Microbial Therapy and Breast Cancer Management: Exploring Mechanisms, Clinical Efficacy, and Integration within the One Health Approach
Abstract
1. Introduction
2. Breast Microbiome in Breast Cancer Pathogenesis and Early Detection
3. Microbiome Dynamics in Cancer Management
3.1. Radiotherapy
3.2. Chemotherapy
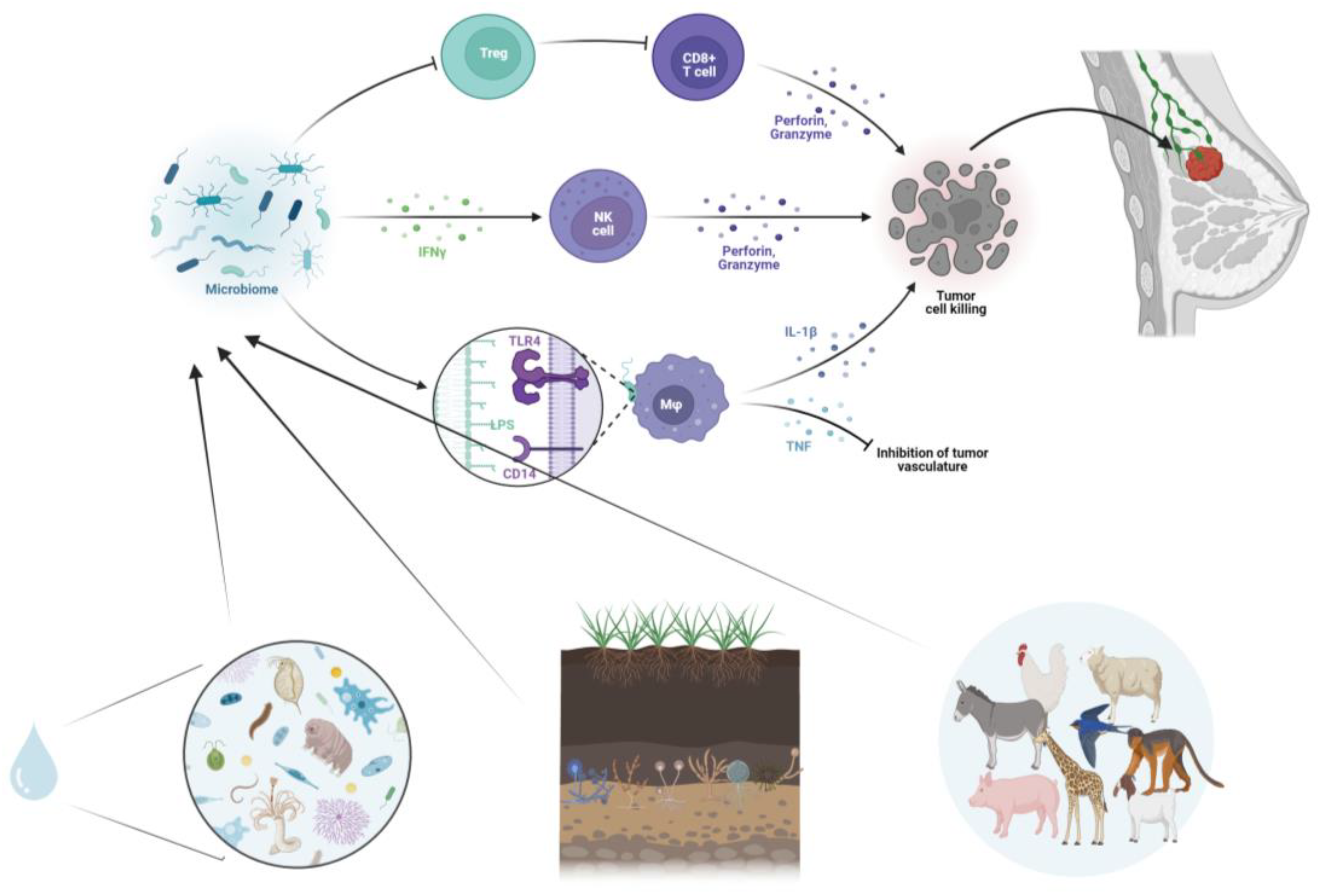
3.3. Cancer Immunotherapy and Microbiome Immunomodulation
3.4. Exploring Breast Microbiome Dynamics regarding Cancer Pathogenesis, Early Detection, and Management (Summary Box)
4. Microbiome-Modulating Interventions
4.1. Bacterial Therapeutics for Tumor Treatment and Immune Modulation
4.2. Probiotics and Prebiotics
4.3. Bacteriotherapy Approaches
4.4. Fungal Microbial Polysaccharides
4.5. Oncolytic Virotherapy
4.6. Phage-Based Immunotherapy
4.7. Microbiome-Modulating Interventions (Summary Box)
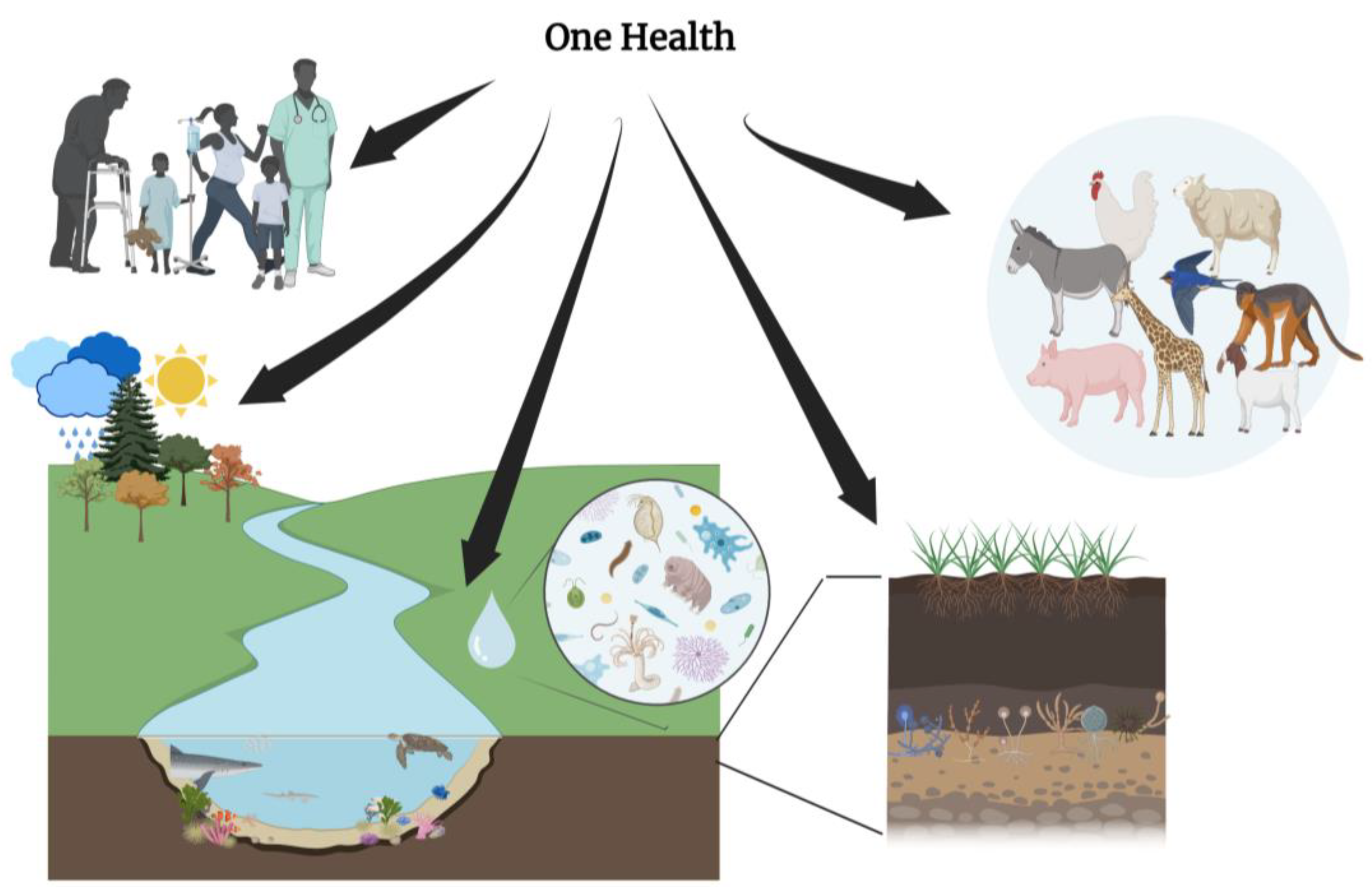
5. Integrating One Health Approach in Cancer Ecology
5.1. Integrating One Health Approach in Cancer Ecology (Summary Box)
6. Conclusions and Future Directions
6.1. Integration of Microbial Therapy within the One Health Approach
6.2. Regulatory Considerations and Ethical Implications
6.3. Future Directions
6.4. Promising Avenues for Further Research and Development
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moi, S.-H.; Lee, Y.-C.; Chuang, L.-Y.; Yuan, S.-S.F.; Ou-Yang, F.; Hou, M.-F.; Yang, C.-H.; Chang, H.-W. Cumulative receiver operating characteristics for analyzing interaction between tissue visfatin and clinicopathologic factors in breast cancer progression. Cancer Cell Int. 2018, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Artusa, V.; Calabrone, L.; Mortara, L.; Peri, F.; Bruno, A. Microbiota-Derived Natural Products Targeting Cancer Stem Cells: Inside the Gut Pharma Factory. Int. J. Mol. Sci. 2023, 24, 4997. [Google Scholar] [CrossRef] [PubMed]
- Xuan, C.; Shamonki, J.M.; Chung, A.; DiNome, M.L.; Chung, M.; Sieling, P.A.; Lee, D.J. Microbial Dysbiosis Is Associated with Human Breast Cancer. PLoS ONE 2014, 9, e83744. [Google Scholar] [CrossRef] [PubMed]
- Nejman, D.; Livyatan, I.; Fuks, G.; Gavert, N.; Zwang, Y.; Geller, L.T.; Rotter-Maskowitz, A.; Weiser, R.; Mallel, G.; Gigi, E.; et al. The human tumor microbiome is composed of tumor type–specific intracellular bacteria. Science 2020, 368, 973–980. [Google Scholar] [CrossRef]
- Dohlman, A.B.; Klug, J.; Mesko, M.; Gao, I.H.; Lipkin, S.M.; Shen, X.; Iliev, I.D. A pan-cancer mycobiome analysis reveals fungal involvement in gastrointestinal and lung tumors. Cell 2022, 185, 3807–3822.e12. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Yao, B.; Dong, T.; Chen, Y.; Yao, J.; Liu, Y.; Li, H.; Bai, H.; Liu, X.; Zhang, Y.; et al. Tumor-resident intracellular microbiota promotes metastatic colonization in breast cancer. Cell 2022, 185, 1356–1372.e26. [Google Scholar] [CrossRef] [PubMed]
- Lakritz, J.R.; Poutahidis, T.; Mirabal, S.; Varian, B.J.; Levkovich, T.; Ibrahim, Y.M.; Ward, J.M.; Teng, E.C.; Fisher, B.; Parry, N.; et al. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget 2015, 6, 9387–9396. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- German, R.; Marino, N.; Hemmerich, C.; Podicheti, R.; Rusch, D.B.; Stiemsma, L.T.; Gao, H.; Xuei, X.; Rockey, P.; Storniolo, A.M. Exploring breast tissue microbial composition and the association with breast cancer risk factors. Breast Cancer Res. 2023, 25, 82. [Google Scholar] [CrossRef]
