Abrupt Photoperiod Changes Differentially Modulate Hepatic Antioxidant Response in Healthy and Obese Rats: Effects of Grape Seed Proanthocyanidin Extract (GSPE)
Abstract
:1. Introduction
2. Results
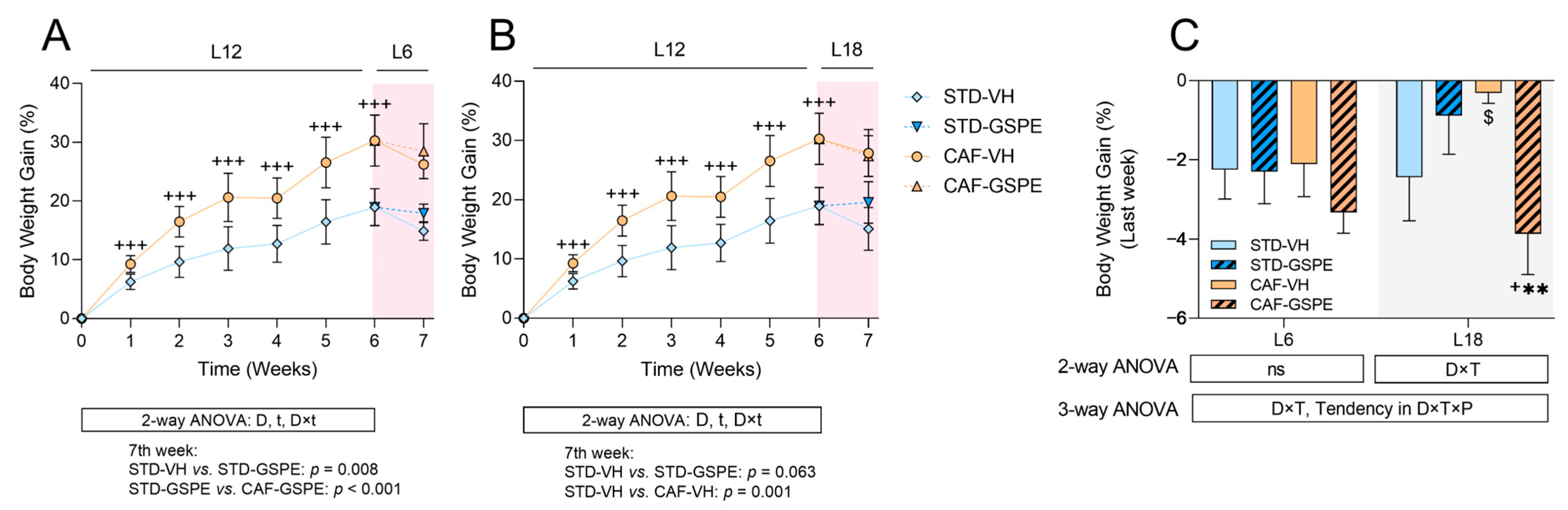
2.1. Abrupt Photoperiod Changes Altered Body Weight Gain, While Treatment with GSPE Partially Improved Serum Biochemical Parameters Affected by the CAF
2.2. CAF Highly Impacted Hepatic Biochemical Parameters, with GSPE Reducing Liver Weight and Total Lipid Content in the L6 Condition
2.3. GSPE Treatment Increased Antioxidant Response Gene Expression in CAF-Fed Rats in a Photoperiod-Dependent Manner
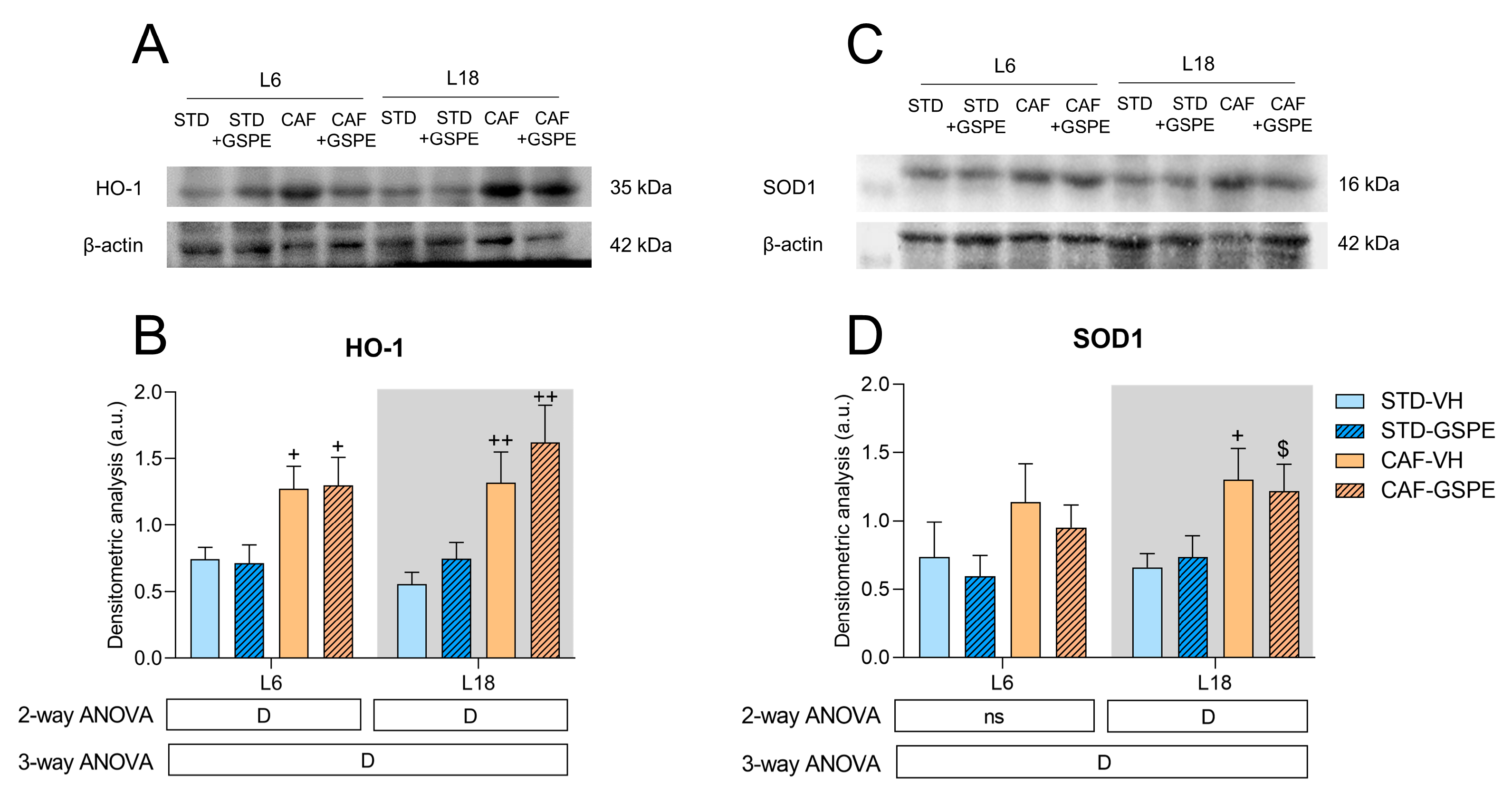
2.4. CAF Significantly Altered Both HO-1 and SOD1 Expressions after an Abrupt Change in Photoperiod, and GSPE could Modulate HO-1 Expression in a Photoperiod-Dependent Manner
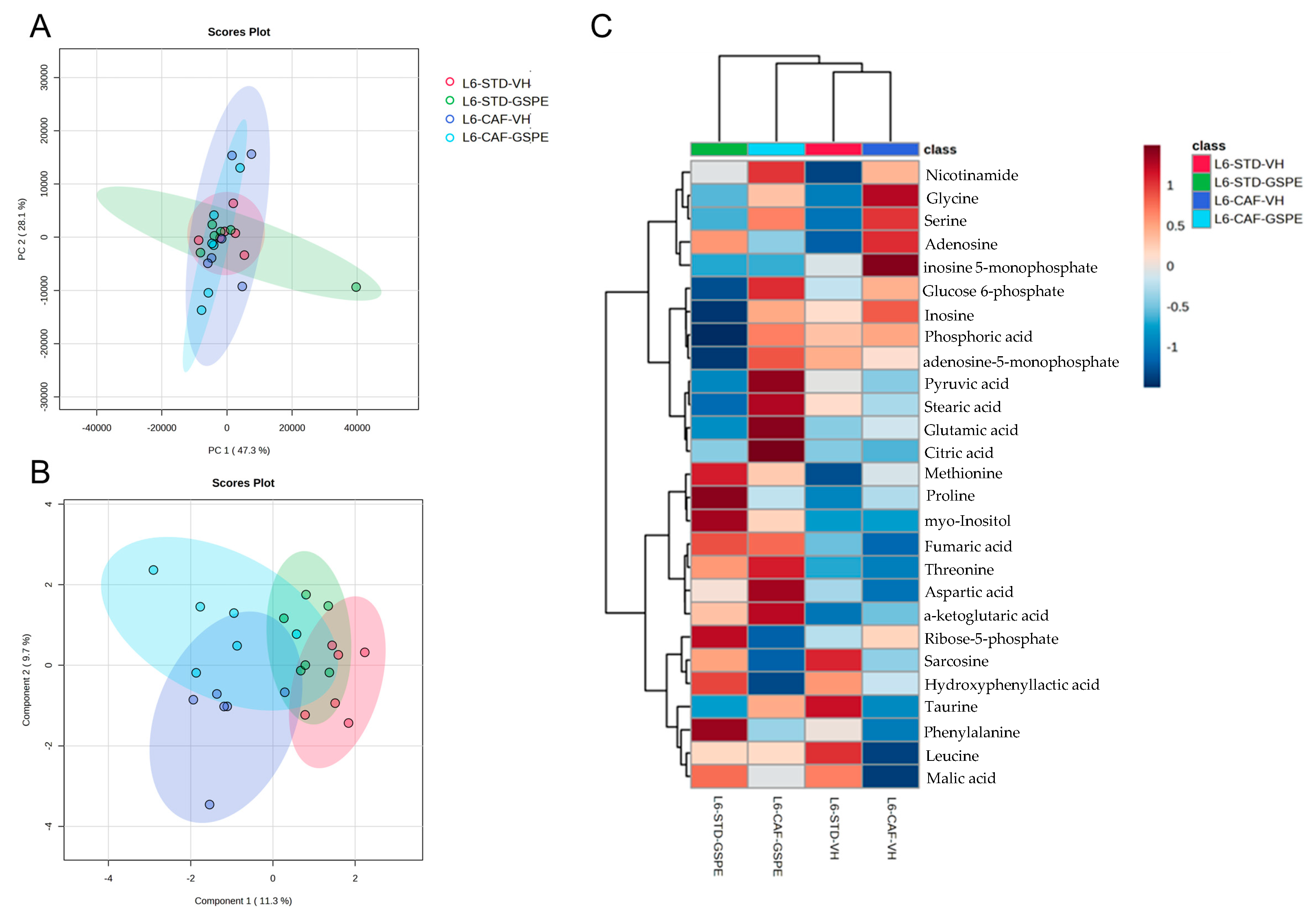
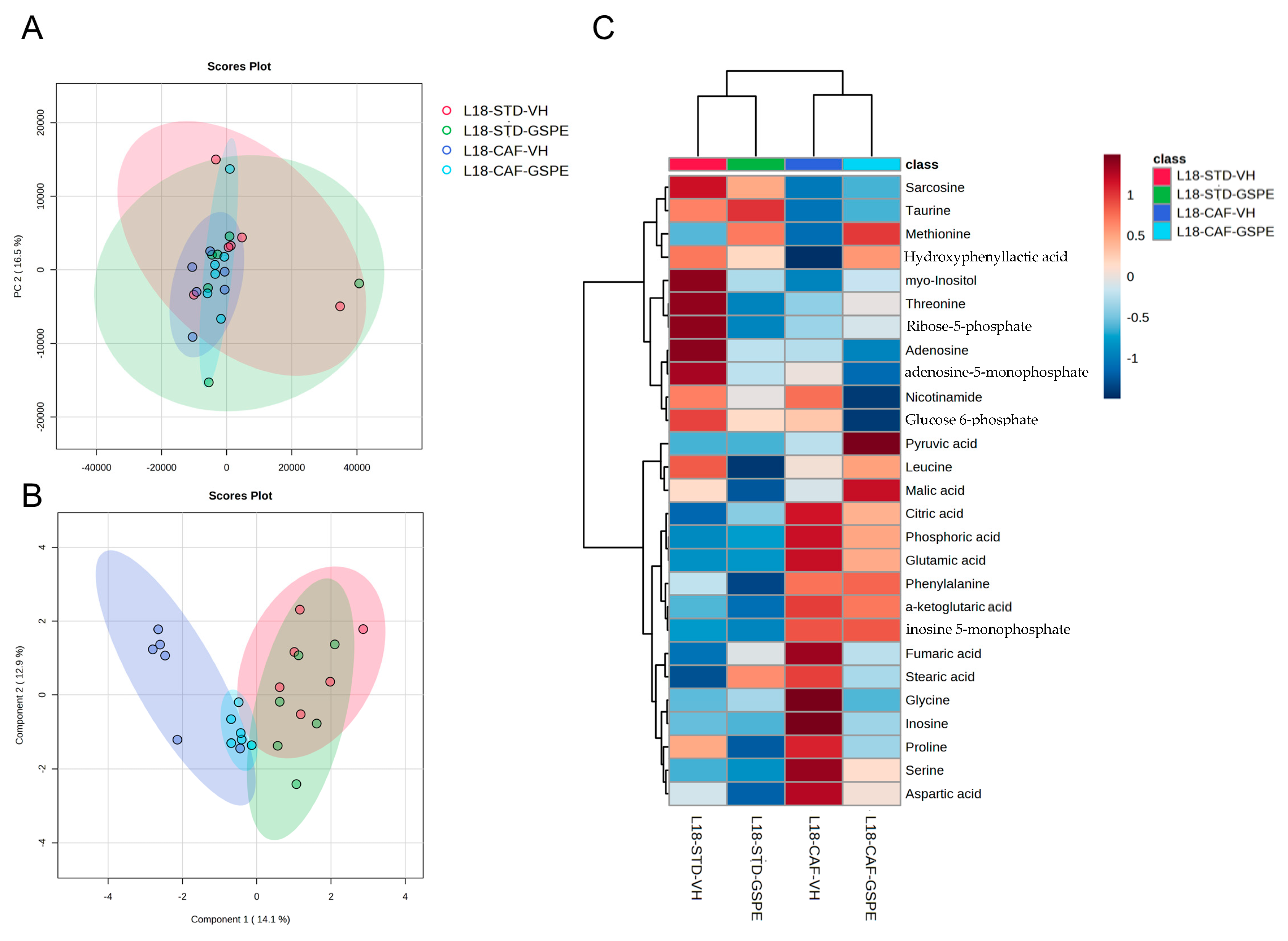
2.5. GSPE Improved Liver Antioxidant Metabolic Profile of CAF-Fed Rats after an Abrupt Change in Photoperiod
3. Discussion
4. Materials and Methods
4.1. Grape Seed Proanthocyanidins Extract (GSPE)
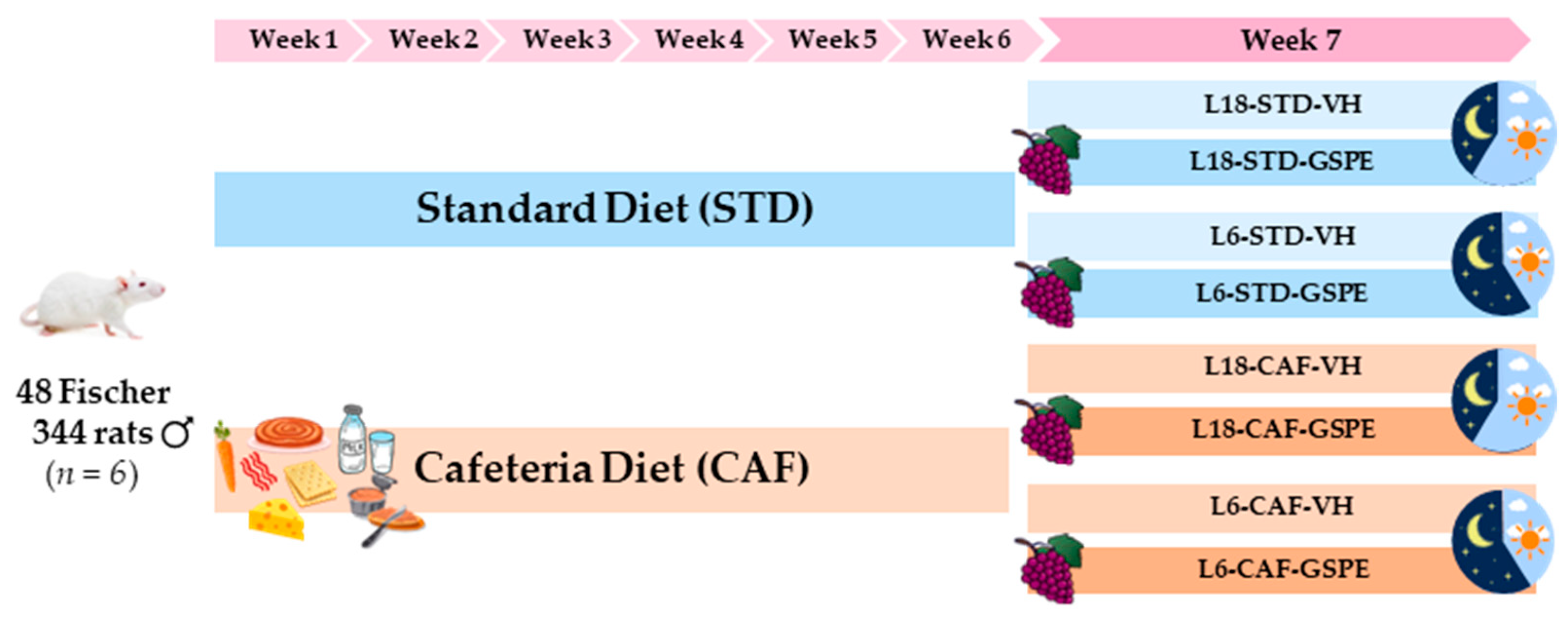
4.2. Experimental Procedure in Rats
4.3. Dosage Information/Dosage Regimen
4.4. Hepatic RNA Extraction
4.5. cDNA Synthesis and Gene-Expression Analysis
4.6. Serum Biochemical Analysis
4.7. Protein Extraction and Western Blot Analysis
4.8. Extraction and Measurement of the Concentration of Lipids in the Liver
4.9. Metabolomics Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Golombek, D.A.; Rosenstein, R.E. Physiology of Circadian Entrainment. Physiol. Rev. 2010, 90, 1063–1102. [Google Scholar] [CrossRef] [PubMed]
- Kuhlman, S.J.; Craig, L.M.; Duffy, J.F. Introduction to Chronobiology. Cold Spring Harb. Perspect. Biol. 2018, 10, a033613. [Google Scholar] [CrossRef] [PubMed]
- Ayyar, V.S.; Sukumaran, S. Circadian Rhythms: Influence on Physiology, Pharmacology, and Therapeutic Interventions. J. Pharmacokinet. Pharmacodyn. 2021, 48, 321–338. [Google Scholar] [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2009, 72, 551–577. [Google Scholar] [CrossRef]
- Korf, H.W. Signaling Pathways to and from the Hypophysial Pars Tuberalis, an Important Center for the Control of Seasonal Rhythms. Gen. Comp. Endocrinol. 2018, 258, 236–243. [Google Scholar] [CrossRef]
- Tsai, L.-L.; Tsai, Y.-C.; Hwang, K.; Huang, Y.-W.; Tzeng, J.-E. Repeated Light-Dark Shifts Speed up Body Weight Gain in Male F344 Rats. Am. J. Physiol. Endocrinol. Metab. 2005, 289, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Jochum, S.B.; Shaikh, M.; Wilber, S.; Zhang, L.; Hayden, D.M.; Forsyth, C.B.; Voigt, R.M.; Bishehsari, F.; Keshavarzian, A.; et al. Circadian Misalignment by Environmental Light/Dark Shifting Causes Circadian Disruption in Colon. PLoS ONE 2021, 16, e0251604. [Google Scholar] [CrossRef]
- Walker, W.H.; Walton, J.C.; DeVries, A.C.; Nelson, R.J. Circadian Rhythm Disruption and Mental Health. Transl. Psychiatry 2020, 10, 28. [Google Scholar] [CrossRef]
