Exploring the Potential of Olfactory Receptor Circulating RNA Measurement for Preeclampsia Prediction and Its Linkage to Mild Gestational Hypothyroidism
Abstract
1. Introduction
2. Results
2.1. Differentially Expressed Genes in Transcriptome Libraries
2.2. Olfactory Receptor Family
2.3. Levothyroxine Effect Analysis in Olfactory Receptors
2.4. Preeclampsia Onset Prediction Using Olfactory Receptors
3. Discussion
4. Methods
4.1. Population
4.2. Transcriptome Libraries
4.3. Blood Sample Collection and Processing
4.4. RNA Extraction and cDNA Synthesis
4.5. Transcriptome Library Construction
4.6. Preeclampsia Onset Prediction
4.7. Levothyroxine Effect Analysis
4.8. Bioinformatic and Statistical Analysis
4.8.1. Bioinformatic
4.8.2. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahadik, K.; Choudhary, P.; Roy, P.K. Study of thyroid function in pregnancy, its feto-maternal outcome; a prospective observational study. BMC Pregnancy Childbirth 2020, 20, 769. [Google Scholar] [CrossRef] [PubMed]
- Sitoris, G.; Veltri, F.; Kleynen, P.; Cogan, A.; Belhomme, J.; Rozenberg, S.; Pepersack, T.; Poppe, K. The Impact of Thyroid Disorders on Clinical Pregnancy Outcomes in a Real-World Study Setting. Thyroid 2020, 30, 106–115. [Google Scholar] [CrossRef]
- Toloza, F.J.K.; Derakhshan, A.; Mannisto, T.; Bliddal, S.; Popova, P.V.; Carty, D.M.; Chen, L.; Taylor, P.; Mosso, L.; Oken, E.; et al. Association between maternal thyroid function and risk of gestational hypertension and pre-eclampsia: A systematic review and individual-participant data meta-analysis. Lancet Diabetes Endocrinol. 2022, 10, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Turunen, S.; Vaarasmaki, M.; Mannisto, T.; Hartikainen, A.L.; Lahesmaa-Korpinen, A.M.; Gissler, M.; Suvanto, E. Pregnancy and Perinatal Outcome Among Hypothyroid Mothers: A Population-Based Cohort Study. Thyroid 2019, 29, 135–141. [Google Scholar] [CrossRef]
- Nazarpour, S.; Ramezani Tehrani, F.; Simbar, M.; Azizi, F. Thyroid dysfunction and pregnancy outcomes. Iran. J. Reprod. Med. 2015, 13, 387–396. [Google Scholar]
- Bjornsdottir, S.; Clarke, B.; Makitie, O.; Sandstrom, A.; Tiblad, E.; Spelman, T.; Kampe, O. Women with Chronic Hypoparathyroidism Have Low Risk of Adverse Pregnancy Outcomes. J. Clin. Endocrinol. Metab. 2021, 106, 3312–3319. [Google Scholar] [CrossRef] [PubMed]
- Cleary-Goldman, J.; Malone, F.D.; Lambert-Messerlian, G.; Sullivan, L.; Canick, J.; Porter, T.F.; Luthy, D.; Gross, S.; Bianchi, D.W.; D’Alton, M.E. Maternal thyroid hypofunction and pregnancy outcome. Obstet. Gynecol. 2008, 112, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Männistö, T.; Vääräsmäki, M.; Pouta, A.; Hartikainen, A.L.; Ruokonen, A.; Surcel, H.M.; Bloigu, A.; Järvelin, M.R.; Suvanto, E. Thyroid dysfunction and autoantibodies during pregnancy as predictive factors of pregnancy complications and maternal morbidity in later life. J. Clin. Endocrinol. Metab. 2010, 95, 1084–1094. [Google Scholar] [CrossRef] [PubMed]
- Maraka, S.; Ospina, N.M.; O’Keeffe, D.T.; Espinosa De Ycaza, A.E.; Gionfriddo, M.R.; Erwin, P.J.; Coddington, C.C.; Stan, M.N.; Murad, M.H.; Montori, V.M. Subclinical Hypothyroidism in Pregnancy: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 580–590. [Google Scholar] [CrossRef]
- Di Girolamo, R.; Liberati, M.; Silvi, C.; D’Antonio, F. Levothyroxine Supplementation in Euthyroid Pregnant Women with Positive Autoantibodies: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 13, 759064. [Google Scholar] [CrossRef]
