Buckwheat (Fagopyrum esculentum) Hulls Are a Rich Source of Fermentable Dietary Fibre and Bioactive Phytochemicals
Abstract
:1. Introduction
2. Results
2.1. Buckwheat Hulls Composition
Buckwheat Hulls Are a Rich Source of Insoluble Dietary Fibre (Non-Starch Polysacchrides) (NSPs)
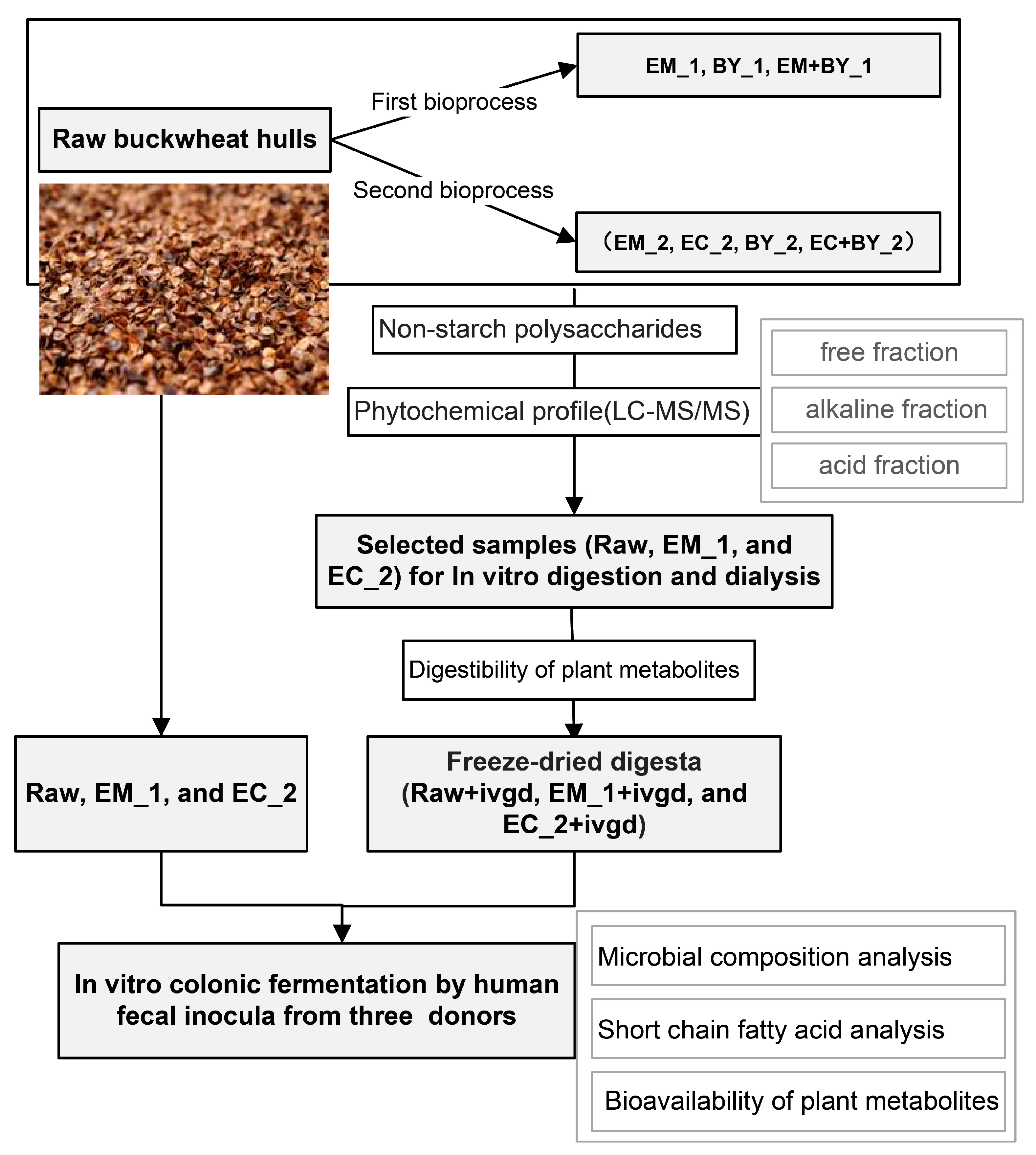
2.2. Bioprocessing of the Buckwheat Hulls
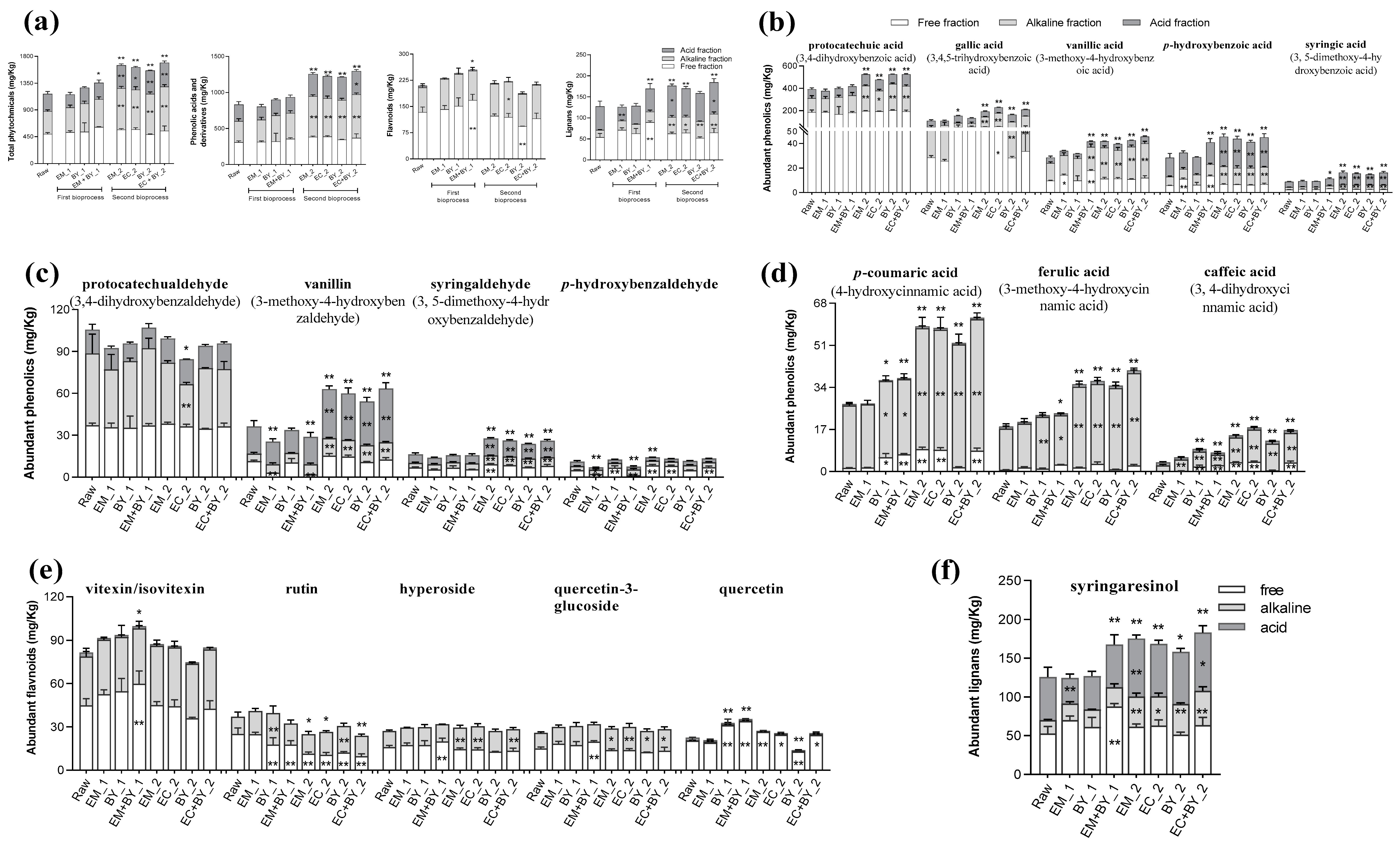
2.2.1. Bioprocessing Treatments of Buckwheat Hulls Promote Increases in NSP Content
2.2.2. Bioprocessing Treatments of Buckwheat Hulls Increased the Extractability of Several Plant Metabolites
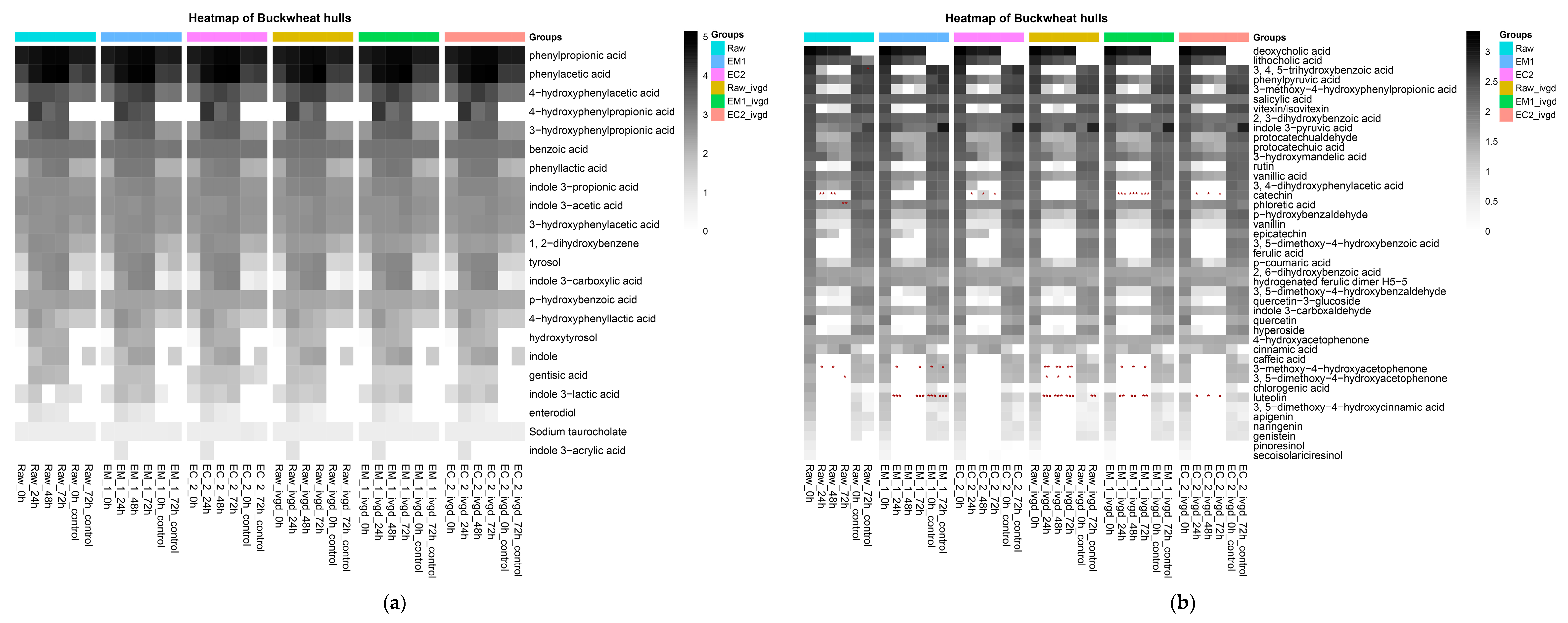
2.2.3. Bioprocessing of Buckwheat Hulls Improved the Release of Plant Metabolites in the Upper Gastrointestinal Tract during In Vitro Digestion
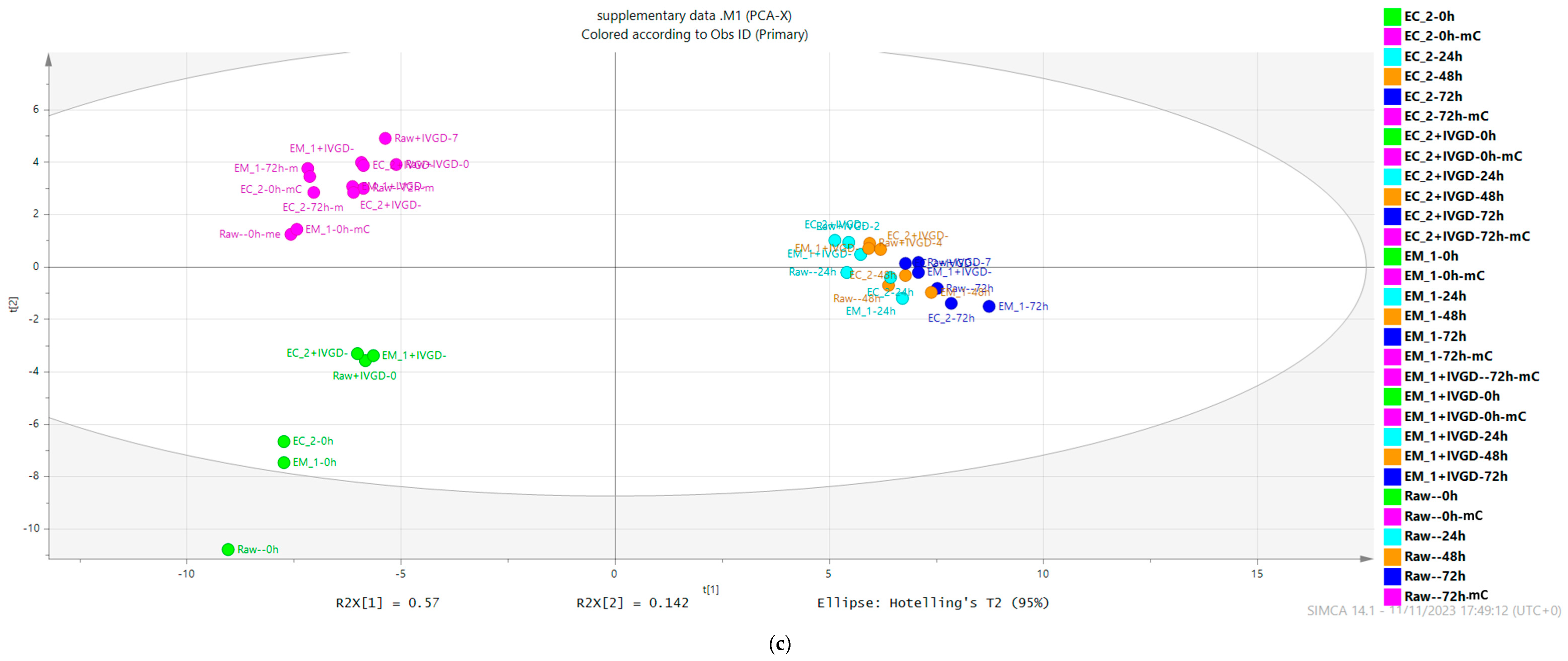
2.2.4. The Main Microbial Metabolism Transformation of Buckwheat Hulls Components Occurs within the First 24 h
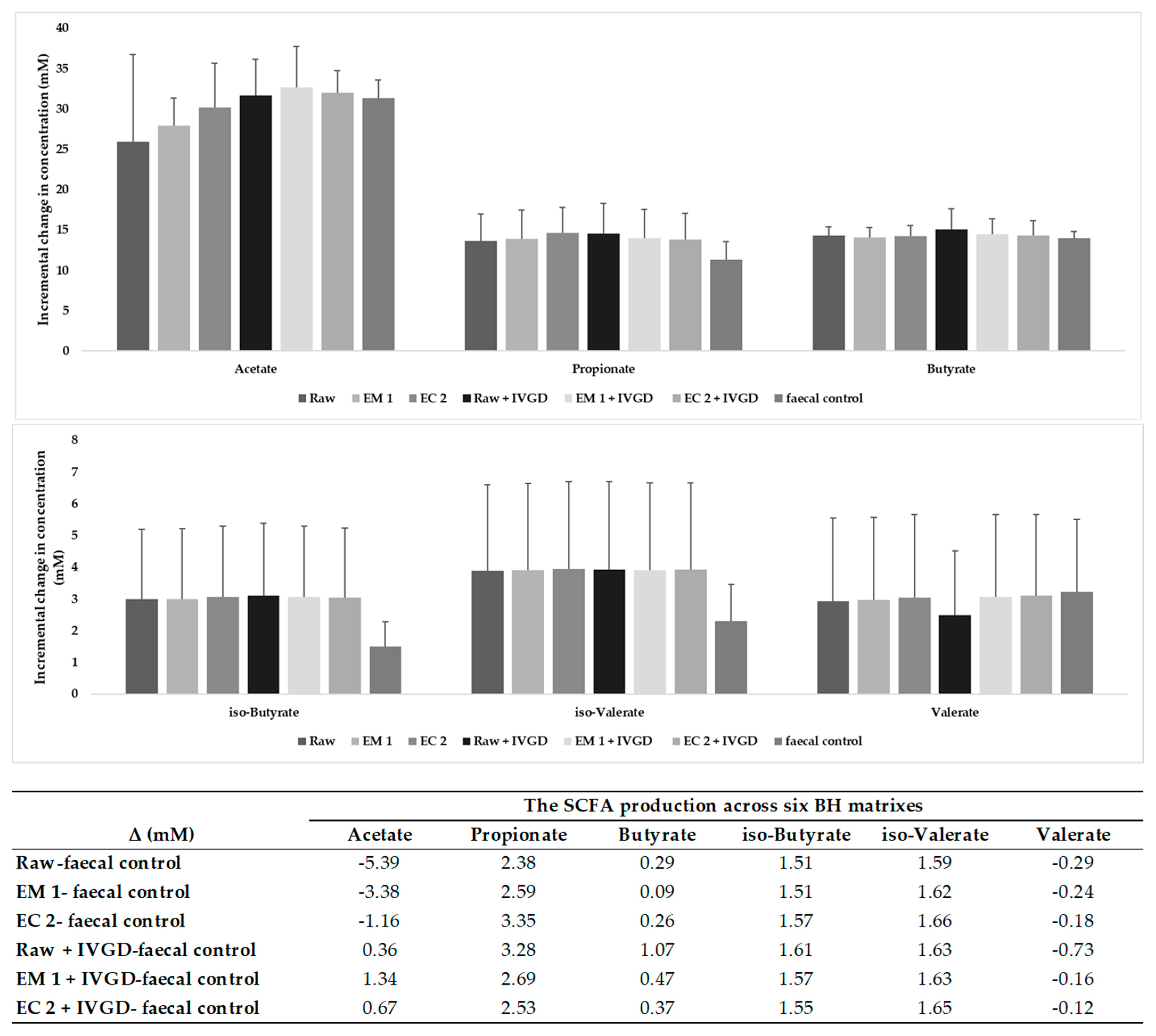
2.2.5. Buckwheat Hulls Are Fermentable Sources of Dietary Fibre
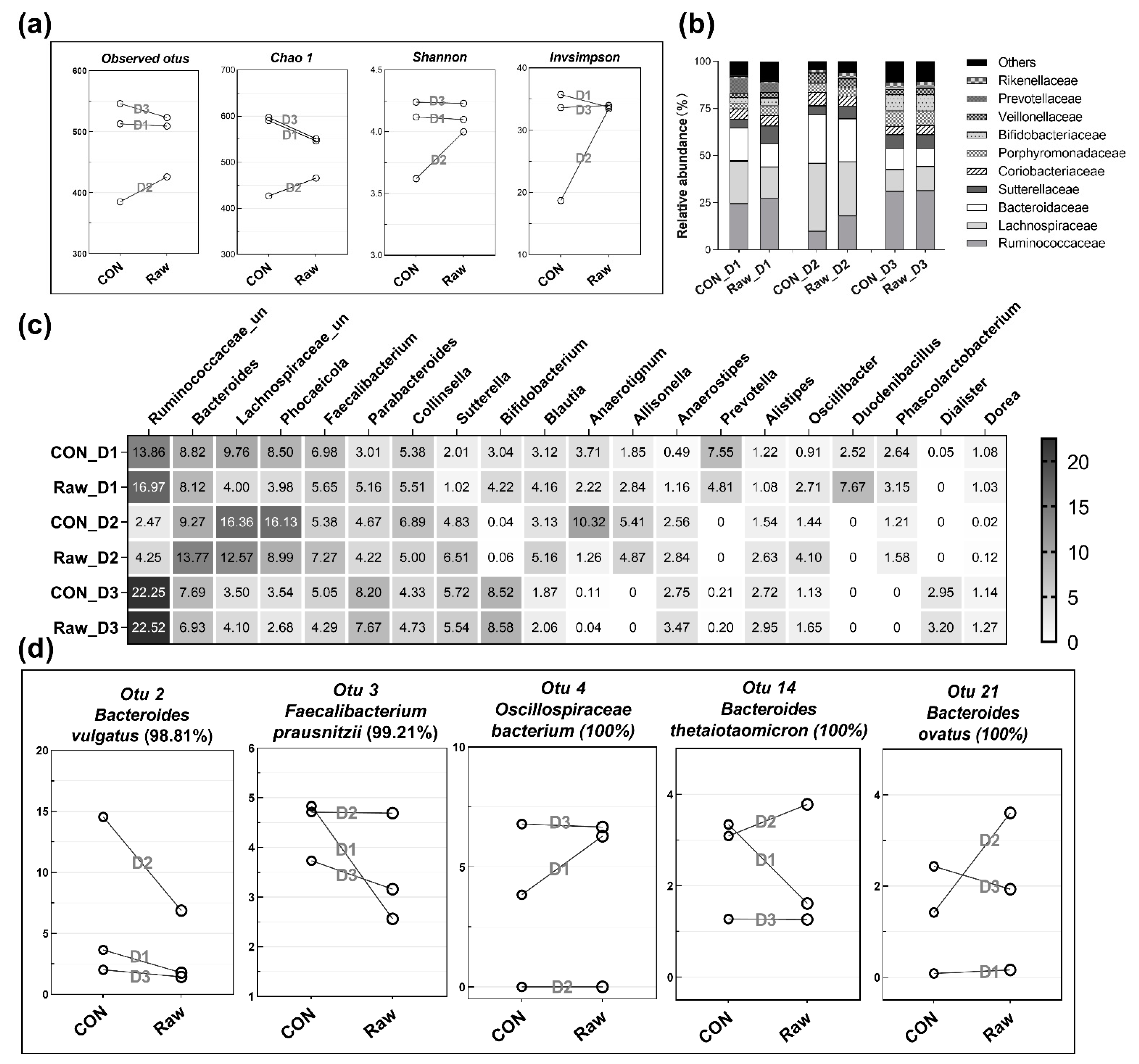
2.2.6. Variations in Bacterial Composition at the Genus and OTU Levels Occur in a Donor-Dependent Manner in Response to Buckwheat Hull Metabolism
3. Discussion
4. Materials and Methods
4.1. Standards and Reagents
4.2. Bioprocessing of Buckwheat Hulls
4.3. Macronutrient Analysis
4.4. Extraction of Phenolic Compounds
4.5. Simulated Gastrointestinal Digestion and Dialysis
4.6. LC-MS Analysis
4.7. In Vitro Colonic Fermentation
