Potential Application of Self-Assembled Peptides and Proteins in Breast Cancer and Cervical Cancer
Abstract
1. Introduction
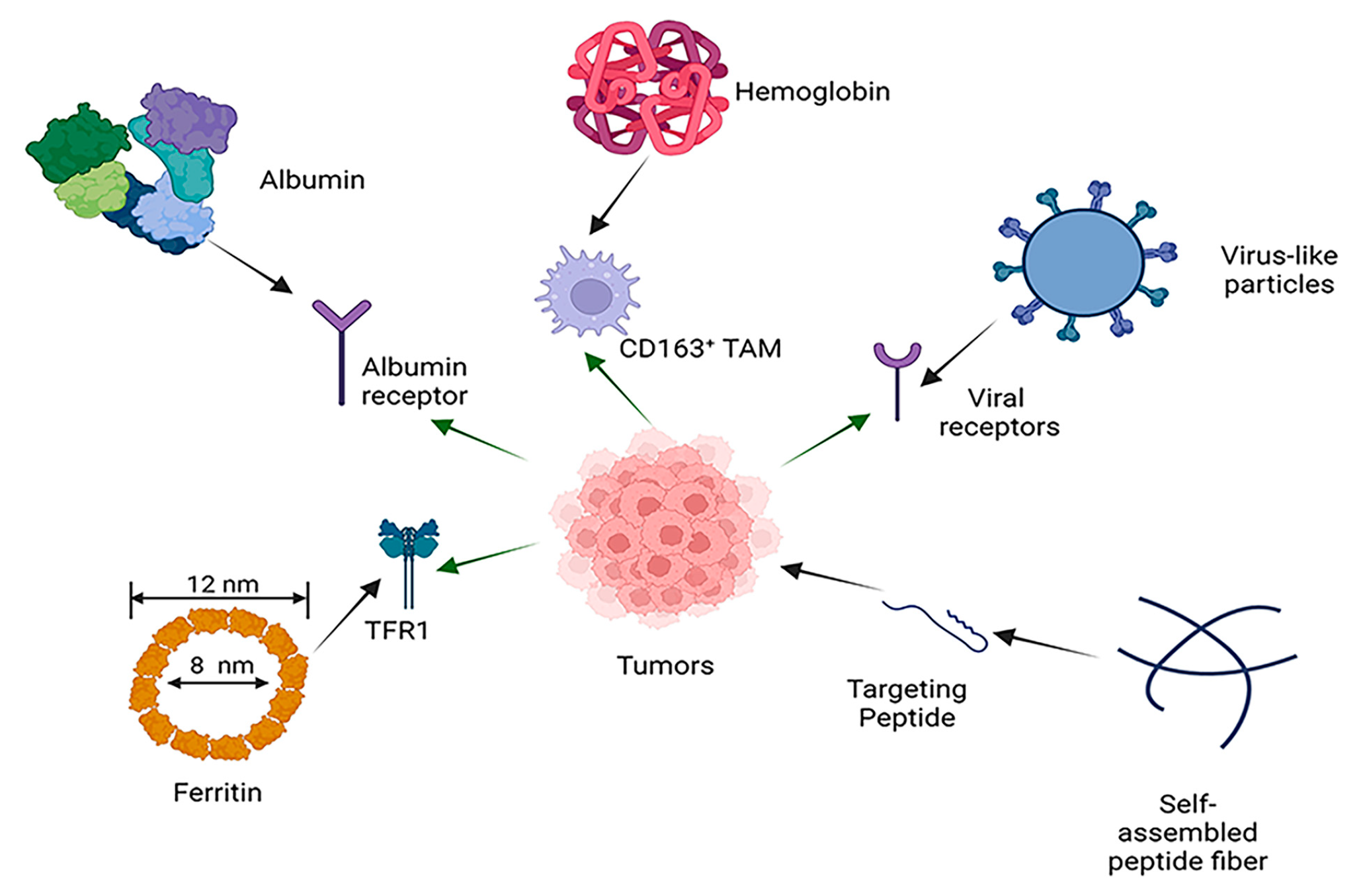
2. Common Self-Assembled Proteins and Peptides in Cancer Therapy
2.1. Albumin
2.2. Ferritin
2.3. Virus-Like Particles
2.4. Self-Assembled Peptides
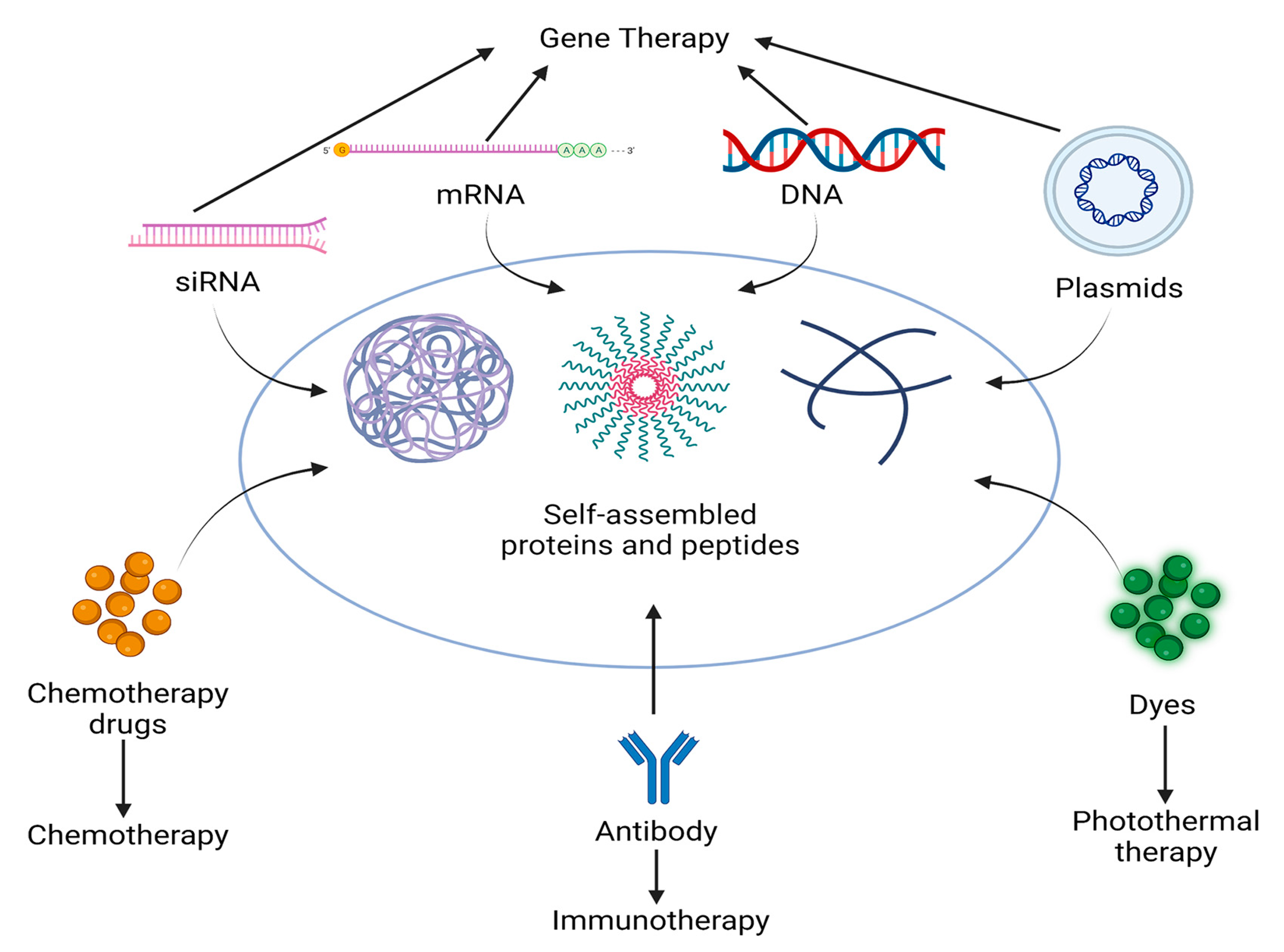
3. Classes of Drugs That Can Be Delivered by Self-Assembled Proteins and Peptides
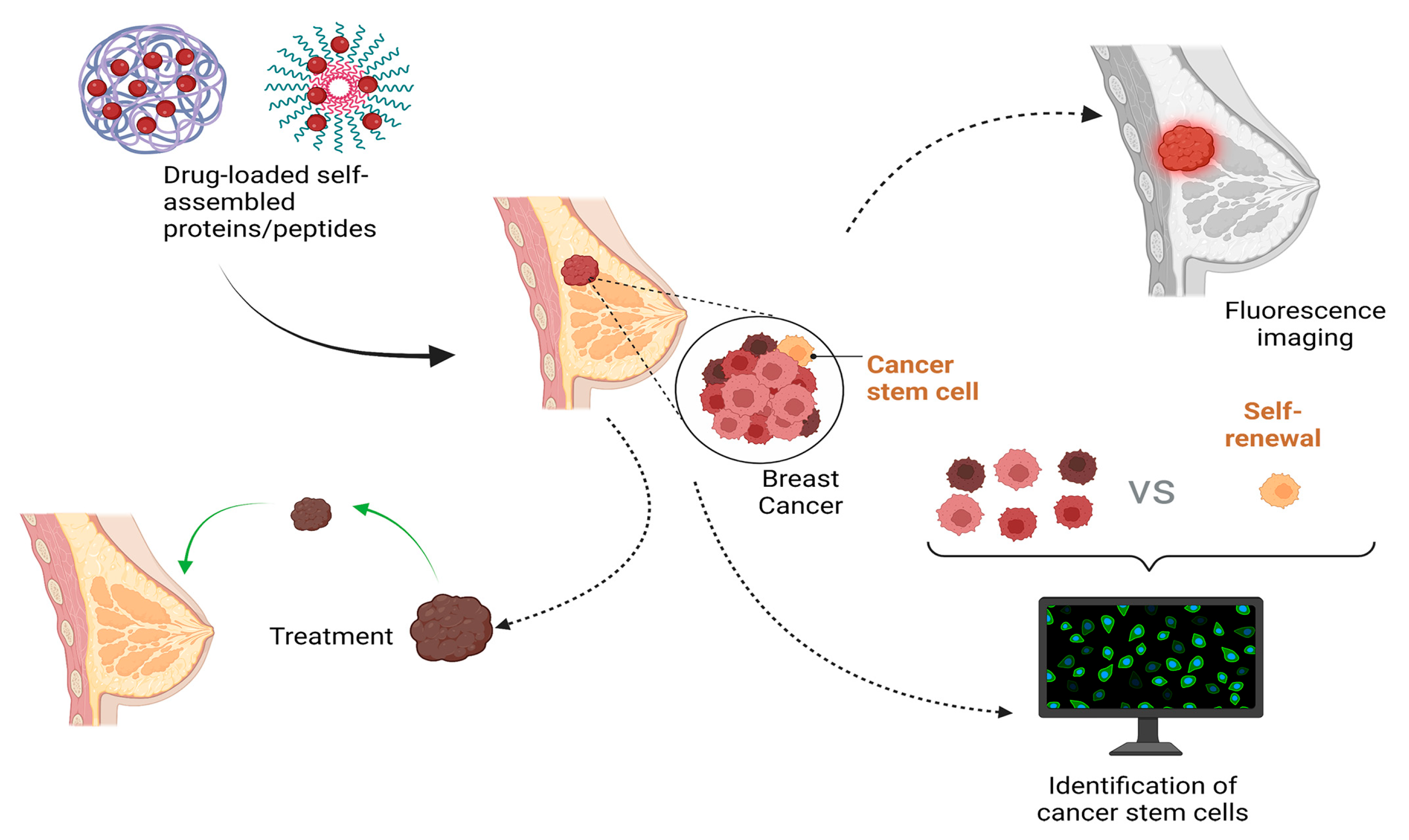
4. Potential Applications of Self-Assembled Proteins and Peptides in BC and CC
5. Discussion
6. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, H.; Xu, J.; Yang, J.; Wang, Z.; Xu, P.; Hao, Q.; Luo, W.; Li, S.; Li, Z.; Xue, X.; et al. On-demand targeting nanotheranostics with stimuli-responsive releasing property to improve delivery efficiency to cancer. Biomaterials 2022, 290, 121852. [Google Scholar] [CrossRef]
- Haddadzadegan, S.; Dorkoosh, F.; Bernkop-Schnürch, A. Oral delivery of therapeutic peptides and proteins: Technology landscape of lipid-based nanocarriers. Adv. Drug Deliv. Rev. 2022, 182, 114097. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal-Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nanomicro. Lett. 2020, 12, 103. [Google Scholar] [CrossRef]
- Abbas, M.; Zou, Q.; Li, S.; Yan, X. Self-Assembled Peptide- and Protein-Based Nanomaterials for Antitumor Photodynamic and Photothermal Therapy. Adv. Mater. 2017, 29, 1605021. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnology 2021, 19, 159. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Pal, D.; Sharma, R.; Garg, V.K.; Goel, N.; Koundal, D.; Zaguia, A.; Koundal, S.; Belay, A. Global Increase in Breast Cancer Incidence: Risk Factors and Preventive Measures. Biomed. Res. Int. 2022, 2022, 9605439. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.; Deng, Y.; Deng, X.; Lou, W.; Wei, B.; Xiang, D.; Hu, J.; Zheng, Y.; Xu, P.; et al. Global Burden of Female Breast Cancer: Age-Period-Cohort Analysis of Incidence Trends From 1990 to 2019 and Forecasts for 2035. Front. Oncol. 2022, 12, 891824. [Google Scholar] [CrossRef]
- Gaudet, M.M.; Deubler, E.; Diver, W.R.; Puvanesarajah, S.; Patel, A.V.; Gansler, T.; Sherman, M.E.; Gapstur, S.M. Breast cancer risk factors by mode of detection among screened women in the Cancer Prevention Study-II. Breast Cancer Res. Treat. 2021, 186, 791–805. [Google Scholar] [CrossRef]
- Sethuraman, A.; Brown, M.; Krutilina, R.; Wu, Z.H.; Seagroves, T.N.; Pfeffer, L.M.; Fan, M. BHLHE40 confers a pro-survival and pro-metastatic phenotype to breast cancer cells by modulating HBEGF secretion. Breast Cancer Res. 2018, 20, 117. [Google Scholar] [CrossRef]
- Li, X.; Deng, S.; Pang, X.; Song, Y.; Luo, S.; Jin, L.; Pan, Y. LncRNA NEAT1 Silenced miR-133b Promotes Migration and Invasion of Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 3616. [Google Scholar] [CrossRef]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Drokow, E.K.; Effah, C.Y.; Agboyibor, C.; Sasu, E.; Amponsem-Boateng, C.; Akpabla, G.S.; Ahmed, H.A.W.; Sun, K. The Impact of Video-Based Educational Interventions on Cervical Cancer, Pap Smear and HPV Vaccines. Front. Public Health 2021, 9, 681319. [Google Scholar] [CrossRef]
- Sun, M.; Zhou, D.; Wu, J.; Zhou, J.; Xu, J. Sdy-1 Executes Antitumor Activity in HepG2 and HeLa Cancer Cells by Inhibiting the Wnt/β-Catenin Signaling Pathway. Mar. Drugs 2022, 20, 125. [Google Scholar] [CrossRef]
- Karimi, M.; Bahrami, S.; Ravari, S.B.; Zangabad, P.S.; Mirshekari, H.; Bozorgomid, M.; Shahreza, S.; Sori, M.; Hamblin, M.R. Albumin nanostructures as advanced drug delivery systems. Expert Opin. Drug Deliv. 2016, 13, 1609–1623. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X. Simple bioconjugate chemistry serves great clinical advances: Albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 2016, 45, 1432–1456. [Google Scholar] [CrossRef]
- Brekken, R.A.; Sage, E.H. SPARC, a matricellular protein: At the crossroads of cell-matrix communication. Matrix Biol. 2001, 19, 816–827. [Google Scholar] [CrossRef]
- Motamed, K. SPARC (osteonectin/BM-40). Int. J. Biochem. Cell Biol. 1999, 31, 1363–1366. [Google Scholar] [CrossRef]
- Podhajcer, O.L.; Benedetti, L.G.; Girotti, M.R.; Prada, F.; Salvatierra, E.; Llera, A.S. The role of the matricellular protein SPARC in the dynamic interaction between the tumor and the host. Cancer Metastasis Rev. 2008, 27, 691–705. [Google Scholar] [CrossRef]
- Shi, Q.; Bao, S.; Song, L.; Wu, Q.; Bigner, D.D.; Hjelmeland, A.B.; Rich, J.N. Targeting SPARC expression decreases glioma cellular survival and invasion associated with reduced activities of FAK and ILK kinases. Oncogene 2007, 26, 4084–4094. [Google Scholar] [CrossRef]
- Lv, W.; Yang, K.; Yu, J.; Wu, Y.; Zhang, M.; Liu, Z.; Wang, X.; Zhou, J.; Ma, H.; Yi, R.; et al. A generalizable strategy for crosslinkable albumin-based hydrogels and application as potential anti-tumor nanoplatform. J. Biomater. Appl. 2023, 37, 1813–1822. [Google Scholar] [CrossRef]
- Watt, R.K. The many faces of the octahedral ferritin protein. Biometals 2011, 24, 489–500. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.J.; Ryan, J.C.; Niemi, E.C.; Lebrón, J.A.; Björkman, P.J.; Arase, H.; Torti, F.M.; Torti, S.V.; Nakamura, M.C.; et al. Binding and uptake of H-ferritin are mediated by human transferrin receptor-1. Proc. Natl. Acad. Sci. USA 2010, 107, 3505–3510. [Google Scholar] [CrossRef] [PubMed]
- Habashy, H.O.; Powe, D.G.; Staka, C.M.; Rakha, E.A.; Ball, G.; Green, A.R.; Aleskandarany, M.; Paish, E.C.; Douglas Macmillan, R.; Nicholson, R.I.; et al. Transferrin receptor (CD71) is a marker of poor prognosis in breast cancer and can predict response to tamoxifen. Breast Cancer Res. Treat. 2010, 119, 283–293. [Google Scholar] [CrossRef]
- Kondo, K.; Noguchi, M.; Mukai, K.; Matsuno, Y.; Sato, Y.; Shimosato, Y.; Monden, Y. Transferrin receptor expression in adenocarcinoma of the lung as a histopathologic indicator of prognosis. Chest 1990, 97, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Cao, C.; Pan, Y.; Lu, D.; Yang, D.; Feng, J.; Song, L.; Liang, M.; Yan, X. Magnetoferritin nanoparticles for targeting and visualizing tumour tissues. Nat. Nanotechnol. 2012, 7, 459–464. [Google Scholar] [CrossRef]