- Lu, H.Q.; Hu, R. Lasting Effects of Intrauterine Exposure to Preeclampsia on Offspring and the Underlying Mechanism. Am. J. Perinatol. Rep. 2019, 9, e275–e291. [Google Scholar] [CrossRef] [PubMed]
- Paschou, S.A.; Bletsa, E.; Stampouloglou, P.K.; Tsigkou, V.; Valatsou, A.; Stefanaki, K.; Kazakou, P.; Spartalis, M.; Spartalis, E.; Oikonomou, E.; et al. Thyroid disorders and cardiovascular manifestations: An update. Endocrine 2022, 75, 672–683. [Google Scholar] [CrossRef]
- Fommei, E.; Iervasi, G. The role of thyroid hormone in blood pressure homeostasis: Evidence from short-term hypothyroidism in humans. J. Clin. Endocrinol. Metab. 2002, 87, 1996–2000. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jonklaas, J.; Bianco, A.C.; Bauer, A.J.; Burman, K.D.; Cappola, A.R.; Celi, F.S.; Cooper, D.S.; Kim, B.W.; Peeters, R.P.; Rosenthal, M.S.; et al. Guidelines for the treatment of hypothyroidism: Prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 2014, 24, 1670–1751. [Google Scholar] [CrossRef]
- Massolt, E.T.; Meima, M.E.; Swagemakers, S.M.A.; Leeuwenburgh, S.; van den Hout-van Vroonhoven, M.; Brigante, G.; Kam, B.L.R.; van der Spek, P.J.; van IJcken, W.F.J.; Visser, T.J.; et al. Thyroid State Regulates Gene Expression in Human Whole Blood. J. Clin. Endocrinol. Metab. 2018, 103, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Bochukova, E.; Schoenmakers, N.; Agostini, M.; Schoenmakers, E.; Rajanayagam, O.; Keogh, J.M.; Henning, E.; Reinemund, J.; Gevers, E.; Sarri, M.; et al. A mutation in the thyroid hormone receptor alpha gene. N. Engl. J. Med. 2012, 366, 243–249. [Google Scholar] [CrossRef]
- Mizuma, H.; Murakami, M.; Mori, M. Thyroid hormone activation in human vascular smooth muscle cells: Expression of type II iodothyronine deiodinase. Circ. Res. 2001, 88, 313–318. [Google Scholar] [CrossRef] [PubMed]
- White, P.; Dauncey, M.J. Differential expression of thyroid hormone receptor isoforms is strikingly related to cardiac and skeletal muscle phenotype during postnatal development. J. Mol. Endocrinol. 1999, 23, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Neggazi, S.; Hamlat, N.; Canaple, L.; Gauthier, K.; Samarut, J.; Bricca, G.; Aouichat-Bouguerra, S.; Beylot, M. TRalpha inhibits arterial renin-angiotensin system expression and prevents cholesterol accumulation in vascular smooth muscle cells. Ann. Endocrinol. 2019, 80, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Herring, P.T. The Thyroid Gland in Health and Disease. Nature 1917, 100, 202–203. [Google Scholar] [CrossRef][Green Version]
- McConnell, R.J.; Menendez, C.E.; Smith, F.R.; Henkin, R.I.; Rivlin, R.S. Defects of taste and smell in patients with hypothyroidism. Am. J. Med. 1975, 59, 354–364. [Google Scholar] [CrossRef] [PubMed]
- Baskoy, K.; Ay, S.A.; Altundag, A.; Kurt, O.; Salihoglu, M.; Deniz, F.; Tekeli, H.; Yonem, A.; Hummel, T. Is There Any Effect on Smell and Taste Functions with Levothyroxine Treatment in Subclinical Hypothyroidism? PLoS ONE 2016, 11, e0149979. [Google Scholar] [CrossRef] [PubMed]
- Beard, M.D.; Mackay-Sim, A. Loss of sense of smell in adult, hypothyroid mice. Dev. Brain Res. 1987, 433, 181–189. [Google Scholar] [CrossRef]
