Metabolites-Based Network Pharmacology to Preliminarily Verify In Vitro Anti-Inflammatory Effect of Ardisiacrispin B
Abstract
:1. Introduction
2. Results
2.1. Analysis of Metabolites in Plasma after Administration of Ardisiacrispin B
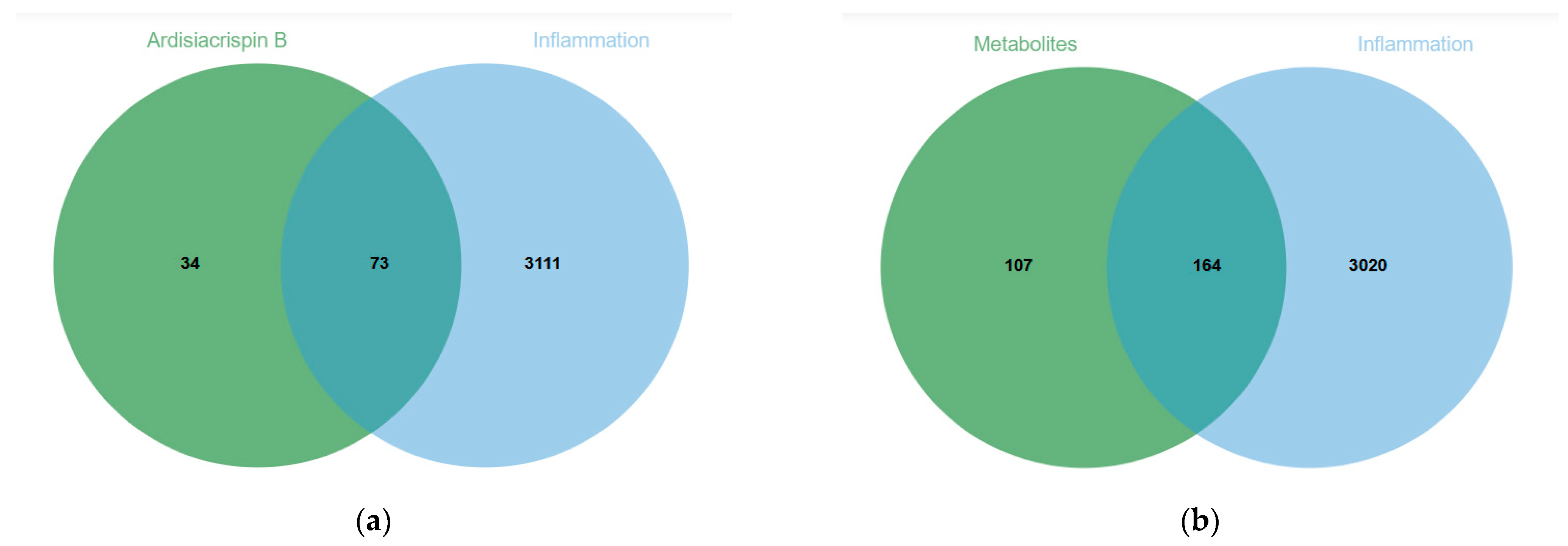
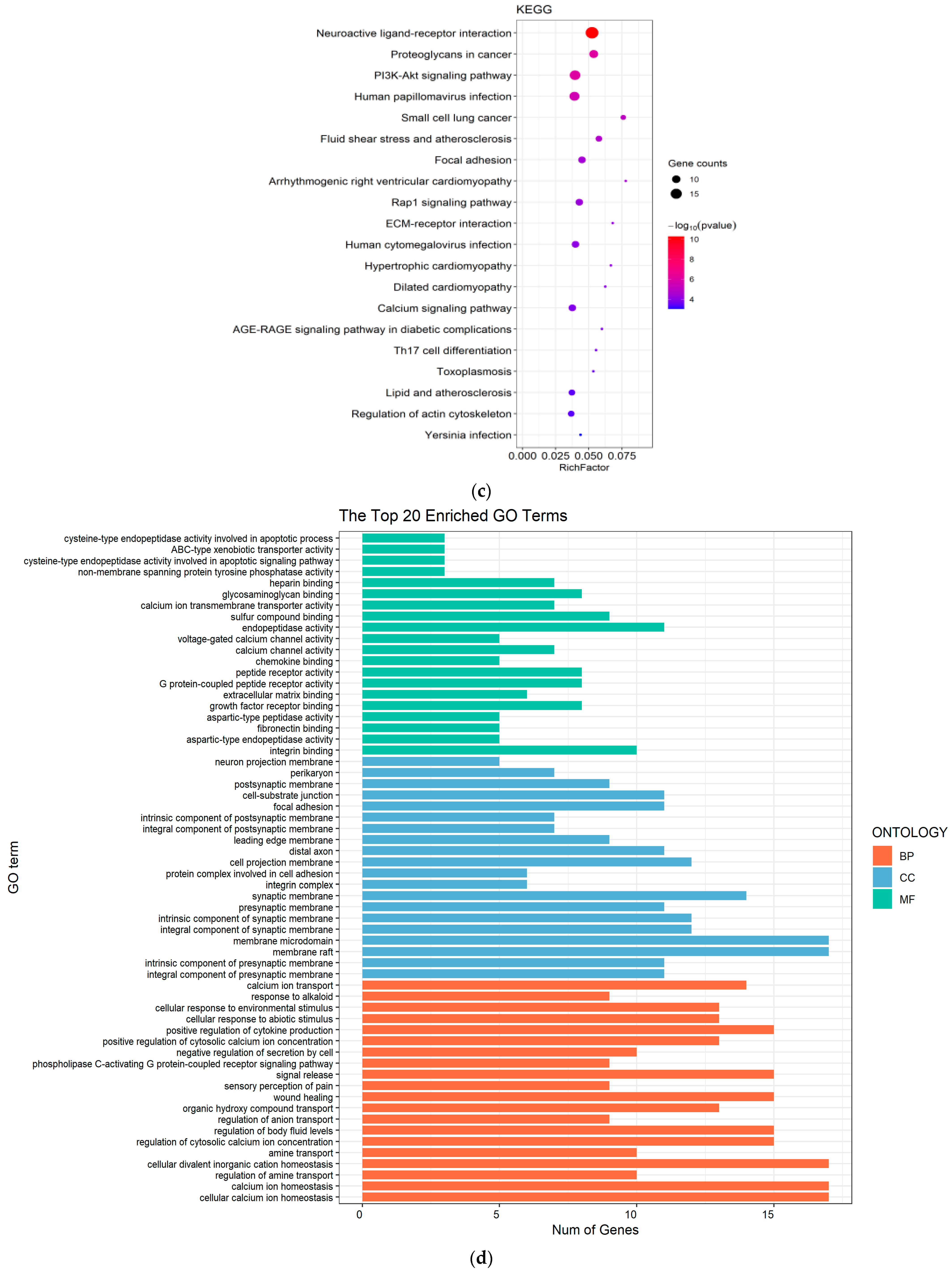
2.2. Molecular-Mechanism Analysis of the Anti-Inflammatory Effect of Ardisiacrispin B and Its Metabolites
2.3. Anti-Inflammatory Effect
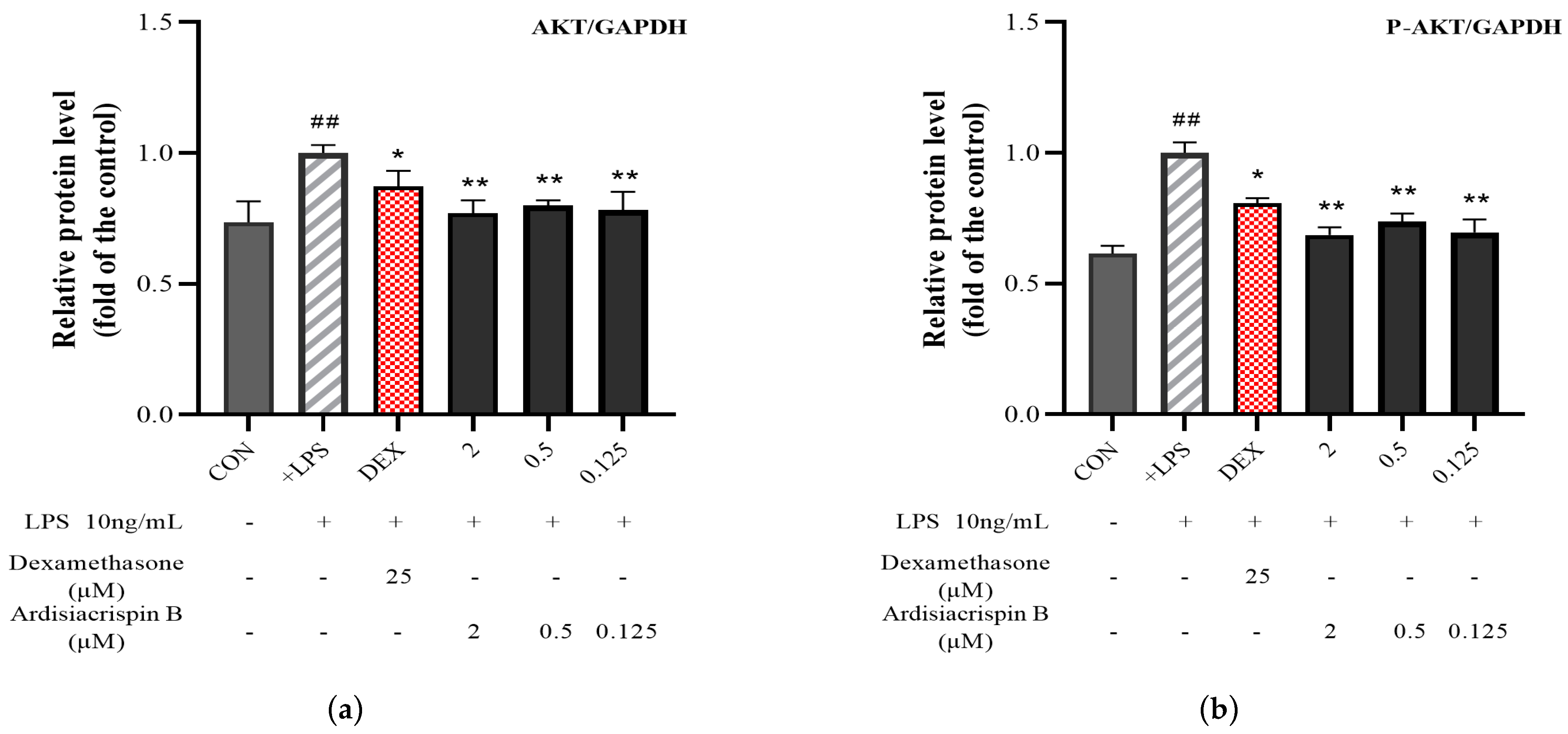
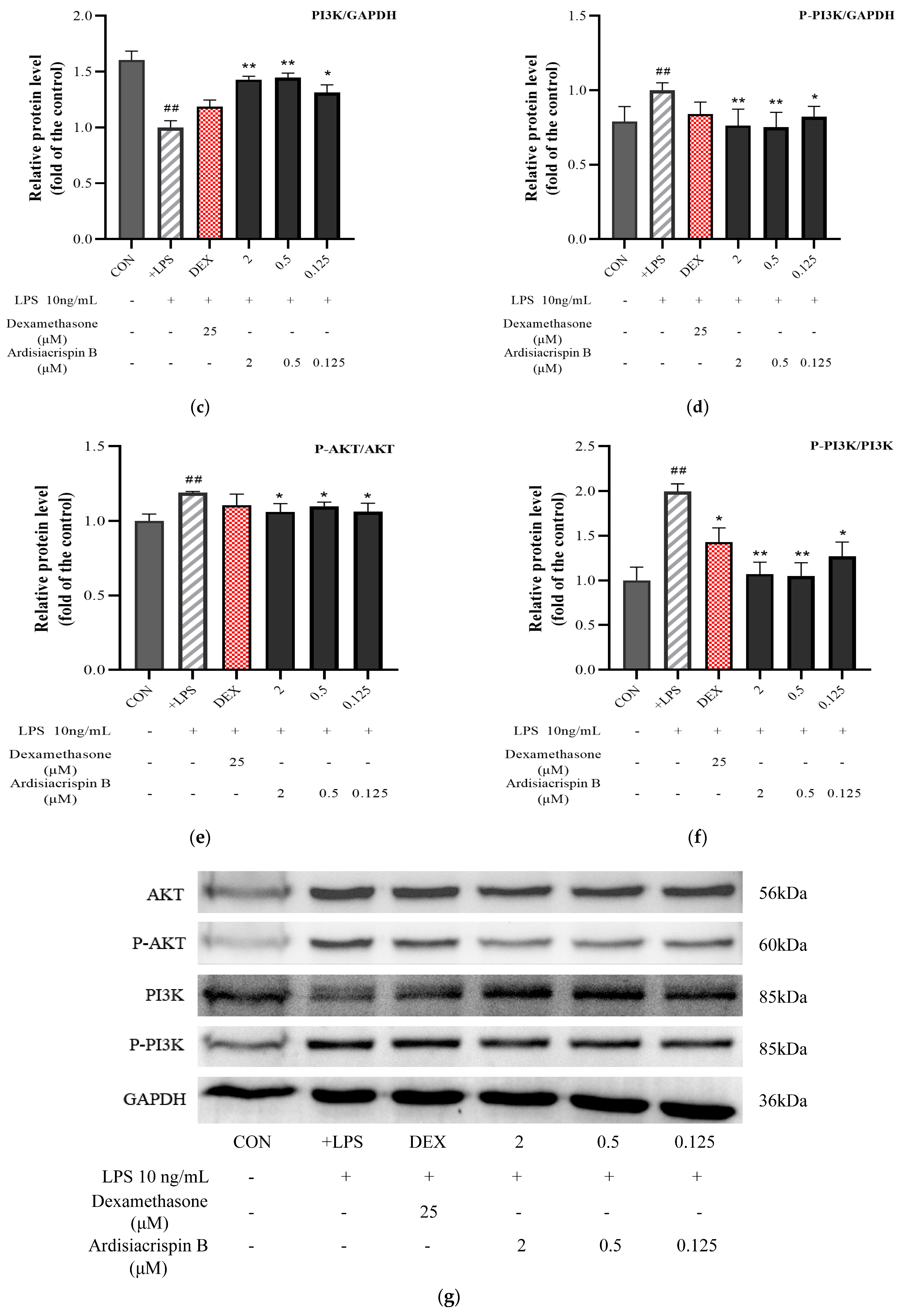
2.4. Protein Expressions of AKT, P-AKT, PI3K, and P-PI3K
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Cells
4.3. Isolation of Ardisiacrispin B
4.4. Plasma-Metabolite Research
4.4.1. Preparation of Plasma-Containing Drugs
4.4.2. Instrument Conditions
4.5. Network Pharmacology
4.6. Anti-Inflammatory Mechanisms
4.6.1. Determination of Cell Viability
4.6.2. Detection of the Contents of NO, TNF-α, and IL-1β in Cells
4.6.3. Western Blot
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tao, Q.; Li, J. Progress on the research of inflammation and disease. J. Tradit. Chin. Vet. Med. 2023, 42, 34–43. [Google Scholar]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Wu, G.; Li, L.; Gan, J. Study on chemical constituents and anti-inflammatory activities of sesterterpenoids of Dactylospongia elegans from the South China Sea. Nat. Prod. Res. Dev. 2023, 35, 1357–1363. [Google Scholar]
- Jing, B.; Liu, M.; Yang, L.; Cai, H.Y.; Chen, J.B.; Li, Z.X.; Kou, X.; Wu, Y.Z.; Qin, D.J.; Zhou, L.; et al. Characterization of naturally occurring pentacyclic triterpenes as novel inhibitors of deubiquitinating protease USP7 with anticancer activity in vitro. Acta Pharmacol. Sin. 2018, 39, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Xiao, J.; Xiao, S.; Cui, P.; Liu, X. Progress in the study of natural pentacyclic triterpenoids with antibacterial activity. Chin. Tradit. Pat. Med. 2023, 45, 1231–1240. [Google Scholar]
- Xiao, S.; Wang, H.; Wang, Q.; Han, X.; Xu, R.; Meng, K.; Tian, Z.; Zhang, L.; Zhou, D. Recent progresses on pentacyclic triterpene-based antiviral agents. Sin. Chim. 2015, 45, 865–883. [Google Scholar]
- Zhou, W.; Huang, Y.; Hu, X.; Yuan, J.; Song, Z.; Liu, B.; Li, L. Chemical constituents of triterpenoids from Sabia Discolor Dunnandwith Hypoglycemic Activity. J. Nanning Norm. Univ. (Nat. Sci. Ed.) 2023, 40, 73–81. [Google Scholar]