- Khoshnejad, M.; Parhiz, H.; Shuvaev, V.V.; Dmochowski, I.J.; Muzykantov, V.R. Ferritin-based drug delivery systems: Hybrid nanocarriers for vascular immunotargeting. J. Control Release 2018, 282, 13–24. [Google Scholar] [CrossRef]
- Zhen, Z.; Tang, W.; Chen, H.; Lin, X.; Todd, T.; Wang, G.; Cowger, T.; Chen, X.; Xie, J. RGD-modified apoferritin nanoparticles for efficient drug delivery to tumors. ACS Nano 2013, 7, 4830–4837. [Google Scholar] [CrossRef]
- Ma, Y.; Li, R.; Dong, Y.; You, C.; Huang, S.; Li, X.; Wang, F.; Zhang, Y. tLyP-1 Peptide Functionalized Human H Chain Ferritin for Targeted Delivery of Paclitaxel. Int. J. Nanomed. 2021, 16, 789–802. [Google Scholar] [CrossRef]
- Sitia, L.; Bonizzi, A.; Mazzucchelli, S.; Negri, S.; Sottani, C.; Grignani, E.; Rizzuto, M.A.; Prosperi, D.; Sorrentino, L.; Morasso, C.; et al. Selective Targeting of Cancer-Associated Fibroblasts by Engineered H-Ferritin Nanocages Loaded with Navitoclax. Cells 2021, 10, 328. [Google Scholar] [CrossRef]
- Hyman, P.; Trubl, G.; Abedon, S.T. Virus-Like Particle: Evolving Meanings in Different Disciplines. Phage 2021, 2, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, M.O.; Zha, L.; Cabral-Miranda, G.; Bachmann, M.F. Major findings and recent advances in virus-like particle (VLP)-based vaccines. Semin. Immunol. 2017, 34, 123–132. [Google Scholar] [CrossRef]
- Bryan, J.T.; Buckland, B.; Hammond, J.; Jansen, K.U. Prevention of cervical cancer: Journey to develop the first human papillomavirus virus-like particle vaccine and the next generation vaccine. Curr. Opin. Chem. Biol. 2016, 32, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Mirghani, H.; Sturgis, E.M.; Aupérin, A.; Monsonego, J.; Blanchard, P. Is there an increased risk of cancer among spouses of patients with an HPV-related cancer: A systematic review. Oral Oncol. 2017, 67, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.L. Cancer Prevention: HPV Vaccination. Semin. Oncol. Nurs. 2016, 32, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Rolih, V.; Caldeira, J.; Bolli, E.; Salameh, A.; Conti, L.; Barutello, G.; Riccardo, F.; Magri, J.; Lamolinara, A.; Parra, K.; et al. Development of a VLP-Based Vaccine Displaying an xCT Extracellular Domain for the Treatment of Metastatic Breast Cancer. Cancers 2020, 12, 1492. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, B.; Al-Barwani, F.; Pelham, S.J.; Young, K.; Ward, V.K.; Young, S.L. Multi-target chimaeric VLP as a therapeutic vaccine in a model of colorectal cancer. J. Immunother. Cancer 2017, 5, 69. [Google Scholar] [CrossRef]
- Nooraei, S.; Bahrulolum, H.; Hoseini, Z.S.; Katalani, C.; Hajizade, A.; Easton, A.J.; Ahmadian, G. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J. Nanobiotechnology 2021, 19, 59. [Google Scholar] [CrossRef]
- Hu, H.; Steinmetz, N.F. Cisplatin Prodrug-Loaded Nanoparticles Based on Physalis Mottle Virus for Cancer Therapy. Mol. Pharm. 2020, 17, 4629–4636. [Google Scholar] [CrossRef]
- Charlton Hume, H.K.; Vidigal, J.; Carrondo, M.J.T.; Middelberg, A.P.J.; Roldão, A.; Lua, L.H.L. Synthetic biology for bioengineering virus-like particle vaccines. Biotechnol. Bioeng. 2019, 116, 919–935. [Google Scholar] [CrossRef] [PubMed]
- La Manna, S.; Di Natale, C.; Florio, D.; Marasco, D. Peptides as Therapeutic Agents for Inflammatory-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2714. [Google Scholar] [CrossRef]
- La Manna, S.; Di Natale, C.; Onesto, V.; Marasco, D. Self-Assembling Peptides: From Design to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 12662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, W.; Yang, L.; Zhang, A.; Zhang, Y.; Liu, J.; Liu, J. Injectable and pH-responsive self-assembled peptide hydrogel for promoted tumor cell uptake and enhanced cancer chemotherapy. Biomater. Sci. 2022, 10, 854–862. [Google Scholar] [CrossRef]
- Daso, R.E.; Banerjee, I.A. Self-Assembled Peptide-Based Biocomposites for Near-Infrared Light Triggered Drug Release to Tumor Cells. Biotechnol. J. 2020, 15, e2000128. [Google Scholar] [CrossRef] [PubMed]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Yang, T.; Li, S.; Xu, G.; Liang, H.; Yang, F. Developing an Anticancer Platinum(II) Compound Based on the Uniqueness of Human Serum Albumin. J. Med. Chem. 2023, 66, 5669–5684. [Google Scholar] [CrossRef]
- Ferrado, J.B.; Perez, A.A.; Visentini, F.F.; Islan, G.A.; Castro, G.R.; Santiago, L.G. Formation and characterization of self-assembled bovine serum albumin nanoparticles as chrysin delivery systems. Colloids Surf. B Biointerfaces 2019, 173, 43–51. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J. Control Release 2020, 318, 1–15. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, H.; Zheng, B.; Wang, H.; Qi, X.; Wang, S.; Liu, Z.; Sun, L.; Liu, Y.; Qin, X.; et al. Combined Self-Assembled Hendeca-Arginine Nanocarriers for Effective Targeted Gene Delivery to Bladder Cancer. Int. J. Nanomed. 2022, 17, 4433–4448. [Google Scholar] [CrossRef]
- Guan, S.; Munder, A.; Hedtfeld, S.; Braubach, P.; Glage, S.; Zhang, L.; Lienenklaus, S.; Schultze, A.; Hasenpusch, G.; Garrels, W.; et al. Self-assembled peptide-poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat. Nanotechnol. 2019, 14, 287–297. [Google Scholar] [CrossRef]
- Jeong, S.G.; Ryu, Y.C.; Hwang, B.H. Synergistic gene delivery by self-assembled nanocomplexes using fusion peptide and calcium phosphate. J. Control Release 2021, 338, 284–294. [Google Scholar] [CrossRef]
- Liu, B.Y.; He, X.Y.; Zhuo, R.X.; Cheng, S.X. Reversal of tumor malignization and modulation of cell behaviors through genome editing mediated by a multi-functional nanovector. Nanoscale 2018, 10, 21209–21218. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Sun, Y.; Liang, X.; Gu, X.; Ning, J.; Xu, Y.; Chen, S.; Pan, L. Emerging new therapeutic antibody derivatives for cancer treatment. Signal. Transduct. Target. Ther. 2022, 7, 39. [Google Scholar] [CrossRef]
- Wu, S.Y.; Wu, F.G.; Chen, X. Antibody-Incorporated Nanomedicines for Cancer Therapy. Adv. Mater. 2022, 34, e2109210. [Google Scholar] [CrossRef]
- Stuchinskaya, T.; Moreno, M.; Cook, M.J.; Edwards, D.R.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using antibody-phthalocyanine-gold nanoparticle conjugates. Photochem. Photobiol. Sci. 2011, 10, 822–831. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.I.; Lukianov, C.I.; Champion, J.A. Self-assembled protein nanocarrier for intracellular delivery of antibody. J. Control Release 2017, 249, 1–10. [Google Scholar] [CrossRef]