- Gunbey, E.; Karli, R.; Gokosmanoglu, F.; Duzgun, B.; Ayhan, E.; Atmaca, H.; Unal, R. Evaluation of olfactory function in adults with primary hypothyroidism. Int. Forum Allergy Rhinol. 2015, 5, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Pruszewicz, A.; Lacka, K.; Karlik, M. Olfaction in congenital hypothyroidism. Otolaryngol. Pol. 2002, 56, 577–581. [Google Scholar] [PubMed]
- Malnic, B.; Godfrey, P.A.; Buck, L.B. The human olfactory receptor gene family. Proc. Natl. Acad. Sci. USA 2004, 101, 2584–2589. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, P.A.; Malnic, B.; Buck, L.B. The mouse olfactory receptor gene family. Proc. Natl. Acad. Sci. USA 2004, 101, 2156–2161. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.; Axel, R. A novel multigene family may encode odorant receptors: A molecular basis for odor recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef] [PubMed]
- de Lima Hirata, A.H.; de Jesus Rocha, L.A.; da Silva, V.A.; de Almeida, R.J.; Bacigalupo, L.D.S.; Varela, P.; Martins, L.; Pesquero, J.B.; Delle, H.; Camacho, C.P. Circulating RNA Transcriptome of Pregnant Women with TSH Just Above the Trimester-Specific Reference and its Correlation with the Hypertensive Phenotype. Sci. Rep. 2020, 10, 6439. [Google Scholar] [CrossRef]
- Flegel, C.; Manteniotis, S.; Osthold, S.; Hatt, H.; Gisselmann, G. Expression profile of ectopic olfactory receptors determined by deep sequencing. PLoS ONE 2013, 8, e55368. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Nanduri, J.; Wang, N.; Kumar, G.K.; Bindokas, V.; Paul, B.D.; Chen, X.; Fox, A.P.; Vignane, T.; Filipovic, M.R.; et al. Hypoxia sensing requires H(2)S-dependent persulfidation of olfactory receptor 78. Sci. Adv. 2023, 9, eadf3026. [Google Scholar] [CrossRef] [PubMed]
- Demircan Sezer, S.; Küçük, M.; Nergiz Avcıoğlu, S.; Zafer, E.; Altinkaya, S.O.; Bıçakçı, B.; Yenisey, C.; Yuksel, H.; Kurt Ömürlü, İ. Comparison of maternal and umbilical cord blood HIF-1α and nitric oxide levels in early and late onset preeclamptic pregnancies. Gynecol. Endocrinol. 2015, 31, 945–948. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.J.; Su, X.; Wang, B.; Matthews, T.; Nanduri, J.; Prabhakar, N.R. Role of olfactory receptor78 in carotid body-dependent sympathetic activation and hypertension in murine models of chronic intermittent hypoxia. J. Neurophysiol. 2021, 125, 2054–2067. [Google Scholar] [CrossRef] [PubMed]
- Poll, B.G.; Xu, J.; Gupta, K.; Shubitowski, T.B.; Pluznick, J.L. Olfactory receptor 78 modulates renin but not baseline blood pressure. Physiol. Rep. 2021, 9, e15017. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Protzko, R.J.; Gevorgyan, H.; Peterlin, Z.; Sipos, A.; Han, J.; Brunet, I.; Wan, L.X.; Rey, F.; Wang, T.; et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc. Natl. Acad. Sci. USA 2013, 110, 4410–4415. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Nakashima, N.; Nakashima, K.; Takano, M. Olfactory receptor 78 is expressed in hypothalamic vasopressin/oxytocin neurons, parenchymal microglia and choroidal macrophages in mice. Mol. Brain 2022, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Shepard, B.D.; Pluznick, J.L. How does your kidney smell? Emerging roles for olfactory receptors in renal function. Pediatr. Nephrol. 2016, 31, 715–723. [Google Scholar] [CrossRef]
- Pluznick, J.L.; Zou, D.J.; Zhang, X.; Yan, Q.; Rodriguez-Gil, D.J.; Eisner, C.; Wells, E.; Greer, C.A.; Wang, T.; Firestein, S.; et al. Functional expression of the olfactory signaling system in the kidney. Proc. Natl. Acad. Sci. USA 2009, 106, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Simonides, W.S.; Mulcahey, M.A.; Redout, E.M.; Muller, A.; Zuidwijk, M.J.; Visser, T.J.; Wassen, F.W.; Crescenzi, A.; da-Silva, W.S.; Harney, J.; et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J. Clin. Investig. 2008, 118, 975–983. [Google Scholar] [CrossRef]
