Quantitative Proteomics of Maternal Blood Plasma in Isolated Intrauterine Growth Restriction
Abstract
:1. Introduction
2. Results
2.1. Features of Pregnancy Course and Outcomes with IUGR
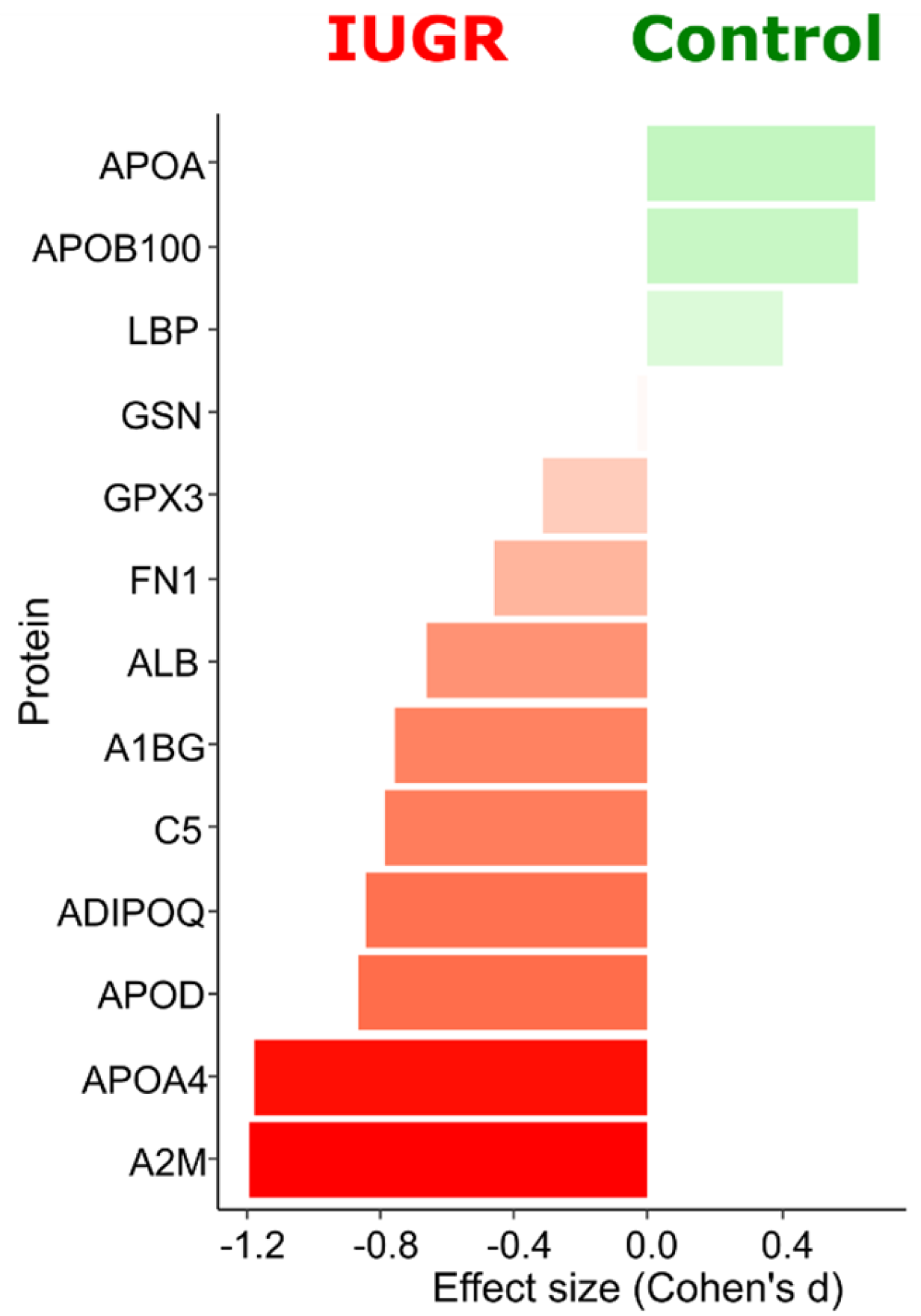
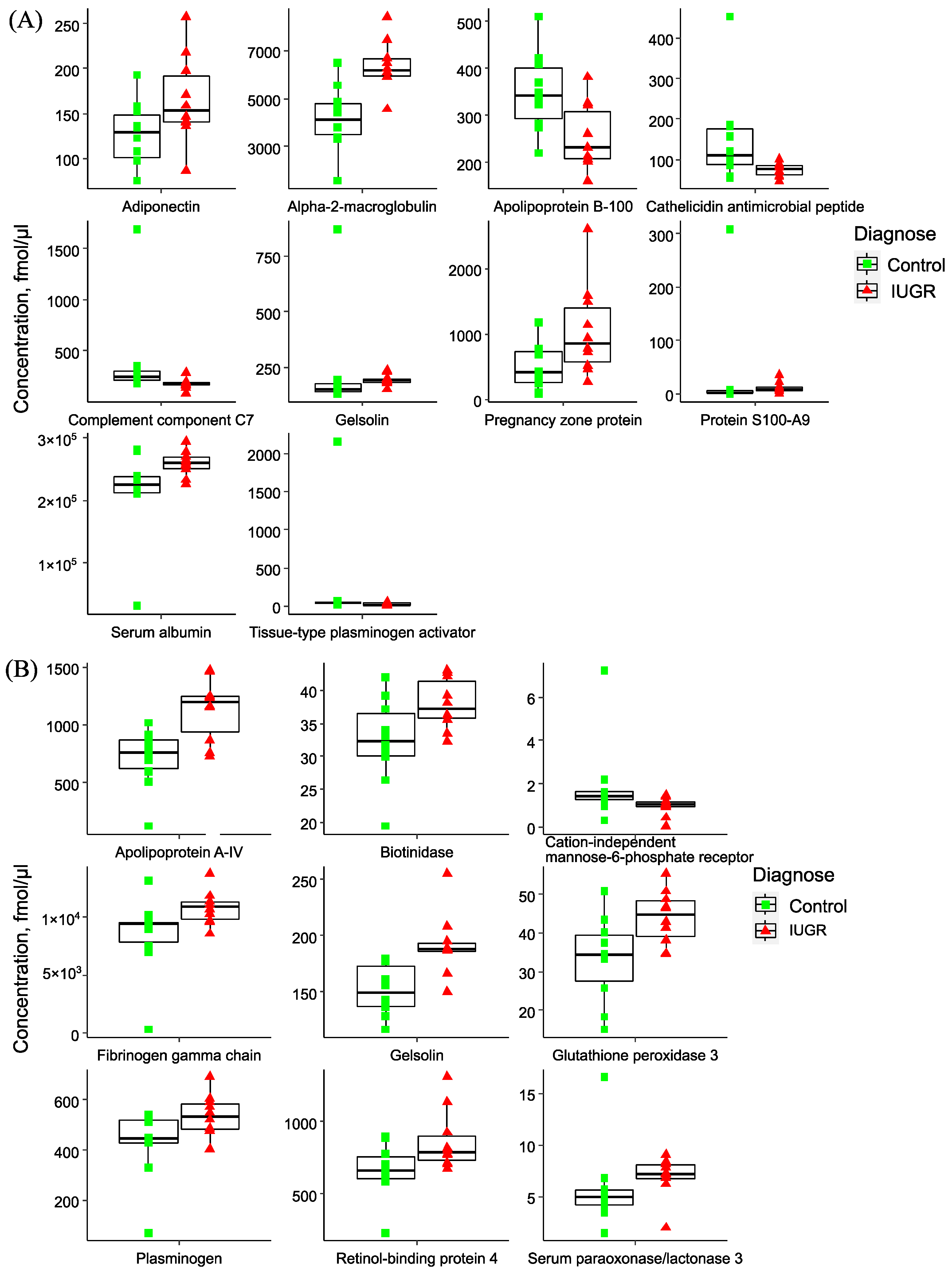
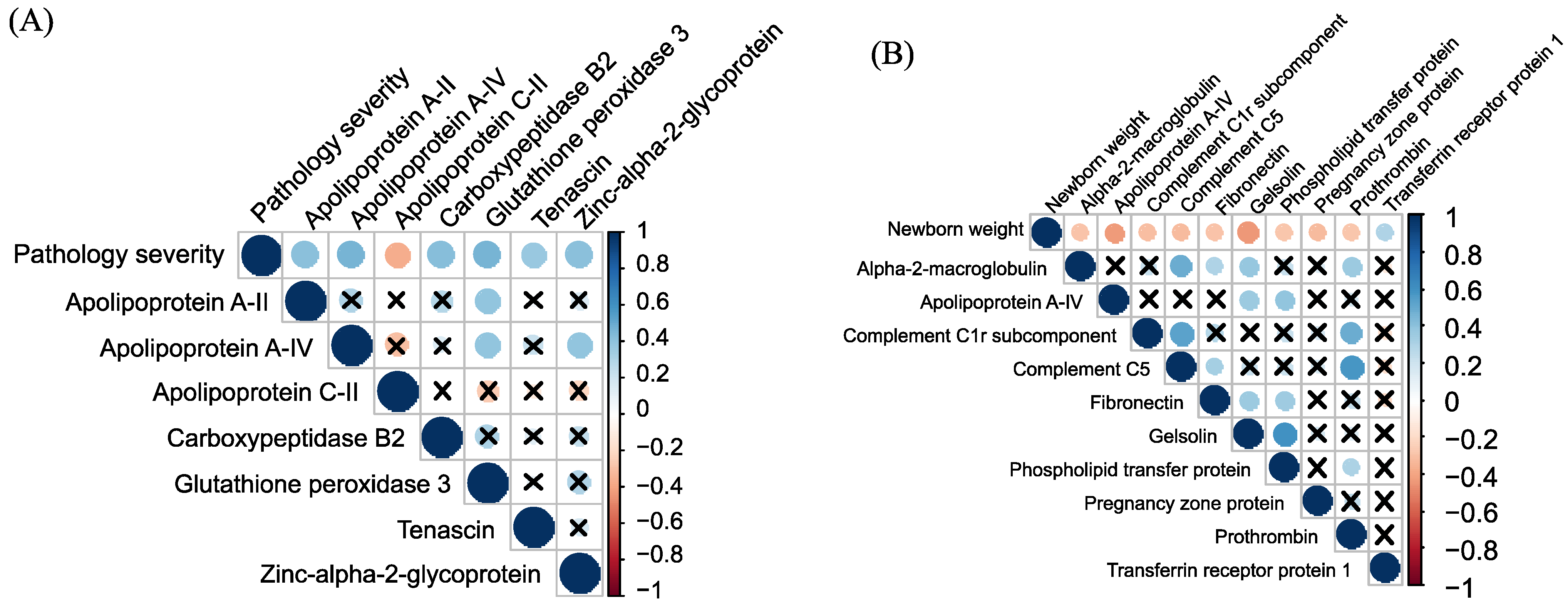
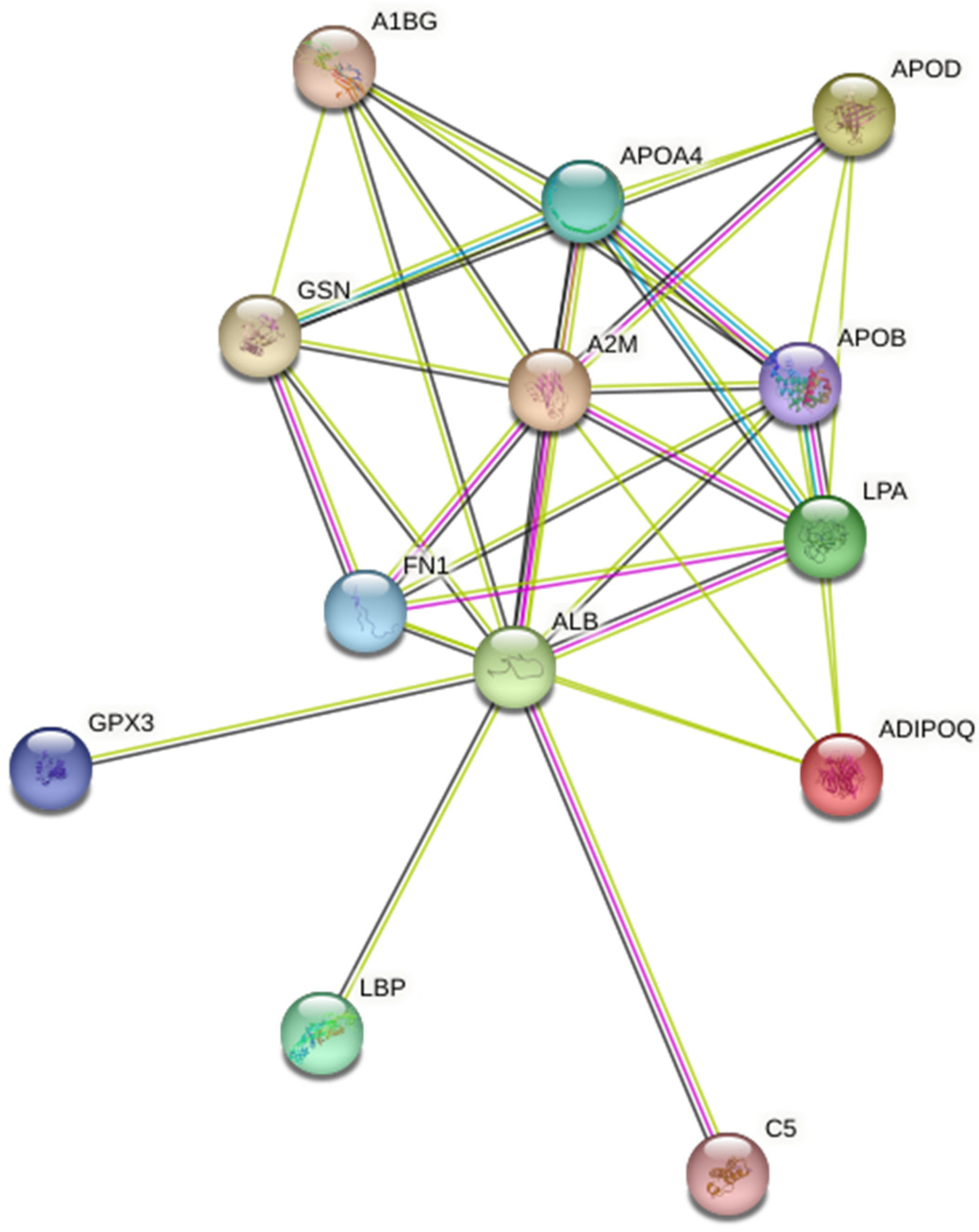
2.2. Quantitative Analysis of Plasma Proteins in Maternal Blood
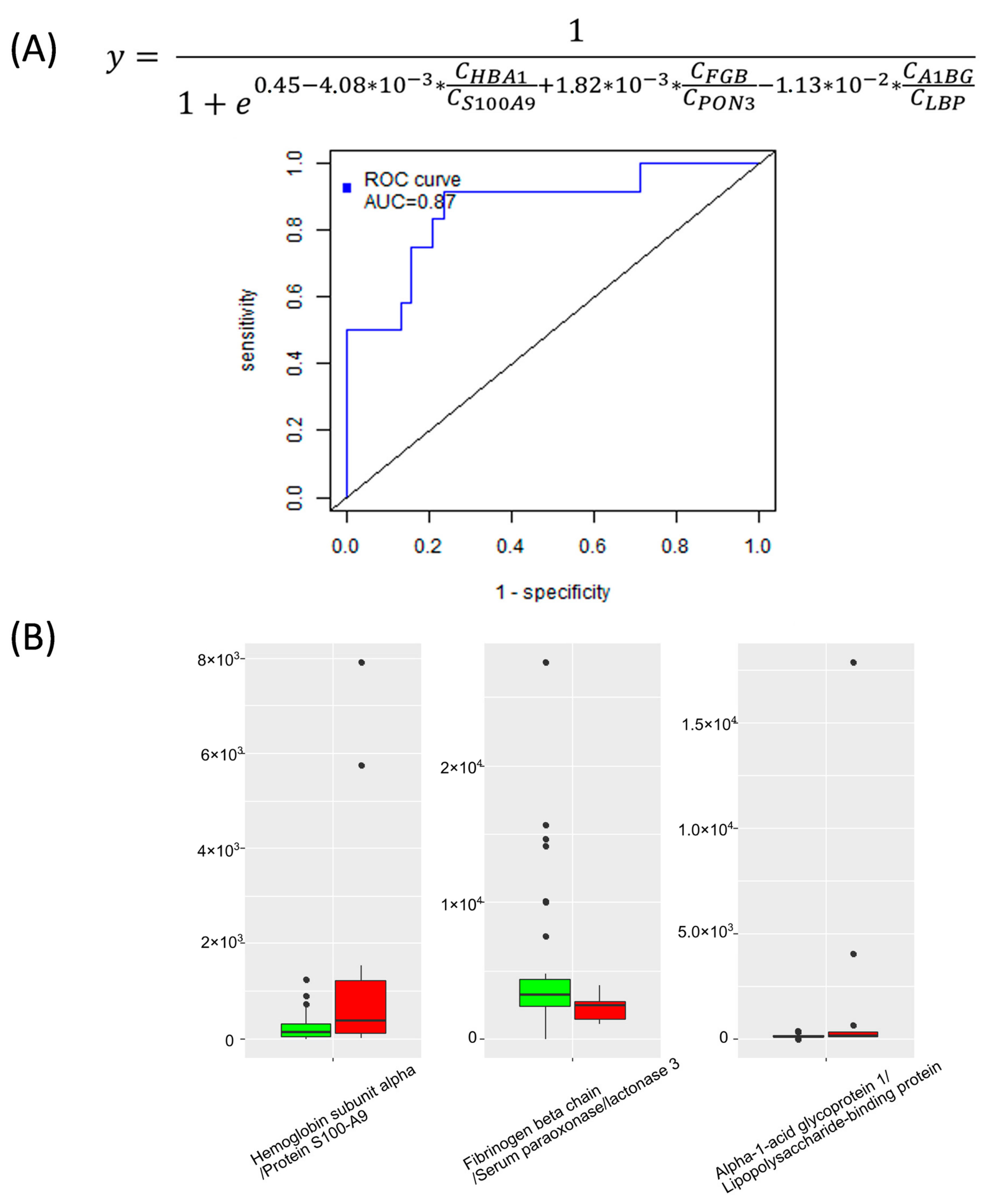
2.3. Binary Classifiers Based on Plasma Proteins Level in Maternal Blood
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Maternal Plasma Collection
4.3. Plasma Sample Preparation for Quantitative Analysis
4.4. Quantitative Analysis of 125 Plasma Proteins by LC-MRM MS
4.5. Statistical Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- King, V.J.; Bennet, L.; Stone, P.R.; Clark, A.; Gunn, A.J.; Dhillon, S.K. Fetal growth restriction and stillbirth: Biomarkers for identifying at risk fetuses. Front. Physiol. 2022, 13, 959750. [Google Scholar] [CrossRef]
- Biks, G.A.; Blencowe, H.; Hardy, V.P.; Geremew, B.M.; Angaw, D.A.; Wagnew, A.; Abebe, S.M.; Guadu, T.; Martins, J.S.D.; Fisker, A.B.; et al. Birthweight data completeness and quality in population-based surveys: EN-INDEPTH study. Popul. Health Metr. 2021, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Lees, C.C.; Stampalija, T.; Baschat, A.; da Silva Costa, F.; Ferrazzi, E.; Figueras, F.; Hecher, K.; Poon, L.C.; Salomon, L.J.; Unterscheider, J. ISUOG Practice Guidelines: Diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet. Gynecol. 2020, 56, 298–312. [Google Scholar] [CrossRef] [PubMed]
- Albu, A.R.; Anca, A.F.; Horhoianu, V.V.; Horhoianu, I.A. Predictive factors for intrauterine growth restriction. J. Med. Life 2014, 7, 165–171. [Google Scholar] [PubMed]
- Pedroso, M.A.; Palmer, K.R.; Hodges, R.J.; da Costa, F.S.; Rolnik, D.L. Uterine artery doppler in screening for preeclampsia and fetal growth restriction. Rev. Bras. Ginecol. Obstet. 2018, 40, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Kwiatkowska, E.; Rzepka, R.; Torbe, A.; Dolegowska, B. Ischemic placental syndrome—Prediction and new disease monitoring. J. Matern. Neonatal Med. 2016, 29, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, B. Placental origins of preeclampsia: Challenging the current hypothesis. Hypertension 2008, 51, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.V.; Fedorov, I.S.; Brzhozovskiy, A.G.; Bugrova, A.E.; Chagovets, V.V.; Volochaeva, M.V.; Starodubtseva, N.L.; Frankevich, V.E.; Nikolaev, E.N.; Shmakov, R.G.; et al. Mirnas and their gene targets—A clue to differentiate pregnancies with small for gestational age newborns, intrauterine growth restriction, and preeclampsia. Diagnostics 2021, 11, 729. [Google Scholar] [CrossRef]
- Chen, W.; Wei, Q.; Liang, Q.; Song, S.; Li, J. Diagnostic capacity of sFlt-1/PlGF ratio in fetal growth restriction: A systematic review and meta-analysis. Placenta 2022, 127, 37–42. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Papageorghiou, A.T.; Kennedy, S.H.; Villar, J. Novel biomarkers for predicting intrauterine growth restriction: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2013, 120, 681–694. [Google Scholar] [CrossRef]
