Nanocomposite Materials Based on Polylactide and Gold Complex Compounds for Absorbed Dose Diagnostics in BNCT
Abstract
1. Introduction
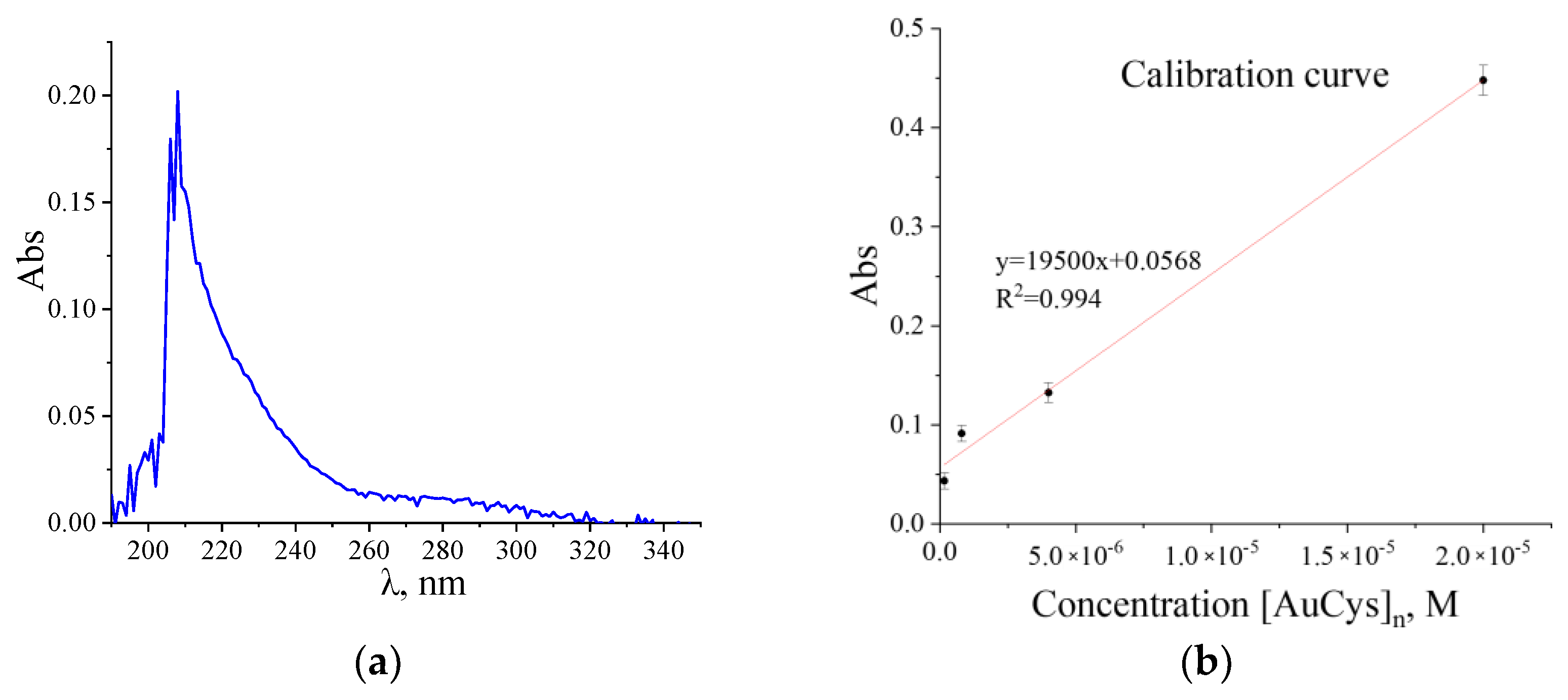
2. Results and Discussion
- (1)
- Polylactide swelled in water for the first week, and there was practically no decrease in molecular weight during this period.
- (2)
- After the end of the first period, hydrolysis of PLA ester bonds occurred during the next 2 weeks, resulting in a 20% decrease in Mn, with low molecular weight hydrolysis products remaining within the material.
- (3)
- At the third stage, a drop in the degradation rate was observed due to the shift of thermodynamic equilibrium towards high molecular weight compounds, which was in turn due to the increased concentration of low molecular weight compounds in the surrounding aqueous medium.
3. Materials and Methods
3.1. Materials
3.1.1. PLA-Based Film Materials
3.1.2. Obtaining Nanoporous PLA Films
3.1.3. [AuCys]n Synthesis
3.1.4. Obtaining PLA-[AuCys]n Composites
3.2. Research Methods
3.2.1. UV-Vis Spectroscopy
3.2.2. Thermogravimetric Analysis
3.2.3. Differential Scanning Calorimetry
3.2.4. Electron Microscopy
3.2.5. Atomic Force Microscopy
3.2.6. Analysis of Molecular Weight Characteristics of PLA
3.2.7. Dynamic Light Scattering (DLS)
3.2.8. Statistical Analysis
3.3. Cytotoxicity Assay
3.3.1. Cell Lines
3.3.2. MTT Test
3.3.3. Colony Forming Assay
4. Conclusions
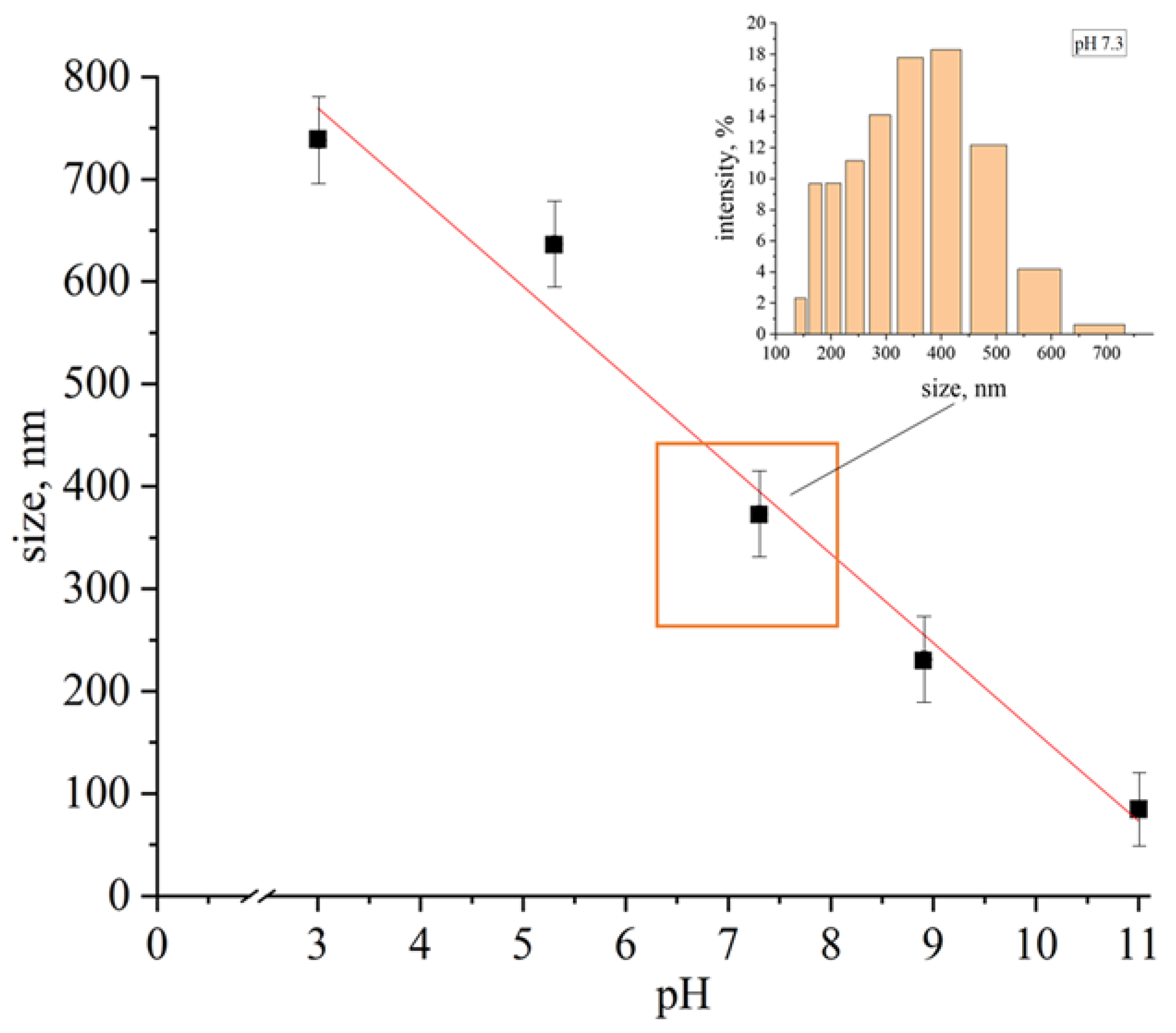
- The optimization of conditions for the synthesis of a cysteine–gold complex compound was carried out. The method of controlling the particle size and stability of gold complex compounds by changing the pH was shown for the first time. With increasing pH, the stability of the solution increased from several minutes to weeks. Moreover, there was a linear decrease in particle size from hundreds to tens of nanometers due to the formation of a soluble form of the [Au(SR)2]− type complex in alkaline medium.