- Procopciuc, L.M.; Caracostea, G.; Hazi, G.; Nemeti, G.; Stamatian, F. D2-Thr92Ala, thyroid hormone levels and biochemical hypothyroidism in preeclampsia. Gynecol. Endocrinol. 2017, 33, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Koo, J. Olfactory receptors in non-chemosensory tissues. BMB Rep. 2012, 45, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Massberg, D.; Hatt, H. Human Olfactory Receptors: Novel Cellular Functions Outside of the Nose. Physiol. Rev. 2018, 98, 1739–1763. [Google Scholar] [CrossRef] [PubMed]
- Boeldt, D.S.; Bird, I.M. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J. Endocrinol. 2017, 232, R27–R44. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, Z.; Li, B.; Guo, S.; Yao, M. Expression and Clinical Significance of Serum 25-OH-D in pregnant women with SCH (Subclinical Hypothyroidism) and GDM (Gestational Diabetes Mellitus). Pak. J. Med. Sci. 2018, 34, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
- Daemen, A.; Manning, G. HER2 is not a cancer subtype but rather a pan-cancer event and is highly enriched in AR-driven breast tumors. Breast Cancer Res. 2018, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, G.; Kaltenberger, S.; Fiala, C.; Knofler, M.; Pollheimer, J. ERBB2 gene amplification increases during the transition of proximal EGFR(+) to distal HLA-G(+) first trimester cell column trophoblasts. Placenta 2015, 36, 803–808. [Google Scholar] [CrossRef]
- Mourouzis, I.; Tzovaras, A.; Armonis, B.; Ardavanis, A.; Skondra, M.; Misitzis, J.; Pectasides, D.; Pantos, C. Are Thyroid Hormone and Tumor Cell Proliferation in Human Breast Cancers Positive for HER2 Associated? Int. J. Endocrinol. 2015, 2015, 765406. [Google Scholar] [CrossRef]
- Oki, N.; Matsuo, H.; Nakago, S.; Murakoshi, H.; Laoag-Fernandez, J.B.; Maruo, T. Effects of 3,5,3′-triiodothyronine on the invasive potential and the expression of integrins and matrix metalloproteinases in cultured early placental extravillous trophoblasts. J. Clin. Endocrinol. Metab. 2004, 89, 5213–5221. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Spencer, T.E.; Wu, G. Mechanisms for the establishment and maintenance of pregnancy: Synergies from scientific collaborations. Biol. Reprod. 2018, 99, 225–241. [Google Scholar] [CrossRef]
- Kim, S.H.; Yoon, Y.C.; Lee, A.S.; Kang, N.; Koo, J.; Rhyu, M.R.; Park, J.H. Expression of human olfactory receptor 10J5 in heart aorta, coronary artery, and endothelial cells and its functional role in angiogenesis. Biochem. Biophys. Res. Commun. 2015, 460, 404–408. [Google Scholar] [CrossRef]
- Liou, L.; Hornburg, M.; Robertson, D.S. Global FDR control across multiple RNAseq experiments. Bioinformatics 2023, 39, btac718. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Tarca, A.L.; Pataki, B.A.; Romero, R.; Sirota, M.; Guan, Y.; Kutum, R.; Gomez-Lopez, N.; Done, B.; Bhatti, G.; Yu, T.; et al. Crowdsourcing assessment of maternal blood multi-omics for predicting gestational age and preterm birth. Cell Rep. Med. 2021, 2, 100323. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, 3.4.1 (2017-06-30) ed.; R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 14 February 2020).