- Schilperoort, M.; Bravenboer, N.; Lim, J.; Mletzko, K.; Busse, B.; van Ruijven, L.; Kroon, J.; Rensen, P.C.N.; Kooijman, S.; Winter, E.M. Circadian Disruption by Shifting the Light-Dark Cycle Negatively Affects Bone Health in Mice. FASEB J. 2020, 34, 1052–1064. [Google Scholar] [CrossRef]
- Xie, X.; Zhao, B.; Huang, L.; Shen, Q.; Ma, L.; Chen, Y.; Wu, T.; Fu, Z. Effects of Altered Photoperiod on Circadian Clock and Lipid Metabolism in Rats. Chronobiol. Int. 2017, 34, 1094–1104. [Google Scholar] [CrossRef]
- Lewis, P.; Oster, H.; Korf, H.W.; Foster, R.G.; Erren, T.C. Food as a Circadian Time Cue—Evidence from Human Studies. Nat. Rev. Endocrinol. 2020, 16, 213–223. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, J.S.; Branecky, K.L.; Yang, W.; Ellacott, K.L.J.; Niswender, K.D.; Yamazaki, S. High-Fat Diet Acutely Affects Circadian Organisation and Eating Behavior. Eur. J. Neurosci. 2013, 37, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sato, S. Circadian Regulation of Metabolism: Commitment to Health and Diseases. Endocrinology 2023, 164, bqad086. [Google Scholar] [CrossRef]
- Albrecht, U. The Circadian Clock, Metabolism and Obesity. Obes. Rev. 2017, 18, 25–33. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef] [PubMed]
- Manna, P.; Jain, S.K. Obesity, Oxidative Stress, Adipose Tissue Dysfunction, and the Associated Health Risks: Causes and Therapeutic Strategies. Metab. Syndr. Relat. Disord. 2015, 13, 423–444. [Google Scholar] [CrossRef]
- McClean, C.; Davison, G.W. Circadian Clocks, Redox Homeostasis, and Exercise: Time to Connect the Dots? Antioxidants 2022, 11, 256. [Google Scholar] [CrossRef]
- Cortés-Espinar, A.J.; Ibarz-Blanch, N.; Soliz-Rueda, J.R.; Bonafos, B.; Feillet-Coudray, C.; Casas, F.; Bravo, F.I.; Calvo, E.; Ávila-Román, J.; Mulero, M. Rhythm and ROS: Hepatic Chronotherapeutic Features of Grape Seed Proanthocyanidin Extract Treatment in Cafeteria Diet-Fed Rats. Antioxidants 2023, 12, 1606. [Google Scholar] [CrossRef]
- Lu, M.-C.; Ji, J.-A.; Jiang, Z.-Y.; You, Q.-D. The Keap1-Nrf2-ARE Pathway As a Potential Preventive and Therapeutic Target: An Update. Med. Res. Rev. 2016, 36, 924–963. [Google Scholar] [CrossRef]
- Pekovic-Vaughan, V.; Gibbs, J.; Yoshitane, H.; Yang, N.; Pathiranage, D.; Guo, B.; Sagami, A.; Taguchi, K.; Bechtold, D.; Loudon, A.; et al. The Circadian Clock Regulates Rhythmic Activation of the NRF2/Glutathione-Mediated Antioxidant Defense Pathway to Modulate Pulmonary Fibrosis. Genes. Dev. 2014, 28, 548–560. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. NAD(P)H:Quinone Acceptor Oxidoreductase 1 (NQO1), a Multifunctional Antioxidant Enzyme and Exceptionally Versatile Cytoprotector. Arch. Biochem. Biophys. 2010, 501, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.Y.; Jin, S.H.; Cho, I.J.; Ki, S.H. Nrf2-ARE Pathway Regulates Induction of Sestrin-2 Expression. Free Radic. Biol. Med. 2012, 53, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme Oxygenase-1, Oxidation, Inflammation, and Atherosclerosis. Front. Pharmacol. 2012, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, L.; Yao, K. The Antioxidative Function of Alpha-Ketoglutarate and Its Applications. BioMed Res. Int. 2018, 2018, 3408467. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding Oxidants and Antioxidants: Classical Team with New Players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Earle-payne, K.; Kidd, M.T. Taurine as a Natural Antioxidant: From Direct Antioxidant Effects to Protective Action in Various Toxicological Models. Antioxidants 2021, 10, 1876. [Google Scholar] [CrossRef]
