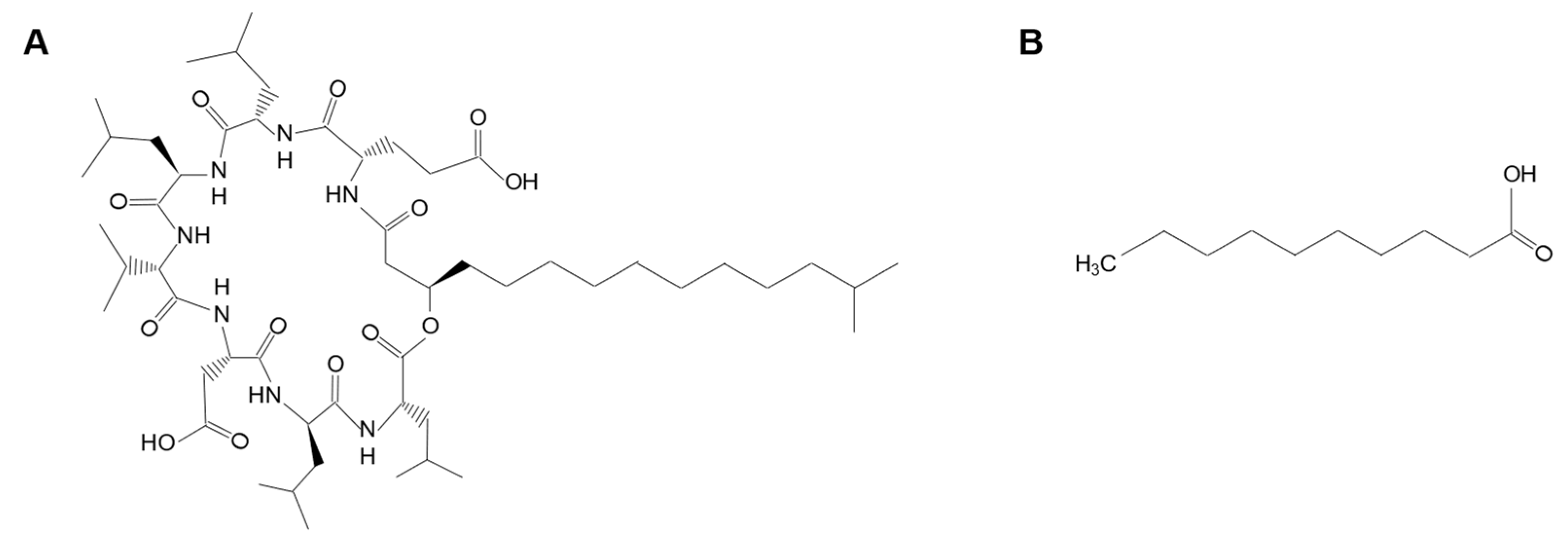
4.1. Chemicals
4.2. Strains and Growth Conditions
4.3. C. albicans Susceptibility Testing
4.4. C. albicans Growth Curves
4.5. Adhesion of C. albicans to Polystyrene
4.6. Analysis of Plasma Membrane Permeabilization
4.7. Statistical Analysis
5. Conclusions
- PSZ in combination with SU and CA leads to the decreased growth of C. albicans. The level of growth inhibition is dependent on the types of alterations in the ergosterol or SLs biosynthesis pathways of C. albicans strains;
- The strain most susceptible to the combination of PSZ and SU is C. albicans that lacks the ERG11 gene. The most susceptible to the combination of PSZ and CA is the strain of C. albicans that lacks both FEN1 and FEN12 genes;
- PSZ in combination with SU causes the significantly decreased adhesion of all investigated C. albicans strains. The combination of PSZ with CA does not lead to the impaired adhesion of C. albicans with the deletion of genes involved in SLs biosynthesis, and for C. albicans ERG11 gene mutants, the effect is less dramatic comparing to a combined therapy of PSZ and SU;
- The combination of PSZ with SU and CA does not correspond with increased C. albicans PM permeabilization compared to control conditions. Nevertheless, the formation of cell clumps was observed due to the use of PSZ with SU and CA. Therefore, the synergy between PSZ and SU or CA is based on a reduction in the adhesion of C. albicans, rather than PM permeabilization.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PSZ | posaconazole; |
| Erg11p | lanosterol 14α-demethylase; |
| PM | plasma membrane; |
| SU | surfactin; |
| CA | capric acid; |
| SLs | sphingolipids; |
| PI | propidium iodide. |
References
- Skiest, D.J.; Vazquez, J.A.; Anstead, G.M.; Graybill, J.R.; Reynes, J.; Ward, D.; Hare, R.; Boparai, N.; Isaacs, R. Posaconazole for the treatment of azole-refractory oropharyngeal and esophageal candidiasis in subjects with HIV infection. Clin. Infect Dis. 2007, 44, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Madison, V.; Chau, A.S.; Loebenberg, D.; Palermo, R.E.; McNicholas, P.M. Three-dimensional models of wild-type and mutated forms of cytochrome P450 14alpha-sterol demethylases from Aspergillus fumigatus and Candida albicans provide insights into posaconazole binding. Antimicrob. Agents Chemother. 2004, 48, 568–574. [Google Scholar] [CrossRef]
- Aggarwal, R.; Sumran, G. An insight on medicinal attributes of 1,2,4-triazoles. Eur. J. Med. Chem. 2020, 205, 112652. [Google Scholar] [CrossRef] [PubMed]
- Law, D.; Moore, C.B.; Denning, D.W. Activity of SCH 56592 compared with those of fluconazole and itraconazole against Candida spp. Antimicrob. Agents Chemother. 1997, 41, 2310–2311. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Messer, S.A.; Hollis, R.J.; Jones, R.N.; Pfaller, M.A. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 2003, 41, 3623–3626. [Google Scholar] [CrossRef] [PubMed]
- Ianas, V.; Matthias, K.R.; Klotz, S.A. Role of posaconazole in the treatment of oropharyngeal candidiasis. Infect. Drug Resist. 2010, 3, 45–51. [Google Scholar] [PubMed]