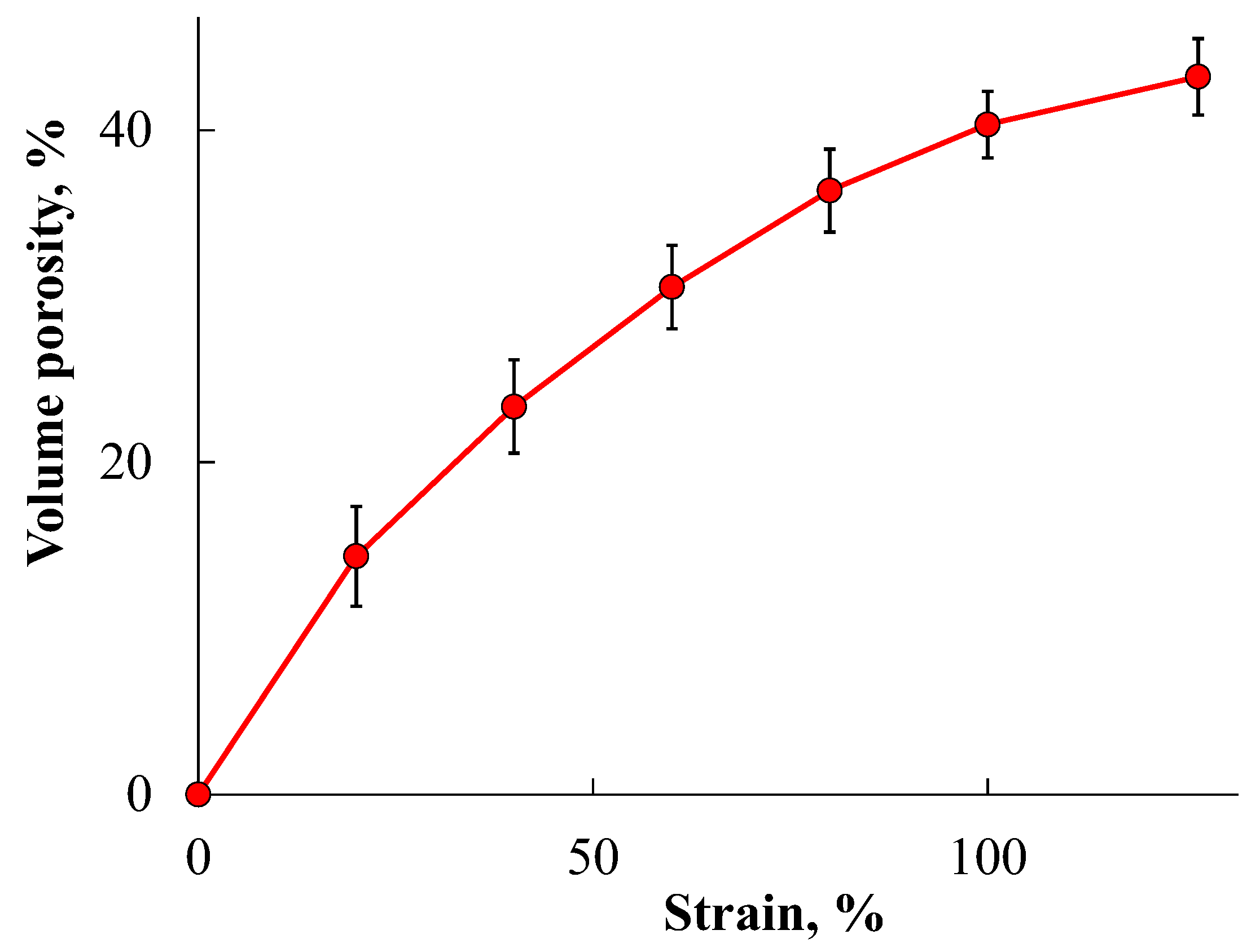
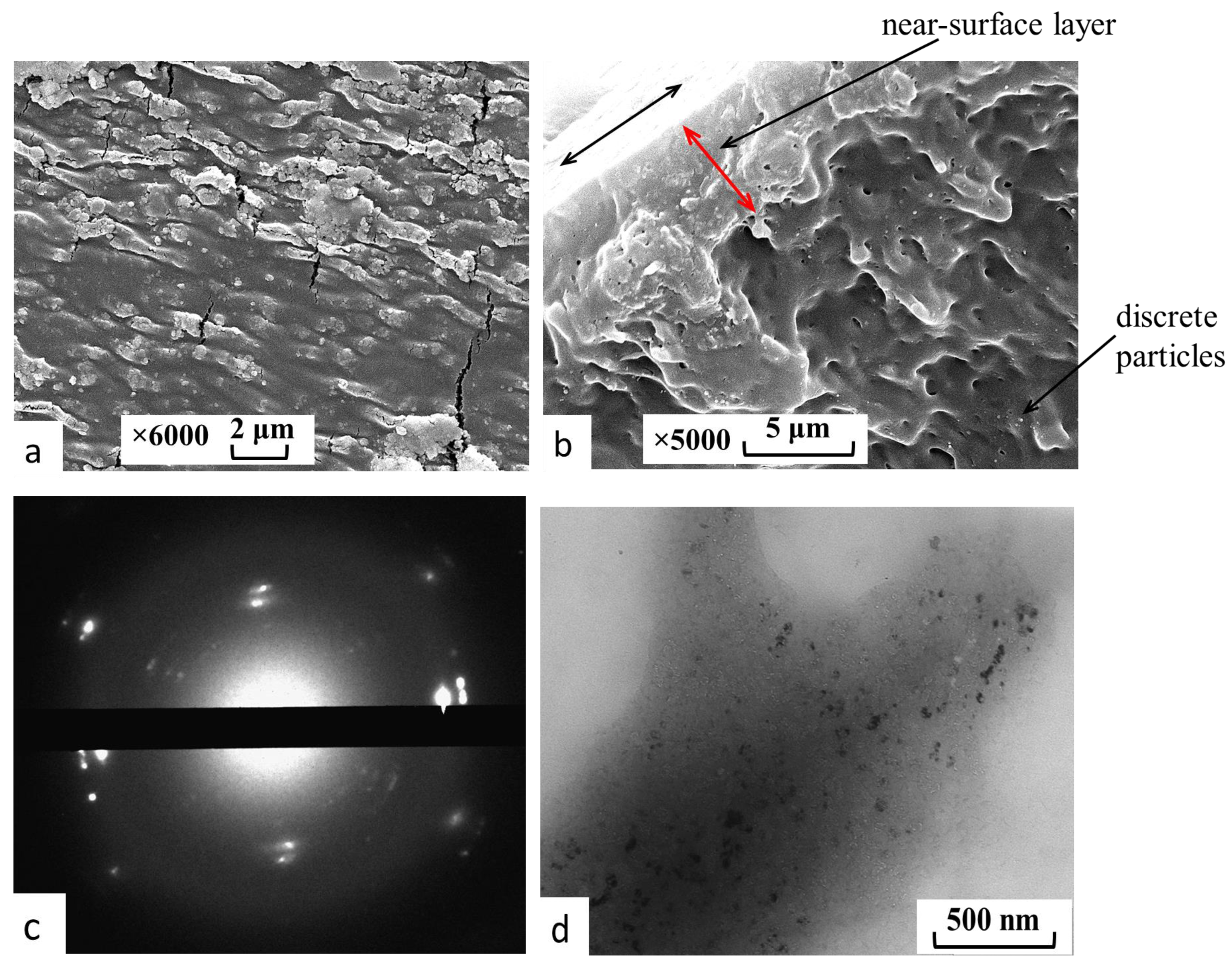
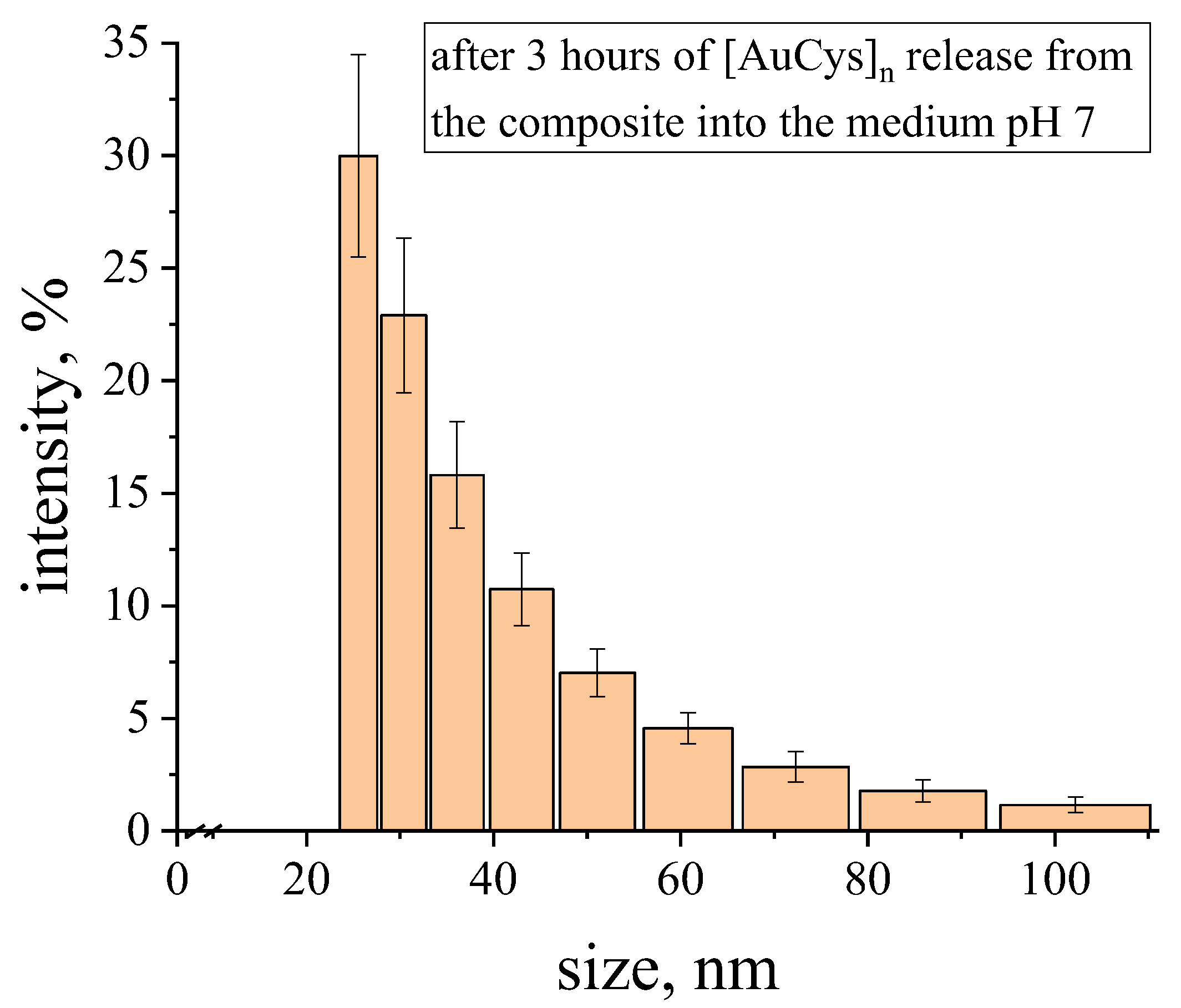
- The content, size and character of the distribution of gold complex compounds synthesized in the volume of nanoporous PLA matrices was determined. The ability to control the formation of gold complex salt particles within the pore size of the polymer was demonstrated for the first time. It was found that the synthesis of gold complex salts in the macroscopic volume led to the formation of particles of about 350 nm in solution without polymer stabilization, while the particles were 5–20 nm in size when directly synthesized in the pores of the polymer matrix obtained by the crazing mechanism. The sizes of the formed gold complex compound particles were retained after their release from the polymer pores. The results obtained are important for application in BNCT dosimetry, since particles smaller than 100 nm are desirable for this task.
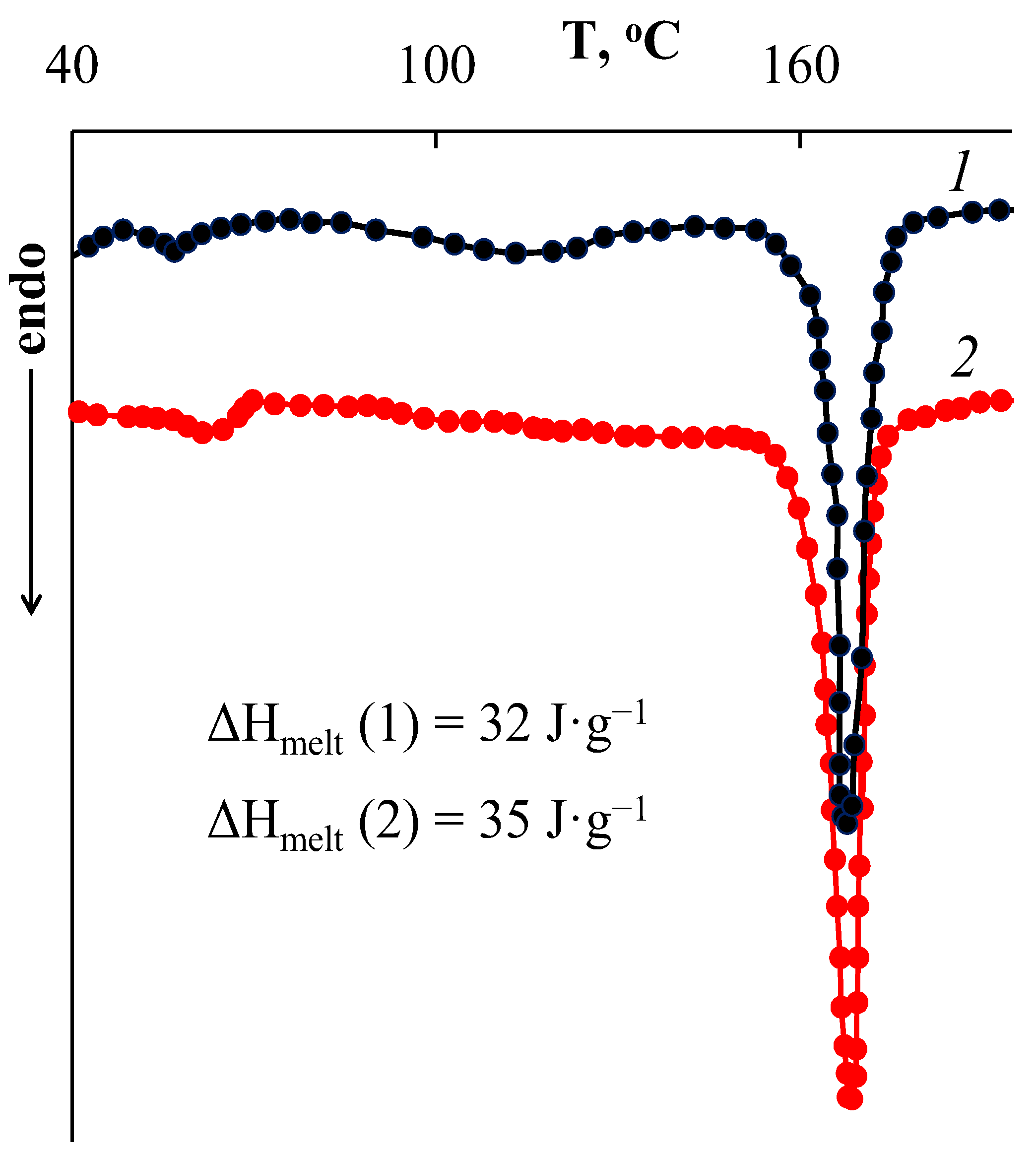
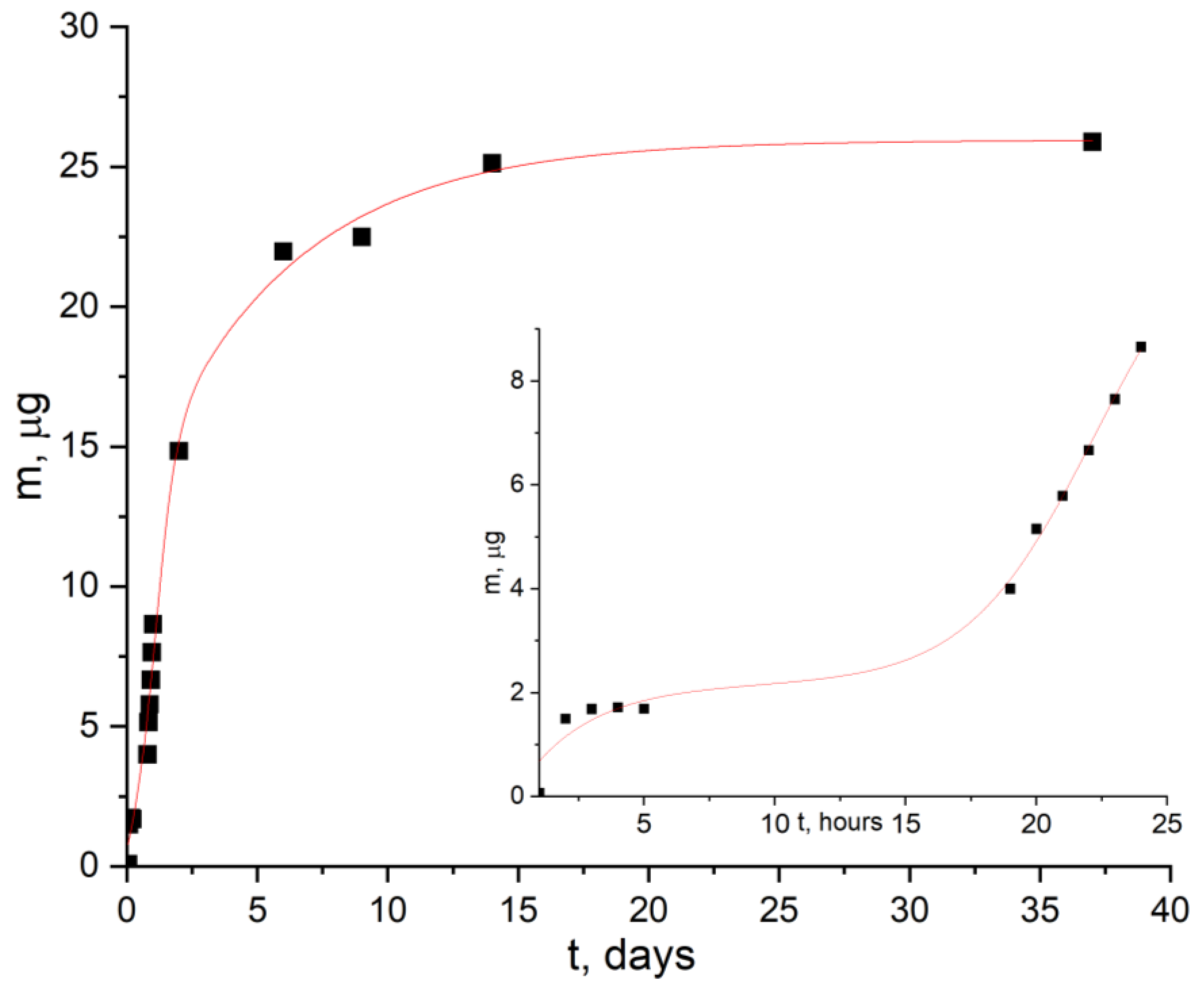
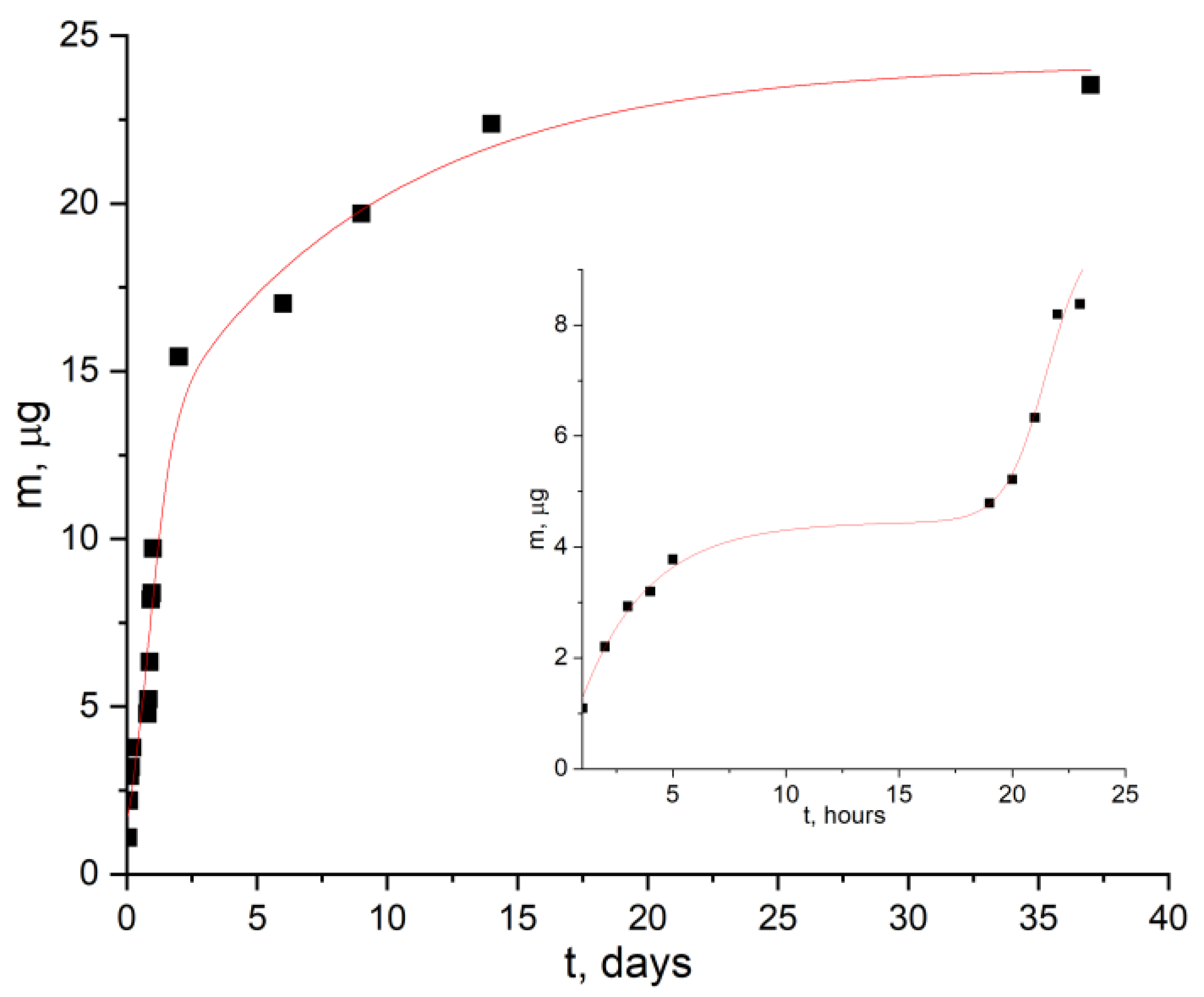
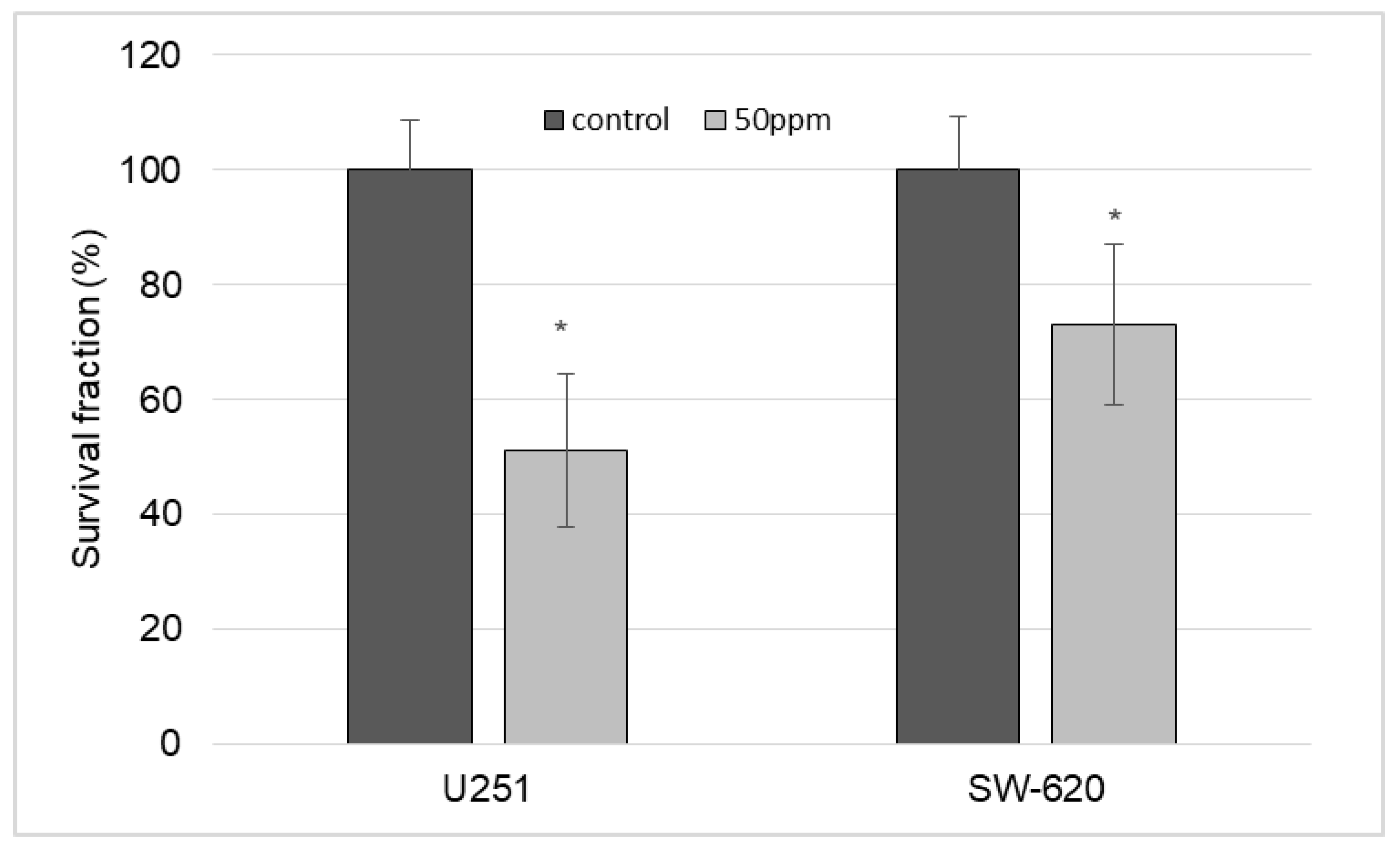
- The possibility of prolonged release of gold complex compound from the obtained composite was shown. The release of the filler from the polymer matrix volume had a delayed start—this process began only after several hours and was characterized by an effective rate constant of 1 µg/h. At the same time, in vitro studies showed that the concentration of 6.25 µg/mL was reliably safe and did not reduce the survival of U251 and SW-620 cells. Simultaneously with the release of the filler, decomposition of the PLA matrix occurred.