Menadione Contribution to the In Vitro Radical Scavenging Potential of Phytochemicals Naringenin and Lignin
Abstract
1. Introduction
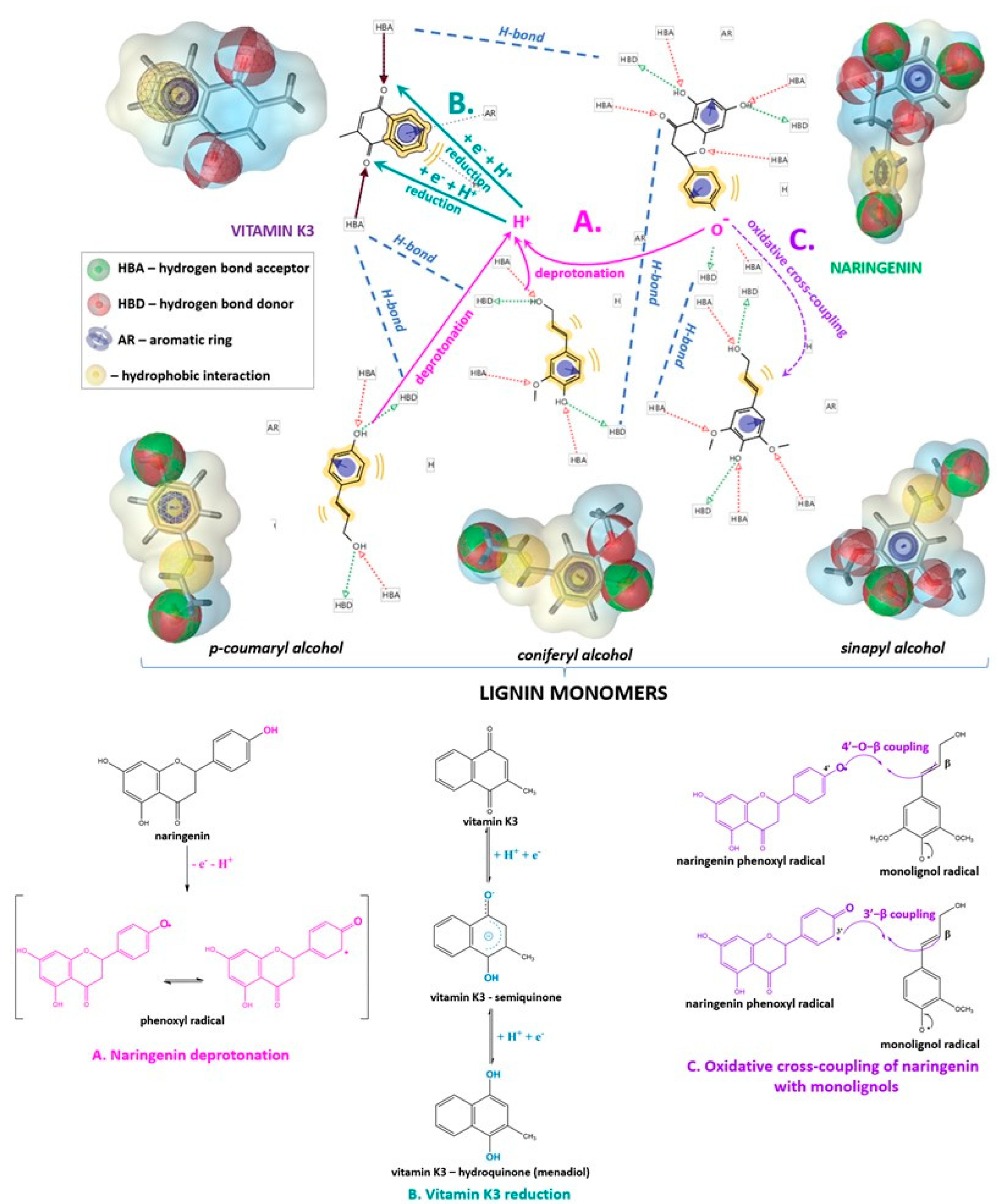
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Radical Scavenging Potential
3.3. Intermolecular Interactions and Molecular Docking
3.4. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popa, D.-S.; Bigman, G.; Rusu, M.E. The Role of Vitamin K in Humans: Implication in Aging and Age-Associated Diseases. Antioxidants 2021, 10, 566. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.V.; Nayak, V.L.; Ramakrishna, S.; Mallavadhani, U.V. Novel menadione hybrids: Synthesis, anticancer activity, and cell-based studies. Chem. Biol. Drug Des. 2018, 91, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Xu, W.; Mai, K.; Zhang, W.; Feng, X.; Liufu, Z. Effects of dietary menadione on the activity of antioxidant enzymes in abalone, Haliotis discus hannai Ino. Chin. J. Oceanol. Limnol. 2012, 30, 118–123. [Google Scholar] [CrossRef]
- Bajor, M.; Graczyk-Jarzynka, A.; Marhelava, K.; Kurkowiak, M.; Rahman, A.; Aura, C.; Russell, N.; Zych, A.O.; Firczuk, M.; Winiarska, M.; et al. Triple Combination of Ascorbate, Menadione and the Inhibition of Peroxiredoxin-1 Produces Synergistic Cytotoxic Effects in Triple-Negative Breast Cancer Cells. Antioxidants 2020, 9, 320. [Google Scholar] [CrossRef]
- Lee, M.H.; Yang, J.Y.; Cho, Y.; Woo, H.J.; Kwon, H.J.; Kim, D.H.; Park, M.; Moon, C.; Yeon, M.J.; Kim, H.W.; et al. Inhibitory Effects of Menadione on Helicobacter pylori Growth and Helicobacter pylori-Induced Inflammation via NF-κB Inhibition. Int. J. Mol. Sci. 2019, 20, 1169. [Google Scholar] [CrossRef]
- Afianda, F.; Wiraswati, H.L.; Alisjahbana, B.; Fauziah, N. Antimalarial Effects of Menadione on Plasmodium falciparum FCR-3 Strains: In Vitro Study. IOSR J. Pharm. Biol. Sci. 2020, 15, 56–60. [Google Scholar]
- Kapadia, G.J.; Soares, I.A.O.; Rao, G.S.; Badoco, F.R.; Furtado, R.A.; Correa, M.B.; Tavares, D.C.; Cunha, W.R.; Magalhães, L.G. Antiparasitic activity of menadione (vitamin K3) against Schistosoma mansoni in BABL/c mice. Acta Trop. 2017, 167, 163–173. [Google Scholar] [CrossRef]
- de Souza, A.C.; Ribeiro, R.C.B.; Costa, D.C.S.; Pauli, F.P.; Pinho, D.R.; de Moraes, M.G.; da Silva, F.C.; Forezi, L.S.M.; Ferreira, V.F. Menadione: A platform and a target to valuable compounds synthesis. Beilstein J. Org. Chem. 2022, 18, 381–419. [Google Scholar] [CrossRef]
- Combs, G.F., Jr.; McClung, J.P.; Vitamin, K. The Vitamins, Fundamental Aspects in Nutrition and Health; Academic Press: London, UK, 2017; pp. 243–265. [Google Scholar] [CrossRef]