4.7.1. Human Faecal Incubation
4.7.2. Microbial Metabolites Analysis
4.7.3. Short Chain Fatty Acids (SCFAs) Analysis
4.7.4. Microbial Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dwivedi, S.; Sahrawat, K.; Upadhyaya, H.; Ortiz, R. Food, nutrition and agrobiodiversity under global climate change. Adv. Agron. 2013, 120, 1–128. [Google Scholar]
- Massawe, F.; Mayes, S.; Cheng, A. Crop diversity: An unexploited treasure trove for food security. Trends Plant Sci. 2016, 21, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Mijatović, D.; Van Oudenhoven, F.; Eyzaguirre, P.; Hodgkin, T. The role of agricultural biodiversity in strengthening resilience to climate change: Towards an analytical framework. Int. J. Agric. Sustain. 2013, 11, 95–107. [Google Scholar] [CrossRef]
- Neacsu, M.; Duncan, S. Exploiting Underutilised crops. Food Sci. Technol. 2023, 37, 36–39. [Google Scholar]
- Mayes, S.; Massawe, F.; Alderson, P.; Roberts, J.; Azam-Ali, S.; Hermann, M. The potential for underutilized crops to improve security of food production. J. Exp. Bot. 2012, 63, 1075–1079. [Google Scholar] [CrossRef]
- Agostoni, C.; Bresson, J.-L.; Fairweather Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A. Scientific Opinion on Dietary Reference Values for carbohydrates and dietary fibre. EFSA J. 2010, 8, 1462. [Google Scholar]
- Mozaffarian, D.; Hao, T.; Rimm, E.B.; Willett, W.C.; Hu, F. Changes in diet and lifestyle and long-term weight gain in women and men. N. Engl. J. Med. 2011, 364, 2392–2404. [Google Scholar] [CrossRef]