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khokhlov, V.F.; Kulakov, V.N.; Shano, I.N.; Nasonova, T.A.; Mitin, V.N.; Dobrynina, O.A. Method of Photon-Capture Tumor Therapy. RF Patent No. 2270045, 24 June 2004. [Google Scholar]
- Koryakin, S.N.; Yadrovskaya, V.A.; Beketov, E.E.; Isaeva, E.V.; Ulyanenko, S.E.; Uspenskiy, S.A.; Khabarov, V.N.; Selyanin, M.A. The study of hyaluronic acid compounds for neutron capture and photon activation therapies. Cent. Eur. J. Biol. 2014, 9, 922–930. [Google Scholar] [CrossRef][Green Version]
- Taskaev, S.Y. Method of Measuring Absorbed Dose in Boron-Neutron-Capture Therapy of Malignant Tumors. RF Patent No. 2606337, 25 November 2015. [Google Scholar]
- Zaboronok, A.; Taskaev, S.; Volkova, O.; Mechetina, L.; Kasatova, A.; Sycheva, T.; Nakai, K.; Kasatov, D.; Makarov, A.; Kolesnikov, I.; et al. Gold Nanoparticles Permit In Situ Absorbed Dose Evaluation in Boron Neutron Capture Therapy for Malignant Tumors. Pharmaceutics 2021, 13, 1490. [Google Scholar] [CrossRef]
- Morozov, K.V.; Kolyvanova, M.A.; Kartseva, M.E.; Shishmakova, E.M.; Dement’eva, O.V.; Isagulieva, A.K.; Salpagarov, M.H.; Belousov, A.V.; Rudoy, V.M.; Shtil, A.A.; et al. Radiosensitization by Gold Nanoparticles: Impact of the Size, Dose Rate, and Photon Energy. Nanomaterials 2020, 10, 952. [Google Scholar] [CrossRef]
- Aquino, S.F.; Stuckey, D.C. Bioavailability and Toxicity of Metal Nutrients during Anaerobic Digestion. J. Environ. Eng. 2007, 133, 28–35. [Google Scholar] [CrossRef]
- Stanaćev, V.S.; Milošević, N.; Stanaćev, V.Ž.; Puvača, N.; Milić, D.; Pavlovski, Z. Chelating forms of microelements in poultry nutrition. World’s Poult. Sci. J. 2014, 70, 105–112. [Google Scholar] [CrossRef]