- Tampo, Y.; Yonaha, M. Enzymatic and molecular aspects of the antioxidant effect of menadione in hepatic microsomes. Arch. Biochem. Biophys. 1996, 334, 163–174. [Google Scholar] [CrossRef]
- Talcott, R.E.; Smith, M.T.; Giannini, D.D. Inhibition of microsomal lipid peroxidation by naphthoquinones: Structure-activity relationships and possible mechanisms of action. Arch. Biochem. Biophys. 1985, 241, 88–94. [Google Scholar] [CrossRef]
- Mukai, K.; Itoh, S.; Morimoto, H. Stopped-flow kinetic study of vitamin E regeneration with biological hydroquinones (reduced forms of ubiquinone, vitamin K, and tocopherol quinone) in solution. J. Biol. Chem. 1992, 267, 22277–22281. [Google Scholar] [CrossRef] [PubMed]
- Vervoort, L.M.; Ronden, J.E.; Thijssen, H.H. The potent antioxidant activity of the vitamin K cycle in microsomal lipid peroxidation. Biochem. Pharmacol. 1997, 54, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Abraham, M.H.; Acree, W.E. Descriptors for vitamin K3 (menadione); calculation of biological and physicochemical properties. J. Mol. Liq. 2021, 330, 115707. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Fang, B.; Xu, J.; Dong, X.; Yang, L.; Zhang, Z.; Guo, S.; Ding, B. Effects of Vitamin A and K3 on Immune Function and Intestinal Antioxidant Capacity of Aged Laying Hens. Braz. J. Poult. Sci. 2022, 24, 001–010. [Google Scholar] [CrossRef]
- Booth, S.L. Roles for vitamin K beyond coagulation. Annu. Rev. Nutr. 2009, 29, 89–110. [Google Scholar] [CrossRef]
- Fizesan, I.; Rusu, M.E.; Georgiu, C.; Pop, A.; Stefan, M.-G.; Muntean, D.M.; Mirel, S.; Vostinaru, O.; Kiss, B.; Popa, D.-S. Antitussive, Antioxidant, and Anti-Inflammatory Effects of a Walnut (Juglans regia L.) Septum Extract Rich in Bioactive Compounds. Antioxidants 2021, 10, 119. [Google Scholar] [CrossRef]
- He, T.; Hatem, E.; Vernis, L.; Lei, M.; Huang, M.-E. PRX1 knockdown potentiates vitamin K3 toxicity in cancer cells: A potential new therapeutic perspective for an old drug. J. Exp Clin. Cancer Res. 2015, 34, 152. [Google Scholar] [CrossRef]
- Lamson, D.W.; Gu, Y.H.; Plaza, S.M.; Brignall, M.S.; Brinton, C.A.; Sadlon, A.E. The Vitamin C: Vitamin K3 System—Enhancers and Inhibitors of the Anticancer Effect. Altern. Med. Rev. 2010, 15, 345–351. [Google Scholar]
- Bonilla-Porras, A.R.; Jimenez-Del-Rio, M.; Velez-Pardo, C. Vitamin K3 and vitamin C alone or in combination induced apoptosis in leukemia cells by a similar oxidative stress signalling mechanism. Cancer Cell Int. 2011, 11, 19. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Karwowski, B.T. Vitamin K Contribution to DNA Damage—Advantage or Disadvantage? A Human Health Response. Nutrients 2022, 14, 4219. [Google Scholar] [CrossRef]