- Tzeng, A.; Sangwan, N.; Jia, M.; Liu, C.-C.; Keslar, K.S.; Downs-Kelly, E.; Fairchild, R.L.; Al-Hilli, Z.; Grobmyer, S.R.; Eng, C. Human breast microbiome correlates with prognostic features and immunological signatures in breast cancer. Genome Med. 2021, 13, 60. [Google Scholar] [CrossRef]
- Urbaniak, C.; Gloor, G.B.; Brackstone, M.; Scott, L.; Tangney, M.; Reid, G. The Microbiota of Breast Tissue and Its Association with Breast Cancer. Appl. Environ. Microbiol. 2016, 82, 5039–5048. Available online: https://pubmed.ncbi.nlm.nih.gov/27342554/ (accessed on 1 November 2023). [CrossRef] [PubMed]
- Urbaniak, C.; Cummins, J.; Brackstone, M.; Macklaim, J.M.; Gloor, G.B.; Baban, C.K.; Scott, L.; O’Hanlon, D.M.; Burton, J.P.; Francis, K.P.; et al. Microbiota of Human Breast Tissue. Appl. Environ. Microbiol. 2014, 80, 3007–3014. [Google Scholar] [CrossRef] [PubMed]
- Maroof, H.; Hassan, Z.M.; Mobarez, A.M.; Mohamadabadi, M.A. Lactobacillus acidophilus Could Modulate the Immune Response against Breast Cancer in Murine Model. J. Clin. Immunol. 2012, 32, 1353–1359. [Google Scholar] [CrossRef]
- Bernardo, G.; Le Noci, V.; Ottaviano, E.; De Cecco, L.; Camisaschi, C.; Guglielmetti, S.; Di Modica, M.; Gargari, G.; Bianchi, F.; Indino, S.; et al. Reduction of Staphylococcus epidermidis in the mammary tumor microbiota induces antitumor immunity and decreases breast cancer aggressiveness. Cancer Lett. 2023, 555, 216041. [Google Scholar] [CrossRef]
- Cai, Z.; Sanchez, A.; Shi, Z.; Zhang, T.; Liu, M.; Zhang, D. Activation of Toll-like Receptor 5 on Breast Cancer Cells by Flagellin Suppresses Cell Proliferation and Tumor Growth. Cancer Res. 2011, 71, 2466–2475. [Google Scholar] [CrossRef]
- Zhu, J.; Liao, M.; Yao, Z.; Liang, W.; Li, Q.; Liu, J.; Yang, H.; Ji, Y.; Wei, W.; Tan, A.; et al. Breast cancer in postmenopausal women is associated with an altered gut metagenome. Microbiome 2018, 6, 136. [Google Scholar] [CrossRef]
- Yang, J.; Lagishetty, V.; Kurnia, P.; Henning, S.M.; Ahdoot, A.I.; Jacobs, J.P. Microbial and Chemical Profiles of Commercial Kombucha Products. Nutrients 2022, 14, 670. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.Y.; Stearns, V. Innovating and expanding weight loss strategies for breast cancer survivors. Oncotarget 2021, 12, 521–524. [Google Scholar] [CrossRef]
- Efficacy Study of Clinical Nutrition to Treat Lung Neoplasms and Breast Carcinoma. Available online: https://clinicaltrials.gov/study/NCT02603016 (accessed on 5 January 2024).
- Clinical Trial of Trametes Versicolor in Women with Breast Cancer. Available online: https://www.clinicaltrials.gov/study/NCT00680667?cond=Cancer&term=Coriolus%20versicolor&rank=1 (accessed on 5 January 2024).
- Viker, K.B.; Steele, M.B.; Iankov, I.D.; Concilio, S.C.; Ammayappan, A.; Bolon, B.; Jenks, N.J.; Goetz, M.P.; Panagioti, E.; Federspiel, M.J.; et al. Preclinical safety assessment of MV-s-NAP, a novel oncolytic measles virus strain armed with an H. pylori immunostimulatory bacterial transgene. Mol. Ther.-Methods Clin. Dev. 2022, 26, 532–546. [Google Scholar] [CrossRef]
- Study to Investigate Efficacy of a Novel Probiotic on the Bacteriome and Mycobiome of Breast Cancer. Available online: https://clinicaltrials.gov/study/NCT04362826 (accessed on 5 January 2024).