- Li, M.; Meares, C.F. Synthesis, metal chelate stability studies, and enzyme digestion of a peptide-linked DOTA derivative and its corresponding radiolabeled immunoconjugates. Bioconjug. Chem. 1993, 4, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kontoghiorghes, G.J. Advances on Chelation and Chelator Metal Complexes in Medicine. Int. J. Mol. Sci. 2020, 21, 2499. [Google Scholar] [CrossRef]
- Flora, G.; Mittal, M.; Flora, S. Medical Countermeasures—Chelation Therapy. In Handbook of Arsenic Toxicology; Academic Press: Cambridge, MA, USA, 2015; pp. 589–626. [Google Scholar]
- Sadler, P.J.; Sue, R.E. The chemistry of gold drugs. Met.-Based Drugs 1994, 1, 107–144. [Google Scholar] [CrossRef]
- Simon, T.M.; Kunishima, D.H.; Vibert, G.J.; Lorber, A. Screening trial with the coordinated gold compound auranofin using mouse lymphocyte leukemia P388. Cancer 1979, 44, 1965–1975. [Google Scholar] [CrossRef]
- Shapiro, D.L.; Masci, J.R. Treatment of HIV associated psoriatic arthritis with oral gold. J. Rheumatol. 1996, 23, 1818–1820. [Google Scholar] [PubMed]
- Chen, L.Q.; Fang, L.; Ling, J.; Ding, C.Z.; Kang, B.; Huang, C.Z. Nanotoxicity of Silver Nanoparticles to Red Blood Cells: Size Dependent Adsorption, Uptake, and Hemolytic Activity. Chem. Res. Toxicol. 2015, 28, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Rothen-Rutishauser, B.M.; Schürch, S.; Haenni, B.; Kapp, N.; Gehr, P. Interaction of fine particles and nanoparticles with red blood cells visualized with advanced microscopic techniques. Environ. Sci. Technol. 2006, 40, 4353–4359. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, N.; Ceylan, H.; Guler, M.O.; Tekinay, A.B. Intracellular Accumulation of Gold Nanoparticles Leads to Inhibition of Macropinocytosis to Reduce the Endoplasmic Reticulum Stress. Sci. Rep. 2017, 7, 40493. [Google Scholar] [CrossRef] [PubMed]