- Jabbari, M.; Jabbari, A. Antioxidant potential and DPPH radical scavenging kinetics of water-insoluble flavonoid naringenin in aqueous solution of micelles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 392–399. [Google Scholar] [CrossRef]
- Saiqing, T.; Zhen, R.; Axue, M.; Dong, W.; Jiushe, K. Effect of vitamin K on wound healing: A systematic review and meta-analysis based on preclinical studies. Front. Pharmacol. 2022, 13, 1063349. [Google Scholar] [CrossRef]
- Farajtabar, A.; Gharib, F. Spectral analysis of naringenin deprotonation in aqueous ethanol solutions. Chem. Pap. 2013, 67, 538–545. [Google Scholar] [CrossRef]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, J.; Jesudoss, V.A.; Menon, V.P.; Namasivayam, N. Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol. Mech. Methods 2012, 22, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.Z.; Ying, J.; Zhang, J.; Wang, X.F.; Hu, Y.H.; Liang, Y.P.; Liu, Q.; Xu, G.H. Naringenin pre-treatment inhibits neuroapoptosis and ameliorates cognitive impairment in rats exposed to isoflurane anesthesia by regulating the PI3/AKT/PTEN signalling pathway and suppressing NF-kappab-mediated inflammation. Int. J. Mol. Med. 2016, 38, 1271–1280. [Google Scholar] [CrossRef]
- Ali, R.; Shahid, A.; Ali, N.; Hasan, S.K.; Majed, F.; Sultana, S. Amelioration of benzo[a]pyrene-induced oxidative stress and pulmonary toxicity by naringenin in Wistar rats: A plausible role of COX-2 and NF-kappab. Hum. Exp. Toxicol. 2017, 36, 349–364. [Google Scholar] [CrossRef]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri, W.A., Jr. Naringenin inhibits superoxide anion-induced inflammatory pain: Role of oxidative stress, cytokines, Nrf-2 and the NO-cGMP-PKG-KATP channel signaling pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef]
- Park, S.; Lim, W.; Bazer, F.W.; Song, G. Naringenin induces mitochondria-mediated apoptosis and endoplasmic reticulum stress by regulating MAPK and AKT signal transduction pathways in endometriosis cells. Mol. Hum. Reprod. 2017, 23, 842–854. [Google Scholar] [CrossRef]
- Rashmi, R.; Magesh, S.B.; Ramkumar, K.M.; Suryanarayanan, S.; Rao, M.V.S. Antioxidant Potential of Naringenin Helps to Protect Liver Tissue from Streptozotocin-Induced Damage. Rep. Biochem. Mol. Biol. 2018, 7, 76–84. [Google Scholar]
- Ivanova, D.; Toneva, M.; Simeonov, E.; Nikolova, B.; Semkova, S.; Antov, G.; Yaneva, Z. Newly Synthesized Lignin Microparticles as Bioinspired Oral Drug-Delivery Vehicles: Flavonoid-Carrier Potential and In Vitro Radical-Scavenging Activity. Pharmaceutics 2023, 15, 1067. [Google Scholar] [CrossRef] [PubMed]