- Shen, H.; Gao, Q.; Liu, T.; Wang, H.; Zhang, R.; Zhou, J.; Ding, S.; Ye, Y.; Sun, Z. Self-Assembled Tocopherol-Albumin Nanoparticles with Full Biocompatibility for Chemo-photothermal Therapy against Breast Cancer. Curr. Drug Deliv. 2022, 19, 49–63. [Google Scholar] [CrossRef]
- Wendel Naumann, R.; Leath, C.A., 3rd. Advances in immunotherapy for cervical cancer. Curr. Opin. Oncol. 2020, 32, 481–487. [Google Scholar] [CrossRef]
- Gupta, N.; Kumar, H.; Gupta, S.; Basavarajaiah, S.M.; Saini, K. A Concise Review on Natural Products and Their Derivatives for Breast Cancer Treatment. Chem. Biodivers 2023, 20, e202300688. [Google Scholar] [CrossRef]
- Kupeli Akkol, E.; Bardakci, H.; Barak, T.H.; Aschner, M.; Seker Karatoprak, G.; Khan, H.; Hussain, Y. Herbal Ingredients in the Prevention of Breast Cancer: Comprehensive Review of Potential Molecular Targets and Role of Natural Products. Oxid. Med. Cell Longev. 2022, 2022, 6044640. [Google Scholar] [CrossRef]
- Pandolfi, L.; Bellini, M.; Vanna, R.; Morasso, C.; Zago, A.; Carcano, S.; Avvakumova, S.; Bertolini, J.A.; Rizzuto, M.A.; Colombo, M.; et al. H-Ferritin Enriches the Curcumin Uptake and Improves the Therapeutic Efficacy in Triple Negative Breast Cancer Cells. Biomacromolecules 2017, 18, 3318–3330. [Google Scholar] [CrossRef]
- Cui, T.; Zhang, S.; Sun, H. Co-delivery of doxorubicin and pH-sensitive curcumin prodrug by transferrin-targeted nanoparticles for breast cancer treatment. Oncol. Rep. 2017, 37, 1253–1260. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Diaz, L.A.; Ruiz-Pacheco, J.A.; Elsawy, M.A.; Reyes-Martinez, J.E.; Enriquez-Rodriguez, A.I. Self-Assembling Peptides as an Emerging Platform for the Treatment of Metabolic Syndrome. Int. J. Nanomed. 2020, 15, 10349–10370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, Y.; Eldi, P.; Guo, X.; Hayball, J.D.; Garg, S.; Albrecht, H. Targeting prostate cancer cells with hybrid elastin-like polypeptide/liposome nanoparticles. Int. J. Nanomed. 2018, 13, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Sabra, S.A.; Sheweita, S.A.; Haroun, M.; Ragab, D.; Eldemellawy, M.A.; Xia, Y.; Goodale, D.; Allan, A.L.; Elzoghby, A.O.; Rohani, S. Magnetically Guided Self-Assembled Protein Micelles for Enhanced Delivery of Dasatinib to Human Triple-Negative Breast Cancer Cells. J. Pharm. Sci. 2019, 108, 1713–1725. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.; Mazzucchelli, S.; Galbiati, E.; Sommaruga, S.; Fiandra, L.; Truffi, M.; Rizzuto, M.A.; Colombo, M.; Tortora, P.; Corsi, F.; et al. Protein nanocages for self-triggered nuclear delivery of DNA-targeted chemotherapeutics in Cancer Cells. J. Control Release 2014, 196, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Luo, J.; Fowler, W.L.; Li, Y.; Lee, J.S.; Xing, L.; Cheng, R.H.; Wang, L.; Lam, K.S. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials 2009, 30, 6006–6016. [Google Scholar] [CrossRef] [PubMed]
- Bonofiglio, D.; Giordano, C.; De Amicis, F.; Lanzino, M.; Ando, S. Natural Products as Promising Antitumoral Agents in Breast Cancer: Mechanisms of Action and Molecular Targets. Mini. Rev. Med. Chem. 2016, 16, 596–604. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Meng, X.; Gan, R.Y.; Zhang, J.J.; Li, H.B. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef]
- Spradlin, J.N.; Hu, X.; Ward, C.C.; Brittain, S.M.; Jones, M.D.; Ou, L.; To, M.; Proudfoot, A.; Ornelas, E.; Woldegiorgis, M.; et al. Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. Nat. Chem. Biol. 2019, 15, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, J.; Cui, R.; Lin, J.; Ding, X. Curcumin in Treating Breast Cancer: A Review. J. Lab. Autom. 2016, 21, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xi, Y. MicroRNAs mediate therapeutic and preventive effects of natural agents in breast cancer. Chin. J. Nat. Med. 2016, 14, 881–887. [Google Scholar] [CrossRef]
- Ahmed, F.; Ijaz, B.; Ahmad, Z.; Farooq, N.; Sarwar, M.B.; Husnain, T. Modification of miRNA Expression through plant extracts and compounds against breast cancer: Mechanism and translational significance. Phytomedicine 2020, 68, 153168. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.Y.J.; El-Sahli, S.; Wang, L. The Potential of Natural Products in the Treatment of Triple-negative Breast Cancer. Curr. Cancer Drug Targets 2022, 22, 388–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Q.; Yu, L.; Zhu, J.; Cao, Y.; Gao, X. The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J. Ethnopharmacol. 2021, 264, 113249. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xia, L.; Li, J. Potential Mechanisms of Plant-Derived Natural Products in the Treatment of Cervical Cancer. Biomolecules 2021, 11, 1539. [Google Scholar] [CrossRef]
- Tao, T.; Zhang, P.; Zeng, Z.; Wang, M. Advances in autophagy modulation of natural products in cervical cancer. J. Ethnopharmacol. 2023, 314, 116575. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. Evaluation of antioxidant and anticancer activity of copper oxide nanoparticles synthesized using medicinally important plant extracts. Biomed. Pharmacother. 2017, 89, 1067–1077. [Google Scholar] [CrossRef]
- Hung, C.Y.; Lee, C.H.; Chiou, H.L.; Lin, C.L.; Chen, P.N.; Lin, M.T.; Hsieh, Y.H.; Chou, M.C. Praeruptorin-B Inhibits 12-O-Tetradecanoylphorbol-13-Acetate-Induced Cell Invasion by Targeting AKT/NF-kappaB via Matrix Metalloproteinase-2/-9 Expression in Human Cervical Cancer Cells. Cell. Physiol. Biochem. 2019, 52, 1255–1266. [Google Scholar] [CrossRef]
- Jaglanian, A.; Tsiani, E. Rosemary Extract Inhibits Proliferation, Survival, Akt, and mTOR Signaling in Triple-Negative Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 810. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Zhuang, J.; Gao, C.; Li, H.; Liu, L.; Feng, F.; Zhou, C.; Yao, K.; Deng, L.; et al. The Modulatory Properties of Astragalus membranaceus Treatment on Triple-Negative Breast Cancer: An Integrated Pharmacological Method. Front. Pharmacol. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Wu, C.; Qiu, S.; Liu, P.; Ge, Y.; Gao, X. Rhizoma Amorphophalli inhibits TNBC cell proliferation, migration, invasion and metastasis through the PI3K/Akt/mTOR pathway. J. Ethnopharmacol. 2018, 211, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Gu, P.Y.; Fang, L.W.; Huang, Y.L.; Lin, C.F.; Liou, C.J. Sophoraflavanone G from Sophora flavescens induces apoptosis in triple-negative breast cancer cells. Phytomedicine 2019, 61, 152852. [Google Scholar] [CrossRef] [PubMed]