- Ao, Y.; Feng, C.; Wu, Y.; Wu, J.; Wu, J. Protective effect of total triterpenoids from Ganderma lucidium on acetaminophen induced liver injury in rats. Strait Pharm. J. 2022, 34, 8–12. [Google Scholar]
- Sheng, H.; Sun, H. Synthesis, biology and clinical significance of pentacyclic triterpenes: A multi-target approach to prevention and treatment of metabolic and vascular diseases. Nat. Prod. Rep. 2011, 28, 543–593. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef]
- Zhang, B.W.; Xing, Y.; Wen, C.; Yu, X.X.; Sun, W.L.; Xiu, Z.L.; Dong, Y.S. Pentacyclic triterpenes as α-glucosidase and α-amylase inhibitors: Structure-activity relationships and the synergism with acarbose. Bioorg. Med. Chem. Lett. 2017, 27, 5065–5070. [Google Scholar] [CrossRef] [PubMed]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China 2020: Part One; China Medical Science Press: Beijing, China, 2020; p. 143. [Google Scholar]
- Qiu, D. Chinese Herbal Medicine Miao Medicine Volume; Guizhou Science and Technology Publishing House Co., Ltd.: Guiyang, China, 2005; pp. 267–269. [Google Scholar]
- Zhou, W. Study on the Pharmacodynamic Material Basis and Mechanism of the Anti Acute Lung Injury Effect of Chinese Medicine Ardisia crenata Sims in Rats. Ph.D. Thesis, Guizhou Medical University, Guiyang, China, 2023. [Google Scholar]
- Li, Y.; Xia, B.; Long, Q.; Zhang, G.; Zha, J.; Wang, A.; Wang, Y. Determination of the content of the main active components of Ardisiae Crenatae Radix. Lishizhen Med. Mater. Med. Res. 2011, 22, 1929–1931. [Google Scholar]
- Shen, Y.; Chen, J.; Xu, J.; Guan, B.; Luo, Z. Effect of Bergenin on the expression of IL-6, TNF-α and NF-κB in RAW264.7 cells induced by lipopolysaccharide. J. Chin. Med. Mater. 2012, 35, 1660–1662. [Google Scholar]
- Arfan, M.; Amin, H.; Khan, N.; Khan, I.; Saeed, M.; Khan, M.A.; Fazal-ur-Rehman. Analgesic and anti-inflammatory activities of 11-O-galloylbergenin. J. Ethnopharmacol. 2010, 131, 502–504. [Google Scholar] [CrossRef] [PubMed]
- Mbaveng, A.T.; Ndontsa, B.L.; Kuete, V.; Nguekeu, Y.M.M.; Çelik, İ.; Mbouangouere, R.; Tane, P.; Efferth, T. A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine 2018, 43, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Kong, W.L.; Tu, P.F. Chemical and pharmacological advances of study on Lysimachia. Chin. J. Chin. Mater. Med. 2004, 29, 295–298. [Google Scholar]