- Engineering Gut Microbiome to Target Breast Cancer. Available online: https://clinicaltrials.gov/study/NCT03358511 (accessed on 5 January 2024).
- To Evaluate the Clinical Efficacy of Probiotics in Patients with the Breast Cancer. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT06039644 (accessed on 5 January 2024).
- Orall Administered Probiotics to Improve the Quality of the Vaginal Flora of Women with Breast Cancer and Chemotherapy. Available online: https://clinicaltrials.gov/study/NCT01723592 (accessed on 5 January 2024).
- Naderi, N.; Mosahebi, A.; Williams, N.R. Microorganisms and Breast Cancer: An In-Depth Analysis of Clinical Studies. Pathogens 2023, 13, 6. [Google Scholar] [CrossRef]
- Treatment of Patients with Cancer with Genetically Modified Salmonella Typhimurium Bacteria. Available online: https://clinicaltrials.gov/study/NCT00004988?cond=NCT00004988&rank=1 (accessed on 5 January 2024).
- GRACE-Trial: A Randomized Active-Controlled Trial for Vulvovaginal Atrophy in Breast Cancer Patients on Endocrine Therapy. (GRACE). Available online: https://clinicaltrials.gov/study/NCT05562518?cond=NCT05562518&rank=1 (accessed on 5 January 2024).
- Clinical Trial of Neoadjuvant Chemotherapy with Atezolizumab or Placebo in Patients with Triple-Negative Breast Cancer Followed After Surgery by Atezolizumab or Placebo. Available online: https://clinicaltrials.gov/study/NCT03281954 (accessed on 5 January 2024).
- Effects of Probiotics on the Gut Microbiome and Immune System in Operable Stage I-III Breast or Lung Cancer. Available online: https://clinicaltrials.gov/study/NCT04857697?cond=NCT04857697&rank=1 (accessed on 5 January 2024).
- Lakey, J.H.; Slatin, S.L. Pore-Forming Colicins and Their Relatives. In Pore-Forming Toxins; Van Der Goot, F.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 131–161. [Google Scholar]
- Kaur, S.; Kaur, S. Bacteriocins as Potential Anticancer Agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Gautam, A.; Raghava, G.P.S.; Korpole, S. Anticancer properties of a defensin like class IId bacteriocin Laterosporulin10. Sci. Rep. 2017, 7, 46541. [Google Scholar] [CrossRef]
- Hidaka, A.; Hamaji, Y.; Sasaki, T.; Taniguchi, S.; Fujimori, M. Exogeneous Cytosine Deaminase Gene Expression in Bifidobacterium breveI-53-8w for Tumor-Targeting Enzyme/Prodrug Therapy. Biosci. Biotechnol. Biochem. 2007, 71, 2921–2926. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; de Carvalho, T.C.; Parshikov, I.; dos Santos, R.A.; Emery, F.; Furtado, N.C. Cytotoxicity of lapachol metabolites produced by probiotics. Lett. Appl. Microbiol. 2014, 59, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Karami, P.; Goli, H.R.; Abediankenari, S.; Chandani, S.R.; Jafari, N.; Ghasemi, M.; Ahanjan, M. Anti-tumor effects of Bacteroides fragilis and Bifidobacterium bifidum culture supernatants on mouse breast cancer. Gene Rep. 2023, 33, 101815. [Google Scholar] [CrossRef]
- Aarnoutse, R.; Ziemons, J.; Hillege, L.E.; de Vos-Geelen, J.; de Boer, M.; Bisschop, S.M.P.; Vriens, B.E.P.J.; Vincent, J.; van de Wouw, A.J.; Le, G.N.; et al. Changes in intestinal microbiota in postmenopausal oestrogen receptor-positive breast cancer patients treated with (neo)adjuvant chemotherapy. NPJ Breast Cancer 2022, 8, 89. [Google Scholar] [CrossRef]
- Bilenduke, E.; Sterrett, J.D.; Ranby, K.W.; Borges, V.F.; Grigsby, J.; Carr, A.L.; Kilbourn, K.; Lowry, C.A. Impacts of breast cancer and chemotherapy on gut microbiome, cognitive functioning, and mood relative to healthy controls. Sci. Rep. 2022, 12, 19547. [Google Scholar] [CrossRef]
- Horigome, A.; Okubo, R.; Hamazaki, K.; Kinoshita, T.; Katsumata, N.; Uezono, Y.; Xiao, J.; Matsuoka, Y. Association between blood omega-3 polyunsaturated fatty acids and the gut microbiota among breast cancer survivors. Benef. Microbes 2019, 10, 751–758. [Google Scholar] [CrossRef]
- Terrisse, S.; Derosa, L.; Iebba, V.; Ghiringhelli, F.; Vaz-Luis, I.; Kroemer, G.; Fidelle, M.; Christodoulidis, S.; Segata, N.; Thomas, A.M.; et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 2021, 28, 2778–2796. [Google Scholar] [CrossRef]
- Okubo, R.; Kinoshita, T.; Katsumata, N.; Uezono, Y.; Xiao, J.; Matsuoka, Y.J. Impact of chemotherapy on the association between fear of cancer recurrence and the gut microbiota in breast cancer survivors. Brain Behav. Immun. 2019, 85, 186–191. [Google Scholar] [CrossRef]
- Martín, B.R.; Rodríguez, E.J.F.; Galve, M.I.R.; Hernández, J.J.C. Study of Chemotherapy-Induced Cognitive Impairment in Women with Breast Cancer. Int. J. Environ. Res. Public Health 2020, 17, 8896. [Google Scholar] [CrossRef]
- Williams, L.J.; Fletcher, E.; Douglas, A.; Anderson, E.D.C.; McCallum, A.; Simpson, C.R.; Smith, J.; Moger, T.A.; Peltola, M.; Mihalicza, P.; et al. Retrospective cohort study of breast cancer incidence, health service use and outcomes in Europe: A study of feasibility. Eur. J. Public Health 2017, 28, 327–332. [Google Scholar] [CrossRef]
- Jim, H.S.; Phillips, K.M.; Chait, S.; Faul, L.A.; Popa, M.A.; Lee, Y.-H.; Hussin, M.G.; Jacobsen, P.B.; Small, B.J. Meta-Analysis of Cognitive Functioning in Breast Cancer Survivors Previously Treated with Standard-Dose Chemotherapy. J. Clin. Oncol. 2012, 30, 3578–3587. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Ng, T.; Shwe, M.; Ho, H.K.; Foo, K.M.; Cham, M.T.; Lee, J.A.; Fan, G.; Tan, Y.P.; Yong, W.S.; et al. Association of proinflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: A multi-centered, prospective, cohort study. Ann. Oncol. 2015, 26, 1446–1451. [Google Scholar] [CrossRef]
- Shadad, A.K. Gastrointestinal radiation injury: Symptoms, risk factors and mechanisms. World J. Gastroenterol. 2013, 19, 185. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.D.; Kim, H.J.; Seo, J.G.; Kang, S.W.; Bae, J.-W. Impact of Pelvic Radiotherapy on Gut Microbiota of Gynecological Cancer Patients Revealed by Massive Pyrosequencing. PLoS ONE 2013, 8, e82659. [Google Scholar] [CrossRef] [PubMed]
- Shiao, S.L.; Kershaw, K.M.; Limon, J.J.; You, S.; Yoon, J.; Ko, E.Y.; Guarnerio, J.; Potdar, A.A.; McGovern, D.P.; Bose, S.; et al. Commensal bacteria and fungi differentially regulate tumor responses to radiation therapy. Cancer Cell 2021, 39, 1202–1213.e6. [Google Scholar] [CrossRef]
- Uribe-Herranz, M.; Rafail, S.; Beghi, S.; Gil-De-Gómez, L.; Verginadis, I.; Bittinger, K.; Pustylnikov, S.; Pierini, S.; Perales-Linares, R.; Blair, I.A.; et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Investig. 2019, 130, 466–479. [Google Scholar] [CrossRef]