- Shoelson, S.E.; Herrero, L.; Naaz, A.J.G. Obesity, inflammation, and insulin resistance. Gastroenterology 2007, 132, 2169–2180. [Google Scholar] [CrossRef]
- Gorinstein, S.; Lojek, A.; Číž, M.; Pawelzik, E.; Delgado-Licon, E.; Medina, O.J.; Moreno, M.; Salas, I.A.; Goshev, I. Comparison of composition and antioxidant capacity of some cereals and pseudocereals. Int. J. Food Sci. Technol. 2008, 43, 629–637. [Google Scholar] [CrossRef]
- Gorinstein, S.; Vargas, O.J.M.; Jaramillo, N.O.; Salas, I.A.; Ayala, A.L.M.; Arancibia-Avila, P.; Toledo, F.; Katrich, E.; Trakhtenberg, S.J. The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. EFR Technol. 2007, 225, 321–328. [Google Scholar] [CrossRef]
- Morales, D.; Miguel, M.; Garcés-Rimón, M. Pseudocereals: A novel source of biologically active peptides. Crit. Rev. Food Sci. Nutr. 2021, 61, 1537–1544. [Google Scholar] [CrossRef]
- Kim, S.J.; Sohn, H.B.; Suh, J.T.; Kim, G.H.; Hong, S.Y.; Chang, D.C.; Kim, K.D.; Koo, B.C.; Kim, Y.H. Domestic and overseas status of buckwheat production and future trends. J. Korean Soc. Int. Agric. 2017, 29, 226–233. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- Bonafaccia, G.; Gambelli, L.; Fabjan, N.; Kreft, I. Trace elements in flour and bran from common and tartary buckwheat. Food Chem. 2003, 83, 1–5. [Google Scholar] [CrossRef]