- Huang, X.; Yang, R. Progress in the triterpenoids from the Genus Lysimachia L. J. Trop. Subtrop. Bot. 2007, 15, 175–182. [Google Scholar]
- Ni, M.; Han, L. Chemical constituents of Ardisia crenata. Chin. J. Chin. Mater. Med. 1988, 13, 33–34; discussion 59. [Google Scholar]
- Liu, D. Studies on the Bioactive Constituents of Ardisia crenata Sims and Bulbophillum odoratissimum Lindl. Ph.D. Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2004. [Google Scholar]
- Cai, J. Study on Chemical Constituents and Antitumor Activity of Ardisia crenata Root Ardisia crenata Sims and Cajanus cajan Leaf. Ph.D. Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2012. [Google Scholar]
- Maotian, W.; Xiongtai, G.; Xiuwen, H.; Shanhai, H. A new triterpenoid saponin from Ardisia crenata. Planta Med. 1992, 58, 205–207. [Google Scholar] [CrossRef]
- Jia, Z.; Koike, K.; Nikaido, T.; Ohmoto, T. Two novel triterpenoid pentasaccharides with an unusual glycosyl glycerol side chain from Ardisia crenata. Tetrahedron 1994, 50, 11853–11864. [Google Scholar] [CrossRef]
- Jia, Z.; Koike, K.; Ohmoto, T.; Ni, M. Triterpenoid saponins from Ardisia crenata. Phytochemistry 1994, 37, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Koike, K.; Nikaido, T.; Ohmoto, T.; Ni, M. Triterpenoid saponins from Ardisia crenata and their inhibitory activity on cAMP phosphodiesterase. Chem. Pharm. Bull. 1994, 42, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.F.; Xu, J.F.; Feng, Z.M.; Zhang, P.C. Cytotoxic triterpenoid saponins from the roots of Ardisia crenata. J. Asian Nat. Prod. Res. 2008, 10, 833–839. [Google Scholar] [CrossRef]
- Song, N.N.; Yang, L.M.; Zhang, M.J.; An, R.F.; Liu, W.; Huang, X.F. Triterpenoid saponins and phenylpropanoid glycoside from the roots of Ardisia crenata and their cytotoxic activities. Chin. J. Nat. Med. 2021, 19, 63–69. [Google Scholar] [CrossRef]
- Ye, H.; Zhou, Y.; Liao, Z.; Yin, X.; Wei, X.; Lei, C.; Zhou, Y. Isolation and identification of triterpenoids from the roots of Ardisia crenata var. bicolor. J. Chin. Med. Mater. 2022, 45, 346–350. [Google Scholar]
- Han, L.; Ni, M. Chemical constituents of Ardisia crenata. Chin. J. Chin. Mater. Med. 1989, 14, 33–35; discussion 58–59. [Google Scholar]
- Li, X.; Shi, H.; Ding, J.; Feng, T.; Liu, X.; Liu, C.; Zhou, Y. Analysis of chemical constituents as flavonoids and coumarins in Radix Ardisiae from different sources. Chin. Pharm. 2021, 32, 443–452. [Google Scholar]