- Marini, F.; Binder, H. pcaExplorer: An R/Bioconductor package for interacting with RNA-seq principal components. BMC Bioinform. 2019, 20, 331. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Galili, T. Dendextend: An R package for visualizing, adjusting and comparing trees of hierarchical clustering. Bioinformatics 2015, 31, 3718–3720. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Galen, R.S.; Gambino, S.R. Beyond Normality: The Predictive Value and Efficiency of Medical Diagnoses; Wiley: New York, NY, USA, 1975; p. 237. [Google Scholar]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]

| Healthy-Thyroid Pregnancy | Non-Treated Mild Gestational Hypothyroidism | Preeclampsia | p-Value | |
|---|---|---|---|---|
| Clinical history | ||||
| Age (years) | 23 (22–26) | 35 (22–38) | 31 (29–33) | 0.192 |
| Gestational age (weeks) | 38.7 (38.4–40.7) | 41 (37.2–41.0) | 34.5 (32.7–39.8) | 0.164 |
| Previous abortions | yes (66.7%) | no (100%) | yes (66.7%) | 0.165 |
| Twinning | no (100%) | yes (33.3%) | no (100%) | 0.325 |
| Cesarean birth | 1 (33.3%) | 2 (66.7%) | 3 (100%) | 0.223 |
| Vaginal delivery | 2 (66.7%) | 1 (33.3%) | - | 0.223 |
| Primigravida | 1 (33.3%) | 2 (66.7%) | - | 0.223 |
| Multigravida | 2 (66.7%) | 1 (33.3%) | 3 (100%) | 0.223 |
| Newborn weight (g) | 3515 (2695–3850) | 3290 (2595–3600) | 2730 (2575–3190) | 0.488 |
| Physical exam | ||||
| Weight (Kg) | 70.0 (66.0–70.6) | 87.5 (63.0–92.0) | 86.8 (67.0–102.0) | 0.396 |
| SBP (mmHg) * | 110 (110–120) | 118 (90–120) | 140 | 0.038 |
| DBP (mmHg) * | 60 (60–80) | 68 (60–80) | 110 (100–110) | 0.008 |
| Laboratory tests | ||||
| TSH (µIU/mL) * | 1.94 (1.69–2.86) | 4.36 (3.70–4.49) | 3.12 (1.74–3.29) | 0.049 |