- Smith, G.C.S. Developing Novel Tests to Screen for Fetal Growth Restriction. Trends Mol. Med. 2021, 27, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Gordijn, S.J.; Beune, I.M.; Thilaganathan, B.; Papageorghiou, A.; Baschat, A.A.; Baker, P.N.; Silver, R.M.; Wynia, K.; Ganzevoort, W. Consensus definition of fetal growth restriction: A Delphi procedure. Ultrasound Obstet. Gynecol. 2016, 48, 333–339. [Google Scholar] [CrossRef]
- MacHado Nardozza, L.M.; Junior, E.A.; Barbosa, M.M.; Rabachini Caetano, A.C.; Re Lee, D.J.; Moron, A.F. Fetal growth restriction: Current knowledge to the general Obs/Gyn. Arch. Gynecol. Obstet. 2012, 286, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Kumar, S. Circulating biomarkers associated with placental dysfunction and their utility for predicting fetal growth restriction. Clin. Sci. 2023, 137, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Pijnenborg, R.; Vercruysse, L.; Romero, R. The “great Obstetrical Syndromes” are associated with disorders of deep placentation. Am. J. Obstet. Gynecol. 2011, 204, 193–201. [Google Scholar] [CrossRef]
- Aplin, J.D.; Myers, J.E.; Timms, K.; Westwood, M. Tracking placental development in health and disease. Nat. Rev. Endocrinol. 2020, 16, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, I.; Leavey, K.; Benton, S.J.; Grynspan, D.; Bainbridge, S.A.; Cox, B.J. Placental transcriptional and histologic subtypes of normotensive fetal growth restriction are comparable to preeclampsia. Am. J. Obstet. Gynecol. 2019, 220, 110.e1–110.e21. [Google Scholar] [CrossRef]
- Salomon, L.J.; Alfirevic, Z.; Da Silva Costa, F.; Deter, R.L.; Figueras, F.; Ghi, T.; Glanc, P.; Khalil, A.; Lee, W.; Napolitano, R.; et al. ISUOG Practice Guidelines: Ultrasound assessment of fetal biometry and growth. Ultrasound Obstet. Gynecol. 2019, 53, 715–723. [Google Scholar] [CrossRef]
- Osuchukwu, O.O.; Reed, D.J. Small for Gestational Age; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Hocquette, A.; Durox, M.; Wood, R.; Klungsøyr, K.; Szamotulska, K.; Berrut, S.; Rihs, T.; Kyprianou, T.; Sakkeus, L.; Lecomte, A.; et al. International versus national growth charts for identifying small and large-for-gestational age newborns: A population-based study in 15 European countries. Lancet Reg. Health–Eur. 2021, 8, 100167. [Google Scholar] [CrossRef]
- Vasaks, B.; Koenen, V.; Koster, M.P.H.; Hukkelhoven, C.W.P.M.; Franx, A.; Hanson, M.A.; Visser, G.H.A. Human fetal growth is constrained below optimal for perinatal survival. Ultrasound Obstet. Gynecol. 2015, 45, 162–167. [Google Scholar] [CrossRef]
- Abbatiello, S.E.; Schilling, B.; Mani, D.R.; Zimmerman, L.J.; Hall, S.C.; MacLean, B.; Albertolle, M.; Allen, S.; Burgess, M.; Cusack, M.P.; et al. Large-scale interlaboratory study to develop, analytically validate and apply highly multiplexed, quantitative peptide assays to measure cancer-relevant proteins in plasma. Mol. Cell. Proteom. 2015, 14, 2357–2374. [Google Scholar] [CrossRef] [PubMed]
- Percy, A.J.; Mohammed, Y.; Yang, J.; Borchers, C.H. A standardized kit for automated quantitative assessment of candidate protein biomarkers in human plasma. Bioanalysis 2015, 7, 2991–3004. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, P.; Roome, S.; Borchers, C.H.; Goodlett, D.R.; Mohammed, Y. An Update on MRMAssayDB: A Comprehensive Resource for Targeted Proteomics Assays in the Community. J. Proteome Res. 2021, 20, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Domanski, D.; Percy, A.J.; Yang, J.; Chambers, A.G.; Hill, J.S.; Freue, G.V.C.; Borchers, C.H. MRM-based multiplexed quantitation of 67 putative cardiovascular disease biomarkers in human plasma. Proteomics 2012, 12, 1222–1243. [Google Scholar] [CrossRef] [PubMed]
