NXC736 Attenuates Radiation-Induced Lung Fibrosis via Regulating NLRP3/IL-1β Signaling Pathway
Abstract
1. Introduction
2. Results
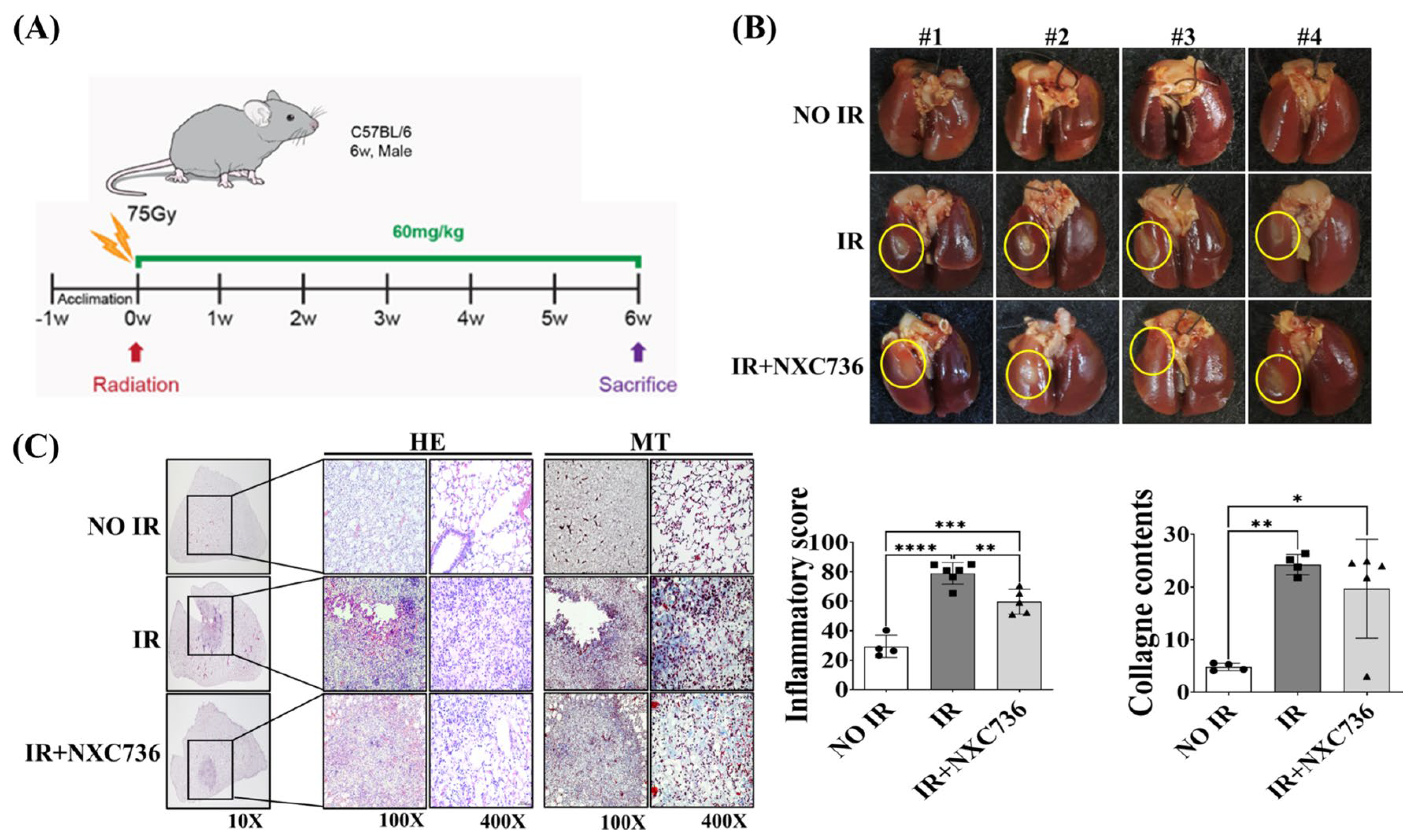
2.1. Treatment with NXC76 Changed Gross Morphological Findings