- Wilking, M.; Ndiaye, M.; Mukhtar, H.; Ahmad, N. Circadian Rhythm Connections to Oxidative Stress: Implications for Human Health. Antioxid. Redox Signal. 2013, 19, 192–208. [Google Scholar] [CrossRef]
- Wible, R.S.; Ramanathan, C.; Sutter, C.H.; Olesen, K.M.; Kensler, T.W.; Liu, A.C.; Sutter, T.R. NRF2 Regulates Core and Stabilizing Circadian Clock Loops, Coupling Redox and Timekeeping in Mus Musculus. eLife 2018, 7, e31656. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Zhang, D.; Jin, T.; Cai, D.J.; Wu, Q.; Lu, Y.; Liu, J.; Klaassen, C.D. Diurnal Variation of Hepatic Antioxidant Gene Expression in Mice. PLoS ONE 2012, 7, e44237. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef]
- Devi, A.; Jolitha, A.B.; Ishii, N. Grape Seed Proanthocyanidin Extract (GSPE) and Antioxidant Defense in the Brain of Adult Rats. Med. Sci. Monit. 2006, 12, BR124-9. [Google Scholar]
- Puiggròs, F.; Llópiz, N.; Ardévol, A.; Bladé, C.; Arola, L.; Salvadó, M.J. Grape Seed Procyanidins Prevent Oxidative Injury by Modulating the Expression of Antioxidant Enzyme Systems. J. Agric. Food Chem. 2005, 53, 6080–6086. [Google Scholar] [CrossRef] [PubMed]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Chronic Administration of Grape-Seed Polyphenols Attenuates the Development of Hypertension and Improves Other Cardiometabolic Risk Factors Associated with the Metabolic Syndrome in Cafeteria Diet-Fed Rats. Br. J. Nutr. 2017, 117, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome with Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Latre, A.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.-J.; Bladé, C.; Arola, L. Dietary Proanthocyanidins Modulate BMAL1 Acetylation, Nampt Expression and NAD Levels in Rat Liver. Sci. Rep. 2015, 5, 10954. [Google Scholar] [CrossRef]
- Ribas-Latre, A.; Del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Arola, L.; Bladé, C. Dietary Proanthocyanidins Modulate Melatonin Levels in Plasma and the Expression Pattern of Clock Genes in the Hypothalamus of Rats. Mol. Nutr. Food Res. 2015, 59, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Soliz-Rueda, J.R.; López-Fernández-Sobrino, R.; Torres-Fuentes, C.; Bravo, F.I.; Suárez, M.; Mulero, M.; Muguerza, B. Metabolism Disturbance by Light/Dark Cycle Switching Depends on the Rat Health Status: The Role of Grape Seed Flavanols. Food Funct. 2023, 14, 6443–6454. [Google Scholar] [CrossRef]
- Kolbe, I.; Oster, H. Chronodisruption, Metabolic Homeostasis, and the Regulation of Inflammation in Adipose Tissues. Yale J. Biol. Med. 2019, 92, 317–325. [Google Scholar] [PubMed]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative Stress, Plant Natural Antioxidants, and Obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [PubMed]
- Lalanza, J.F.; Snoeren, E.M.S. The Cafeteria Diet: A Standardized Protocol and Its Effects on Behavior. Neurosci. Biobehav. Rev. 2021, 122, 92–119. [Google Scholar] [CrossRef]