- Fedoros, E.I.; Orlov, A.A.; Zherebker, A.; Gubareva, E.A.; Maydin, M.A.; Konstantinov, A.I.; Krasnov, K.A.; Karapetian, R.N.; Izotova, E.I.; Pigarev, S.E.; et al. Novel water-soluble lignin derivative BP-Cx-1: Identification of components and screening of potential targets in silico and in vitro. Oncotarget 2018, 9, 18578–18593. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, M.; Koziara, Z.; Suliborska, K.; Chrzanowski, W.; Wormstone, M.; Namieśnik, J.; Bartoszek, A. Interactions between polyphenolic antioxidants quercetin and naringenin dictate the distinctive redox-related chemical and biological behaviour of their mixtures. Sci. Rep. 2021, 11, 12282. [Google Scholar] [CrossRef] [PubMed]
- Baranowska, M.; Suliborska, K.; Todorovic, V.; Kusznierewicz, B.; Chrzanowski, W.; Sobajic, S.; Bartoszek, A. Interactions between bioactive components determine antioxidant, cytotoxic and nutrigenomic activity of cocoa powder extract. Free. Radic. Biol. Med. 2020, 154, 48–61. [Google Scholar] [CrossRef]
- Shahidi, F.; Janitha, P.; Wanasundara, P. Phenolic antioxidants. Crit. Rev. Food Sci. Nutrit. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical Scavenging Mechanisms of Phenolic Compounds: A Quantitative Structure-Property Relationship (QSPR) Study. Front. Nutr. Sec. Food Chem. 2022, 9, 882458. [Google Scholar] [CrossRef]
- Duan, X.; Wang, X.; Chen, J.; Liu, G.; Liu, Y. Structural properties and antioxidation activities of lignins isolated from sequential two-step formosolv fractionation. RSC Adv. 2022, 12, 24242–24251. [Google Scholar] [CrossRef]
- Tavares, D.; Cavali, M.; Tanobe, V.d.O.A.; Torres, L.A.Z.; Rozendo, A.S.; Zandoná Filho, A.; Soccol, C.R.; Woiciechowski, A.L. Lignin from Residual Sawdust of Eucalyptus spp.—Isolation, Characterization, and Evaluation of the Antioxidant Properties. Biomass 2022, 2, 195–208. [Google Scholar] [CrossRef]
- Jabbari, M.; Mir, H.; Kanaani, A.; Ajloo, D. Kinetic solvent effects on the reaction between flavonoid naringenin and 2,2-diphenyl-1-picrylhydrazyl radical in different aqueous solutions of ethanol: An experimental and theoretical study. J. Mol. Liq. 2014, 196, 381–391. [Google Scholar] [CrossRef]
- Shubina, V.S.; Kozina, V.I.; Shatalin, Y.V. Comparison of Antioxidant Properties of a Conjugate of Taxifolin with Glyoxylic Acid and Selected Flavonoids. Antioxidants 2021, 10, 1262. [Google Scholar] [CrossRef]
- Skroza, D.; Šimat, V.; Vrdoljak, L.; Jolić, N.; Skelin, A.; Čagalj, M.; Frleta, R.; Generalić Mekinić, I. Investigation of Antioxidant Synergisms and Antagonisms among Phenolic Acids in the Model Matrices Using FRAP and ORAC Methods. Antioxidants 2022, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chakraborty, R.; Raychaudhuri, U. Determination of pH-dependent antioxidant activity of palm (Borassus flabellifer) polyphenol compounds by photoluminol and DPPH methods: A comparison of redox reaction sensitivity. 