- Qi, X. Study on the Chmistry Constituents and Bioactivity of Ardisia crenata Sims and Pholidota cantonensis Rolfe. Master’s Thesis, Henan University, Zhengzhou, China, 2012. [Google Scholar]
- Zhao, O.; Du, Y.; Ban, D.; Wei, W. Study on bioactive components of Ardisia crispa (Thunb.) DC. of Miao Nationality Herba (Ⅰ). Hubei Agric. Sci. 2013, 52, 4723–4725, 4747. [Google Scholar]
- Chen, S.; Hu, W.; Huang, Y.; Jiang, X.; Wang, Z. Preliminary analysis on chemical compositions of Ardisia crenata. Anhui Agric. Sci. Bull. 2007, 13, 152–153. [Google Scholar]
- Zhen, H. Separation of saponins from Ardisia crenata by RP-HPLC. Chin. Tradit. Herb. Drugs 2009, 40, 898–900. [Google Scholar]
- Yang, S.; Yu, Z.; Wang, L.; Yuan, T.; Wang, X.; Zhang, X.; Wang, J.; Lv, Y.; Du, G. The natural product bergenin ameliorates lipopolysaccharide-induced acute lung injury by inhibiting NF-kappaB activition. J. Ethnopharmacol. 2017, 200, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Jansakul, C.; Baumann, H.; Kenne, L.; Samuelsson, G. Ardisiacrispin A and B, two utero-contracting saponins from Ardisia crispa. Planta Med. 1987, 53, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y. Microbial Transformation of Ardisiacrispin A and B. Master’s Thesis, Shenyang Pharmaceutical University, Shenyang, China, 2007. [Google Scholar]
- Han, M.; Sha, X.; Wu, Y.; Fang, X. Oral absorption of ginsenoside Rb1 using in vitro and in vivo models. Planta Med. 2006, 72, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.J.; Zhang, W.D.; Zhang, C.; Liu, R.H.; Shen, Y.H.; Li, H.L.; Wang, X.L.; Wang, X.W.; Zhu, J.B.; Chen, C.L. Quantitative determination of the anticancer agent tubeimoside I in rat plasma by liquid chromatography coupled with mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007, 845, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xiong, Y. Advances in studies on absorption characteristics of pentacyclic triterpenes. Chin. Tradit. Herb. Drugs 2009, 40, 1505–1508. [Google Scholar]
- Ban, Y.; Chen, R.; Zhang, Y.; Zhang, L.; Liu, W.; Zhu, G.; Tang, L. Advances in studies on oral absorption and metabolism of pentacyclic triterpenoids. Chem. Reag. 2021, 43, 906–916. [Google Scholar]
- Hattori, M.; Sakamoto, T.; Kobashi, K.; Namba, T. Metabolism of glycyrrhizin by human intestinal flora. Planta Med. 1983, 48, 38–42. [Google Scholar] [CrossRef]