- Carrillo-Muñoz, A.J.; Quindós, G.; Ruesga, M.; Alonso, R.; del Valle, O.; Hernández-Molina, J.M.; McNicholas, P.; Loebenberg, D.; Santos, P. Antifungal activity of posaconazole compared with fluconazole and amphotericin B against yeasts from oropharyngeal candidiasis and other infections. J. Antimicrob. Chemother. 2005, 5, 317–319. [Google Scholar] [CrossRef]
- Ceballos-Garzon, A.; Peñuela, A.; Valderrama-Beltrán, S.; Vargas-Casanova, Y.; Ariza, B.; Parra-Giraldo, C.M. Emergence and circulation of azole-resistant C. albicans, C. auris and C. parapsilosis bloodstream isolates carrying Y132F, K143R or T220L Erg11p substitutions in Colombia. Front. Cell Infect Microbiol. 2023, 13, 1136217. [Google Scholar] [CrossRef]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef]
- Suchodolski, J.; Derkacz, D.; Muraszko, J.; Panek, J.J.; Jezierska, A.; Łukaszewicz, M.; Krasowska, A. Fluconazole and Lipopeptide Surfactin Interplay During Candida albicans Plasma Membrane and Cell Wall Remodeling Increases Fungal Immune System Exposure. Pharmaceutics 2020, 12, 314. [Google Scholar] [CrossRef]
- Ceresa, C.; Rinaldi, M.; Chiono, V.; Carmagnola, I.; Allegrone, G.; Fracchia, L. Lipopeptides from Bacillus subtilis AC7 inhibit adhesion and biofilm formation of Candida albicans on silicone. Antonie Leeuwenhoek 2016, 109, 1375. [Google Scholar] [CrossRef] [PubMed]
- Rahman, L.; Sarwar, Y.; Khaliq, S.; Inayatullah Abbas, W.; Mobeen, A.; Ullah, A.; Hussain, S.Z.; Khan, W.S.; Kyriazi, M.E.; Hussain, I.; et al. Surfactin-Conjugated Silver Nanoparticles as an Antibacterial and Antibiofilm Agent against Pseudomonas aeruginosa. ACS Appl. Mater. Interfaces 2023, 15, 43321–43331. [Google Scholar] [CrossRef] [PubMed]
- Moryl, M.; Spętana, M.; Dziubek, K.; Paraszkiewicz, K.; Różalska, S.; Płaza, G.A.; Różalski, A. Antimicrobial, antiadhesive and antibiofilm potential of lipopeptides synthesised by Bacillus subtilis, on uropathogenic bacteria. Acta Biochim. Pol. 2015, 62, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, N.M. New advances in fatty acids as antimalarial, antimycobacterial and antifungal agents. Prog. Lipid Res. 2008, 47, 50–61. [Google Scholar] [CrossRef]
- Murzyn, A.; Krasowska, A.; Stefanowicz, P.; Dziadkowiec, D.; Łukaszewicz, M. Capric acid secreted by S. boulardii inhibits C. albicans filamentous growth, adhesion and biofilm formation. PLoS ONE 2010, 5, 12050. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.; Derkacz, D.; Bernat, P.; Krasowska, A. Capric acid secreted by Saccharomyces boulardii influences the susceptibility of Candida albicans to fluconazole and amphotericin B. Sci. Rep. 2021, 11, 6519. [Google Scholar] [CrossRef] [PubMed]
- Alabi, P.E.; Gautier, C.; Murphy, T.P.; Gu, X.; Lepas, M.; Aimanianda, V.; Sello, J.K.; Ene, I.V. Small molecules restore azole activity against drug-tolerant and drug-resistant Candida isolates. mBio 2023, 14, 0047923. [Google Scholar] [CrossRef]