- Buyukdere, Y.; Gulec, A.; Akyol, A. Cafeteria Diet Increased Adiposity in Comparison to High Fat Diet in Young Male Rats. PeerJ 2019, 7, e6656. [Google Scholar] [CrossRef]
- Carillon, J.; Romain, C.; Bardy, G.; Fouret, G.; Feillet-Coudray, C.; Gaillet, S.; Lacan, D.; Cristol, J.-P.; Rouanet, J.-M. Cafeteria Diet Induces Obesity and Insulin Resistance Associated with Oxidative Stress but Not with Inflammation: Improvement by Dietary Supplementation with a Melon Superoxide Dismutase. Free Radic. Biol. Med. 2013, 65, 254–261. [Google Scholar] [CrossRef]
- Fonken, L.K.; Workman, J.L.; Walton, J.C.; Weil, Z.M.; Morris, J.S.; Haim, A.; Nelson, R.J. Light at Night Increases Body Mass by Shifting the Time of Food Intake. Proc. Natl. Acad. Sci. USA 2010, 107, 18664–18669. [Google Scholar] [CrossRef]
- Espinoza Gallardo, A.C.; López-Espinoza, A.; Vázquez-Cisneros, L.C.; Zepeda-Salvador, A.P.; Santillano-Herrera, D. Light-Dark Cycle Inversion Effect on Food Intake and Body Weight in Rats. Nutr. Hosp. 2021, 38, 495–501. [Google Scholar] [CrossRef]
- Arble, D.M.; Ramsey, K.M.; Bass, J.; Turek, F.W. Circadian Disruption and Metabolic Disease: Findings from Animal Models. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 785–800. [Google Scholar] [CrossRef]
- Tavolaro, F.M.; Thomson, L.M.; Ross, A.W.; Morgan, P.J.; Helfer, G. Photoperiodic Effects on Seasonal Physiology, Reproductive Status and Hypothalamic Gene Expression in Young Male F344 Rats. J. Neuroendocrinol. 2015, 27, 79–87. [Google Scholar] [CrossRef]
- Ross, A.W.; Russell, L.; Helfer, G.; Thomson, L.M.; Dalby, M.J.; Morgan, P.J. Photoperiod Regulates Lean Mass Accretion, but Not Adiposity, in Growing F344 Rats Fed a High Fat Diet. PLoS ONE 2015, 10, e0119763. [Google Scholar] [CrossRef] [PubMed]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Caimari, A.; Arola, L. Cherry Consumption out of Season Alters Lipid and Glucose Homeostasis in Normoweight and Cafeteria-Fed Obese Fischer 344 Rats. J. Nutr. Biochem. 2019, 63, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Arreaza-Gil, V.; Escobar-Martínez, I.; Suárez, M.; Bravo, F.I.; Muguerza, B.; Arola-Arnal, A.; Torres-Fuentes, C. Gut Seasons: Photoperiod Effects on Fecal Microbiota in Healthy and Cafeteria-Induced Obese Fisher 344 Rats. Nutrients 2022, 14, 722. [Google Scholar] [CrossRef]
- Zhou, J.; Mao, L.; Xu, P.; Wang, Y. Effects of (−)-Epigallocatechin Gallate (EGCG) on Energy Expenditure and Microglia-Mediated Hypothalamic Inflammation in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 1681. [Google Scholar] [CrossRef] [PubMed]
- del Bas, J.M.; Guirro, M.; Boqué, N.; Cereto, A.; Ras, R.; Crescenti, A.; Caimari, A.; Canela, N.; Arola, L. Alterations in Gut Microbiota Associated with a Cafeteria Diet and the Physiological Consequences in the Host. Int. J. Obes. 2018, 42, 746–754. [Google Scholar] [CrossRef]
- Kumar, S.; Alagawadi, K.; Rao, M.R. Effect of Argyreia Speciosa Root Extract on Cafeteria Diet-Induced Obesity in Rats. Indian. J. Pharmacol. 2011, 43, 163. [Google Scholar] [CrossRef] [PubMed]