- Paek, I.B.; Moon, Y.; Kim, J.; Ji, H.Y.; Kim, S.A.; Sohn, D.H.; Kim, J.B.; Lee, H.S. Pharmacokinetics of a ginseng saponin metabolite compound K in rats. Biopharm. Drug Dispos. 2006, 27, 39–45. [Google Scholar] [CrossRef]
- Zhou, W.; Feng, M.Q.; Li, J.Y.; Zhou, P. Studies on the preparation, crystal structure and bioactivity of ginsenoside compound K. J. Asian Nat. Prod. Res. 2006, 8, 519–527. [Google Scholar] [CrossRef]
- Choi, K.; Choi, C. Proapoptotic ginsenosides compound K and Rh enhance Fas-induced cell death of human astrocytoma cells through distinct apoptotic signaling pathways. Cancer Res. Treat. 2009, 41, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gao, H.; Feng, X.; Bi, C.; Zhang, J.; Yin, J. Ginsenoside Rh2 impedes proliferation and migration and induces apoptosis by regulating NF-κB, MAPK, and PI3K/Akt/mTOR signaling pathways in osteosarcoma cells. J. Biochem. Mol. Toxicol. 2020, 34, e22597. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Wan, J.Y.; Zeng, J.; Huang, W.H.; Sava-Segal, C.; Li, L.; Niu, X.; Wang, Q.; Wang, C.Z.; Yuan, C.S. Effects of compound K, an enteric microbiome metabolite of ginseng, in the treatment of inflammation associated colon cancer. Oncol. Lett. 2018, 15, 8339–8348. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Martinez, M.; Cabail, M.Z. The PI3K/Akt Pathway in Meta-Inflammation. Int. J. Mol. Sci. 2022, 23, 15330. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, K.; Cai, X.; Yang, B.; He, Q.; Wang, J.; Weng, Q. Targeting PI3K/AKT signaling for treatment of idiopathic pulmonary fibrosis. Acta Pharm. Sin. B 2022, 12, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Wu, Y.; He, P.; Fan, Y.; Zhong, X.; Zheng, H.; Luo, T. PI3K/AKT/mTOR-Targeted Therapy for Breast Cancer. Cells 2022, 11, 2508. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Xiao, X.; Li, S.; Lyu, X.; Gong, H.; Tan, S.; Dong, L.; Sanders, Y.Y.; Zhang, X. Aspirin alleviates pulmonary fibrosis through PI3K/AKT/mTOR-mediated autophagy pathway. Exp. Gerontol. 2023, 172, 112085. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Luo, T.; Shi, H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. Mol. Biomed. 2022, 3, 47. [Google Scholar] [CrossRef]
- Duan, H.; Li, M.; Liu, J.; Sun, J.; Wu, C.; Chen, Y.; Guo, X.; Liu, X. An Integrated Approach Based on Network Analysis Combined with Experimental Verification Reveals PI3K/Akt/Nrf2 Signaling Is an Important Way for the Anti-Myocardial Ischemia Activity of Yi-Qi-Tong-Luo Capsule. Front. Pharmacol. 2022, 13, 794528. [Google Scholar] [CrossRef]