- El Hasasna, H.; Athamneh, K.; Al Samri, H.; Karuvantevida, N.; Al Dhaheri, Y.; Hisaindee, S.; Ramadan, G.; Al Tamimi, N.; AbuQamar, S.; Eid, A.; et al. Rhus coriaria induces senescence and autophagic cell death in breast cancer cells through a mechanism involving p38 and ERK1/2 activation. Sci. Rep. 2015, 5, 13013. [Google Scholar] [CrossRef] [PubMed]
- Rios-Fuller, T.J.; Ortiz-Soto, G.; Lacourt-Ventura, M.; Maldonado-Martinez, G.; Cubano, L.A.; Schneider, R.J.; Martinez-Montemayor, M.M. Ganoderma lucidum extract (GLE) impairs breast cancer stem cells by targeting the STAT3 pathway. Oncotarget 2018, 9, 35907–35921. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, H.; Sun, H.; Qu, J.; Zhao, B.; Hu, X.; Li, W.; Qian, Z.; Yu, X.; Kang, F.; et al. Extracts of Cordyceps sinensis inhibit breast cancer growth through promoting M1 macrophage polarization via NF-kappaB pathway activation. J. Ethnopharmacol. 2020, 260, 112969. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.; Cui, J.; Xiao, T.; Jiang, D. Paeoniflorin inhibits proliferation and invasion of breast cancer cells through suppressing Notch-1 signaling pathway. Biomed. Pharmacother. 2016, 78, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Ou, T.T.; Yang, M.Y.; Huang, C.C.; Wang, C.J. Nelumbo nucifera Gaertn leaves extract inhibits the angiogenesis and metastasis of breast cancer cells by downregulation connective tissue growth factor (CTGF) mediated PI3K/AKT/ERK signaling. J. Ethnopharmacol. 2016, 188, 111–122. [Google Scholar] [CrossRef]
- El Hasasna, H.; Saleh, A.; Al Samri, H.; Athamneh, K.; Attoub, S.; Arafat, K.; Benhalilou, N.; Alyan, S.; Viallet, J.; Al Dhaheri, Y.; et al. Rhus coriaria suppresses angiogenesis, metastasis and tumor growth of breast cancer through inhibition of STAT3, NFkappaB and nitric oxide pathways. Sci. Rep. 2016, 6, 21144. [Google Scholar] [CrossRef]
- Yang, J.; Fa, J.; Li, B. Apoptosis Induction of Epifriedelinol on Human Cervical Cancer Cell Line. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 80–86. [Google Scholar] [CrossRef][Green Version]
- Das, A.; Harshadha, K.; Dhinesh Kannan, S.K.; Hari Raj, K.; Jayaprakash, B. Evaluation of Therapeutic Potential of Eugenol-A Natural Derivative of Syzygium aromaticum on Cervical Cancer. Asian Pac. J. Cancer Prev. 2018, 19, 1977–1985. [Google Scholar] [CrossRef]
- Moreira, T.F.; Sorbo, J.M.; Souza, F.O.; Fernandes, B.C.; Ocampos, F.M.M.; de Oliveira, D.M.S.; Arcaro, C.A.; Assis, R.P.; Barison, A.; Miguel, O.G.; et al. Emodin, Physcion, and Crude Extract of Rhamnus sphaerosperma var. pubescens Induce Mixed Cell Death, Increase in Oxidative Stress, DNA Damage, and Inhibition of AKT in Cervical and Oral Squamous Carcinoma Cell Lines. Oxid. Med. Cell. Longev. 2018, 2018, 2390234. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, V.R.; Renjith, R.S.; Mukherjee, A.; Anil, S.R.; Sreekumar, J.; Jyothi, A.N. Comparative Study on the Chemical Structure and In Vitro Antiproliferative Activity of Anthocyanins in Purple Root Tubers and Leaves of Sweet Potato (Ipomoea batatas). J. Agric. Food Chem. 2019, 67, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Q.; Yang, L.; Xia, X.; Zhu, R.; Chen, S.; Wang, M.; Cheng, L.; Wu, X.; Wang, S. Curcumin-Loaded TPGS/F127/P123 Mixed Polymeric Micelles for Cervical Cancer Therapy: Formulation, Characterization, and InVitro and InVivo Evaluation. J. Biomed. Nanotechnol. 2017, 13, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Tadi, P. Aromatase Inhibitors; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Quintanilla Rodriguez, B.S.; Correa, R. Raloxifene; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ogino, M.H.; Tadi, P. Cyclophosphamide; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ballard, T.; Chargui, S. Pamidronate; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Greenblatt, K.; Khaddour, K. Trastuzumab; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Farrar, M.C.; Jacobs, T.F. Tamoxifen; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Farooq, M.; Patel, S.P. Fulvestrant; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mandapati, A.; Lukong, K.E. Triple negative breast cancer: Approved treatment options and their mechanisms of action. J. Cancer Res. Clin. Oncol. 2023, 149, 3701–3719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, H.; Zhang, J.; Bao, G.; Zhang, G.; Wang, H.; Wang, X. Effectiveness and safety of pegylated liposomal doxorubicin versus epirubicin as neoadjuvant or adjuvant chemotherapy for breast cancer: A real-world study. BMC Cancer 2021, 21, 1301. [Google Scholar] [CrossRef] [PubMed]
- Nicoletto, R.E.; Ofner, C.M., 3rd. Cytotoxic mechanisms of doxorubicin at clinically relevant concentrations in breast cancer cells. Cancer Chemother. Pharmacol. 2022, 89, 285–311. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Pang, D.; Yang, H.; Li, W.; Wang, S.; Cui, S.; Liao, N.; Wang, Y.; Wang, C.; Chang, Y.C.; et al. Efficacy, Safety, and Tolerability of Pertuzumab, Trastuzumab, and Docetaxel for Patients With Early or Locally Advanced ERBB2-Positive Breast Cancer in Asia: The PEONY Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e193692. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Z.; Liu, Z.; Yang, B.; Yang, H.; Tang, J.; Wang, K.; Liu, Y.; Wang, H.; Fu, P.; et al. Neoadjuvant pyrotinib, trastuzumab, and docetaxel for HER2-positive breast cancer (PHEDRA): A double-blind, randomized phase 3 trial. BMC Med. 2022, 20, 498. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Z.Y.; Li, J.; Lin, Y.; Liu, Z.; Cao, Y.; Zhang, G.; Gao, H.F.; Yang, M.; Yang, C.Q.; et al. Neoadjuvant docetaxel plus carboplatin vs epirubicin plus cyclophosphamide followed by docetaxel in triple-negative, early-stage breast cancer (NeoCART): Results from a multicenter, randomized controlled, open-label phase II trial. Int. J. Cancer 2022, 150, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Valero, V. Primary chemotherapy with docetaxel for the management of breast cancer. Oncology (Williston Park) 2002, 16, 35–43. [Google Scholar] [PubMed]
- Norum, J. Adjuvant cyclophosphamide, methotrexate, fluorouracil (CMF) in breast cancer—Is it cost-effective? Acta Oncol. 2000, 39, 33–39. [Google Scholar] [CrossRef] [PubMed]