- Kononikhin, A.S.; Zakharova, N.V.; Semenov, S.D.; Bugrova, A.E.; Brzhozovskiy, A.G.; Indeykina, M.I.; Fedorova, Y.B.; Kolykhalov, I.V.; Strelnikova, P.A.; Ikonnikova, A.Y.; et al. Prognosis of Alzheimer’s Disease Using Quantitative Mass Spectrometry of Human Blood Plasma Proteins and Machine Learning. Int. J. Mol. Sci. 2022, 23, 7907. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Dai, D.L.; Wilson-McManus, J.; Smith, D.; Francis, G.A.; Borchers, C.H.; McManus, B.M.; Hill, J.S.; Cohen Freue, G.V. Multiplexed LC–ESI–MRM-MS-based Assay for Identification of Coronary Artery Disease Biomarkers in Human Plasma. Proteom.-Clin. Appl. 2019, 13, 1700111. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Gies, A.; Weigl, K.; Tikk, K.; Benner, A.; Schrotz-King, P.; Borchers, C.H.; Brenner, H. Evaluation and validation of plasma proteins using two different protein detection methods for early detection of colorectal cancer. Cancers 2019, 11, 1426. [Google Scholar] [CrossRef]
- Starodubtseva, N.L.; Tokareva, A.O.; Rodionov, V.V.; Brzhozovskiy, A.G.; Bugrova, A.E.; Chagovets, V.V.; Kometova, V.V.; Kukaev, E.N.; Soares, N.C.; Kovalev, G.I.; et al. Integrating Proteomics and Lipidomics for Evaluating the Risk of Breast Cancer Progression: A Pilot Study. Biomedicines 2023, 11, 1786. [Google Scholar] [CrossRef]
- Pacora, P.; Romero, R.; Jung, E.; Gudicha, D.W.; Hernandez-Andrade, E.; Musilova, I.; Kacerovsky, M.; Jaiman, S.; Erez, O.; Hsu, C.D.; et al. Reduced fetal growth velocity precedes antepartum fetal death. Ultrasound Obstet. Gynecol. 2021, 57, 942–952. [Google Scholar] [CrossRef]
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal morbidities of fetal growth restriction: Pathophysiology and impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef]
- Fung, C.; Zinkhan, E. Short- and Long-Term Implications of Small for Gestational Age. Obstet. Gynecol. Clin. N. Am. 2021, 48, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Dudink, I.; Hüppi, P.S.; Sizonenko, S.V.; Castillo-Melendez, M.; Sutherland, A.E.; Allison, B.J.; Miller, S.L. Altered trajectory of neurodevelopment associated with fetal growth restriction. Exp. Neurol. 2022, 347, 113885. [Google Scholar] [CrossRef] [PubMed]
- Crispi, F.; Miranda, J.; Gratacós, E. Long-term cardiovascular consequences of fetal growth restriction: Biology, clinical implications, and opportunities for prevention of adult disease. Am. J. Obstet. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Erez, O.; Maymon, E.; Xu, Z.; Pacora, P.; Done, B.; Hassan, S.S.; Tarca, A.L.; Arbor, A.; Lansing, E. The Maternal Plasma Proteome Changes as a Function of Gestational Age in Normal Pregnancy: A Longitudinal Study. Am. J. Obstet. Gynecol. 2015, 155, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.; Youssef, L.; Miranda, J.; Crovetto, F.; Estanyol, J.M.; Fernandez, G.; Crispi, F.; Gratacós, E. Maternal proteomic profiling reveals alterations in lipid metabolism in late-onset fetal growth restriction. Sci. Rep. 2020, 10, 21033. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Simões, R.V.; Paules, C.; Cañueto, D.; Pardo-Cea, M.A.; García-Martín, M.L.; Crovetto, F.; Fuertes-Martin, R.; Domenech, M.; Gómez-Roig, M.D.; et al. Metabolic profiling and targeted lipidomics reveals a disturbed lipid profile in mothers and fetuses with intrauterine growth restriction. Sci. Rep. 2018, 8, 13614. [Google Scholar] [CrossRef] [PubMed]