- Meng, L.Q.; Yang, F.Y.; Wang, M.S.; Shi, B.K.; Chen, D.X.; Chen, D.; Zhou, Q.; He, Q.B.; Ma, L.X.; Cheng, W.L.; et al. Quercetin protects against chronic prostatitis in rat model through NF-κB and MAPK signaling pathways. Prostate 2018, 78, 790–800. [Google Scholar] [CrossRef]
- Li, M.; Lu, G.; Hu, J.; Shen, X.; Ju, J.; Gao, Y.; Qu, L.; Xia, Y.; Chen, Y.; Bai, Y. EVA1A/TMEM166 Regulates Embryonic Neurogenesis by Autophagy. Stem Cell Rep. 2016, 6, 396–410. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Pan, Y.; Yu, J.; Wang, B.; Dai, G.; Ying, X. Decursin alleviates the aggravation of osteoarthritis via inhibiting PI3K-Akt and NF-kB signal pathway. Int. Immunopharmacol. 2021, 97, 107657. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Ding, S.; Zhang, X.; Di, W.; Wang, X.; Zhang, H.; Chen, Y.; Zhang, Y.; Hu, Y. To Investigate the Occurrence and Development of Colorectal Cancer Based on the PI3K/AKT/mTOR Signaling Pathway. Front. Biosci. (Landmark Ed.) 2023, 28, 37. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Z.; Zhu, Y. Progress in mechanisms research of lipoplysaccharide-induced acute lung injury. Chin. Med. Pharm. 2011, 1, 47–49. [Google Scholar]
- Lu, L.; Zhang, L. Development on pathogenesis of lipopolysaccharide-induced acute lung injury. Med. Recapitul. 2010, 16, 170–173. [Google Scholar]
- Song, Z. Research progress of dexamethasone in the mechanism of lung protection in acute lung injury. J. Mod. Med. Health 2017, 33, 2143–2146. [Google Scholar]
- Meduri, G.U.; Golden, E.; Freire, A.X.; Taylor, E.; Zaman, M.; Carson, S.J.; Gibson, M.; Umberger, R. Methylprednisolone infusion in early severe ARDS: Results of a randomized controlled trial. Chest 2007, 131, 954–963. [Google Scholar] [CrossRef]
- Zha, J. Study on the Quality Evaluation of Hmong Medicine Ardisia crenata Sims. Master’s Thesis, Guiyang Medical College, Guiyang, China, 2012. [Google Scholar]
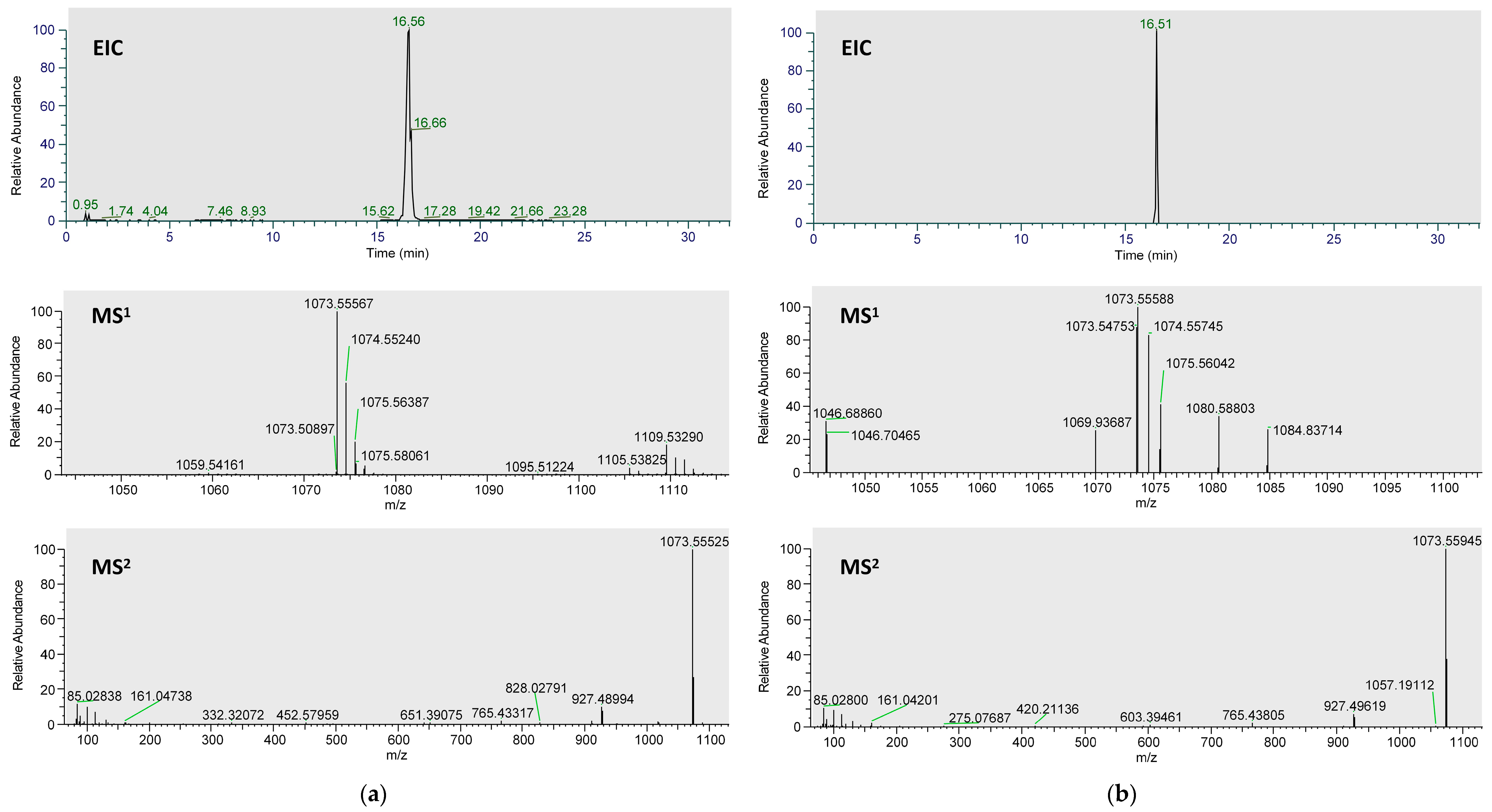
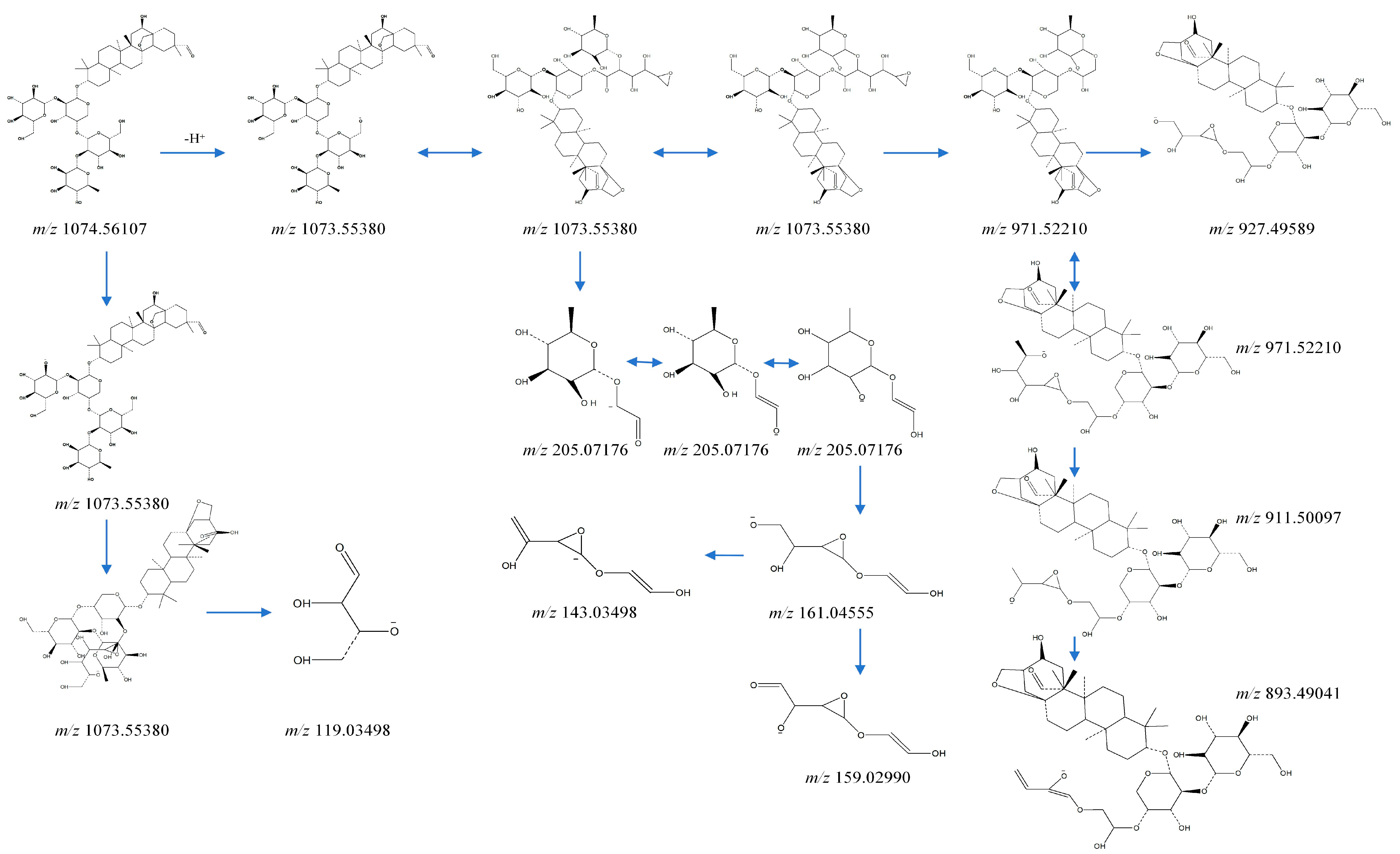



| Name | Formula | RT | MS 1 | ppm | MS 2 | Area | Metabolic Type |
|---|---|---|---|---|---|---|---|
| Ardisiac rispin B (M) | C53H86O22 | 16.49 | [M−H]− 1073.5550 | 1.21 | 119.0323, 143.0320, 159.0268, 161.0420, 205.0695, 893.4904,911.4976, 927.4962, 971.5221 | 1.92 × 105 | Prototype |