- Guo, X.-D.; Wu, C.-S.; Ma, Y.-J.; Parry, J.; Xu, Y.-Y.; Liu, H.; Wang, M.J. Comparison of milling fractions of tartary buckwheat for their phenolics and antioxidant properties. Food Res. Int. 2012, 49, 53–59. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Cazin, J.-C.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Erdman, J.W., Jr.; Balentine, D.; Arab, L.; Beecher, G.; Dwyer, J.T.; Folts, J.; Harnly, J.; Hollman, P.; Keen, C.L.; Mazza, G. Flavonoids and heart health. In Proceedings of the ILSI North America Flavonoids Workshop, Washington, DC, USA, 31 May–1 June 2005; Volume 137, pp. 718S–737S. [Google Scholar]
- Dong, L.; Qin, C.; Li, Y.; Wu, Z.; Liu, L. Oat phenolic compounds regulate metabolic syndrome in high fat diet-fed mice via gut microbiota. Food Biosci. 2022, 50, 101946. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Krumina-Zemture, G.; Beitane, I.; Gramatina, I. Amino acid and dietary fibre content of pea and buckwheat flours. Food Sci. 2016, 1, 84–90. [Google Scholar]
- Van Loon, L.J.C. Leucine as a pharmaconutrient in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Multari, S.; Neacsu, M.; Scobbie, L.; Cantlay, L.; Duncan, G.; Vaughan, N.; Stewart, D.; Russell, W.R. Nutritional and phytochemical content of high-protein crops. J. Agric. Food Chem. 2016, 64, 7800–7811. [Google Scholar] [CrossRef] [PubMed]
- Steadman, K.; Burgoon, M.; Lewis, B.; Edwardson, S.; Obendorf, R. Buckwheat seed milling fractions: Description, macronutrient composition and dietary fibre. J. Cereal Sci. 2001, 33, 271–278. [Google Scholar] [CrossRef]