- van Outryve, S.; Schrijvers, D.; van den Brande, J.; Wilmes, P.; Bogers, J.; van Marck, E.; Vermorken, J.B. Methotrexate-associated liver toxicity in a patient with breast cancer: Case report and literature review. Neth. J. Med. 2002, 60, 216–222. [Google Scholar] [PubMed]
- Hamed, K.M.; Dighriri, I.M.; Baomar, A.F.; Alharthy, B.T.; Alenazi, F.E.; Alali, G.H.; Alenazy, R.H.; Alhumaidi, N.T.; Alhulayfi, D.H.; Alotaibi, Y.B.; et al. Overview of Methotrexate Toxicity: A Comprehensive Literature Review. Cureus 2022, 14, e29518. [Google Scholar] [CrossRef]
- Pinedo, H.M.; Peters, G.F. Fluorouracil: Biochemistry and pharmacology. J. Clin. Oncol. 1988, 6, 1653–1664. [Google Scholar] [CrossRef]
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256. [Google Scholar] [CrossRef]
- Bardia, A.; Aftimos, P.; Bihani, T.; Anderson-Villaluz, A.T.; Jung, J.; Conlan, M.G.; Kaklamani, V.G. EMERALD: Phase III trial of elacestrant (RAD1901) vs endocrine therapy for previously treated ER+ advanced breast cancer. Future Oncol. 2019, 15, 3209–3218. [Google Scholar] [CrossRef]
- Sanchez, K.G.; Nangia, J.R.; Schiff, R.; Rimawi, M.F. Elacestrant and the Promise of Oral SERDs. J. Clin. Oncol. 2022, 40, 3227–3229. [Google Scholar] [CrossRef]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Gomez Moreno, H.L.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar] [CrossRef]
- Andrikopoulou, A.; Chatzinikolaou, S.; Panourgias, E.; Kaparelou, M.; Liontos, M.; Dimopoulos, M.A.; Zagouri, F. The emerging role of capivasertib in breast cancer. Breast 2022, 63, 157–167. [Google Scholar] [CrossRef]
- Huitric, E.; Verhasselt, P.; Andries, K.; Hoffner, S.E. In vitro antimycobacterial spectrum of a diarylquinoline ATP synthase inhibitor. Antimicrob. Agents Chemother. 2007, 51, 4202–4204. [Google Scholar] [CrossRef]
- Peloquin, C.A.; Davies, G.R. The Treatment of Tuberculosis. Clin Pharmacol Ther 2021, 110, 1455–1466. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.V. Current status and future prospects for new therapies for pulmonary tuberculosis. Curr. Opin. Pulm. Med. 2006, 12, 167–171. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, R.J.; Spigelman, M. New drugs for tuberculosis: Current status and future prospects. Clin. Chest Med. 2005, 26, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Deng, J.; Yuan, Y.; Chen, W.; Sun, W.; Wang, Y.; Huang, H.; Liang, B.; Ming, T.; Wen, J.; et al. Advances in Trop2-targeted therapy: Novel agents and opportunities beyond breast cancer. Pharmacol. Ther. 2022, 239, 108296, Erratum in Pharmacol. Ther. Phys. 2023, 243, 108336. [Google Scholar] [CrossRef]
- Wilson, K.E.; Bachawal, S.V.; Willmann, J.K. Intraoperative Resection Guidance with Photoacoustic and Fluorescence Molecular Imaging Using an Anti-B7-H3 Antibody-Indocyanine Green Dual Contrast Agent. Clin. Cancer Res. 2018, 24, 3572–3582. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Liu, W.L.; Luo, X.J.; Shi, J.P.; Zeng, Y.Z.; Chen, W.L.; Huang, W.H.; Zhu, Y.Y.; Gao, W.L.; Li, R.H.; et al. Novel Self-Assembled Multifunctional Nanoprobes for Second-Near-Infrared-Fluorescence-Image-Guided Breast Cancer Surgery and Enhanced Radiotherapy Efficacy. Adv. Sci. 2023, 10, e2205294. [Google Scholar] [CrossRef]
- Tang, M.; Zeng, L.; Zeng, Z.; Liu, J.; Yuan, J.; Wu, D.; Lu, Y.; Zi, J.; Ye, M. Proteomics study of colorectal cancer and adenomatous polyps identifies TFR1, SAHH, and HV307 as potential biomarkers for screening. J. Proteom. 2021, 243, 104246. [Google Scholar] [CrossRef]
- Candelaria, P.V.; Leoh, L.S.; Penichet, M.L.; Daniels-Wells, T.R. Antibodies Targeting the Transferrin Receptor 1 (TfR1) as Direct Anti-cancer Agents. Front. Immunol. 2021, 12, 607692. [Google Scholar] [CrossRef]
- Ji, P.; Wang, X.; Yin, J.; Mou, Y.; Huang, H.; Ren, Z. Selective delivery of curcumin to breast cancer cells by self-targeting apoferritin nanocages with pH-responsive and low toxicity. Drug Deliv. 2022, 29, 986–996. [Google Scholar] [CrossRef]
- Deshpande, S.; Masurkar, N.D.; Girish, V.M.; Desai, M.; Chakraborty, G.; Chan, J.M.; Drum, C.L. Thermostable exoshells fold and stabilize recombinant proteins. Nat. Commun. 2017, 8, 1442. [Google Scholar] [CrossRef]
- Sadeghi, S.; Masurkar, N.D.; Vallerinteavide Mavelli, G.; Deshpande, S.; Kok Yong Tan, W.; Yee, S.; Kang, S.A.; Lim, Y.P.; Kai-Hua Chow, E.; Drum, C.L. Bioorthogonal Catalysis for Treatment of Solid Tumors Using Thermostable, Self-Assembling, Single Enzyme Nanoparticles and Natural Product Conversion with Indole-3-acetic Acid. ACS Nano 2022, 16, 10292–10301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Qiu, T.; Jiang, D.; Xu, H.; Zou, L.; Yang, Q.; Chen, C.; Jiao, B. SGCE Promotes Breast Cancer Stem Cells by Stabilizing EGFR. Adv. Sci. 2020, 7, 1903700. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, Y.; Huang, X.; Li, L.; Han, B.; Cao, Y.; Zhao, J. Self-Assembling Peptide-Based Multifunctional Nanofibers for Electrochemical Identification of Breast Cancer Stem-like Cells. Anal. Chem. 2019, 91, 7531–7537. [Google Scholar] [CrossRef] [PubMed]
- Derakhshan, F.; Reis-Filho, J.S. Pathogenesis of Triple-Negative Breast Cancer. Annu. Rev. Pathol. 2022, 17, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Borri, F.; Granaglia, A. Pathology of triple negative breast cancer. Semin Cancer Biol. 2021, 72, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Tanioka, N.; Shimizu, H.; Omori, E.; Takahashi, T.; Yamaoka, M.; Morimatsu, H. Role of the Transcription Factor BTB and CNC Homology 1 in a Rat Model of Acute Liver Injury Induced by Experimental Endotoxemia. Acta Med. Okayama 2021, 75, 363–372. [Google Scholar] [CrossRef]
- Lee, J.; Yesilkanal, A.E.; Wynne, J.P.; Frankenberger, C.; Liu, J.; Yan, J.; Elbaz, M.; Rabe, D.C.; Rustandy, F.D.; Tiwari, P.; et al. Effective breast cancer combination therapy targeting BACH1 and mitochondrial metabolism. Nature 2019, 568, 254–258. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Zhao, J.; Rong, H.; Chen, Y.; Xiong, M.; Ye, X.; Yu, S.; Hu, H. Coordinated regulation of BACH1 and mitochondrial metabolism through tumor-targeted self-assembled nanoparticles for effective triple negative breast cancer combination therapy. Acta Pharm. Sin. B 2022, 12, 3934–3951. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, N.W.; Kim, H.; Kim, D.H.; Chae, J.W.; Lee, W.; Song, G.Y.; Cho, C.W.; Kim, D.D.; Lee, J.Y. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carbohydr. Polym. 2021, 253, 117187. [Google Scholar] [CrossRef]