| M1 | C6H12O6 | 0.91 | [M+Na]+ 203.0516 | −4.92 | 68.0204, 85.5360, 120.4583, 134.0188, 143.0314 | 2.68 × 107 | Oxidation |
| M2 | C6H12O7 | 0.94 | [M−H]− 195.0501 | −4.61 | 75.0075, 129.0175, 160.8396 | 6.43 × 106 | Oxidation, oxidation |
| M3 | C12H22O11 | 1.04 | [M+Na]+ 365.1044 | −2.74 | 185.0415, 203.0537 | 5.78 × 105 | Oxidation, glucoside conjugation |
| M4 | C6H10O10S | 1.89 | [M+H]+ 275.0077 | 3.64 | 156.9802, 174.9911, 193.0011, 256.9966 | 7.06 × 106 | Oxidation, sulfation |
| M5 | C6H12O9S | 2.04 | [M+H]+ 261.0283 | 3.45 | 138.9711, 146.9967, 156.9816, 174.9911, 193.0011, 235.0140 | 2.98 × 106 | Oxidation, sulfation |
| M6 | C6H10O2 | 2.51 | [M+NH4]+ 132.1015 | −3.03 | 69.0703, 86.0972, 104.9639 | 3.51 × 107 | Dehydration, nitro reduction |
| M7 | C12H20O7 | 3.05 | [M+NH4]+ 294.1538 | −3.06 | 97.0292, 127.0338, 132.1025, 230.1390, 258.1338, 276.1437 | 1.22 × 106 | Dehydration, nitro reduction |
| M8 | C6H10O5 | 6.68 | [M+FA−H]− 207.0514 | 1.93 | 96.9587, 127.8675, 159.8575, 162.8349 | 1.71 × 105 | Dealkylation |
| M9 | C6H10O4 | 6.86 | [M+Na]+ 169.0475 | 2.37 | 56.9656, 84.9605, 108.9596 | 1.35 × 105 | Dehydration |
| M10 | C11H20N2O5 | 7.62 | [M+H]+ 261.1452 | 3.06 | 86.0971, 132.1014, 198.1128, 244.1171 | 6.36 × 106 | Dehydration, ornithine conjugation |
| M11 | C8H15NO4 | 8.15 | [M−H]− 188.0922 | −3.19 | 116.0705, 141.8659, 159.8768 | 7.66 × 105 | Nitro reduction, Glycine conjugation |
| M12 | C55H84O22 | 16.55 | [M+H]+ 1097.5559 | 2.92 | 275.0751, 421.1329, 643.2072, 951.4934 | 1.84 × 106 | Dehydration, acetylation |
| M13 | C30H48O4 | 18.19 | [M+Na]+ 495.3459 | 3.03 | 80.9486, 184.0734, 495.3459 | 9.16 × 105 | Dealkylation |
| M14 | C30H44O2 | 18.20 | [M+H]+ 437.3426 | 2.74 | 95.0862, 107.0856, 119.0856, 407.3309, 419.3309 | 1.51 × 105 | Dehydration, dehydration |
| M15 | C30H46O3 | 18.20 | [M+H]+ 455.3540 | 4.39 | 95.0861, 119.0854, 145.1015, 187.1483, 437.3434 | 1.47 × 105 | Dehydration |
| M16 | C12H20O12 | 18.56 | [M+Na]+ 379.0838 | −2.11 | 149.0223, 222.9697, 253.0187, 266.9596, 365.1435 | 4.16 × 105 | Glucuronide conjugation |
| M17 | C35H58N2O6 | 20.08 | [M−H+HAc]− 661.4452 | 2.87 | 179.1073, 230.0919, 262.0817, 301.2181, 319.2280, 341.2104 | 8.65 × 106 | Hydration, ornithine conjugation |
| M18 | C22H42O6 | 22.90 | [M+Na]+ 425.2887 | 3.29 | 90.9772, 220.9344, 288.9230 | 6.03 × 106 | Palmitoyl conjugation |