- Pecks, U.; Caspers, R.; Schiessl, B.; Bauerschlag, D.; Piroth, D.; Maass, N.; Rath, W. The evaluation of the oxidative state of low-density lipoproteins in intrauterine growth restriction and preeclampsia. Hypertens. Pregnancy 2012, 31, 156–165. [Google Scholar] [CrossRef]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and cardiovascular disease: Biomarker and potential therapeutic target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef]
- Herrera, E. Lipid metabolism in pregnancy and its consequences in the fetus and newborn. Endocrine 2002, 19, 43–55. [Google Scholar] [CrossRef]
- Alahakoon, T.I.; Medbury, H.J.; Williams, H.; Lee, V.W. Lipid profiling in maternal and fetal circulations in preeclampsia and fetal growth restriction-A prospective case control observational study. BMC Pregnancy Childbirth 2020, 20, 61. [Google Scholar] [CrossRef]
- Lu, Y.; Jia, Z.; Su, S.; Han, L.; Meng, L.; Tang, G.; Wang, J.; Zhang, C.; Xie, X.; Zhang, Y.; et al. Establishment of trimester-specific reference intervals of serum lipids and the associations with pregnancy complications and adverse perinatal outcomes: A population-based prospective study. Ann. Med. 2021, 53, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Wölter, M.; Okai, C.A.; Smith, D.S.; Ruß, M.; Rath, W.; Pecks, U.; Borchers, C.H.; Glocker, M.O. Maternal Apolipoprotein B100 Serum Levels are Diminished in Pregnancies with Intrauterine Growth Restriction and Differentiate from Controls. Proteom.-Clin. Appl. 2018, 12, e1800017. [Google Scholar] [CrossRef] [PubMed]
- Lo Vasco, V.R.; Salmaso, R.; Zanardo, V.; Businaro, R.; Visentin, S.; Trevisanuto, D.; Cosmi, E. Fetal aorta wall inflammation in ultrasound-detected aortic intima/media thickness and growth retardation. J. Reprod. Immunol. 2011, 91, 103–107. [Google Scholar] [CrossRef] [PubMed]
- López-Bermejo, A.; Fernández-Real, J.M.; Garrido, E.; Rovira, R.; Brichs, R.; Genaró, P.; Bach, C.; Cabrero, D.; Kihara, S.; Funahashi, T.; et al. Maternal soluble tumour necrosis factor receptor type 2 (sTNFR2) and adiponectin are both related to blood pressure during gestation and infant’s birthweight. Clin. Endocrinol. 2004, 61, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.R.; Semple, R.K. Hypoadiponectinemia—Cause or consequence of human “insulin resistance”? J. Clin. Endocrinol. Metab. 2010, 95, 1544–1554. [Google Scholar] [CrossRef] [PubMed]
- Mazaki-Tovi, S.; Kanety, H.; Pariente, C.; Hemi, R.; Wiser, A.; Schiff, E.; Sivan, E. Maternal serum adiponectin levels during human pregnancy. J. Perinatol. 2007, 27, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Rosario, F.J.; Schumacher, M.A.; Jiang, J.; Kanai, Y.; Powell, T.L.; Jansson, T. Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J. Physiol. 2012, 590, 1495–1509. [Google Scholar] [CrossRef] [PubMed]
- Aye, I.L.M.H.; Powell, T.L.; Jansson, T. Review: Adiponectin-The missing link between maternal adiposity, placental transport and fetal growth? Placenta 2013, 34, S40–S45. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, J.; Zhang, Y.; Liu, S.; Yang, J.; Yin, T. Regulation and Function of Chemokines at the Maternal–Fetal Interface. Front. Cell Dev. Biol. 2022, 10, 826053. [Google Scholar] [CrossRef]