- Anson, N.M.; Selinheimo, E.; Havenaar, R.; Aura, A.-M.; Mattila, I.; Lehtinen, P.; Bast, A.; Poutanen, K.; Haenen, G.R.M.M. Bioprocessing of wheat bran improves in vitro bioaccessibility and colonic metabolism of phenolic compounds. J. Agric. Food Chem. 2009, 57, 6148–6155. [Google Scholar] [CrossRef] [PubMed]
- Kuijsten, A.; Arts, I.C.; Vree, T.B.; Hollman, P.C.H. Pharmacokinetics of enterolignans in healthy men and women consuming a single dose of secoisolariciresinol diglucoside. J. Nutr. 2005, 135, 795–801. [Google Scholar] [CrossRef]
- Nesbitt, P.D.; Lam, Y.; Thompson, L.U. Human metabolism of mammalian lignan precursors in raw and processed flaxseed. Am. J. Clin. Nutr. 1999, 69, 549–555. [Google Scholar] [CrossRef]
- Duncan, S.H.; Conti, E.; Ricci, L.; Walker, A.W. Links between diet, intestinal anaerobes, microbial metabolites and health. Biomedicines 2023, 11, 1338. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Z.; Duncan, G.J.; Morris, A.; Scobbie, L.; Henderson, D.; Morrice, P.; Russell, W.R.; Duncan, S.H.; Neacsu, M. Bioprocessing of Hempseed (Cannabis sativa L.) Food By-Products Increased Nutrient and Phytochemical In Vitro Bioavailability during Digestion and Microbial Fermentation. Appl. Sci. 2023, 13, 5781. [Google Scholar] [CrossRef]
- Lamothe, L.M.; Cantu-Jungles, T.M.; Chen, T.; Green, S.; Naqib, A.; Srichuwong, S.; Hamaker, B.R. Boosting the value of insoluble dietary fiber to increase gut fermentability through food processing. Food Funct. 2021, 12, 10658–10666. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, K.; Kerckhof, F.M.; Verspreet, J.; Courtin, C.M.; Van de Wiele, T. Inter-individual differences determine the outcome of wheat bran colonization by the human gut microbiome. Environ. Microbiol. 2017, 19, 3251–3267. [Google Scholar] [CrossRef] [PubMed]
- De Paepe, K.; Verspreet, J.; Courtin, C.M.; Van de Wiele, T. Microbial succession during wheat bran fermentation and colonisation by human faecal microbiota as a result of niche diversification. ISME J. 2020, 14, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, Y.; Li, X.; Sun, B. Dynamic balancing of intestinal short-chain fatty acids: The crucial role of bacterial metabolism. Trends Food Sci. Technol. 2020, 100, 118–130. [Google Scholar] [CrossRef]
- Duncan, S.H.; Russell, W.R.; Quartieri, A.; Rossi, M.; Parkhill, J.; Walker, A.W.; Flint, H.J. Wheat bran promotes enrichment within the human colonic microbiota of butyrate-producing bacteria that release ferulic acid. Environ. Microbiol. 2016, 18, 2214–2225. [Google Scholar] [CrossRef] [PubMed]
- Russell, W.R.; Scobbie, L.; Labat, A.; Duthie, G.G. Selective bio-availability of phenolic acids from Scottish strawberries. Mol. Nutr. Food Res. 2009, 53, S85–S91. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Elsheikh, E.A.E.; Fadul, I.; El Tinay, A. Effect of cooking on anti-nutritional factors and in vitro protein digestibility (IVPD) of faba bean grown with different nutritional regimes. Food Chem. 2000, 68, 211–212. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2017.