- Britt, R.D., Jr.; Velten, M.; Locy, M.L.; Rogers, L.K.; Tipple, T.E. The thioredoxin reductase-1 inhibitor aurothioglucose attenuates lung injury and improves survival in a murine model of acute respiratory distress syndrome. Antioxid. Redox Signal. 2014, 20, 2681–2691. [Google Scholar] [CrossRef] [PubMed]
- Gromer, S.; Arscott, L.D.; Williams, C.H., Jr.; Schirmer, R.H.; Becker, K. Human placenta thioredoxin reductase. Isolation of the selenoenzyme, steady state kinetics, and inhibition by therapeutic gold compounds. J. Biol. Chem. 1998, 273, 20096–20101. [Google Scholar] [CrossRef]
- Kean, W.F.; Kean, I.R. Clinical pharmacology of gold. Inflammopharmacology 2008, 16, 112–125. [Google Scholar] [CrossRef]
- Chang, H.Y.; Tseng, Y.T.; Yuan, Z.; Chou, H.L.; Chen, C.H.; Hwang, B.J.; Tsai, M.C.; Chang, H.T.; Huang, C.C. The effect of ligand-ligand interactions on the formation of photoluminescent gold nanoclusters embedded in Au(i)-thiolate supramolecules. Phys. Chem. Chem. Phys. PCCP 2017, 19, 12085–12093. [Google Scholar] [CrossRef] [PubMed]
- Trofimchuk, E.S.; Moskvina, M.A.; Ivanova, O.A.; Potseleev, V.V.; Demina, V.A.; Nikonorova, N.I.; Chvalun, S.N. Porous polylactide prepared by the delocalized crazing as a template for nanocomposite materials. Mendeleev Commun. 2020, 30, 171–173. [Google Scholar] [CrossRef]
- Khavpachev, M.A.; Trofimchuk, E.S.; Nikonorova, N.I.; Garina, E.S.; Moskvina, M.A.; Efimov, A.V.; Demina, V.A.; Bakirov, A.V.; Sedush, N.G.; Potseleev, V.V.; et al. Bioactive Polylactide Fibrous Materials Prepared by Crazing Mechanism. Macromol. Mater. Eng. 2020, 305, 2000163. [Google Scholar] [CrossRef]
- Mironov, I.V.; Kharlamova, V.Y. On the Complexation of Gold(I) with Glutathione in Aqueous Solutions. J. Solut. Chem. 2020, 49, 583–597. [Google Scholar] [CrossRef]