- Martín-González, M.Z.; Palacios, H.; Rodríguez, M.A.; Arola, L.; Aragonès, G.; Muguerza, B. Beneficial Effects of a Low-Dose of Conjugated Linoleic Acid on Body Weight Gain and Other Cardiometabolic Risk Factors in Cafeteria Diet-Fed Rats. Nutrients 2020, 12, 408. [Google Scholar] [CrossRef]
- de Melo, A.F.; Moreira, C.C.L.; Sales, C.F.; Rentz, T.; Raposo, H.F.; Garófalo, M.A.R.; Botion, L.M.; Kettelhut, I.d.C.; de Oliveira, H.C.F.; Chaves, V.E. Increase in Liver Cytosolic Lipases Activities and VLDL-TAG Secretion Rate Do Not Prevent the Non-Alcoholic Fatty Liver Disease in Cafeteria Diet-Fed Rats. Biochimie 2018, 150, 16–22. [Google Scholar] [CrossRef]
- Chambel, S.S.; Santos-Gonçalves, A.; Duarte, T.L. The Dual Role of Nrf2 in Nonalcoholic Fatty Liver Disease: Regulation of Antioxidant Defenses and Hepatic Lipid Metabolism. BioMed Res. Int. 2015, 2015, 597134. [Google Scholar] [CrossRef]
- Saran, A.R.; Dave, S.; Zarrinpar, A. Circadian Rhythms in the Pathogenesis and Treatment of Fatty Liver Disease. Gastroenterology 2020, 158, 1948–1966.e1. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J.; et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.M.; Colom-Pellicer, M.; Blanco, J.; Calvo, E.; Aragonès, G.; Mulero, M. Grape-Seed Procyanidin Extract (GSPE) Seasonal-Dependent Modulation of Glucose and Lipid Metabolism in the Liver of Healthy F344 Rats. Biomolecules 2022, 12, 839. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Jordan, H.; Martín-González, M.Z.; Suárez, M.; Aragonès, G.; Muguerza, B.; Rodríguez, M.A.; Bladé, C. The Disruption of Liver Metabolic Circadian Rhythms by a Cafeteria Diet Is Sex-Dependent in Fischer 344 Rats. Nutrients 2020, 12, 1085. [Google Scholar] [CrossRef]
- Budkowska, M.; Cecerska-Heryć, E.; Marcinowska, Z.; Siennicka, A.; Dołęgowska, B. The Influence of Circadian Rhythm on the Activity of Oxidative Stress Enzymes. Int. J. Mol. Sci. 2022, 23, 14275. [Google Scholar] [CrossRef]
- Early, J.O.; Menon, D.; Wyse, C.A.; Cervantes-Silva, M.P.; Zaslona, Z.; Carroll, R.G.; Palsson-McDermott, E.M.; Angiari, S.; Ryan, D.G.; Corcoran, S.E.; et al. Circadian Clock Protein BMAL1 Regulates IL-1β in Macrophages via NRF2. Proc. Natl. Acad. Sci. USA 2018, 115, E8460–E8468. [Google Scholar] [CrossRef] [PubMed]
- Hybertson, B.M.; Gao, B. Role of the Nrf2 Signaling System in Health and Disease. Clin. Genet. 2014, 86, 447–452. [Google Scholar] [CrossRef]
- Niu, L.; Shao, M.; Liu, Y.; Hu, J.; Li, R.; Xie, H.; Zhou, L.; Shi, L.; Zhang, R.; Niu, Y. Reduction of Oxidative Damages Induced by Titanium Dioxide Nanoparticles Correlates with Induction of the Nrf2 Pathway by GSPE Supplementation in Mice. Chem. Biol. Interact. 2017, 275, 133–144. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, H.; Tan, X.; Yang, D.; Lv, Z.; Jiang, H.; Lu, J.; Baiyun, R.; Zhang, Z. GSPE Reduces Lead-Induced Oxidative Stress by Activating the Nrf2 Pathway and Suppressing MiR153 and GSK-3β in Rat Kidney. Oncotarget 2017, 8, 42226–42237. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.; Chen, D.; He, J.; Zheng, P.; Luo, Y.; Yu, B.; Huang, Z. Dietary Grape Seed Proanthocyanidin Extract Supplementation Improves Antioxidant Capacity and Lipid Metabolism in Finishing Pigs. Anim. Biotechnol. 2023, 34, 1–11. [Google Scholar] [CrossRef]