- Richardson, A.; Calder, A.; Stewart, C.; Smith, A. Simultaneous determination of volatile and non-volatile acidic fermentation products of anaerobes by capillary gas chromatography. Lett. Appl. Microbiol. 1989, 9, 5–8. [Google Scholar] [CrossRef]
| Nutrient Composition | Content (%) | Amino Acid Composition | |||
|---|---|---|---|---|---|
| EAA | Content (%) | Non-EAA | Content (%) | ||
| Moisture | 10.8 ± 0.0 | Leucine | 0.l9 | Glutamic acid | 0.33 |
| Ash | 1.7 ± 0.0 | Tryptophan | 0.15 | Aspartic acid | 0.25 |
| Protein | 4.05 ± 0.05 | Threonine | 0.l4 | Cysteic acid | 0.23 |
| Total fat | 0.1 ± 0.0 | Valine | 0.13 | Glycine | 0.16 |
| Total starch | 2.55 ± 0.0 | Phenylalanine | 0.11 | Serine | 0.15 |
| Resistant starch | n.d. | Histidine | 0.11 | Proline | 0.14 |
| Total NSPs | Isoleucine | 0.11 | Alanine | 0.13 | |
| -Insoluble | 31.1 ± 0.2 | Lysine | 0.09 | Arginine | 0.1 |
| -Soluble | 0.30 ± 0.05 | Methionine | 0.02 | Tyrosine | 0.04 |
| Cystine | n.d. | ||||
| NSPs | Raw | First Bioprocess | Second Bioprocess | |||||
|---|---|---|---|---|---|---|---|---|
| EM_1 | BY_1 | EM+BY_1 | EM_2 | EC_2 | BY_2 | EC+BY_2 | ||
| Insoluble (%) | ||||||||
| Xylose | 15.78 ± 0.27 | 19.73 ± 0.65 ** | 19.50 ± 1.82 | 18.94 ± 0.07 | 20.51 ± 0.23 ** | 20.37 ± 0.33 ** | 18.26 ± 0.63 | 18.72 ± 0.44 |
| Glucose | 9.67 ± 0.34 | 20.07 ± 1.35 ** | 16.83 ± 0.62 | 14.79 ± 0.18 | 10.89 ± 1.17 | 11.04 ± 1.28 | 10.27 ± 0.56 | 10.39 ± 0.82 |
| Arabinose | 0.72 ± 0.01 | 0.82 ± 0.04 ** | 0.82 ± 0.00 | 0.80 ± 0.02 | 0.83 ± 0.01 ** | 0.84 ± 0.01 ** | 0.78 ± 0.04 | 0.76 ± 0.01 |
| Galactose | 0.61 ± 0.01 | 0.67 ± 0.03 ** | 0.63 ± 0.04 | 0.65 ± 0.00 | 0.70 ± 0.01 ** | 0.71 ± 0.00 ** | 0.67 ± 0.03 | 0.66 ± 0.01 |
| Rhamnose | 0.33 ± 0.01 | 0.43 ± 0.03 ** | 0.40 ± 0.02 | 0.39 ± 0.01 | 0.37 ± 0.02 | 0.38 ± 0.00 * | 0.37 ± 0.02 | 0.37 ± 0.01 |
| Mannose | 0.24 ± 0.01 | 0.30 ± 0.04 ** | 0.42 ± 0.03 | 0.40 ± 0.01 | 0.26 ± 0.01 | 0.27 ± 0.00 | 0.33 ± 0.02 | 0.33 ± 0.02 |
| Fucose | 0.07 ± 0.00 | 0.07 ± 0.00 ** | 0.10 ± 0.03 | 0.07 ± 0.01 | 0.08 ± 0.00 ** | 0.08 ± 0.00 ** | 0.07 ± 0.00 | 0.08 ± 0.01 |
| Uronic Acid | 3.68 ± 0.11 | 4.31 ± 0.01 | 3.90 ± 0.12 | 4.06 ± 0.02 | 4.05 ± 0.18 | 3.75 ± 0.56 | 3.77 ± 0.07 | 4.34 ± 0.53 |
| Total | 31.09 ± 0.22 | 46.41 ± 2.12 ** | 42.60 ± 1.14 | 40.09 ± 0.25 | 37.68 ± 1.26 ** | 37.43 ± 0.68 ** | 34.51 ± 1.21 | 35.64 ± 0.57 |
| Soluble (%) | ||||||||
| Xylose | 0.06 ± 0.01 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.05 ± 0.03 | 0.02 ± 0.02 | 0.04 ± 0.04 | 0.04 ± 0.02 | 0.01 ± 0.02 |
| Glucose | 0.09 ± 0.01 | 0.09 ± 0.03 | 0.07 ± 0.01 | 0.09 ± 0.04 | 0.04 ± 0.02 | 0.06 ± 0.04 | 0.07 ± 0.02 | 0.03 ± 0.00 |
| Arabinose | 0.02 ± 0.00 | 0.03 ± 0.00 * | 0.04 ± 0.01 | 0.03 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.01 * | 0.03 ± 0.01 | 0.03 ± 0.01 |
| Galactose | 0.02 ± 0.00 | 0.00 ± 0.00 | 0.04 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.03 |
| Rhamnose | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.02 |
| Mannose | 0.00 ± 0.00 | 0.06 ± 0.00 ** | 0.10 ± 0.00 | 0.08 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.02 |
| Fucose | 0.03 ± 0.00 | 0.05 ± 0.01 | 0.04 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.02 | 0.04 ± 0.01 | 0.04 ± 0.00 | 0.03 ± 0.00 |
| Uronic Acid | 0.09 ± 0.03 | 0.02 ± 0.00 ** | 0.15 ± 0.03 | 0.04 ± 0.01 | 0.01 ± 0.00 ** | 0.01 ± 0.00 ** | 0.09 ± 0.03 | 0.01 ± 0.00 |
| Total | 0.30 ± 0.05 | 0.31 ± 0.06 | 0.54 ± 0.01 | 0.34 ± 0.07 | 0.12 ± 0.04 | 0.18 ± 0.10 | 0.31 ± 0.09 | 0.15 ± 0.08 |
| Plant Metabolites (mg/kg) | Raw | EM_1 | EC_2 | |||
|---|---|---|---|---|---|---|
| Free Fraction Content (Initial) | Content after In Vitro Digestion | Free Fraction Content (Initial) | Content after In Vitro Digestion | Free Fraction Content (Initial) | Content after In Vitro Digestion | |