- Lee, A.; Djamgoz, M.B.A. Triple negative breast cancer: Emerging therapeutic modalities and novel combination therapies. Cancer Treat. Rev. 2018, 62, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, R.; Wu, X.; Sun, Y.; Yao, M.; Li, J.; Xu, Y.; Gu, J. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. Faseb. J. 2005, 19, 1978–1985. [Google Scholar] [CrossRef]
- Jiang, P.; Gao, W.; Ma, T.; Wang, R.; Piao, Y.; Dong, X.; Wang, P.; Zhang, X.; Liu, Y.; Su, W.; et al. CD137 promotes bone metastasis of breast cancer by enhancing the migration and osteoclast differentiation of monocytes/macrophages. Theranostics 2019, 9, 2950–2966. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zeng, Y.; Shi, S.; Xu, L.; Zhang, H.; Pathak, J.L.; Pan, Y. Design of polyaspartic acid peptide-poly (ethylene glycol)-poly (ε-caprolactone) nanoparticles as a carrier of hydrophobic drugs targeting cancer metastasized to bone. Int. J. Nanomed. 2017, 12, 3561–3575. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ma, S.; Xu, D.; Meng, X.; Lei, N.; Liu, C.; Zhao, Y.; Qi, Y.; Cheng, Z.; Wang, F. Peptide-functionalized therapeutic nanoplatform for treatment orthotopic triple negative breast cancer and bone metastasis. Nanomedicine 2023, 50, 102669. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Lv, G.; Xu, F.H.; Ma, L.L.; Yao, Y.M. Comprehensive Survey of Clinical Trials Registration for Melanoma Immunotherapy in the ClinicalTrials.gov. Front. Pharmacol. 2019, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Si, Y.; Wen, Y.; Kelly, S.H.; Chong, A.S.; Collier, J.H. Intranasal delivery of adjuvant-free peptide nanofibers elicits resident CD8(+) T cell responses. J. Control Release 2018, 282, 120–130. [Google Scholar] [CrossRef]
- Li, S.; Zhu, W.; Ye, C.; Sun, W.; Xie, H.; Yang, X.; Zhang, Q.; Ma, Y. Local mucosal immunization of self-assembled nanofibers elicits robust antitumor effects in an orthotopic model of mouse genital tumors. Nanoscale 2020, 12, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Wang, M.; Yang, T.; Li, M.; Liang, Z.; Chen, C.; Zhang, L.; Xue, C.; Sun, B.; Mao, C. Self-assembled flagella protein nanofibers induce enhanced mucosal immunity. Biomaterials 2022, 288, 121733. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Xu, Y.; Mao, L.; Ou, R.; Ding, Z.; Zhang, X.; Tang, J.; Li, B.; Jia, Z.; Tian, Z.; et al. Heat shock protein 110 improves the antitumor effects of the cytotoxic T lymphocyte epitope E7(49-57) in mice. Cancer Biol. Ther. 2010, 9, 134–141. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C.; Liu, F.; Zhou, Y.; Luo, L.; Qiao, H. RGD Peptide-Based Target Drug Delivery of Doxorubicin Nanomedicine. Drug Dev. Res. 2017, 78, 283–291. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, F.; Ni, B.; Jing, T.; Tang, J. Tumor targeting nanoparticle E7(49-57)-HSP110-RGD elicits potent anti-tumor immune response in a CD8-dependent manner in cervical cancer-bearing mouse model. Hum. Vaccin. Immunother. 2021, 17, 3529–3538. [Google Scholar] [CrossRef]
- Predina, J.D.; Keating, J.; Newton, A.; Corbett, C.; Xia, L.; Shin, M.; Frenzel Sulyok, L.; Deshpande, C.; Litzky, L.; Nie, S.; et al. A clinical trial of intraoperative near-infrared imaging to assess tumor extent and identify residual disease during anterior mediastinal tumor resection. Cancer 2019, 125, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Wang, J.; Li, Y.; Zhang, Y.; Yu, L.; Xu, T.; Guo, H.; Zhang, Y.; Wang, X.; Wang, X.; et al. Tumor Noninvasive and Target Embolization Therapy Platform by Intravenous Injection Based on Acidic Microenvironment-Responsive Hyperbranched Poly(amino acid)s. ACS Cent. Sci. 2020, 6, 1977–1986. [Google Scholar] [CrossRef]
- Li, L.; Liang, N.; Wang, D.; Yan, P.; Kawashima, Y.; Cui, F.; Sun, S. Amphiphilic Polymeric Micelles Based on Deoxycholic Acid and Folic Acid Modified Chitosan for the Delivery of Paclitaxel. Int. J. Mol. Sci. 2018, 19, 3132. [Google Scholar] [CrossRef]
- Luan, X.; Yuan, H.; Song, Y.; Hu, H.; Wen, B.; He, M.; Zhang, H.; Li, Y.; Li, F.; Shu, P.; et al. Reappraisal of anticancer nanomedicine design criteria in three types of preclinical cancer models for better clinical translation. Biomaterials 2021, 275, 120910. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, C.; Shuaizhen, Q.; Yu, R.; Zheng, Y. Design of integrin α(v)β(3) targeting self-assembled protein nanoparticles with RGD peptide. Biomed. Pharmacother. 2020, 128, 110236. [Google Scholar] [CrossRef]
- Lv, M.Y.; Xiao, W.Y.; Zhang, Y.P.; Jin, L.L.; Li, Z.H.; Lei, Z.; Cheng, D.B.; Jin, S.D. In situ self-assembled peptide enables effective cancer immunotherapy by blockage of CD47. Colloids Surf. B Biointerfaces 2022, 217, 112655. [Google Scholar] [CrossRef]
- Padežnik, T.; Oleksy, A.; Cokan, A.; Takač, I.; Sobočan, M. Changes in the Extracellular Matrix in Endometrial and Cervical Cancer: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 5463. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Sheng, B.; Zeng, Q.; Yao, W.; Jiang, Q. Correlation between MMP2 expression in lung cancer tissues and clinical parameters: A retrospective clinical analysis. BMC Pulm. Med. 2020, 20, 283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Huang, Q.; Xiao, W.; Zhao, Y.; Pi, J.; Xu, H.; Zhao, H.; Xu, J.; Evans, C.E.; Jin, H. Advances in Anti-Tumor Treatments Targeting the CD47/SIRPα Axis. Front. Immunol. 2020, 11, 18. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X.; Yang, Y.; Li, Y.; Wu, S. LSD1 silencing contributes to enhanced efficacy of anti-CD47/PD-L1 immunotherapy in cervical cancer. Cell Death Dis. 2021, 12, 282. [Google Scholar] [CrossRef]
- Volkova, L.V.; Pashov, A.I.; Omelchuk, N.N. Cervical Carcinoma: Oncobiology and Biomarkers. Int. J. Mol. Sci. 2021, 22, 12571. [Google Scholar] [CrossRef]
- Huang, H.; Ding, Y.; Sun, X.S.; Nguyen, T.A. Peptide hydrogelation and cell encapsulation for 3D culture of MCF-7 breast cancer cells. PLoS ONE 2013, 8, e59482. [Google Scholar] [CrossRef]
- Mi, K.; Wang, G.; Liu, Z.; Feng, Z.; Huang, B.; Zhao, X. Influence of a self-assembling peptide, RADA16, compared with collagen I and Matrigel on the malignant phenotype of human breast-cancer cells in 3D cultures and in vivo. Macromol. Biosci. 2009, 9, 437–443. [Google Scholar] [CrossRef]