- Liu, K.; Luo, M.; Wei, S. The Bioprotective Effects of Polyphenols on Metabolic Syndrome against Oxidative Stress: Evidences and Perspectives. Oxid. Med. Cell. Longev. 2019, 2019, 6713194. [Google Scholar] [CrossRef]
- Truzzi, F.; Tibaldi, C.; Zhang, Y.; Dinelli, G.; D′Amen, E. An Overview on Dietary Polyphenols and Their Biopharmaceutical Classification System (Bcs). Int. J. Mol. Sci. 2021, 22, 5514. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Latre, A.; Del Bas, J.M.; Baselga-Escudero, L.; Casanova, E.; Arola-Arnal, A.; Salvadó, M.J.; Bladé, C.; Arola, L. Dietary Proanthocyanidins Modulate the Rhythm of BMAL1 Expression and Induce RORα Transactivation in HepG2 Cells. J. Funct. Foods 2015, 13, 336–344. [Google Scholar] [CrossRef]
- Rodríguez, R.M.; Cortés-Espinar, A.J.; Soliz-Rueda, J.R.; Feillet-Coudray, C.; Casas, F.; Colom-Pellicer, M.; Aragonès, G.; Avila-Román, J.; Muguerza, B.; Mulero, M.; et al. Time-of-Day Circadian Modulation of Grape-Seed Procyanidin Extract (GSPE) in Hepatic Mitochondrial Dynamics in Cafeteria-Diet-Induced Obese Rats. Nutrients 2022, 14, 774. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Pan, X.; Wei, G.; Hua, Y. Research Progress of Glutathione Peroxidase Family (GPX) in Redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef] [PubMed]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of Inflammation by the Antioxidant Haem Oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Costa Silva, R.C.M.; Correa, L.H.T. Heme Oxygenase 1 in Vertebrates: Friend and Foe. Cell Biochem. Biophys. 2022, 80, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Milani, P.; Gagliardi, S.; Cova, E.; Cereda, C. SOD1 Transcriptional and Posttranscriptional Regulation and Its Potential Implications in ALS. Neurol. Res. Int. 2011, 2011, 458427. [Google Scholar] [CrossRef]
- Toivonen, J.M.; Manzano, R.; Oliván, S.; Zaragoza, P.; García-Redondo, A.; Osta, R. MicroRNA-206: A Potential Circulating Biomarker Candidate for Amyotrophic Lateral Sclerosis. PLoS ONE 2014, 9, e89065. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Suarez, M.; Torres, J.L.; Salvado, M.J.; Arola, L.; Arola-Arnal, A. Resveratrol and EGCG Bind Directly and Distinctively to MiR-33a and MiR-122 and Modulate Divergently Their Levels in Hepatic Cells. Nucleic Acids Res. 2014, 42, 882–892. [Google Scholar] [CrossRef]
- Baselga-Escudero, L.; Blade, C.; Ribas-Latre, A.; Casanova, E.; Salvadó, M.-J.; Arola, L.; Arola-Arnal, A. Chronic Supplementation of Proanthocyanidins Reduces Postprandial Lipemia and Liver MiR-33a and MiR-122 Levels in a Dose-Dependent Manner in Healthy Rats. J. Nutr. Biochem. 2014, 25, 151–156. [Google Scholar] [CrossRef]
- Arola-Arnal, A.; Bladé, C. Proanthocyanidins Modulate MicroRNA Expression in Human HepG2 Cells. PLoS ONE 2011, 6, e25982. [Google Scholar] [CrossRef]
- Manocchio, F.; Soliz-Rueda, J.R.; Ribas-Latre, A.; Bravo, F.I.; Arola-Arnal, A.; Suarez, M.; Muguerza, B. Grape Seed Proanthocyanidins Modulate the Hepatic Molecular Clock via MicroRNAs. Mol. Nutr. Food Res. 2022, 66, e2200443. [Google Scholar] [CrossRef] [PubMed]