| protocatechuic acid | 184.81 ± 21.00 | 300.18 ± 3.46 | 185.37 ± 8.24 | 268.99 ± 1.59 ** | 193.21 ± 3.07 | 346.67 ± 6.28 ** |
| protocatechualdehyde | 37.16 ± 1.54 | 29.04 ± 0.69 | 35.80 ± 2.91 | 30.90 ± 1.56 | 36.18 ± 1.63 | 33.11 ± 0.61 ** |
| 3, 4, 5-trihydroxybenzoic acid | 28.50 ± 1.67 | 1.10 ± 0.18 | 25.65 ± 1.27 | 1.17 ± 0.39 | 57.38 ± 1.53 | 4.82 ± 0.33 ** |
| vanillin | 11.40 ± 0.78 | 5.07 ± 0.17 | 3.11 ± 0.08 | 10.08 ± 0.61 ** | 14.57 ± 1.20 | 8.89 ± 0.75 * |
| p-hydroxybenzoic acid | 9.55 ± 0.43 | 19.85 ± 0.09 | 14.16 ± 0.81 | 21.98 ± 0.13 ** | 11.30 ± 0.95 | 26.36 ± 0.32 ** |
| 3, 5-dimethoxy-4-hydroxybenzaldehyde | 6.85 ± 0.46 | 6.42 ± 0.09 | 5.49 ± 0.41 | 7.08 ± 0.45 | 8.57 ± 0.39 | 7.09 ± 0.49 |
| vanillic acid | 5.67 ± 0.23 | 9.54 ± 0.10 | 10.99 ± 0.94 | 9.89 ± 0.34 | 6.47 ± 0.19 | 12.65 ± 0.29 ** |
| p-hydroxybenzaldehyde | 4.97 ± 0.39 | 4.24 ± 0.08 | 2.14 ± 0.18 | 8.32 ± 0.55 * | 8.03 ± 0.57 | 8.55 ± 0.17 ** |
| salicylic acid | 4.47 ± 0.32 | 6.03 ± 0.14 | 4.10 ± 0.20 | 5.27 ± 0.27 * | 5.57 ± 1.10 | 7.36 ± 0.26 ** |
| chlorogenic acid | 2.32 ± 0.25 | 1.41 ± 0.08 | 2.93 ± 0.49 | 0.46 ± 0.05 ** | 1.85 ± 0.20 | 0.40 ± 0.06 ** |
| 3, 5-dimethoxy-4-hydroxybenzoic acid | 2.26 ± 0.13 | 3.64 ± 0.19 | 2.77 ± 0.18 | 3.49 ± 0.06 | 2.68 ± 0.22 | 4.17 ± 0.18 * |
| 3-hydroxymandelic acid | 2.00 ± 0.81 | 8.71 ± 0.49 | 2.61 ± 0.13 | 13.17 ± 0.02 ** | n.d. | 13.89 ± 0.28 ** |
| 2-hydroxybenzyl alcohol | 1.91 ± 0.13 | 1.03 ± 0.05 | 2.01 ± 0.13 | 1.15 ± 0.14 | 1.33 ± 0.15 | 1.39 ± 0.10 * |
| p-coumaric acid | 1.46 ± 0.11 | 7.10 ± 0.32 | 1.56 ± 0.11 | 12.26 ± 0.36 ** | 8.81 ± 0.82 | 14.48 ± 0.36 ** |
| phenylacetic acid | 1.12 ± 0.06 | 6.06 ± 0.60 | 2.94 ± 0.23 | 7.57 ± 0.66 | n.d. | 7.45 ± 0.31 |
| ferulic acid | 0.69 ± 0.02 | 1.59 ± 0.04 | 1.58 ± 0.37 | 2.59 ± 0.16 * | 3.01 ± 0.84 | 3.95 ± 0.23 ** |
| 3, 4-dihydroxyphenylacetic acid | n.d. | 7.47 ± 0.76 | n.d. | 7.44 ± 0.49 | 6.98 ± 0.69 | 10.73 ± 0.43 * |
| 4-hydroxyphenylacetic acid | n.d. | 4.17 ± 0.11 | n.d. | 6.82 ± 0.45 * | n.d. | 9.66 ± 0.65 ** |
| caffeic acid | n.d. | 0.80 ± 0.03 | 0.26 ± 0.04 | 1.13 ± 0.03 ** | 3.89 ± 0.36 | 1.95 ± 0.11 ** |
| 2, 3-dihydroxybenzoic acid | n.d. | 0.43 ± 0.04 | 0.28 ± 0.24 | 0.59 ± 0.02 * | 1.87 ± 0.10 | 2.32 ± 0.08 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Fan, S.; Duncan, G.J.; Morris, A.; Henderson, D.; Morrice, P.; Russell, W.R.; Duncan, S.H.; Neacsu, M. Buckwheat (Fagopyrum esculentum) Hulls Are a Rich Source of Fermentable Dietary Fibre and Bioactive Phytochemicals. Int. J. Mol. Sci. 2023, 24, 16310. https://doi.org/10.3390/ijms242216310
Zhang Z, Fan S, Duncan GJ, Morris A, Henderson D, Morrice P, Russell WR, Duncan SH, Neacsu M. Buckwheat (Fagopyrum esculentum) Hulls Are a Rich Source of Fermentable Dietary Fibre and Bioactive Phytochemicals. International Journal of Molecular Sciences. 2023; 24(22):16310. https://doi.org/10.3390/ijms242216310
Chicago/Turabian StyleZhang, Zhihong, Songtao Fan, Gary J. Duncan, Amanda Morris, Donna Henderson, Philip Morrice, Wendy R. Russell, Sylvia H. Duncan, and Madalina Neacsu. 2023. "Buckwheat (Fagopyrum esculentum) Hulls Are a Rich Source of Fermentable Dietary Fibre and Bioactive Phytochemicals" International Journal of Molecular Sciences 24, no. 22: 16310. https://doi.org/10.3390/ijms242216310
APA StyleZhang, Z., Fan, S., Duncan, G. J., Morris, A., Henderson, D., Morrice, P., Russell, W. R., Duncan, S. H., & Neacsu, M. (2023). Buckwheat (Fagopyrum esculentum) Hulls Are a Rich Source of Fermentable Dietary Fibre and Bioactive Phytochemicals. International Journal of Molecular Sciences, 24(22), 16310. https://doi.org/10.3390/ijms242216310