- Mi, K.; Xing, Z. CD44(+)/CD24(-) breast cancer cells exhibit phenotypic reversion in three-dimensional self-assembling peptide RADA16 nanofiber scaffold. Int. J. Nanomed. 2015, 10, 3043–3053. [Google Scholar] [CrossRef]
- Clough, H.C.; O’Brien, M.; Zhu, X.; Miller, A.F.; Saiani, A.; Tsigkou, O. Neutrally charged self-assembling peptide hydrogel recapitulates in vitro mechanisms of breast cancer progression. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112200. [Google Scholar] [CrossRef]
- Patrick, C.W., Jr. Tissue engineering strategies for adipose tissue repair. Anat. Rec. 2001, 263, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Zhao, J.F.; Xue, H.Y.; Li, D. Facial aesthetic fat graft retention rates after filtration, centrifugation, or sedimentation processing techniques measured using three-dimensional surface imaging devices. Chin. Med. J. 2019, 132, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhou, T.; Tian, L.; Xia, Z.; Xu, F. Self-Assembling RADA16-I Peptide Hydrogel Scaffold Loaded with Tamoxifen for Breast Reconstruction. Biomed. Res. Int. 2017, 2017, 3656193. [Google Scholar] [CrossRef] [PubMed]

| Serial Number | Name | Cancer | Signaling Pathway | References |
|---|---|---|---|---|
| 1 | Rosemary extract | BC | AKT/mTOR | [81] |
| 2 | Astragalus polysaccharides | BC | PIK3CG/AKT/BCL2 | [82] |
| 3 | Ethanol extract of Rhizoma Amorphophalli | BC | PI3K/AKT/mTOR | [83] |
| 4 | Sophoraflavanone G | BC | MAPK (AKT) | [84] |
| 5 | Rhus coriaria ethanolic extract | BC | ERK1/2, p38 | [85] |
| 6 | Ganoderma lucidum extract | BC | IL-6/JAK2/STAT3 (JAK1/STAT3) | [86] |
| 7 | Extracts of Cordyceps sinensis | BC | NF-κB (macrophage) | [87] |
| 8 | Paeoniflorin | BC | Notch1 | [88] |
| 9 | Nelumbo nucifera Gaertn. leaf extract | BC | PI3K/AKT/ERK (CTGF) | [89] |
| 10 | Ethanolic extract of Rhus coriaria | BC | NF-κB, STAT3 | [90] |
| 11 | Aster tataricus, Vitex peduncularis Wall | CC | Caspase-3, -8, and -9, Bcl-2, -xL, survivin | [91] |
| 12 | Syzygium aromaticum | CC | Bax, PARP, caspase-3, ROS, Bcl-2, XIAP | [92] |
| 13 | Rhamnus sphaerosperma var. pubescens | CC | NO-, O2-, HOCl/OCl-, p-Akt | [93] |
| 14 | Root tubers and leaves of Ipomoea batatas | CC | CFP/YFP | [94] |
| 15 | Curcuma zedoaria | CC | p15, p53, Bax, cyclin D1, Bcl-2, MMP-2, -9, β-catenin, TCF7, c-Myc | [95] |
| Serial Number | Name | Indications | Adverse Effects | Contraindications | References |
|---|---|---|---|---|---|
| 1 | Aromatase inhibitors | Adjuvant treatment of postmenopausal women with hormone receptor-positive early breast cancer. | Ulcers and blisters are common skin manifestations. | Hypersensitivity. | [96] |
| 2 | Raloxifene | Indicated for a risk reduction in invasive breast cancer in postmenopausal women, demonstrating a high risk for invasive breast cancer or women with osteoporosis. | Hot flashes, flu-like symptoms, muscle spasms, arthralgia, and infection. | Past medical history of deep venous thrombosis, renal vein thrombosis, pulmonary embolism, malignancy, active smoking, or any thrombophilia (factor V Leiden deficiency, prothrombin gene mutation G20210A, antiphospholipid syndrome, antithrombin deficiency, and protein c and s deficiency). | [97] |
| 3 | Cyclophosphamide | Use in the treatment of malignant lymphomas at stages III and IV. | Bladder and gonadal toxicity, hemorrhagic cystitis, amenorrhea, myelosuppression, alopecia, and spells of nausea and vomiting. | Patients with allergies or hypersensitivity reactions to the drug or any of its metabolites. | [98] |
| 4 | Pamidronate | Moderate or severe hypercalcemia of malignancy, moderate-to-severe Paget’s disease of bone, osteolytic bone metastases of breast cancer, and osteolytic lesions of multiple myeloma | Hypocalcemia and resulting secondary hyperparathyroidism, acute phase response, musculoskeletal pain, various ocular events, and osteonecrosis of the jaw. | Those with hypersensitivity to bisphosphonates or mannitol. | [99] |
| 5 | Trastuzumab | HER2-positive breast cancer: adjuvant therapy. | Pregnancy disruption and cardiac dysfunction. | Cardiotoxicity, usually manifested as a decrease in the left ventricular ejection fraction (LVEF). | [100] |
| 6 | Tamoxifen | Treatment of breast cancer in both females and males. | Uterine malignancies, pulmonary embolism, and stroke in patients who are at high risk for cancer or who have ductal carcinoma in situ. | The drug or any component in its formulation or concomitantly with warfarin. | [101] |
| 7 | Fulvestrant | HR-positive and HER2-negative breast cancer. | Pain at the injection site and hot flashes. | Women who are pregnant or breastfeeding. | [102] |
| 8 | Doxorubicin | For acute leukemia (lymphoblastic and granulocytic), malignant lymphoma, breast cancer, lung cancer (small cell and non-small cell lung cancer), ovarian cancer, bone and soft tissue sarcoma, nephroblastoma, bladder cancer, thyroid cancer, prostate cancer, squamous carcinoma of the head and neck, testicular cancer, gastric cancer, and liver cancer. | Bone marrow suppression, cardiotoxicity, gastrointestinal reactions: manifested as loss of appetite, nausea, vomiting, but also oral mucosal erythema, ulcers and esophagitis, gastritis, and alopecia. Fever, hemorrhagic erythema, liver function abnormalities, etc. | Gastrointestinal obstruction, jaundice, or hepatic impairment patients; patients with cardiopulmonary failure, chickenpox; or herpes zoster; patients who have been treated with other antitumor drugs or radiation therapy that has caused bone marrow suppression is contraindicated; patients with severe heart disease are contraindicated. contraindicated in pregnant and lactating women. | [104,105] |
| 9 | Docetaxel | Good efficacy in advanced breast cancer, ovarian cancer, and non-small cell lung cancer. | Bone marrow suppression: dose-limiting toxicity is neutropenia; allergic reactions: mild allergic reactions manifested as itching, flushing, rash, drug fever, chills, etc., severe allergic reactions are rare, characterized by bronchospasm, dyspnea, and hypotension; skin reactions: mainly in the hands and feet, but also in the arms, face and chest rash, which may be accompanied by itching; nausea, vomiting, and diarrhea, alopecia, malaise, mucositis, arthralgia, myalgia, injection site reactions, neurotoxicity and cardiovascular toxicity. | Contraindicated in persons who are hypersensitive to the product. It is contraindicated in persons with severe bone marrow suppression, severe hepatic, or renal impairment, and in pregnant and lactating women. | [106,107] |