3 Biotech 2015, 5, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Yaneva, Z.; Beev, G.; Rusenova, N.; Ivanova, D.; Tzanova, M.; Stoeva, D.; Toneva, M. Antimicrobial Potential of Conjugated Lignin/Morin/Chitosan Combinations as a Function of System Complexity. Antibiotics 2022, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Lukeš, V.; Ilčin, M. DFT/B3LYP study of tocopherols and chromans antioxidant action energetics. Chem. Phys. 2007, 336, 51–57. [Google Scholar] [CrossRef]
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of hydroxyl groups: An overlooked influence on antiradical properties of flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef]
- Vaganek, A.; Rimarcik, J.; Lukes, V.; Klein, E. On the energetics of homolytic and heterolytic OAH bond cleavage in flavonoids. Comput. Theor. Chem. 2012, 991, 192–200. [Google Scholar] [CrossRef]
- de Oliveira, A.S.; Brighente, I.M.C.; Lund, R.G.; Llanes, L.C.; Nunes, R.J.; Bretanha, L.C.; Yunes, R.A.; Carvalho, P.H.A.; Ribeiro, J.S. Antioxidant and Antifungal Activity of Naphthoquinones Dimeric Derived from Lawsone. J. Biosci. Med. 2017, 5, 39–48. [Google Scholar] [CrossRef][Green Version]
- Fiorentini, D.; Cipollone, M.; Galli, M.C.; Landi, L. Antioxidant activity of reduced menadione in solvent solution and in model membranes. Free Radic. Res. 1997, 26, 419–429. [Google Scholar] [CrossRef]
- Rencoret, J.; Rosado, M.J.; Kim, H.; Timokhin, V.I.; Gutiérrez, A.; Bausch, F.; Rosenau, T.; Potthast, A.; Ralph, J.; Del Río, J.C. Flavonoids naringenin chalcone, naringenin, dihydrotricin, and tricin are lignin monomers in papyrus. Plant Physiol. 2022, 188, 208–219. [Google Scholar] [CrossRef]
- Beck, R.; Verrax, J.; Dejeans, N.; Taper, H.; Calderon, P.B. Menadione reduction by pharmacological doses of ascorbate induces an oxidative stress that kills breast cancer cells. Int. J. Toxicol. 2009, 28, 33–42. [Google Scholar] [CrossRef]
- Sun, R.C. Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels Chemistry, Extractives, Lignins, Hemicelluloses and Cellulose, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Biela, M.; Kleinová, A.; Klein, E. Phenolic Acids and Their Carboxylate Anions: Thermodynamics of Primary Antioxidant Action. Phytochemistry 2022, 200, 113254. [Google Scholar] [CrossRef] [PubMed]
- Hang, D.T.N.; Hoa, N.T.; Bich, H.N.; Mechler, A.; Vo, Q.V. The Hydroperoxyl Radical Scavenging Activity of Natural Hydroxybenzoic Acids in Oil and Aqueous Environments: Insights into the Mechanism and Kinetics. Phytochemistry 2022, 201, 113281. [Google Scholar] [CrossRef] [PubMed]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Cotrim Marques, S.; Marques