- Girardi, G.; Lingo, J.J.; Fleming, S.D.; Regal, J.F. Essential Role of Complement in Pregnancy: From Implantation to Parturition and Beyond. Front. Immunol. 2020, 11, 1681. [Google Scholar] [CrossRef]
- Tayade, C.; Esadeg, S.; Fang, Y.; Croy, B.A. Functions of alpha 2 macroglobulins in pregnancy. Mol. Cell. Endocrinol. 2005, 245, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Cater, J.H.; Kumita, J.R.; Abdallah, R.Z.; Zhao, G.; Bernardo-Gancedo, A.; Henry, A.; Winata, W.; Chi, M.; Grenyer, B.S.F.; Townsend, M.L.; et al. Human pregnancy zone protein stabilizes misfolded proteins including preeclampsia- and Alzheimer’sassociated amyloid beta peptide. Proc. Natl. Acad. Sci. USA 2019, 116, 6101–6110. [Google Scholar] [CrossRef] [PubMed]
- Löb, S.; Vattai, A.; Kuhn, C.; Schmoeckel, E.; Mahner, S.; Wöckel, A.; Kolben, T.; Keil, C.; Jeschke, U.; Vilsmaier, T. Pregnancy Zone Protein (PZP) is significantly upregulated in the decidua of recurrent and spontaneous miscarriage and negatively correlated to Glycodelin A (GdA). J. Reprod. Immunol. 2021, 143, 103267. [Google Scholar] [CrossRef] [PubMed]
- Löb, S.; Vattai, A.; Kuhn, C.; Mittelberger, J.; Herbert, S.-L.; Wöckel, A.; Schmoeckel, E.; Mahner, S.; Jeschke, U. The Pregnancy Zone Protein (PZP) is significantly downregulated in the placenta of preeclampsia and HELLP syndrome patients. J. Reprod. Immunol. 2022, 153, 103663. [Google Scholar] [CrossRef] [PubMed]
- Fosheim, I.K.; Jacobsen, D.P.; Sugulle, M.; Alnaes-Katjavivi, P.; Fjeldstad, H.E.S.; Ueland, T.; Lekva, T.; Staff, A.C. Serum amyloid A1 and pregnancy zone protein in pregnancy complications and correlation with markers of placental dysfunction. Am. J. Obstet. Gynecol. MFM 2023, 5, 100794. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, P.; Liu, M.; Huang, Z.; Yang, X.; Ding, Y.; Liu, J.; Cheng, X.; Xu, S.; He, M.; et al. Alpha-2-macroglobulin is involved in the occurrence of early-onset pre-eclampsia via its negative impact on uterine spiral artery remodeling and placental angiogenesis. BMC Med. 2023, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Neel, N.R.; Goldenberg, R.L.; Tamura, T.; Cliver, S.P.; Avent, K.; Hoffman, H.J. Maternal alpha-2-macroglobulin levels and fetal growth in Guatemala. Int. J. Gynecol. Obstet. 1992, 38, 25–29. [Google Scholar] [CrossRef]
- Al-Azemi, M.; Raghupathy, R.; Azizieh, F. Pro-inflammatory and anti-inflammatory cytokine profiles in fetal growth restriction. Clin. Exp. Obstet. Gynecol. 2017, 44, 98–103. [Google Scholar] [CrossRef]
- Rashid, C.S.; Bansal, A.; Simmons, R.A. Oxidative stress, intrauterine growth restriction, and developmental programming of type 2 diabetes. Physiology 2018, 33, 348–359. [Google Scholar] [CrossRef]
- Richani, K.; Soto, E.; Romero, R.; Espinoza, J.; Chaiworapongsa, T.; Nien, J.K.; Edwin, S.; Kim, Y.M.; Hong, J.S.; Mazor, M. Normal pregnancy is characterized by systemic activation of the complement system. J. Matern. Neonatal Med. 2005, 17, 239–245. [Google Scholar] [CrossRef]
- Mitchell, M.D.; Peiris, H.N.; Kobayashi, M.; Koh, Y.Q.; Duncombe, G.; Illanes, S.E.; Rice, G.E.; Salomon, C. Placental exosomes in normal and complicated pregnancy. Am. J. Obstet. Gynecol. 2015, 213, S173–S181. [Google Scholar] [CrossRef]