- Guo, H.; Chou, W.-C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- Alexander, J.L.; Wilson, I.D.; Teare, J.; Marchesi, J.R.; Nicholson, J.K.; Kinross, J.M. Gut microbiota modulation of chemotherapy efficacy and toxicity. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Di Modica, M.; Gargari, G.; Regondi, V.; Bonizzi, A.; Arioli, S.; Belmonte, B.; De Cecco, L.; Fasano, E.; Bianchi, F.; Bertolotti, A.; et al. Gut Microbiota Condition the Therapeutic Efficacy of Trastuzumab in HER2-Positive Breast Cancer. Cancer Res. 2021, 81, 2195–2206. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.A.; Nobre, J.G.; Batista, M.V.; Ribeiro, C.; Calle, C.; Cortes, A.; Marhold, M.; Negreiros, I.; Borralho, P.; Brito, M.; et al. Human Microbiota and Breast Cancer—Is There any Relevant Link?—A Literature Review and New Horizons toward Personalised Medicine. Front. Microbiol. 2021, 12, 584332. [Google Scholar] [CrossRef]
- Laborda-Illanes, A.; Sanchez-Alcoholado, L.; Dominguez-Recio, M.E.; Jimenez-Rodriguez, B.; Lavado, R.; Comino-Méndez, I.; Alba, E.; Queipo-Ortuño, M.I. Breast and Gut Microbiota Action Mechanisms in Breast Cancer Pathogenesis and Treatment. Cancers 2020, 12, 2465. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Bai, J.; Ma, C.; Wei, J.; Du, X. The Role of Gut Microbiota in Tumor Immunotherapy. J. Immunol. Res. 2021, 2021, 5061570. [Google Scholar] [CrossRef]
- Haque, S.; Raina, R.; Afroze, N.; Hussain, A.; Alsulimani, A.; Singh, V.; Mishra, B.N.; Kaul, S.; Kharwar, R.N. Microbial dysbiosis and epigenetics modulation in cancer development—A chemopreventive approach. Semin. Cancer Biol. 2022, 86, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Nanda, R.; Chow, L.Q.M.; Dees, E.C.; Berger, R.; Gupta, S.; Geva, R.; Pusztai, L.; Pathiraja, K.; Aktan, G.; Cheng, J.D.; et al. Pembrolizumab in Patients with Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J. Clin. Oncol. 2016, 34, 2460–2467. [Google Scholar] [CrossRef]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: Cohort A of the phase II KEYNOTE-086 study. Ann. Oncol. 2019, 30, 397–404. [Google Scholar] [CrossRef]
- Dirix, L.Y.; Takacs, I.; Jerusalem, G.; Nikolinakos, P.; Arkenau, H.T.; Forero-Torres, A.; Boccia, R.; Lippman, M.E.; Somer, R.; Smakal, M.; et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN Solid Tumor study. Breast Cancer Res. Treat. 2018, 167, 671–686. [Google Scholar] [CrossRef]
- AACR Annual Meeting 2017 Online Proceedings and Itinerary Planner|Presentation. Available online: https://www.abstractsonline.com/pp8/#!/4292/presentation/1296 (accessed on 1 November 2023).
- Emens, L.A.; Adams, S.; Loi, S.; Schneeweiss, A.; Rugo, H.S.; Winer, E.P.; Barrios, C.H.; Dieras, V.; de la Haba-Rodriguez, J.; Gianni, L.; et al. IMpassion130: A Phase III randomized trial of atezolizumab with nab-paclitaxel for first-line treatment of patients with metastatic triple-negative breast cancer (mTNBC). J. Clin. Oncol. 2016, 34, TPS1104. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Kalinsky, K.; Kaklamani, V.G.; D’Adamo, D.R.; Aktan, G.; Tsai, M.L.; O’Regan, R.; Kaufman, P.A.; Wilks, S.; Andreopoulou, E.; et al. A phase Ib/II study of eribulin (ERI) plus pembrolizumab (PEMBRO) in metastatic triple-negative breast cancer (mTNBC) (ENHANCE 1). J. Clin. Oncol. 2020, 38, 1015. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jia, S.; Dong, T.; Zhao, F.; Xu, T.; Yang, Q.; Gong, J.; Fang, M. Metabolomic and Transcriptomic Analysis of MCF-7 Cells Exposed to 23 Chemicals at Human-Relevant Levels: Estimation of Individual Chemical Contribution to Effects. Environ. Health Perspect. 2020, 128, 127008. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Y.; Liu, M.-T.; Tao, T.; Zhu, X.; Fei, F.-Q. The role of gut microbiota in tumorigenesis and treatment. Biomed. Pharmacother. 2021, 138, 111444. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, G.; Le Noci, V.; Di Modica, M.; Montanari, E.; Triulzi, T.; Pupa, S.M.; Tagliabue, E.; Sommariva, M.; Sfondrini, L. The Emerging Role of the Microbiota in Breast Cancer Progression. Cells 2023, 12, 1945. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Lang, T.; Yan, W.; Zhu, X.; Huang, X.; Yin, Q.; Li, Y. Gut Microbiota: Influence on Carcinogenesis and Modulation Strategies by Drug Delivery Systems to Improve Cancer Therapy. Adv. Sci. 2021, 8, 2003542. [Google Scholar] [CrossRef] [PubMed]
- Mendes, I.; Vale, N. How Can the Microbiome Induce Carcinogenesis and Modulate Drug Resistance in Cancer Therapy? Int. J. Mol. Sci. 2023, 24, 11855. [Google Scholar] [CrossRef]
- Trivanović, D.; Pavelić, K.; Peršurić, Ž. Fighting Cancer with Bacteria and Their Toxins. Int. J. Mol. Sci. 2021, 22, 12980. [Google Scholar] [CrossRef]
- Khoshnood, S.; Fathizadeh, H.; Neamati, F.; Negahdari, B.; Baindara, P.; Abdullah, M.A.; Haddadi, M.H. Bacteria-derived chimeric toxins as potential anticancer agents. Front. Oncol. 2022, 12, 953678. [Google Scholar] [CrossRef]
- Browne, K.; Chakraborty, S.; Chen, R.; Willcox, M.D.; Black, D.S.; Walsh, W.R.; Kumar, N. A New Era of Antibiotics: The Clinical Potential of Antimicrobial Peptides. Int. J. Mol. Sci. 2020, 21, 7047. [Google Scholar] [CrossRef]
- Patyar, S.; Joshi, R.; Byrav, D.P.; Prakash, A.; Medhi, B.; Das, B. Bacteria in cancer therapy: A novel experimental strategy. J. Biomed. Sci. 2010, 17, 21. [Google Scholar] [CrossRef] [PubMed]
- Nandi, D.; Parida, S.; Sharma, D. The gut microbiota in breast cancer development and treatment: The good, the bad, and the useful! Gut Microbes 2023, 15, 2221452. [Google Scholar] [CrossRef] [PubMed]
- Allemailem, K.S. Innovative Approaches of Engineering Tumor-Targeting Bacteria with Different Therapeutic Payloads to Fight Cancer: A Smart Strategy of Disease Management. Int. J. Nanomed. 2021, 16, 8159–8184. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wang, C.; Shao, Y.; Wang, Y.; Xing, D.; Geng, Z. Recent advances in bacteria-mediated cancer therapy. Front. Bioeng. Biotechnol. 2022, 10, 1026248. [Google Scholar] [CrossRef]
- Duong, M.T.-Q.; Qin, Y.; You, S.-H.; Min, J.-J. Bacteria-cancer interactions: Bacteria-based cancer therapy. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Du, M.; Chen, Z.; Yuan, Z. Recent Advances in Bacteria-Based Cancer Treatment. Cancers 2022, 14, 4945. [Google Scholar] [CrossRef] [PubMed]
- Freedman, J.C.; Shrestha, A.; McClane, B.A. Clostridium perfringens Enterotoxin: Action, Genetics, and Translational Applications. Toxins 2016, 8, 73. [Google Scholar] [CrossRef]
- Cardillo, F.; Bonfim, M.; Sousa, P.d.S.V.; Mengel, J.; Castello-Branco, L.R.R.; Pinho, R.T. Bacillus Calmette–Guérin Immunotherapy for Cancer. Vaccines 2021, 9, 439. [Google Scholar] [CrossRef]
- Wu, H.; Ganguly, S.; Tollefsbol, T.O. Modulating Microbiota as a New Strategy for Breast Cancer Prevention and Treatment. Microorganisms 2022, 10, 1727. [Google Scholar] [CrossRef]