- Egnatchik, R.A.; Leamy, A.K.; Jacobson, D.A.; Shiota, M.; Young, J.D. ER Calcium Release Promotes Mitochondrial Dysfunction and Hepatic Cell Lipotoxicity in Response to Palmitate Overload. Mol. Metab. 2014, 3, 544–553. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Ory, D.S.; Schaffer, J.E. Palmitate-Induced Apoptosis Can Occur through a Ceramide-Independent Pathway. J. Biol. Chem. 2001, 276, 14890–14895. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhou, X.; Sun, Y.; Hu, L.; Zhu, J.; Shao, C.; Meng, Q.; Shan, A. Threonine, but Not Lysine and Methionine, Reduces Fat Accumulation by Regulating Lipid Metabolism in Obese Mice. J. Agric. Food Chem. 2020, 68, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qian, D.; Wang, Z.; Sun, Y.; Sun, B.; Zhou, X.; Hu, L.; Shan, A.; Ma, Q. Threonine Supplementation Prevents the Development of Fat Deposition in Mice Fed a High-Fat Diet. Food Funct. 2022, 13, 7772–7780. [Google Scholar] [CrossRef] [PubMed]
- Arreaza-Gil, V.; Escobar-Martínez, I.; Mulero, M.; Muguerza, B.; Suárez, M.; Arola-Arnal, A.; Torres-Fuentes, C. Gut Microbiota Influences the Photoperiod Effects on Proanthocyanidins Bioavailability in Diet-Induced Obese Rats. Mol. Nutr. Food Res. 2023, 67, 2200600. [Google Scholar] [CrossRef]
- Mariné-Casadó, R.; Domenech-Coca, C.; del Bas, J.M.; Bladé, C.; Arola, L.; Caimari, A. Intake of an Obesogenic Cafeteria Diet Affects Body Weight, Feeding Behavior, and Glucose and Lipid Metabolism in a Photoperiod-Dependent Manner in F344 Rats. Front. Physiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Gibert-Ramos, A.; Crescenti, A.; Salvadó, M. Consumption of Cherry out of Season Changes White Adipose Tissue Gene Expression and Morphology to a Phenotype Prone to Fat Accumulation. Nutrients 2018, 10, 1102. [Google Scholar] [CrossRef]
- Gibert-Ramos, A.; Ibars, M.; Salvadó, M.J.; Crescenti, A. Response to the Photoperiod in the White and Brown Adipose Tissues of Fischer 344 Rats Fed a Standard or Cafeteria Diet. J. Nutr. Biochem. 2019, 70, 82–90. [Google Scholar] [CrossRef]
- Aragonès, G.; Suárez, M.; Ardid-Ruiz, A.; Vinaixa, M.; Rodríguez, M.A.; Correig, X.; Arola, L.; Bladé, C. Dietary Proanthocyanidins Boost Hepatic NAD + Metabolism and SIRT1 Expression and Activity in a Dose-Dependent Manner in Healthy Rats. Sci. Rep. 2016, 6, 24977. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose Translation from Animal to Human Studies Revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Smedes, F.; Thomasen, T.K. Evaluation of the Bligh & Dyer Lipid Determination Method. Mar. Pollut. Bull. 1996, 32, 681–688. [Google Scholar] [CrossRef]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. Web-Based Inference of Biological Patterns, Functions and Pathways from Metabolomic Data Using MetaboAnalyst. Nat. Protoc. 2011, 6, 743–760. [Google Scholar] [CrossRef]


| Parameter | L6 | L6 ANOVA | L18 | L18 ANOVA | 3-Way ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| STD-VH | STD-GSPE | CAF-VH | CAF-GSPE | STD-VH | STD-GSPE | CAF-VH | CAF-GSPE | ||||