- Auer, J.; Camoin, L.; Guillonneau, F.; Rigourd, V.; Chelbi, S.T.; Leduc, M.; Laparre, J.; Mignot, T.M.; Vaiman, D. Serum profile in preeclampsia and intra-uterine growth restriction revealed by iTRAQ technology. J. Proteom. 2010, 73, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Youssef, L.; Miranda, J.; Blasco, M.; Paules, C.; Crovetto, F.; Palomo, M.; Torramade-Moix, S.; García-Calderó, H.; Tura-Ceide, O.; Dantas, A.P.; et al. Complement and coagulation cascades activation is the main pathophysiological pathway in early-onset severe preeclampsia revealed by maternal proteomics. Sci. Rep. 2021, 11, 3048. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Braekke, K.; Holthe, M.R.; Harsem, N.K.; Fagerhol, M.K.; Staff, A.C. Calprotectin, a marker of inflammation, is elevated in the maternal but not in the fetal circulation in preeclampsia. Am. J. Obstet. Gynecol. 2005, 193, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, J.W.M.; Bevers, E.M.; Lindhout, T. Platelet activation and blood coagulation. Thromb. Haemost. 2002, 88, 186–193. [Google Scholar] [PubMed]
- Herter, J.M.; Rossaint, J.; Zarbock, A. Platelets in inflammation and immunity. J. Thromb. Haemost. 2014, 12, 1764–1775. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G.; Sargent, I.L. Preeclampsia and the systemic inflammatory response. Semin. Nephrol. 2004, 24, 565–570. [Google Scholar] [CrossRef]
- Erez, O.; Romero, R.; Vaisbuch, E.; Kusanovic, J.P.; Mazaki-Tovi, S.; Chaiworapongsa, T.; Gotsch, F.; Mittal, P.; Edwin, S.S.; Nhan-Chang, C.-L.; et al. The pattern and magnitude of “in vivo thrombin generation” differ in women with preeclampsia and in those with SGA fetuses without preeclampsia. J. Matern. Neonatal Med. 2018, 31, 1671–1680. [Google Scholar] [CrossRef]
- De Boer, K.; ten Cate, J.W.; Sturk, A.; Borm, J.J.J.; Treffers, P.E. Enhanced thrombin generation in normal and hypertensive pregnancy. Am. J. Obstet. Gynecol. 1989, 160, 95–100. [Google Scholar] [CrossRef]
- Groom, K.M.; David, A.L. The role of aspirin, heparin, and other interventions in the prevention and treatment of fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S829–S840. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B. Haemostatic changes in pregnancy. Thromb. Res. 2004, 114, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Godtfredsen, A.C.; Sidelmann, J.J.; Dolleris, B.B.; Jørgensen, J.S.; Johansen, E.K.J.; Pedersen, M.F.B.; Palarasah, Y.; Gram, J.B. Fibrinolytic Changes in Women with Preeclampsia. Clin. Appl. Thromb. 2022, 28, 10760296221126172. [Google Scholar] [CrossRef] [PubMed]
- Belo, L.; Santos-Silva, A.; Rumley, A.; Lowe, G.; Pereira-Leite, L.; Quintanilha, A.; Rebelo, I. Elevated tissue plasminogen activator as a potential marker of endothelial dysfunction in pre-eclampsia: Correlation with proteinuria. BJOG Int. J. Obstet. Gynaecol. 2002, 109, 1250–1255. [Google Scholar] [CrossRef]
- Oladosu-Olayiwola, O.; Olawumi, H.; Babatunde, A.; Ijaiya, M.; Durotoye, I.; Biliaminu, S.; Ibraheem, R. Fibrinolytic proteins of normal pregnancy and pre-eclamptic patients in North West Nigeria. Afr. Health Sci. 2018, 18, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Sucak, G.T.; Acar, K.; Sucak, A.; Kirazli, S.; Haznedar, R. Increased global fibrinolytic capacity as a clue for activated fibrinolysis in pre-eclampsia. Blood Coagul. Fibrinolysis 2006, 17, 347–352. [Google Scholar] [CrossRef]