- Eldesouky, H.E.; Salama, E.A.; Li, X.; Hazbun, T.R.; Mayhoub, A.S.; Seleem, M.N. Repurposing approach identifies pitavastatin as a potent azole chemosensitizing agent effective against azole-resistant Candida species. Sci. Rep. 2020, 10, 7525. [Google Scholar] [CrossRef]
- Hunsaker, E.W.; Franz, K.J. Copper potentiates azole antifungal activity in a way that does not involve complex formation. Dalton Trans. 2019, 48, 9654–9662. [Google Scholar] [CrossRef]
- Stenkiewicz-Witeska, J.S.; Ene, I.V. Azole potentiation in Candida species. PLoS Pathog. 2023, 19, 1011583. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.J.; Liu, J.Y.; Ni, P.H.; Wang, S.; Shi, C.; Wei, B.; Ni, Y.X.; Ge, H.L. Erg11 mutations associated with azole resistance in clinical isolates of Candida albicans. FEMS Yeast Res. 2013, 13, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Manastır, L.; Ergon, M.C.; Yücesoy, M. Investigation of mutations in Erg11 gene of fluconazole resistant Candida albicans isolates from Turkish hospitals. Mycoses 2011, 54, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.L.; Souza, C.M.; Pichler, H.; Dewhurst, G.; Schaad, O.; Kajiwara, K.; Wakabayashi, H.; Ivanova, T.; Castillon, G.A.; Piccolis, M.; et al. Functional interactions between sphingolipids and sterols in biological membranes regulating cell physiology. Mol. Biol. Cell 2009, 20, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Nitschke, M.; Araújo, L.V.; Costa, S.G.; Pires, R.C.; Zeraik, A.E.; Fernandes, A.C.; Freire, D.M.; Contiero, J. Surfactin reduces the adhesion of food-borne pathogenic bacteria to solid surfaces. Lett. Appl. Microbiol. 2009, 49, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Lee, J.H.; Beyenal, H.; Lee, J. Fatty acids as antibiofilm and antivirulence agents. Trends Microbiol. 2020, 28, 753–768. [Google Scholar] [CrossRef] [PubMed]
- Bergsson, G.; Arnfinnsson, J.; Steingrimsson, O.; Thormar, H. In vitro killing of Candida albicans by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Nelson, J.; El-Gendy, A.O.; Mansy, M.S.; Ramadan, M.A.; Aziz, R.K. The biosurfactants iturin, lichenysin and surfactin, from vaginally isolated lactobacilli, prevent biofilm formation by pathogenic Candida. FEMS Microbiol. Lett. 2020, 367, 126. [Google Scholar] [CrossRef]
- Willems, H.M.E.; Stultz, J.S.; Coltrane, M.E.; Fortwendel, J.P.; Peters, B.M. Disparate Candida albicans Biofilm Formation in Clinical Lipid Emulsions Due to Capric Acid-Mediated Inhibition. Antimicrob. Agents Chemother. 2019, 63, 1394–1419. [Google Scholar] [CrossRef]
- Fonzi, W.A.; Irwin, M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar] [CrossRef]
- Derkacz, D.; Bernat, P.; Krasowska, A. K143R Amino Acid Substitution in 14-α-Demethylase (Erg11p) Changes Plasma Membrane and Cell Wall Structure of Candida albicans. Int. J. Mol. Sci. 2022, 23, 1631. [Google Scholar] [CrossRef]
- Flowers, S.A.; Colón, B.; Whaley, S.G.; Schuler, M.A.; David Rogers, P. Contribution of clinically derived mutations in ERG11 to azole resistance in Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 450–460. [Google Scholar] [CrossRef]