| FT4 (µIU/mL) | 1 (0.93–1.00) | 1 (0.82–1.00) | 0.84 (0.77–1.14) | 0.797 |
| AntiTPO (UI/mL) | <5 | 5 (<5–7.27) | <5 | 0.368 |
| HTP | GHT | GHT-LT4 | p-Value | |
|---|---|---|---|---|
| Clinical history | ||||
| Age | 25 (18–39) | 23 (19–35) | 30.5 (18–43) | 0.341 |
| Gestational age a | 39.4 (37.1–43.0) | 39.8 (30.0–43.0) | 32.3 (6.8–41.0) | 0.030 |
| Previous abortions | yes (27.3%) | yes (14.3%) | yes (33.3%) | 0.711 |
| Twinning | no (100%) | yes (14.3%) | no (100%) | 0.282 |
| Primigravida | 3 (27.3%) | 3 (42.9%) | 3 (50%) | 0.606 |
| Multigravida | 8 (72.8%) | 4 (57.2%) | 3 (50%) | 0.614 |
| Alcoholist | no (100%) | no (100%) | no (100%) | - |
| Smoker a | no (100%) | no (100%) | yes (33.3%) | 0.038 |
| Physical exam | ||||
| Weight (Kg) | 71.0 (52.0–90.0) | 83.9 (63.0–143.5) | 77.1 (50.9–102.6) | 0.251 |
| SBP (mmHg) | 110 (90–144) | 110 (100–130) | 117 (100–130) | 0.922 |
| DBP (mmHg) | 72 (54–90) | 70 (60–89) | 73 (60–80) | 0.968 |
| Laboratory tests | ||||
| TSH (µIU/mL) | 2 (0.56–2.94) | 4.16 (3.35–7.38) | 1.83 (1.08–4.02) | 0.001 |
| FT4 (µIU/mL) | 0.82 (0.64–1.12) | 0.93 (0.80–1.08) | 0.75 (0.64–0.98) | 0.243 |
| Relative Expression data | ||||
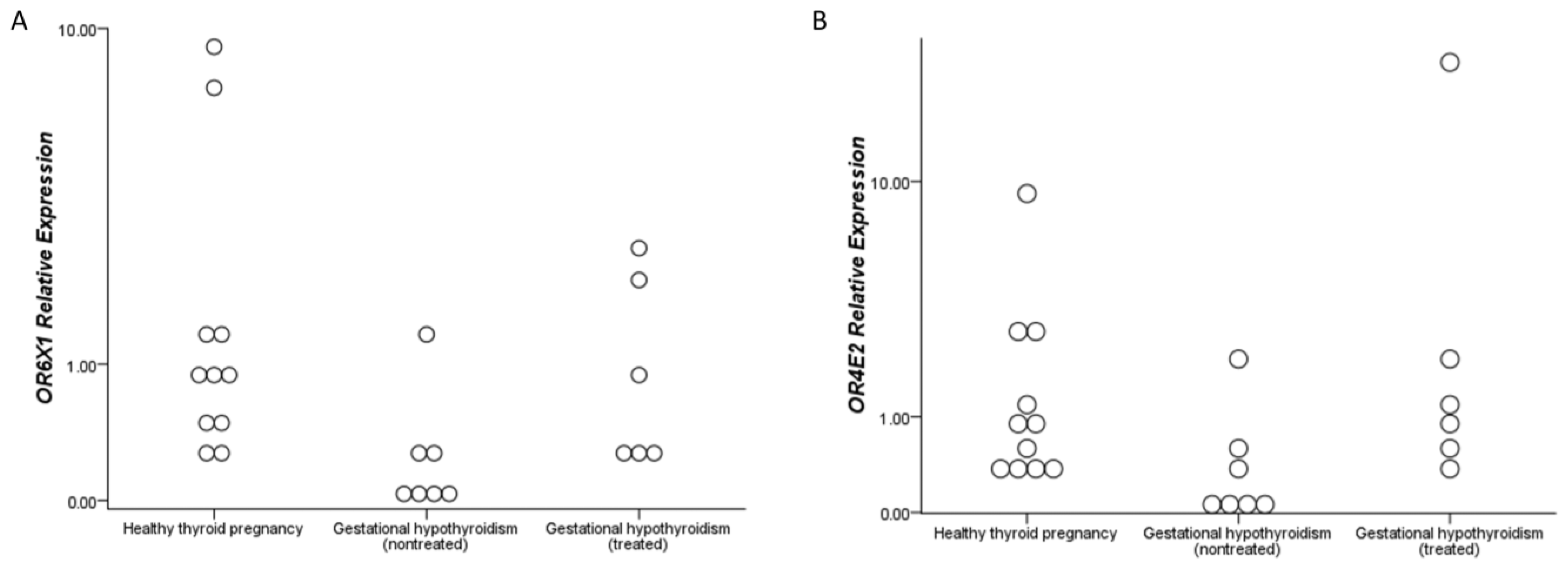
| OR6X1 b,c,d | 0.831 (0.267–9.027) | 0.062 (0.007– 1.352) | 0.637 (0.314–2.605) | 0.002 |
| OR4E2 a,b,c | 0.824 (0.323–9.085) | 0.116 (0.006– 2.101) | 0.957 (0.289–25.107) | 0.014 |
| Parameters | AUC | Cutoff | Expression | chi-Square | Sensitivity | Specificity | PPV | NPV | Accuracy | κ | PL | NL | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X2 | p-Value | ||||||||||||
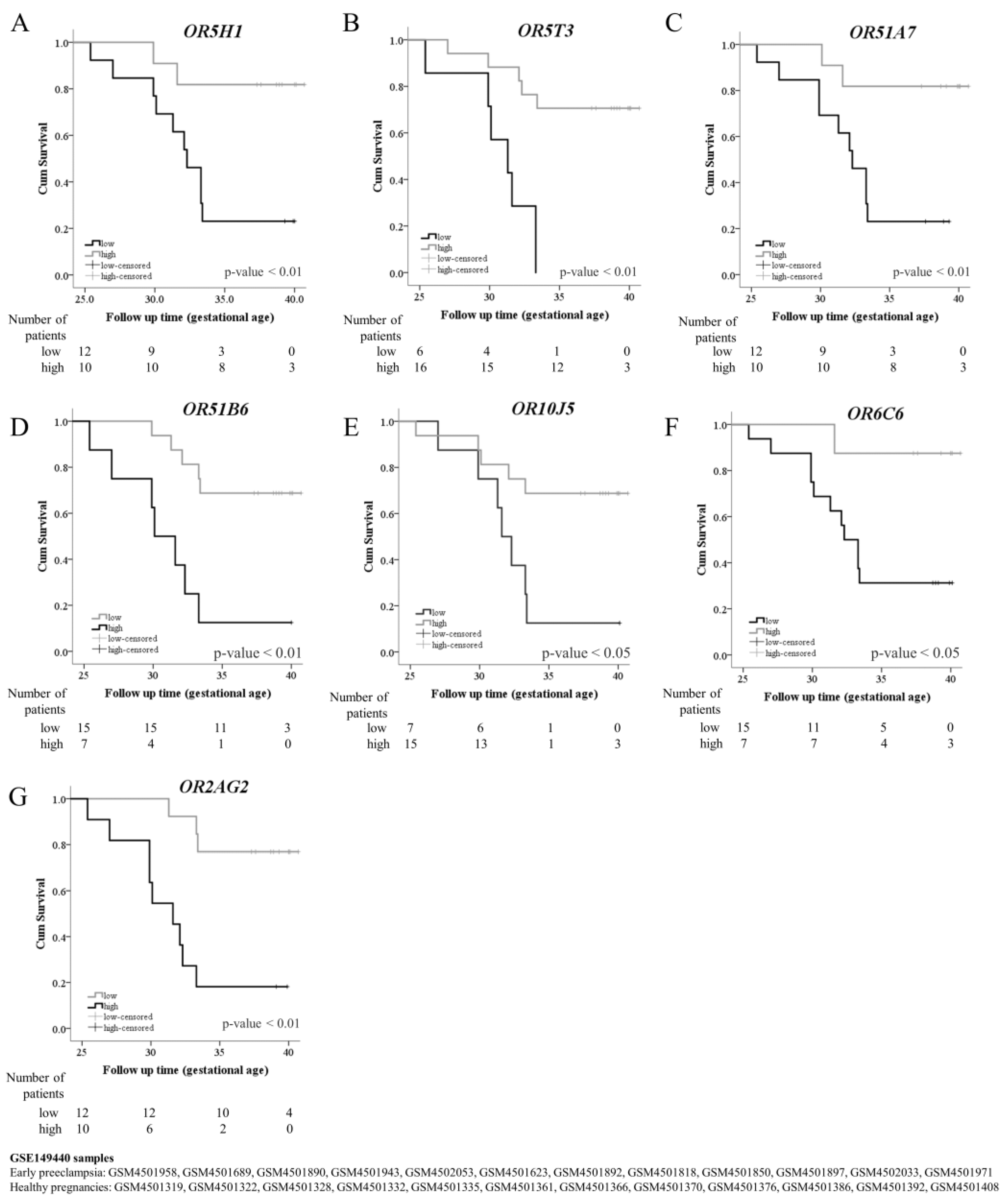
| OR5H1 | 0.847 | 2.60 | Loss | 8.224 | 0.004 | 0.83 | 0.75 | 0.77 | 0.82 | 0.79 | 0.58 | 3.33 | 0.22 |