- Wang, F.; Kohan, A.B.; Lo, C.M.; Liu, M.; Howles, P.; Tso, P. Apolipoprotein A-IV: A protein intimately involved in metabolism. J. Lipid Res. 2015, 56, 1403–1418. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Ko, C.W.; Tso, P.; Bhargava, A. Apolipoprotein A-IV: A multifunctional protein involved in protection against atherosclerosis and diabetes. Cells 2019, 8, 319. [Google Scholar] [CrossRef]
- Xu, X.R.; Wang, Y.; Adili, R.; Ju, L.; Spring, C.M.; Jin, J.W.; Yang, H.; Neves, M.A.D.; Chen, P.; Yang, Y.; et al. Apolipoprotein A-IV binds αIIbβ3 integrin and inhibits thrombosis. Nat. Commun. 2018, 9, 3608. [Google Scholar] [CrossRef]
- Ma, J.; Guo, D.; Miao, W.; Wang, Y.; Yan, L.; Wu, F.; Zhang, C.; Zhang, R.; Zuo, P.; Yang, G.; et al. The value of (18)F-FDG PET/CT-based radiomics in predicting perineural invasion and outcome in non-metastatic colorectal cancer. Abdom. Radiol. 2022, 47, 1244–1254. [Google Scholar] [CrossRef]
- Zeng, Q.; Jiang, H.; Lu, F.; Fu, M.; Bi, Y.; Zhou, Z.; Cheng, J.; Qin, J. Prediction of the immunological and prognostic value of five signatures related to fatty acid metabolism in patients with cervical cancer. Front. Oncol. 2022, 12, 1003222. [Google Scholar] [CrossRef] [PubMed]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 9781118548387. [Google Scholar]
- Nick, T.G.; Campbell, K.M. Logistic regression. Methods Mol. Biol. 2007, 404, 273–301. [Google Scholar] [CrossRef] [PubMed]
- Leite, D.F.B.; de Melo, E.F.; Souza, R.T.; Kenny, L.C.; Cecatti, J.G. Fetal and neonatal growth restriction: New criteria, renew challenges. J. Pediatr. 2018, 203, 462–463. [Google Scholar] [CrossRef] [PubMed]
- Beune, I.M.; Bloomfield, F.H.; Ganzevoort, W.; Embleton, N.D.; Rozance, P.J.; van Wassenaer-Leemhuis, A.G.; Wynia, K.; Gordijn, S.J. Consensus Based Definition of Growth Restriction in the Newborn. J. Pediatr. 2018, 196, 71–76.e1. [Google Scholar] [CrossRef] [PubMed]
- Whiteaker, J.R.; Halusa, G.N.; Hoofnagle, A.N.; Sharma, V.; MacLean, B.; Yan, P.; Wrobel, J.A.; Kennedy, J.; Mani, D.R.; Zimmerman, L.J.; et al. CPTAC Assay Portal: A repository of targeted proteomic assays. Nat. Methods 2014, 11, 703–704. [Google Scholar] [CrossRef]
- Tokareva, A.O.; Chagovets, V.V.; Kononikhin, A.S.; Starodubtseva, N.L.; Frankevich, V.E.; Nikolaev, E.N. Pipeline of mass-spectrometry data processing for diagnostic molecular marker panel obtaining using the example of search markers of breast cancer metastasis. Biomed. Chem. Res. Methods 2021, 4, e00156. [Google Scholar] [CrossRef]
- Tokareva, A.O.; Chagovets, V.V.; Kononikhin, A.S.; Starodubtseva, N.L.; Nikolaev, E.N.; Frankevich, V.E. Comparison of the effectiveness of variable selection method for creating a diagnostic panel of biomarkers for mass spectrometric lipidome analysis. J. Mass Spectrom. 2021, 56, e4702. [Google Scholar] [CrossRef]
| Group 1, Early IUGR (n = 10) | Group 2, Late IUGR (n = 10) | Group 3, Early Control (<32 wks) (n = 10) | Group 4, Late Control (≥32 wks) (n = 10) | Group 5, SGA (n = 10) | p-Value | |
|---|---|---|---|---|---|---|
| Age, years M ± SD (95% Cl) | 34 ± 6 (30–38) | 31 ± 5 (27–34) | 30 ± 4 (27–33) | 30 ± 3 (28–33) | 32 ± 4 (28–35) | ≥0.05 |
| BMI M ± SD (95% CI) | 24 ± 3 (22–26) | 24 ± 3 (22–26) | 28 ± 6 (24–32) | 27 ± 5 (24–31) | 26 ± 5 (22–30) | ≥0.05 |
| IUGR onset, weeks M ± SD (95% CI) | 25 ± 3 (23–27) | 34 ± 2 (33–35) | - | - | 35 ± 2 (34–36) | <0.05 * p1/2 < 0.001 p1/5 < 0.001 |