- Rutkowski, M.R.; Stephen, T.L.; Svoronos, N.; Allegrezza, M.J.; Tesone, A.J.; Perales-Puchalt, A.; Brencicova, E.; Escovar-Fadul, X.; Nguyen, J.M.; Cadungog, M.G.; et al. Microbially Driven TLR5-Dependent Signaling Governs Distal Malignant Progression through Tumor-Promoting Inflammation. Cancer Cell 2014, 27, 27–40. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Eslami-S, Z.; Majidzadeh-A, K.; Halvaei, S.; Babapirali, F.; Esmaeili, R. Microbiome and Breast Cancer: New Role for an Ancient Population. Front. Oncol. 2020, 10, 120. [Google Scholar] [CrossRef]
- Luu, T.H.; Michel, C.; Bard, J.-M.; Dravet, F.; Nazih, H.; Bobin-Dubigeon, C. Intestinal Proportion of Blautia sp. is Associated with Clinical Stage and Histoprognostic Grade in Patients with Early-Stage Breast Cancer. Nutr. Cancer 2017, 69, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.S.; Saeed, A.; Baig, M.; Asif, N.; Masood, N.; Yasmin, A. Anticarcinogenecity of microbiota and probiotics in breast cancer. Int. J. Food Prop. 2018, 21, 655–666. [Google Scholar] [CrossRef]
- Yazdi, M.H.; Mahdavi, M.; Kheradmand, E.; Shahverdi, A.R. The Preventive Oral Supplementation of a Selenium Nanoparticle-enriched Probiotic Increases the Immune Response and Lifespan of 4T1 Breast Cancer Bearing Mice. Arzneimittelforschung 2012, 62, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Aragón, F.; Carino, S.; Perdigón, G.; LeBlanc, A.d.M.d. Inhibition of Growth and Metastasis of Breast Cancer in Mice by Milk Fermented with Lactobacillus casei CRL 431. J. Immunother. 2015, 38, 185–196. [Google Scholar] [CrossRef]
- Chitapanarux, I.; Chitapanarux, T.; Traisathit, P.; Kudumpee, S.; Tharavichitkul, E.; Lorvidhaya, V. Randomized controlled trial of live lactobacillus acidophilus plus bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiat. Oncol. 2010, 5, 31. [Google Scholar] [CrossRef]
- Mego, M.; Chovanec, J.; Vochyanova-Andrezalova, I.; Konkolovsky, P.; Mikulova, M.; Reckova, M.; Miskovska, V.; Bystricky, B.; Beniak, J.; Medvecova, L.; et al. Prevention of irinotecan induced diarrhea by probiotics: A randomized double blind, placebo controlled pilot study. Complement. Ther. Med. 2015, 23, 356–362. [Google Scholar] [CrossRef]
- Hibberd, A.A.; Lyra, A.; Ouwehand, A.C.; Rolny, P.; Lindegren, H.; Cedgård, L.; Wettergren, Y. Intestinal microbiota is altered in patients with colon cancer and modified by probiotic intervention. BMJ Open Gastroenterol. 2017, 4, e000145. [Google Scholar] [CrossRef]
- Theodoropoulos, G.E.; Memos, N.A.; Peitsidou, K.; Karantanos, T.; Spyropoulos, B.G.; Zografos, G. Synbiotics and gastrointestinal function-related quality of life after elective colorectal cancer resection. Ann. Gastroenterol. 2016, 29, 56–62. [Google Scholar] [PubMed]
- Demers, M.; Dagnault, A.; Desjardins, J. A randomized double-blind controlled trial: Impact of probiotics on diarrhea in patients treated with pelvic radiation. Clin. Nutr. 2014, 33, 761–767. [Google Scholar] [CrossRef]
- Österlund, P.; Ruotsalainen, T.; Korpela, R.; Saxelin, M.; Ollus, A.; Valta, P.; Kouri, M.; Elomaa, I.; Joensuu, H. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: A randomised study. Br. J. Cancer 2007, 97, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Meza, L.; Bergerot, P.; Alcantara, M.; Dorff, T.; Lyou, Y.; Frankel, P.; Cui, Y.; Mira, V.; Llamas, M.; et al. Nivolumab plus ipilimumab with or without live bacterial supplementation in metastatic renal cell carcinoma: A randomized phase 1 trial. Nat. Med. 2022, 28, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Toi, M.; Hirota, S.; Tomotaki, A.; Sato, N.; Hozumi, Y.; Anan, K.; Nagashima, T.; Tokuda, Y.; Masuda, N.; Ohsumi, S.; et al. Probiotic Beverage with Soy Isoflavone Consumption for Breast Cancer Prevention: A Case-control Study. Curr. Nutr. Food Sci. 2013, 9, 194–200. [Google Scholar] [CrossRef]
- Juan, Z.; Chen, J.; Ding, B.; Yongping, L.; Liu, K.; Wang, L.; Le, Y.; Liao, Q.; Shi, J.; Huang, J.; et al. Probiotic supplement attenuates chemotherapy-related cognitive impairment in patients with breast cancer: A randomised, double-blind, and placebo-controlled trial. Eur. J. Cancer 2021, 161, 10–22. [Google Scholar] [CrossRef]
- Khazaei, Y.; Basi, A.; Fernandez, M.L.; Foudazi, H.; Bagherzadeh, R.; Shidfar, F. The effects of synbiotics supplementation on reducing chemotherapy-induced side effects in women with breast cancer: A randomized placebo-controlled double-blind clinical trial. BMC Complement. Med. Ther. 2023, 23, 339. [Google Scholar] [CrossRef]
- Nallar, S.C.; Xu, D.-Q.; Kalvakolanu, D.V. Bacteria and genetically modified bacteria as cancer therapeutics: Current advances and challenges. Cytokine 2017, 89, 160–172. [Google Scholar] [CrossRef]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Forbes, N.S. Engineering the perfect (bacterial) cancer therapy. Nat. Rev. Cancer 2010, 10, 785–794. [Google Scholar] [CrossRef]
- Hatakeyama, M. Helicobacter pylori CagA and Gastric Cancer: A Paradigm for Hit-and-Run Carcinogenesis. Cell Host Microbe 2014, 15, 306–316. [Google Scholar] [CrossRef]
- Felgner, S.; Kocijancic, D.; Frahm, M.; Heise, U.; Rohde, M.; Zimmermann, K.; Falk, C.; Erhardt, M.; Weiss, S. Engineered Salmonella enterica serovar Typhimurium overcomes limitations of anti-bacterial immunity in bacteria-mediated tumor therapy. OncoImmunology 2017, 7, e1382791. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Cotter, P.D.; Ross, R.P.; Hill, C. Bioengineering of the model lantibiotic nisin. Bioengineered 2015, 6, 187–192. [Google Scholar] [CrossRef]
- Roberts, N.J.; Zhang, L.; Janku, F.; Collins, A.; Bai, R.-Y.; Staedtke, V.; Rusk, A.W.; Tung, D.; Miller, M.; Roix, J.; et al. Intratumoral injection of Clostridium novyi -NT spores induces antitumor responses. Sci. Transl. Med. 2014, 6, 249ra111. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Gnjatic, S.; Sawhney, N.B. TLR AGONISTS: Are They Good Adjuvants? Cancer J. 2010, 16, 382–391. [Google Scholar] [CrossRef]
- Shanmugam, M.; Abirami, R. Microbial Polysaccharides—Chemistry and Applications. J. Biol. Act. Prod. Nat. 2019, 9, 73–78. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed]
- Lemieszek, M.; Rzeski, W. Anticancer properties of polysaccharides isolated from fungi of the Basidiomycetes class. Contemp. Oncol. 2012, 4, 285–289. [Google Scholar] [CrossRef]
- Chow, L.W.; Lo, C.S.; Loo, W.T.; Hu, X.-C.; Sham, J.S.T. Polysaccharide Peptide Mediates Apoptosis by Up-regulating p21 Gene and Down-regulating Cyclin D1 Gene. Am. J. Chin. Med. 2003, 31, 1–9. [Google Scholar] [CrossRef]