| OR5T3 | 0.819 | 2.28 | Loss | 9.882 | 0.005 | 0.58 | 0.96 | 0.94 | 0.69 | 0.77 | 0.54 | 15.00 | 0.44 |
| OR51A7 | 0.819 | 2.74 | Loss | 8.224 | 0.004 | 0.83 | 0.75 | 0.77 | 0.82 | 0.79 | 0.58 | 3.33 | 0.22 |
| OR51B6 | 0.771 | 3.18 | Gain | 6.750 | 0.027 | 0.58 | 0.92 | 0.88 | 0.69 | 0.75 | 0.50 | 7.00 | 0.45 |
| OR10J5 | 0.764 | 2.83 | Loss | 6.750 | 0.027 | 0.58 | 0.92 | 0.88 | 0.69 | 0.75 | 0.50 | 7.00 | 0.45 |
| OR6C6 | 0.764 | 2.67 | Loss | 6.750 | 0.027 | 0.92 | 0.58 | 0.69 | 0.88 | 0.75 | 0.50 | 2.20 | 0.14 |
| OR2AG2 | 0.785 | 4.70 | Gain | 8.224 | 0.004 | 0.75 | 0.83 | 0.82 | 0.77 | 0.79 | 0.58 | 4.50 | 0.30 |
| OR2W1 | 0.743 | 2.63 | Loss | 12.000 | 0.001 | 0.96 | 0.65 | 0.74 | 0.94 | 0.81 | 0.62 | 2.78 | 0.06 |
| OR5B17 | 0.750 | 2.81 | Loss | 6.750 | 0.027 | 0.58 | 0.92 | 0.88 | 0.69 | 0.75 | 0.50 | 7.00 | 0.45 |
| OR11G2 | 0.771 | 2.97 | Loss | 6.171 | 0.013 | 0.67 | 0.83 | 0.80 | 0.71 | 0.75 | 0.50 | 4.00 | 0.40 |
| OR6C65 | 0.781 | 2.53 | Loss | 6.171 | 0.013 | 0.67 | 0.83 | 0.80 | 0.71 | 0.75 | 0.50 | 4.00 | 0.40 |
| OR11A1 | 0.785 | 3.09 | Loss | 6.000 | 0.014 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.50 | 3.00 | 0.33 |
| ANN (all ORs) | 1.000 | 0.53 | 24.000 | <0.001 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.92 | 25.00 | 0.04 | |
| Olfactory Receptor | Hazard Ratio | 95%CI | p-Value |
|---|---|---|---|
| OR5H1 | 6.08 | 1.32–27.96 | 0.021 |
| OR5T3 | 7.42 | 2.12–26.01 | 0.002 |
| OR51A7 | 6.17 | 1.34–28.39 | 0.019 |
| OR51B6 | 0.18 | 0.06–0.59 | 0.004 |
| OR10J5 | 3.89 | 1.22–12.43 | 0.022 |
| OR6C6 | 7.99 | 1.03–62.21 | 0.047 |