| Ultrasound-based fetal weight estimation, percentile Me [Q1;Q3;] | 2 [1;3] | 1 [0;2] | 43 [21;66] | 59 [45;74] | 2 [1;4] | <0.05 * p1/3 = 0.012 p1/4 = 0.001 p2/3 = 0.001 p2/4 < 0.001 p3/5 = 0.025 p4/5 = 0.004 |
| Uterine artery pulsatility index (UtA PI), left, percentile Me [Q1;Q3;] | 88 [76;99] | 97 [78;99] | 47 [42;78] | 88 [66;94] | 65 [53;74] | ≥0.05 |
| Uterine artery pulsatility index (UtA PI), right, percentile Me [Q1;Q3;] | 91 [62;100] | 95 [55;99] | 71 [42;89] | 94 [75;98] | 58 [36;79] | ≥0.05 |
| Umbilical artery pulsatility index (UA PI), percentile Me [Q1;Q3;] | 99 [84;100] | 83 [54;97] | 67 [52;75] | 68 [27;87] | 52 [37;68] | ≥0.05 |
| Cerebroplacental ratio (CPR), percentile Me [Q1;Q3;] | 1 [1;2] | 16 [2;39] | 33 [27;42] | 62 [36;67] | 54 [34;60] | <0.05 * p1/5 = 0.026 p1/4 = 0.004 |
| Fetal middle cerebral artery pulsatility index (MCA PI), percentile Me [Q1;Q3;] | 12 [2;18] | 17 [3;47] | 28 [19;41] | 89 [52;96] | 49 [31;59] | <0.05 * p1/4 = 0.006 |
| Delivery time, days Me [Q1;Q3;] | 217 [208;250] | 260 [253;268] | 260 [227;262] | 276 [250;282] | 267 [264;270] | <0.05 * p1/5 = 0.008 p1/4 = 0.003 |
| Sensitivity | Specificity | AUC | Variables | β | CI β | Z | P | |
|---|---|---|---|---|---|---|---|---|
| Early IUGR vs. early control | 0.9 | 0.9 | 0.86 | Intercept | −5.08 | −11.04–−1.66 | −2.27 | 0.02 |
| Alpha-2-macroglobulin2 | 1.71 × 10−7 | 6.15 × 10−8–3.54 × 10−7 | 2.42 | 0.02 | ||||
| Late IUGR vs. late control | 0.9 | 0.8 | 0.88 | Intercept | −7.68 | −17.89–−2.37 | −2.02 | 0.04 |
| Alpha-2-macroglobulin * Apolipoprotein A-IV | 1.45 × 10−6 | 4.55 × 10−7–3.39 × 10−6 | 1.98 | 0.047 | ||||
| Late IUGR vs. SGA | 0.8 | 0.8 | 0.8 | Intercept | 5.31 | 1.36–11.39 | 2.15 | 0.03 |
| Antithrombin-III * Apolipoprotein C-I | −2.89 × 10−7 | −6.20 × 10−7–−7.80 × 10−8 | −2.15 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starodubtseva, N.L.; Tokareva, A.O.; Volochaeva, M.V.; Kononikhin, A.S.; Brzhozovskiy, A.G.; Bugrova, A.E.; Timofeeva, A.V.; Kukaev, E.N.; Tyutyunnik, V.L.; Kan, N.E.; et al. Quantitative Proteomics of Maternal Blood Plasma in Isolated Intrauterine Growth Restriction. Int. J. Mol. Sci. 2023, 24, 16832. https://doi.org/10.3390/ijms242316832
Starodubtseva NL, Tokareva AO, Volochaeva MV, Kononikhin AS, Brzhozovskiy AG, Bugrova AE, Timofeeva AV, Kukaev EN, Tyutyunnik VL, Kan NE, et al. Quantitative Proteomics of Maternal Blood Plasma in Isolated Intrauterine Growth Restriction. International Journal of Molecular Sciences. 2023; 24(23):16832. https://doi.org/10.3390/ijms242316832
Chicago/Turabian StyleStarodubtseva, Natalia L., Alisa O. Tokareva, Maria V. Volochaeva, Alexey S. Kononikhin, Alexander G. Brzhozovskiy, Anna E. Bugrova, Angelika V. Timofeeva, Evgenii N. Kukaev, Victor L. Tyutyunnik, Natalia E. Kan, and et al. 2023. "Quantitative Proteomics of Maternal Blood Plasma in Isolated Intrauterine Growth Restriction" International Journal of Molecular Sciences 24, no. 23: 16832. https://doi.org/10.3390/ijms242316832
APA StyleStarodubtseva, N. L., Tokareva, A. O., Volochaeva, M. V., Kononikhin, A. S., Brzhozovskiy, A. G., Bugrova, A. E., Timofeeva, A. V., Kukaev, E. N., Tyutyunnik, V. L., Kan, N. E., Frankevich, V. E., Nikolaev, E. N., & Sukhikh, G. T. (2023). Quantitative Proteomics of Maternal Blood Plasma in Isolated Intrauterine Growth Restriction. International Journal of Molecular Sciences, 24(23), 16832. https://doi.org/10.3390/ijms242316832