| M19 | C22H44O6 | 23.06 | [M+Na]+ 427.3011 | −4.45 | 67.3221, 80.9490, 164.9196, 264.2387 | 5.31 × 105 | Palmitoyl conjugation, reduction |
| M20 | C47H74O16 | 23.09 | [M+H]+ 895.5073 | 2.57 | 83.0501, 111.0449, 129.0567, 215.1252, 583.2816, 783.3730 | 5.75 × 105 | dehydration, reduction |
| M21 | C32H47NO4 | 23.13 | [M+H]+ 510.3557 | −3.92 | 104.1071, 125.0001, 184.0730 | 5.53 × 106 | Dehydration, glycine conjugation |
| 23.85 | [M−H] 508.3423 | −1.77 | 78.9579, 168.0417, 224.0686, 283.2650 | 1.68 × 107 | Dehydration, glycine conjugation | ||
| M22 | C22H40O5 | 23.98 | [M+Na]+ 407.2785 | 4.42 | 67.1376, 96.6027, 113.1913, 142.2898, 182.0834 | 9.91 × 105 | Palmitoyl conjugation, dehydration |
| M23 | C30H46O3 | 26.00 | [M+H]+ 455.3522 | 0.44 | 86.0970, 90.9773, 164.9203, 187.0367, 315.3575, 443.2406 | 1.02 × 106 | Dehydration |
| M24 | C30H44O3 | 26.25 | [M+H]+ 453.3344 | −4.19 | 187.0367, 205.0476 | 1.26 × 106 | Dehydration |
| M25 | C31H48O3 | 26.56 | [M+H]+ 469.3656 | −4.26 | 83.4855, 222.5850, 454.3398 | 1.51 × 107 | Dehydration, methylation |
| M26 | C31H48O4 | 26.99 | [M+H]+ 485.3618 | −1.44 | 219.0620, 336.7990, 381.7993, 467.3460 | 2.90 × 105 | Desaturation, methylation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, W.; Yang, G.; Wen, Y.; Xiao, Q.; Sun, L.; Li, Y.; Gong, Z.; Wang, Y. Metabolites-Based Network Pharmacology to Preliminarily Verify In Vitro Anti-Inflammatory Effect of Ardisiacrispin B. Int. J. Mol. Sci. 2023, 24, 17059. https://doi.org/10.3390/ijms242317059
Zhou W, Yang G, Wen Y, Xiao Q, Sun L, Li Y, Gong Z, Wang Y. Metabolites-Based Network Pharmacology to Preliminarily Verify In Vitro Anti-Inflammatory Effect of Ardisiacrispin B. International Journal of Molecular Sciences. 2023; 24(23):17059. https://doi.org/10.3390/ijms242317059
Chicago/Turabian StyleZhou, Wen, Guixiang Yang, Yushuang Wen, Qian Xiao, Le Sun, Yongjun Li, Zipeng Gong, and Yonglin Wang. 2023. "Metabolites-Based Network Pharmacology to Preliminarily Verify In Vitro Anti-Inflammatory Effect of Ardisiacrispin B" International Journal of Molecular Sciences 24, no. 23: 17059. https://doi.org/10.3390/ijms242317059
APA StyleZhou, W., Yang, G., Wen, Y., Xiao, Q., Sun, L., Li, Y., Gong, Z., & Wang, Y. (2023). Metabolites-Based Network Pharmacology to Preliminarily Verify In Vitro Anti-Inflammatory Effect of Ardisiacrispin B. International Journal of Molecular Sciences, 24(23), 17059. https://doi.org/10.3390/ijms242317059