- Eliza, W.L.; Fai, C.K.; Chung, L.P. Efficacy of Yun Zhi (Coriolus versicolor) on Survival in Cancer Patients: Systematic Review and Meta-Analysis. Recent Pat. Inflamm. Allergy Drug Discov. 2012, 6, 78–87. [Google Scholar] [CrossRef]
- Queiroz, E.A.; Fortes, Z.B.; da Cunha, M.A.; Sarilmiser, H.K.; Dekker, A.M.B.; Öner, E.T.; Dekker, R.F.; Khaper, N. Levan promotes antiproliferative and pro-apoptotic effects in MCF-7 breast cancer cells mediated by oxidative stress. Int. J. Biol. Macromol. 2017, 102, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Alonso, E.N.; Ferronato, M.J.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Guevara, J.A.; Facchinetti, M.M.; Curino, A.C. Antitumoral and antimetastatic activity of Maitake D-Fraction in triple-negative breast cancer cells. Oncotarget 2018, 9, 23396–23412. [Google Scholar] [CrossRef] [PubMed]
- Kodama, N.; Komuta, K.; Nanba, H. Effect of Maitake (Grifola frondosa) D-Fraction on the Activation of NK Cells in Cancer Patients. J. Med. Food 2003, 6, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2016, 15, 660. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Jiang, H.; Cheng, L.; Liu, R. The oncolytic herpes simplex virus vector, G47Δ, effectively targets tamoxifen-resistant breast cancer cells. Oncol. Rep. 2016, 35, 1741–1749. [Google Scholar] [CrossRef]
- Wang, L.-J.; Han, S.-X.; Bai, E.; Zhou, X.; Li, M.; Jing, G.-H.; Zhao, J.; Yang, A.-G.; Zhu, Q. Dose-dependent effect of tamoxifen in tamoxifen-resistant breast cancer cells via stimulation by the ERK1/2 and AKT signaling pathways. Oncol. Rep. 2013, 29, 1563–1569. [Google Scholar] [CrossRef]
- Zeng, W.-G.; Li, J.-J.; Hu, P.; Lei, L.; Wang, J.-N.; Liu, R.-B. An oncolytic herpes simplex virus vector, G47Δ, synergizes with paclitaxel in the treatment of breast cancer. Oncol. Rep. 2013, 29, 2355–2361. [Google Scholar] [CrossRef][Green Version]
- Soliman, H.; Hogue, D.; Han, H.; Mooney, B.; Costa, R.; Lee, M.C.; Niell, B.; Williams, A.; Chau, A.; Falcon, S.; et al. Oncolytic T-VEC virotherapy plus neoadjuvant chemotherapy in nonmetastatic triple-negative breast cancer: A phase 2 trial. Nat. Med. 2023, 29, 450–457. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Wang, H.; Chen, N.G.; Minev, B.R.; Szalay, A.A. Oncolytic vaccinia virus GLV-1h68 strain shows enhanced replication in human breast cancer stem-like cells in comparison to breast cancer cells. J. Transl. Med. 2012, 10, 167. [Google Scholar] [CrossRef]
- Catala, A.; Dzieciatkowska, M.; Wang, G.; Gutierrez-Hartmann, A.; Simberg, D.; Hansen, K.C.; D’alessandro, A.; Catalano, C.E. Targeted Intracellular Delivery of Trastuzumab Using Designer Phage Lambda Nanoparticles Alters Cellular Programs in Human Breast Cancer Cells. ACS Nano 2021, 15, 11789–11805. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Pan, P.; Ye, J.-J.; Zhang, Q.-L.; Zhang, X.-Z. Hybrid M13 bacteriophage-based vaccine platform for personalized cancer immunotherapy. Biomaterials 2022, 289, 121763. [Google Scholar] [CrossRef] [PubMed]
- One Health. Available online: https://www.who.int/news-room/questions-and-answers/item/one-health (accessed on 1 December 2023).
- Kleber, K.T.; Iranpur, K.R.; Perry, L.M.; Cruz, S.M.; Razmara, A.M.; Culp, W.T.N.; Kent, M.S.; Eisen, J.A.; Rebhun, R.B.; Canter, R.J. Using the canine microbiome to bridge translation of cancer immunotherapy from pre-clinical murine models to human clinical trials. Front. Immunol. 2022, 13, 983344. [Google Scholar] [CrossRef] [PubMed]
- Dujon, A.; Brown, J.S.; Destoumieux-Garzón, D.; Vittecoq, M.; Hamede, R.; Tasiemski, A.; Boutry, J.; Tissot, S.; Alix-Panabieres, C.; Pujol, P.; et al. On the need for integrating cancer into the One Health perspective. Evol. Appl. 2021, 14, 2571–2575. [Google Scholar] [CrossRef] [PubMed]
- Kattner, P.; Zeiler, K.; Herbener, V.J.; La Ferla-Brühl, K.; Kassubek, R.; Grunert, M.; Burster, T.; Brühl, O.; Weber, A.S.; Strobel, H.; et al. What Animal Cancers teach us about Human Biology. Theranostics 2021, 11, 6682–6702. [Google Scholar] [CrossRef]
- Dujon, A.M.; Gatenby, R.A.; Bramwell, G.; MacDonald, N.; Dohrmann, E.; Raven, N.; Schultz, A.; Hamede, R.; Gérard, A.-L.; Giraudeau, M.; et al. Transmissible Cancers in an Evolutionary Perspective. iScience 2020, 23, 101269. [Google Scholar] [CrossRef] [PubMed]
- Epstein, B.; Jones, M.; Hamede, R.; Hendricks, S.; McCallum, H.; Murchison, E.P.; Schönfeld, B.; Wiench, C.; Hohenlohe, P.; Storfer, A. Rapid evolutionary response to a transmissible cancer in Tasmanian devils. Nat. Commun. 2016, 7, 12684. [Google Scholar] [CrossRef] [PubMed]
- Kerr, P.J.; Liu, J.; Cattadori, I.; Ghedin, E.; Read, A.F.; Holmes, E.C. Myxoma Virus and the Leporipoxviruses: An Evolutionary Paradigm. Viruses 2015, 7, 1020–1061. [Google Scholar] [CrossRef] [PubMed]
- Di Giallonardo, F.; Holmes, E.C. Viral biocontrol: Grand experiments in disease emergence and evolution. Trends Microbiol. 2015, 23, 83–90. [Google Scholar] [CrossRef]
- Spernovasilis, N.; Tsiodras, S.; Poulakou, G. Emerging and Re-Emerging Infectious Diseases: Humankind’s Companions and Competitors. Microorganisms 2022, 10, 98. [Google Scholar] [CrossRef]
- Giraudeau, M.; Sepp, T.; Ujvari, B.; Ewald, P.W.; Thomas, F. Human activities might influence oncogenic processes in wild animal populations. Nat. Ecol. Evol. 2018, 2, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Sepp, T.; Ujvari, B.; Ewald, P.W.; Thomas, F.; Giraudeau, M. Urban environment and cancer in wildlife: Available evidence and future research avenues. Proc. R. Soc. B Biol. Sci. 2019, 286, 20182434. [Google Scholar] [CrossRef] [PubMed]
- Pesavento, P.A.; Agnew, D.; Keel, M.K.; Woolard, K.D. Cancer in wildlife: Patterns of emergence. Nat. Rev. Cancer 2018, 18, 646–661. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Amato, K.R.; Decaestecker, E.; McKenzie, V.J. Editorial: Impact of anthropogenic environmental changes on animal microbiomes. Front. Ecol. Evol. 2023, 11, 1204035. [Google Scholar] [CrossRef]
- Sonnenburg, E.D.; Sonnenburg, J.L. The ancestral and industrialized gut microbiota and implications for human health. Nat. Rev. Microbiol. 2019, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.A.M.; Chel, H.M.; Thu, M.J.; Bawm, S.; Htun, L.L.; Win, M.M.; Oo, Z.M.; Ohsawa, N.; Lahdenperä, M.; Mohamed, W.M.A.; et al. Anthropogenic interferences lead to gut microbiome dysbiosis in Asian elephants and may alter adaptation processes to surrounding environments. Sci. Rep. 2021, 11, 741. [Google Scholar] [CrossRef]
- Poutahidis, T.; Varian, B.J.; Levkovich, T.; Lakritz, J.R.; Mirabal, S.; Kwok, C.; Ibrahim, Y.M.; Kearney, S.M.; Chatzigiagkos, A.; Alm, E.J.; et al. Dietary Microbes Modulate Transgenerational Cancer Risk. Cancer Res 2015, 75, 1197–1204. [Google Scholar] [CrossRef]