| OR2AG2 | 0.141 | 0.03–0.53 | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirata, A.H.d.L.; Camargo, L.A.d.J.R.; Silva, V.A.d.; Almeida, R.J.d.; Bacigalupo, L.d.S.; Albejante, M.C.; Curi, F.S.d.; Varela, P.; Martins, L.; Pesquero, J.B.; et al. Exploring the Potential of Olfactory Receptor Circulating RNA Measurement for Preeclampsia Prediction and Its Linkage to Mild Gestational Hypothyroidism. Int. J. Mol. Sci. 2023, 24, 16681. https://doi.org/10.3390/ijms242316681
Hirata AHdL, Camargo LAdJR, Silva VAd, Almeida RJd, Bacigalupo LdS, Albejante MC, Curi FSd, Varela P, Martins L, Pesquero JB, et al. Exploring the Potential of Olfactory Receptor Circulating RNA Measurement for Preeclampsia Prediction and Its Linkage to Mild Gestational Hypothyroidism. International Journal of Molecular Sciences. 2023; 24(23):16681. https://doi.org/10.3390/ijms242316681
Chicago/Turabian StyleHirata, Andréa Harumy de Lima, Luiz Antônio de Jesus Rocha Camargo, Valdelena Alessandra da Silva, Robson José de Almeida, Lucas dos Santos Bacigalupo, Maria Clara Albejante, Flavia Salomão d’Avila Curi, Patrícia Varela, Leonardo Martins, João Bosco Pesquero, and et al. 2023. "Exploring the Potential of Olfactory Receptor Circulating RNA Measurement for Preeclampsia Prediction and Its Linkage to Mild Gestational Hypothyroidism" International Journal of Molecular Sciences 24, no. 23: 16681. https://doi.org/10.3390/ijms242316681
APA StyleHirata, A. H. d. L., Camargo, L. A. d. J. R., Silva, V. A. d., Almeida, R. J. d., Bacigalupo, L. d. S., Albejante, M. C., Curi, F. S. d., Varela, P., Martins, L., Pesquero, J. B., Delle, H., & Camacho, C. P. (2023). Exploring the Potential of Olfactory Receptor Circulating RNA Measurement for Preeclampsia Prediction and Its Linkage to Mild Gestational Hypothyroidism. International Journal of Molecular Sciences, 24(23), 16681. https://doi.org/10.3390/ijms242316681