- Howard-Lock, H.E. Structures of gold(i) and silver(i) thiolate complexes of medicinal interest: A review and recent results. Met.-Based Drugs 1999, 6, 201–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Darabi, F.; Marzo, T.; Massai, L.; Scaletti, F.; Michelucci, E.; Messori, L. Reactions of model proteins with aurothiomalate, a clinically established gold(I) drug: The comparison with auranofin. J. Inorg. Biochem. 2015, 149, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; He, L.; McClements, D.J.; Xiao, H. Uptake of Gold Nanoparticles by Intestinal Epithelial Cells: Impact of Particle Size on Their Absorption, Accumulation, and Toxicity. J. Agric. Food Chem. 2015, 63, 8044–8049. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Liu, J.; Tian, M.; Bai, Y.; Bao, Y.; Shu, T.; Su, L.; Zhang, X. An Aggregation-Induced Phosphorescence-Active “Turn-Off” Nanosensor Based on Ferric-Specific Quenching of Luminescent and Water-Soluble Au(I)-Cysteine Nanocomplexes. Anal. Chem. 2020, 92, 6785–6791. [Google Scholar] [CrossRef]
- Fakhouri, H.; Perić, M.; Bertorelle, F.; Dugourd, P.; Dagany, X.; Russier-Antoine, I.; Brevet, P.F.; Bonačić-Koutecký, V.; Antoine, R. Sub-100 nanometer silver doped gold-cysteine supramolecular assemblies with enhanced nonlinear optical properties. Phys. Chem. Chem. Phys. PCCP 2019, 21, 12091–12099. [Google Scholar] [CrossRef] [PubMed]
- Potseleev, V.V.; Trofimchuk, E.S.; Nikonorova, N.I. Kinetics of the release of brilliant green from nanoporous polylactide obtained by a crazing mechanism. Mendeleev Commun. 2021, 31, 515–516. [Google Scholar] [CrossRef]
- Yarysheva, A.Y.; Rukhlya, E.G.; Yarysheva, L.M.; Bagrov, D.V.; Volynskii, A.L.; Bakeev, N.F. The structural evolution of high-density polyethylene during crazing in liquid medium. Eur. Polym. J. 2015, 66, 458–469. [Google Scholar] [CrossRef]