- Kowallik, V.; Das, A.; Mikheyev, A.S. Experimental inheritance of antibiotic acquired dysbiosis affects host phenotypes across generations. Front. Microbiol. 2022, 13, 1030771. [Google Scholar] [CrossRef]
- Dujon, A.M.; Ujvari, B.; Thomas, F. Cancer risk landscapes: A framework to study cancer in ecosystems. Sci. Total. Environ. 2021, 763, 142955. [Google Scholar] [CrossRef]
- Efird, J.T.; Davies, S.W.; O’neal, W.T.; Anderson, E.J. Animal Viruses, Bacteria, and Cancer: A Brief Commentary. Front. Public Health 2014, 2, 14. [Google Scholar] [CrossRef]
- Prüss-Üstün, A.; Wolf, J.; Corvalán, C.F.; Bos, R.; Neira, M.P. Preventing Disease through Healthy Environments: A Global Assessment of the Burden of Disease from Environmental Risks; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- AbdulRaheem, Y. Unveiling the Significance and Challenges of Integrating Prevention Levels in Healthcare Practice. J. Prim. Care Community Health 2023, 14, 21501319231186500. [Google Scholar] [CrossRef] [PubMed]
- About: Health Promotion and Disease Prevention through Population-Based Interventions, Including Action to Address Social Determinants and Health Inequity. Available online: https://www.emro.who.int/about-who/public-health-functions/health-promotion-disease-prevention.html (accessed on 1 December 2023).
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Lee, K.; Raguideau, S.; Sirén, K.; Asnicar, F.; Cumbo, F.; Hildebrand, F.; Segata, N.; Cha, C.-J.; Quince, C. Population-level impacts of antibiotic usage on the human gut microbiome. Nat. Commun. 2023, 14, 1191. [Google Scholar] [CrossRef]
- Wang, W.; Weng, Y.; Luo, T.; Wang, Q.; Yang, G.; Jin, Y. Antimicrobial and the Resistances in the Environment: Ecological and Health Risks, Influencing Factors, and Mitigation Strategies. Toxics 2023, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Péguilhan, R.; Turgeon, N.; Veillette, M.; Baray, J.-L.; Deguillaume, L.; Amato, P.; Duchaine, C. Quantification of antibiotic resistance genes (ARGs) in clouds at a mountain site (puy de Dôme, central France). Sci. Total Environ. 2023, 865, 161264. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, D.H.; Page, S.W. Antimicrobial Stewardship in Veterinary Medicine. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef]
- Roderburg, C.; Loosen, S.H.; Joerdens, M.S.; Demir, M.; Luedde, T.; Kostev, K. Antibiotic therapy is associated with an increased incidence of cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 1285–1293. [Google Scholar] [CrossRef]
- Ibragimova, S.; Ramachandran, R.; Ali, F.R.; Lipovich, L.; Ho, S.B. Dietary Patterns and Associated Microbiome Changes that Promote Oncogenesis. Front. Cell Dev. Biol. 2021, 9, 725821. [Google Scholar] [CrossRef]
- emhj: One Health: Perspectives on Ethical Issues and Evidence from Animal Experiments. Available online: https://www.emro.who.int/emhj-volume-18-2012/issue-11/article-15.html (accessed on 1 December 2023).
- van Herten, J.; Bovenkerk, B.; Verweij, M. One Health as a moral dilemma: Towards a socially responsible zoonotic disease control. Zoonoses Public Health 2018, 66, 26–34. [Google Scholar] [CrossRef]
- Ursell, L.K.; Metcalf, J.L.; Parfrey, L.W.; Knight, R. Defining the human microbiome. Nutr. Rev. 2012, 70 (Suppl. S1), S38–S44. [Google Scholar] [CrossRef]
- Baba, A.I.; Câtoi, C. Comparative Oncology; The Publishing House of the Romanian Academy: Bucharest, Romania, 2007. [Google Scholar]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and Soil Microbial Community: A Review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Woodworth, M.H.; Sitchenko, K.L.; Carpentieri, C.; Friedman-Moraco, R.J.; Wang, T.; Kraft, C.S. Ethical Considerations in Microbial Therapeutic Clinical Trials. New Bioeth. 2017, 23, 210–218. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Ahern, A.; Carbone, C.; Temko, A.; Claesson, M.J.; Gasbarrini, A.; Tortora, G. Gut microbiome, big data and machine learning to promote precision medicine for cancer. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tan, Q.; Fu, Q.; Zhou, Y.; Hu, Y.; Tang, S.; Zhou, Y.; Zhang, J.; Qiu, J.; Lv, Q. Gastrointestinal microbiome and breast cancer: Correlations, mechanisms and potential clinical implications. Breast Cancer 2017, 24, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Wang, X.; Yuan, F.; Li, Y.; Lu, P. Research progress on the effect of gut and tumor microbiota on antitumor efficacy and adverse effects of chemotherapy drugs. Front. Microbiol. 2022, 13, 899111. [Google Scholar] [CrossRef]
- Poff, A.M.; Ari, C.; Seyfried, T.N.; D’agostino, D.P. The Ketogenic Diet and Hyperbaric Oxygen Therapy Prolong Survival in Mice with Systemic Metastatic Cancer. PLoS ONE 2013, 8, e65522. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Soto-Pantoja, D.R.; Gaber, M.; Arnone, A.A.; Bronson, S.M.; Cruz-Diaz, N.; Wilson, A.S.; Clear, K.Y.J.; Ramirez, M.U.; Kucera, G.L.; Levine, E.A.; et al. Diet Alters Entero-Mammary Signaling to Regulate the Breast Microbiome and Tumorigenesis. Cancer Res. 2021, 81, 3890–3904. [Google Scholar] [CrossRef]
- Hou, M.-F.; Ou-Yang, F.; Li, C.-L.; Chen, F.-M.; Chuang, C.-H.; Kan, J.-Y.; Wu, C.-C.; Shih, S.-L.; Shiau, J.-P.; Kao, L.-C.; et al. Comprehensive profiles and diagnostic value of menopausal-specific gut microbiota in premenopausal breast cancer. Exp. Mol. Med. 2021, 53, 1636–1646. [Google Scholar] [CrossRef]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. Available online: https://www.nature.com/articles/s41467-020-16967-2 (accessed on 1 December 2023). [CrossRef]
- Bennet, J.; Brinkman, M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet 1989, 333, 164. [Google Scholar] [CrossRef] [PubMed]
- Mills, H.; Acquah, R.; Tang, N.; Cheung, L.; Klenk, S.; Glassen, R.; Pirson, M.; Albert, A.; Hoang, D.T.; Van, T.N. The Use of Bacteria in Cancer Treatment: A Review from the Perspective of Cellular Microbiology. Emerg. Med. Int. 2022, 2022, 8127137. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Gu, M.-D.; Tang, T. Pseudomonas aeruginosa in Cancer Therapy: Current Knowledge, Challenges and Future Perspectives. Front. Oncol. 2022, 12, 891187. [Google Scholar] [CrossRef] [PubMed]
- Kasinskas, R.W.; Forbes, N.S. Salmonella typhimurium Lacking Ribose Chemoreceptors Localize in Tumor Quiescence and Induce Apoptosis. Cancer Res. 2007, 67, 3201–3209. [Google Scholar] [CrossRef]
| Potential Therapeutic Microbe | Clinical Status | Clinical Phase | Type of Cancer | Mechanisms Involved | References |
|---|---|---|---|---|---|
| Bacillus | Recruiting | N/A | Breast cancer stage I–III | NCT05717972: Evaluate the effects of a fermented tea, kombucha. Once brewed, sugar breaks down from the SCOBY and probiotic bacteria such as Bacillus coagulans, Bacillus subtilis, and Lactobacillus rhamnosus, among others, are released. | [17] |