Nunes, P.; Pedrosa, R.; Leandro, S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius henslowii Sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vásquez, M.J.; Plascencia-Jatomea, M.; Sánchez-Valdes, S.; Tanori-Córdova, J.C.; Castillo-Yañez, F.J.; Quintero-Reyes, I.E.; Graciano-Verdugo, A.Z. Characterization of epigallocatechin-gallate-grafted chitosan nanoparticles and evaluation of their antibacterial and antioxidant potential. Polymers 2021, 13, 1375. [Google Scholar] [CrossRef] [PubMed]



| Radical Scavenging Activity | ABTS Potential | DPPH Potential | ||||
|---|---|---|---|---|---|---|
| System | Experimental | Theoretical | Difference, % | Experimental | Theoretical | Difference, % |
| double combinations | ||||||
| lignin/naringenin | 50.34 | 27.55 | −8.64 | 42.92 | 17.43 | 23.12 |
| vitamin K3/lignin | 53.64 | 18.385 | 45.89 | 40.85 | 23.83 | −14.28 |
| vitamin K3/naringenin | 31.36 | 12.46 | 25.80 | 17.95 | 8.82 | 1.75 |
| triple combination | ||||||
| lignin/naringenin/vitamin K3 | 60.11 | 14.08 | 42.31 | 50.63 | 11.65 | 44.90 |
| Solution Component | Solvent | Concentration/ Volumetric Ratio | pH |
|---|---|---|---|
| naringenin | EtOH | 100 mg/L | 6.84 |
| lignin | Milli-Q water | 500 mg/L | 9.90 |
| vitamin K3 | EtOH | 1.00 M | 7.35 |
| naringenin/vitamin K3 | EtOH | 1:1, v/v | 7.60 |
| naringenin/lignin | EtOH/Milli-Q water | 1:1, v/v | 8.94 |
| vitamin K3/lignin | EtOH/Milli-Q water | 1:1, v/v | 9.80 |
| naringenin/vitamin K3/lignin | EtOH/Milli-Q water | 1:1:1, v/v/v | 9.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaneva, Z.; Ivanova, D.; Toneva, M.; Tzanova, M.; Marutsova, V.; Grozeva, N. Menadione Contribution to the In Vitro Radical Scavenging Potential of Phytochemicals Naringenin and Lignin. Int. J. Mol. Sci. 2023, 24, 16268. https://doi.org/10.3390/ijms242216268
Yaneva Z, Ivanova D, Toneva M, Tzanova M, Marutsova V, Grozeva N. Menadione Contribution to the In Vitro Radical Scavenging Potential of Phytochemicals Naringenin and Lignin. International Journal of Molecular Sciences. 2023; 24(22):16268. https://doi.org/10.3390/ijms242216268
Chicago/Turabian StyleYaneva, Zvezdelina, Donika Ivanova, Monika Toneva, Milena Tzanova, Vanya Marutsova, and Neli Grozeva. 2023. "Menadione Contribution to the In Vitro Radical Scavenging Potential of Phytochemicals Naringenin and Lignin" International Journal of Molecular Sciences 24, no. 22: 16268. https://doi.org/10.3390/ijms242216268
APA StyleYaneva, Z., Ivanova, D., Toneva, M., Tzanova, M., Marutsova, V., & Grozeva, N. (2023). Menadione Contribution to the In Vitro Radical Scavenging Potential of Phytochemicals Naringenin and Lignin. International Journal of Molecular Sciences, 24(22), 16268. https://doi.org/10.3390/ijms242216268