- Yarysheva, A.Y.; Bagrov, D.V.; Bakirov, A.V.; Yarysheva, L.M.; Chvalun, S.N.; Volynskii, A.L. Effect of initial polypropylene structure on its deformation via crazing mechanism in a liquid medium. Eur. Polym. J. 2018, 100, 233–240. [Google Scholar] [CrossRef]
- Trofimchuk, E.S.; Nikonorova, N.I.; Chagarovskii, A.O.; Volynskii, A.L.; Bakeev, N.F. Crystallization of silver chloride in crazed porous polymers. J. Phys. Chem. B 2005, 109, 16278–16283. [Google Scholar] [CrossRef]
- Guo, Y.; Tong, X.; Ji, L.; Wang, Z.; Wang, H.; Hu, J.; Pei, R. Visual detection of Ca2+ based on aggregation-induced emission of Au(I)-Cys complexes with superb selectivity. Chem. Commun. 2015, 51, 596–598. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Corrigan, O.I. Mechanistic aspects of the release of levamisole hydrochloride from biodegradable polymers. J. Control. Release 2000, 69, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.; Schley, J.; Loy, B.; Lind, D.; Hobot, C.; Sparer, R.; Untereker, D. Kinetics and time-temperature equivalence of polymer degradation. Biomacromolecules 2007, 8, 2301–2310. [Google Scholar] [CrossRef] [PubMed]
- Michida, W.; Nagai, A.; Sakuragi, M.; Okobira, T.; Kusakabe, K. Fluorescence Emission Behaviors of the L-Cysteine/Au(I) Complex in a Cyclodextrin-Based Metal-Organic Framework. Processes 2020, 8, 1555. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Filonov, A.; Yaminsky, I.; Akhmetova, A.; Meshkov, G. FemtoScan Online! Why? Nanoindustry 2018, 11, 336–342. [Google Scholar] [CrossRef]





| Equation | Equation Parameters |
|---|---|
| ) | —the amount of released additive in the first stage (explosive effect) —first-order rate constant at the first stage |