| Liver weight (g) | 12.36 ± 0.61 | 12.49 ± 0.59 | 15.29 ± 0.35 ++ | 14.5 ± 0.54 + | D | 13.08 ± 0.59 | 13.22 ± 0.95 | 14.92 ± 0.68 + | 15.1 ± 0.68 $ | D | D |
| Total lipid content (mg lipids/g liver) | 33.71 ± 1.47 | 29.07 ± 4.28 | 74.15 ± 13.15 +++ | 42.15 ± 5.65 ** | D, T, Tendency in D×T | 32.87 ± 3.23 | 31.87 ± 4.9 | 44.41 ± 5.23 && | 55.83 ± 7.57 + | D | D, T×P, D×T×P |
| Cholesterol (µM) | 22.00 ± 3.00 | 23.00 ± 2.00 | 31.00 ± 1.00 + | 33.00 ± 3.00 + | D | 24.00 ± 2.00 | 24.00 ± 2.00 | 34.00 ± 4.00 + | 32.00 ± 4.00 $ | D | D |
| Triglycerides (µM) | 70.00 ± 9.00 | 57.00 ± 8.00 | 65.00 ± 5.00 | 62.00 ± 6.00 | ns | 61.00 ± 6.00 | 55.00 ± 7.00 | 82.00 ± 11.00 $ | 86.00 ± 8.00 ++& | D | D, D×P |
| Phospholipids (µM) | 29.00 ± 1.00 | 25.00 ± 3.00 | 50.00 ± 2.00 ++ | 50.00 ± 5.00 +++ | D | 41.00 ± 7.00 % | 41.00 ± 6.00 & | 52.00 ± 5.00 $ | 52.00 ± 2.00 $ | Tendencyin D | D, P, Tendency in D×P |
| Gene | Accession Number (NCBI) | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) |
|---|---|---|---|
| Nrf2 | NM_001399173.1 | ACATTTCAGTCGCTTGCCCT | TCCTGCCAAACTTGCTCCAT |
| Keap1 | NM_057152.2 | TGGGCGTGGCAGTGCTCAAC | GCCCATCGTAGCCTCCTGCG |
| Sod1 | NM_017050.1 | GGTGGTCCACGAGAAACAAG | CAATCACACCACAAGCCAAG |
| Sod2 | NM_017051.2 | AAGGAGCAAGGTCGCTTACA | ACACATCAATCCCCAGCAGT |
| GPx1 | NM_030826.4 | TGCAATCAGTTCGGACATC | CACCTCGCACTTCTCAAACA |
| GSR | NM_053906.2 | ATCAAGGAGAAGCGGGATG | GCGTAGCCGTGGATGACT |
| Nqo1 | NM_017000.3 | GGGGACATGAACGTCATTCTCT | AGTGGTGACTCCTCCCAGACAG |
| Sesn2 Ppia | NM_001109358.2 NM_017101.1 | TACCTTAGCAGCTTCTGGCG TCAAACACAAATGGTTCCCAGT | AGGTAAGAACACTGGTGGCG ATTCCTGGACCCAAAACGCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés-Espinar, A.J.; Ibarz-Blanch, N.; Soliz-Rueda, J.R.; Calvo, E.; Bravo, F.I.; Mulero, M.; Ávila-Román, J. Abrupt Photoperiod Changes Differentially Modulate Hepatic Antioxidant Response in Healthy and Obese Rats: Effects of Grape Seed Proanthocyanidin Extract (GSPE). Int. J. Mol. Sci. 2023, 24, 17057. https://doi.org/10.3390/ijms242317057
Cortés-Espinar AJ, Ibarz-Blanch N, Soliz-Rueda JR, Calvo E, Bravo FI, Mulero M, Ávila-Román J. Abrupt Photoperiod Changes Differentially Modulate Hepatic Antioxidant Response in Healthy and Obese Rats: Effects of Grape Seed Proanthocyanidin Extract (GSPE). International Journal of Molecular Sciences. 2023; 24(23):17057. https://doi.org/10.3390/ijms242317057
Chicago/Turabian StyleCortés-Espinar, Antonio J., Néstor Ibarz-Blanch, Jorge R. Soliz-Rueda, Enrique Calvo, Francisca Isabel Bravo, Miquel Mulero, and Javier Ávila-Román. 2023. "Abrupt Photoperiod Changes Differentially Modulate Hepatic Antioxidant Response in Healthy and Obese Rats: Effects of Grape Seed Proanthocyanidin Extract (GSPE)" International Journal of Molecular Sciences 24, no. 23: 17057. https://doi.org/10.3390/ijms242317057
APA StyleCortés-Espinar, A. J., Ibarz-Blanch, N., Soliz-Rueda, J. R., Calvo, E., Bravo, F. I., Mulero, M., & Ávila-Román, J. (2023). Abrupt Photoperiod Changes Differentially Modulate Hepatic Antioxidant Response in Healthy and Obese Rats: Effects of Grape Seed Proanthocyanidin Extract (GSPE). International Journal of Molecular Sciences, 24(23), 17057. https://doi.org/10.3390/ijms242317057