- Sharma, S.; Alfatah, M.; Bari, V.K.; Rawal, Y.; Paul, S.; Ganesan, K. Sphingolipid biosynthetic pathway genes FEN1 and SUR4 modulate amphotericin B resistance. Antimicrob. Agents Chemother. 2014, 58, 2409–2414. [Google Scholar] [CrossRef]
- Suchodolski, J.; Feder-Kubis, J.; Krasowska, A. Antifungal activity of ionic liquids based on (-)-menthol: A mechanism study. Microbiol. Res. 2017, 197, 56–64. [Google Scholar] [CrossRef]




| C. albicans Strain | Control | PSZ | PSZ + SU | PSZ + CA |
|---|---|---|---|---|
| SC5314 | 0.55 ± 0.32 | 0.55 ± 0.32 | 0.55 ± 0.32 | 0.55 ± 0.32 |
| KS058 | 5.78 ± 1.10 | 5.78 ± 1.10 | 5.78 ± 1.10 | 5.78 ± 1.10 |
| 10C1B1I1 | 33.76 ± 2.67 * | 33.76 ± 2.67 * | 33.76 ± 2.67 * | 33.76 ± 2.67 * |
| 27A5A33A | 10.04 ± 1.30 | 10.04 ± 1.30 | 10.04 ± 1.30 | 10.04 ± 1.30 |
| 9B4B34A | 3.10 ± 1.08 | 3.10 ± 1.08 | 3.10 ± 1.08 | 3.10 ± 1.08 |
| SN95 | 14.55 ± 2.13 ** | 14.55 ± 2.13 ** | 14.55 ± 2.13 ** | 14.55 ± 2.13 ** |
| fen1Δ/Δ | 12.57 ± 1.73 ** | 12.57 ± 1.73 ** | 12.57 ± 1.73 ** | 12.57 ± 1.73 ** |
| fen12Δ/Δ | 36.43 ± 4.24 * | 36.43 ± 4.24 * | 36.43 ± 4.24 * | 36.43 ± 4.24 * |
| fen1Δ/Δ; fen12Δ/Δ | 4.85 ± 1.22 | 4.85 ± 1.22 | 4.85 ± 1.22 | 4.85 ± 1.22 |
| Strain | Genotype | References |
|---|---|---|
| SC5314 | URA3/URA3 (clinical isolate) | Prof. D. Sanglard [30] |
| KS058 | erg11Δ::SAT1-FLIP/erg11Δ::FRT | [31] |
| 10C1B1I1 | ERG11K143R::FRT/ERG11K143R::FRT | Prof. D. Rogers [32] |
| 27A5A33A | ERG11Y132F,F145L::FRT/ERG11Y132F,F145L::FRT | |
| 9B4B34A | ERG11Y132F,K143R::FRT/ERG11Y132F,K143R::FRT | |
| SN95 | arg4Δ/arg4Δ his1Δ/his1Δ URA3/ura3::imm434 IRO1/iro1::imm434 | Prof. K. Ganesan [33] |
| fen1Δ/Δ | as SN95 but fen1Δ/fen1Δ | |
| fen12Δ/Δ | as SN95 but fen12Δ/fen12Δ | |
| fen1Δ/Δ;fen12Δ/Δ | as SN95 but fen1Δ/fen1Δ;fen12Δ/fen12Δ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derkacz, D.; Grzybowska, M.; Cebula, L.; Krasowska, A. Surfactin and Capric Acid Affect the Posaconazole Susceptibility of Candida albicans Strains with Altered Sterols and Sphingolipids Biosynthesis. Int. J. Mol. Sci. 2023, 24, 17499. https://doi.org/10.3390/ijms242417499
Derkacz D, Grzybowska M, Cebula L, Krasowska A. Surfactin and Capric Acid Affect the Posaconazole Susceptibility of Candida albicans Strains with Altered Sterols and Sphingolipids Biosynthesis. International Journal of Molecular Sciences. 2023; 24(24):17499. https://doi.org/10.3390/ijms242417499
Chicago/Turabian StyleDerkacz, Daria, Monika Grzybowska, Liliana Cebula, and Anna Krasowska. 2023. "Surfactin and Capric Acid Affect the Posaconazole Susceptibility of Candida albicans Strains with Altered Sterols and Sphingolipids Biosynthesis" International Journal of Molecular Sciences 24, no. 24: 17499. https://doi.org/10.3390/ijms242417499
APA StyleDerkacz, D., Grzybowska, M., Cebula, L., & Krasowska, A. (2023). Surfactin and Capric Acid Affect the Posaconazole Susceptibility of Candida albicans Strains with Altered Sterols and Sphingolipids Biosynthesis. International Journal of Molecular Sciences, 24(24), 17499. https://doi.org/10.3390/ijms242417499