| Bacteroides | Active, not recruiting | Phase II | Weight loss in breast cancer patients | NCT04499950: Compare how the ratio between Bacteroides and Firmicutes in microbiome affects weight loss in breast cancer patients. | [18] |
| Coriolus versicolor/Trametes versicolor | Completed | Phase I | Lung neoplasms and breast carcinoma | NCT02603016: Patients will be given maitake mushroom (Grifola frondosa) extract to evaluate treatment efficacy. | [19] |
| Completed | Phase I | Breast cancer | NCT00680667: To determine the side effects and effective dose at which the muschroom extract Coriolus versicolor is effective in treating women with breast cancer between stages I and III following radiation therapy. Results not posted. | [20] | |
| Helicobacter pylori | Recruiting | Phase I | Recruiting | NCT04521764: Genetically engineered measles virus expressing Helicobacter pylori neutrophil-activating protein is administered to breast cancer patients to determine shredding and immune response rate and to record any side effects and therapeutic doses. | [21] |
| Lactobacillus | Not yet recruiting | Phase 2 or phase 3 | Invasive ductal carcinoma (IDC) or invasive lobular carcinoma (ILC) | NCT04362826: Evaluate efficacy of the BIOHM probiotic synthesized of B. breve, S. boulardii, L. acidophilus, and L. rhamnosus microbes. Determine whether any bacteriome and mycobiome profiles of breast tissue are altered following consumption of BIOHM and determine whether quality of life is different for patients that have consumed the probiotic. | [22] |
| Completed | Early phases of clinical trial | Breast adenocarcinoma | NCT03358511: Patients diagnosed with a breast adenocarcinoma administered a dietary supplement known as Primal Defense Ultra® (bearing Saccharomyces, Lactobacillus and Bifidobacterium). Postmenopausal breast cancer patients took the probiotic 3 times a day for 2–4 weeks prior to surgery. Results not yet posted. | [23] | |
| Not yet recruiting | Early phases of clinical trial | Breast cancer patients stage I–III | NCT06039644: Stage I-III breast cancer patients undergoing anthracycline-based and taxane-based chemotherapy are instructed to consume probiotics (various Lactobacillus strains) over a 6-month period to determine whether chemotherapy side effects are improved or even prevented. | [24] | |
| Completed | Phase 2 or phase 3 | Breast cancer patients with vaginal flora score on Nugent scale IV–VI | NCT01723592: Probiotic capsules enclosing four lyophilized Lactobacillus strains were prescribed as a dietary supplement to patients. The study was designed to determine whether the vaginal flora could be improved by at least 2 grades on the Nugent scale. Results indicated that probiotics effectively reduced the Nugent score and were more effective when prescribed during chemotherapy. | [25] | |
| Completed | Early phases of clinical trial | Greater than 25% risk of developing breast cancer (but have never had breast cancer) and/or BRCA1 or BRCA2 positive) | NCT03290651: The hypothesis is that Lactobacilli can restore the breast microbiome of women, displacing any harmful cancer-causing bacteria, and reduce inflammation. | [26] | |
| MV-s-NAP | Recruiting | Phase I | HER2 breast cancer | NCT04521764: Study investigating the side effects and therapeutic dose of MV-s-NAP (modified measles virus) for breast cancer. Laboratory work has shown that the virus abolishes breast cancer cells. | [21] |
| Salmonella | Completed | Phase I | Patients with advanced or metastatic cancer | NCT00004988: Salmonella typhimurium (VNP20009) administered to nonresponsive metastatic melanoma or renal cell carcinoma patients. Observed tumor colonization with no anti-tumor effects. | [27] |
| Not stated | Recruiting | Phase IV | Breast cancer patients with vulvovaginal atrophy | NCT05562518: Through patient-reported outcome measurements, the efficacy of the following combinations of drugs will be evaluated and compared to determine which treatment plan provides the best quality of life for vulvovaginal atrophy in breast cancer.
| [28] |
| According to patient stool analysis | Ongoing | Phase III | Triple-negative breast cancer | EudraCT Number: 2017-002771-25 (NCT03281954). Investigate the gut microbiome population prior to cancer treatment and 30 days following the last treatment to identify the role of gut microbiota in regulating immune response. The impact of the gut microbiota on cancer incidence and its progression will also be evaluated. | [29] |
| Probiotic regimen to be designed according to patient stool analysis prior to treatment | Completed | Early phase I | Breast cancer stage I–III and breast adenocarcinoma | NCT04857697: Breast cancer patients will be administered probiotics, prior to surgery, to determine whether it affects patient outcomes. Dysbiosis and immune system effects will also be evaluated. | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippou, C.; Themistocleous, S.C.; Marangos, G.; Panayiotou, Y.; Fyrilla, M.; Kousparou, C.A.; Pana, Z.-D.; Tsioutis, C.; Johnson, E.O.; Yiallouris, A. Microbial Therapy and Breast Cancer Management: Exploring Mechanisms, Clinical Efficacy, and Integration within the One Health Approach. Int. J. Mol. Sci. 2024, 25, 1110. https://doi.org/10.3390/ijms25021110
Filippou C, Themistocleous SC, Marangos G, Panayiotou Y, Fyrilla M, Kousparou CA, Pana Z-D, Tsioutis C, Johnson EO, Yiallouris A. Microbial Therapy and Breast Cancer Management: Exploring Mechanisms, Clinical Efficacy, and Integration within the One Health Approach. International Journal of Molecular Sciences. 2024; 25(2):1110. https://doi.org/10.3390/ijms25021110
Chicago/Turabian StyleFilippou, Charalampos, Sophia C. Themistocleous, Giorgos Marangos, Yiannis Panayiotou, Maria Fyrilla, Christina A. Kousparou, Zoi-Dorothea Pana, Constantinos Tsioutis, Elizabeth O. Johnson, and Andreas Yiallouris. 2024. "Microbial Therapy and Breast Cancer Management: Exploring Mechanisms, Clinical Efficacy, and Integration within the One Health Approach" International Journal of Molecular Sciences 25, no. 2: 1110. https://doi.org/10.3390/ijms25021110
APA StyleFilippou, C., Themistocleous, S. C., Marangos, G., Panayiotou, Y., Fyrilla, M., Kousparou, C. A., Pana, Z.-D., Tsioutis, C., Johnson, E. O., & Yiallouris, A. (2024). Microbial Therapy and Breast Cancer Management: Exploring Mechanisms, Clinical Efficacy, and Integration within the One Health Approach. International Journal of Molecular Sciences, 25(2), 1110. https://doi.org/10.3390/ijms25021110