| 10 | Methotrexate | It is indicated for systemic use in the treatment of choriocarcinoma of the epithelium, all types of acute leukemia, breast cancer, lung cancer, head and neck cancer, and cervical cancer. High-dose methotrexate supplemented with calcium formyltetrahydrofolate rescue (HDMTX-CFR therapy) has certain efficacy as postoperative adjuvant chemotherapy or systemic therapy for advanced lesions of malignant lymphoma, acute lymphoblastic leukemia, breast cancer, ovarian cancer, small-cell lung cancer, and so on. | Gastrointestinal reactions are mainly stomatitis, mouth and lip ulcers, pharyngitis, nausea, vomiting, gastritis and diarrhea. Bone marrow suppression is mainly manifested as a decline in white blood cells, platelets also have a certain effect, in severe cases, whole blood decline, skin or visceral bleeding can occur. A large number of applications can lead to serum alanine aminotransferase (ALT) elevation, or drug hepatitis, a small amount of persistent application can lead to hepatic cirrhosis. | Causes kidney damage at high doses and can be teratogenic when used early in pregnancy. | [110,111] |
| 11 | Fluorouracil | It is used alone or in combination with other agents in the adjuvant treatment of breast cancer surgery and in the palliative treatment of some non-surgical malignancies, particularly those of the breast and pancreas. The combination of cyclophosphamide and MTX (breast cancer) often results in high response rates and survival rates. | Bone marrow suppression: mainly leukopenia, platelet drop. Loss of appetite, nausea, vomiting, stomatitis, gastritis, abdominal pain and diarrhea and other gastrointestinal reactions; injection of local pain, phlebitis or arterial endarteritis, often alopecia, erythematous dermatitis, skin pigmentation hand-foot syndrome and temporary cerebellar motor disorders, and occasionally affects the function of the heart. | Blood test should be strictly checked during the use of the drug. Keep in a dark place away from light, the temperature should not be lower than 10 °C, and it should not exceed 35 °C. Inflammation in the area of application during the treatment period, the inflammation will subside after stopping the drug. This product can cause severe skin irritation, especially in the sun. | [109,113]. |
| 12 | Orserdu(elacestrant) | Treatment of postmenopausal women or adult men with advanced or metastatic ER-positive, HER2-negative, and ESR1-mutated breast cancer that has progressed after at least one endocrine therapy. | Musculoskeletal pain, nausea, elevated cholesterol, increased AST, increased triglycerides, fatigue, decreased hemoglobin, vomiting, increased ALT, decreased sodium, increased creatinine, decreased appetite, diarrhea, headache, constipation, abdominal pain, hot flashes, and indigestion. | Lactation: breastfeeding is not recommended. Hepatic impairment: avoid use in patients with severe hepatic impairment (Child–Pugh C). Reduce dose in patients with moderate hepatic impairment (Child–Pugh B). | [114] |
| 13 | Truqap(capivasertib) | In combination with fulvestrant for the treatment of adult patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, locally advanced, or metastatic breast cancer with one or more PIK3CA/AKT1/PTEN mutation as detected by an FDA-approved trial, who have progressed after treatment with at least one endocrine-based regimen in metastatic disease, or who have relapsed within 12 months of completing adjuvant therapy. | Musculoskeletal pain, nausea, elevated cholesterol, increased AST, increased triglycerides, fatigue, decreased hemoglobin, vomiting, increased ALT, decreased sodium, increased creatinine, decreased appetite, diarrhea, headache, constipation, abdominal pain, hot flashes, and dyspepsia. | Dyslipidemia: may result in hypercholesterolemia and hypertriglyceridemia. Monitor lipids before and periodically after initiating therapy. Embryo-fetal toxicity: can lead to fetal harm. Advise of potential risks to the fetus and use effective contraception. | [117,118] |
| 14 | Verzenio(abemaciclib) | Patients with advanced or metastatic HR+ and HER-2- breast cancer; in patients whose disease has worsened after hormonal therapy for breast cancer, it may be combined with flugestone; patients with early-stage breast cancer who are receiving chemotherapy for distant metastases; and the combination of abciximil and an aromatase inhibitor may be used as a first-line hormonal treatment option for breast cancer in postmenopausal women. | Diarrhea, neutropenia, nausea, abdominal pain, infection, fatigue, anemia, neutropenia, decreased appetite, vomiting, headache, and thrombocytopenia | Avoid concomitant use of ketoconazole. Decrease concomitant use of VERZENIO administration with other strong CYP3A inhibitors; avoid concomitant use of strong CYP3A inducers; advise against breastfeeding during lactation. | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chen, M.; Geng, Z.; Liu, T.; Li, S.; Yu, Q.; Cao, L.; Liu, D. Potential Application of Self-Assembled Peptides and Proteins in Breast Cancer and Cervical Cancer. Int. J. Mol. Sci. 2023, 24, 17056. https://doi.org/10.3390/ijms242317056
Zhang S, Chen M, Geng Z, Liu T, Li S, Yu Q, Cao L, Liu D. Potential Application of Self-Assembled Peptides and Proteins in Breast Cancer and Cervical Cancer. International Journal of Molecular Sciences. 2023; 24(23):17056. https://doi.org/10.3390/ijms242317056
Chicago/Turabian StyleZhang, Shidong, Meiqi Chen, Zijun Geng, Tianjia Liu, Shuangyang Li, Qixuan Yu, Lingling Cao, and Da Liu. 2023. "Potential Application of Self-Assembled Peptides and Proteins in Breast Cancer and Cervical Cancer" International Journal of Molecular Sciences 24, no. 23: 17056. https://doi.org/10.3390/ijms242317056
APA StyleZhang, S., Chen, M., Geng, Z., Liu, T., Li, S., Yu, Q., Cao, L., & Liu, D. (2023). Potential Application of Self-Assembled Peptides and Proteins in Breast Cancer and Cervical Cancer. International Journal of Molecular Sciences, 24(23), 17056. https://doi.org/10.3390/ijms242317056