| —second stage rate constant | |
| —time of maximum release rate at the second stage data |
| pH | t2, Max, h | ft,max, μg | fb, μg | k1, h−1 | k2, h−1 | R2 |
|---|---|---|---|---|---|---|
| 7 | 27 ± 2 | 25.9 | 2.0 | 0.007 ± 0.001 | 0.11 ± 0.02 | 0.99 |
| 9 | 21 ± 4 | 23.5 | 4.5 | 0.005 ± 0.002 | 0.10 ± 0.02 | 0.98 |
| Model | , mol/L | , ч | R2 | , L/(mol·h) |
|---|---|---|---|---|
| ) | 3.42 | 168 | 0.98 | 2 · 10−8 ± 1 · 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potseleev, V.; Uspenskii, S.; Trofimchuk, E.; Bolshakova, A.; Kasatova, A.; Kasatov, D.; Taskaev, S. Nanocomposite Materials Based on Polylactide and Gold Complex Compounds for Absorbed Dose Diagnostics in BNCT. Int. J. Mol. Sci. 2023, 24, 16492. https://doi.org/10.3390/ijms242216492
Potseleev V, Uspenskii S, Trofimchuk E, Bolshakova A, Kasatova A, Kasatov D, Taskaev S. Nanocomposite Materials Based on Polylactide and Gold Complex Compounds for Absorbed Dose Diagnostics in BNCT. International Journal of Molecular Sciences. 2023; 24(22):16492. https://doi.org/10.3390/ijms242216492
Chicago/Turabian StylePotseleev, Vladislav, Sergey Uspenskii, Elena Trofimchuk, Anastasia Bolshakova, Anna Kasatova, Dmitrii Kasatov, and Sergey Taskaev. 2023. "Nanocomposite Materials Based on Polylactide and Gold Complex Compounds for Absorbed Dose Diagnostics in BNCT" International Journal of Molecular Sciences 24, no. 22: 16492. https://doi.org/10.3390/ijms242216492
APA StylePotseleev, V., Uspenskii, S., Trofimchuk, E., Bolshakova, A., Kasatova, A., Kasatov, D., & Taskaev, S. (2023). Nanocomposite Materials Based on Polylactide and Gold Complex Compounds for Absorbed Dose Diagnostics in BNCT. International Journal of Molecular Sciences, 24(22), 16492. https://doi.org/10.3390/ijms242216492