and Radiation-Induced Inflammatory Cells Infiltration
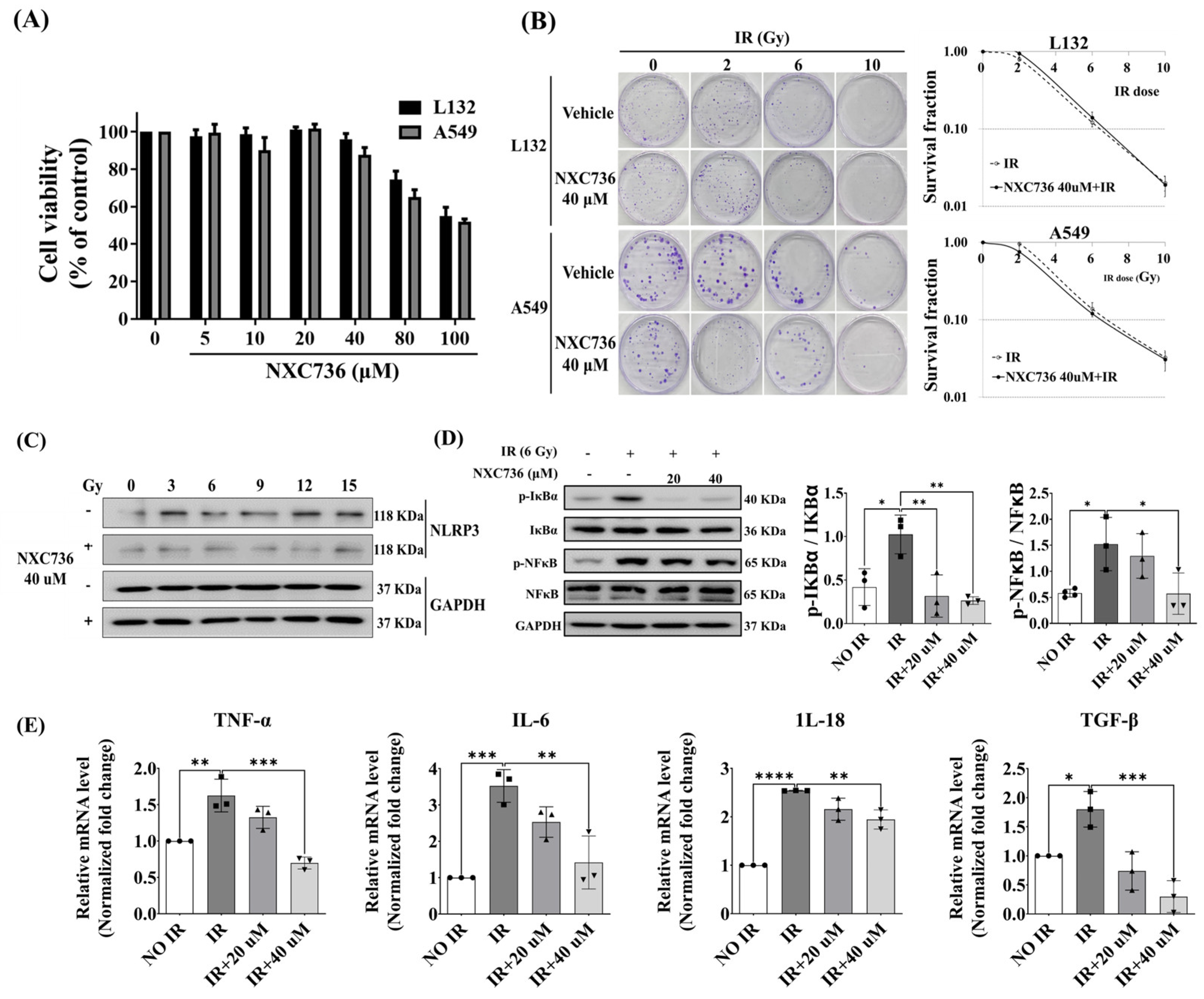
2.2. NXC736 Attenuates RILF and Rescues Lung Function
2.3. NXC736 Treatment Suppressed the Expression of Inflammation-Related Molecules
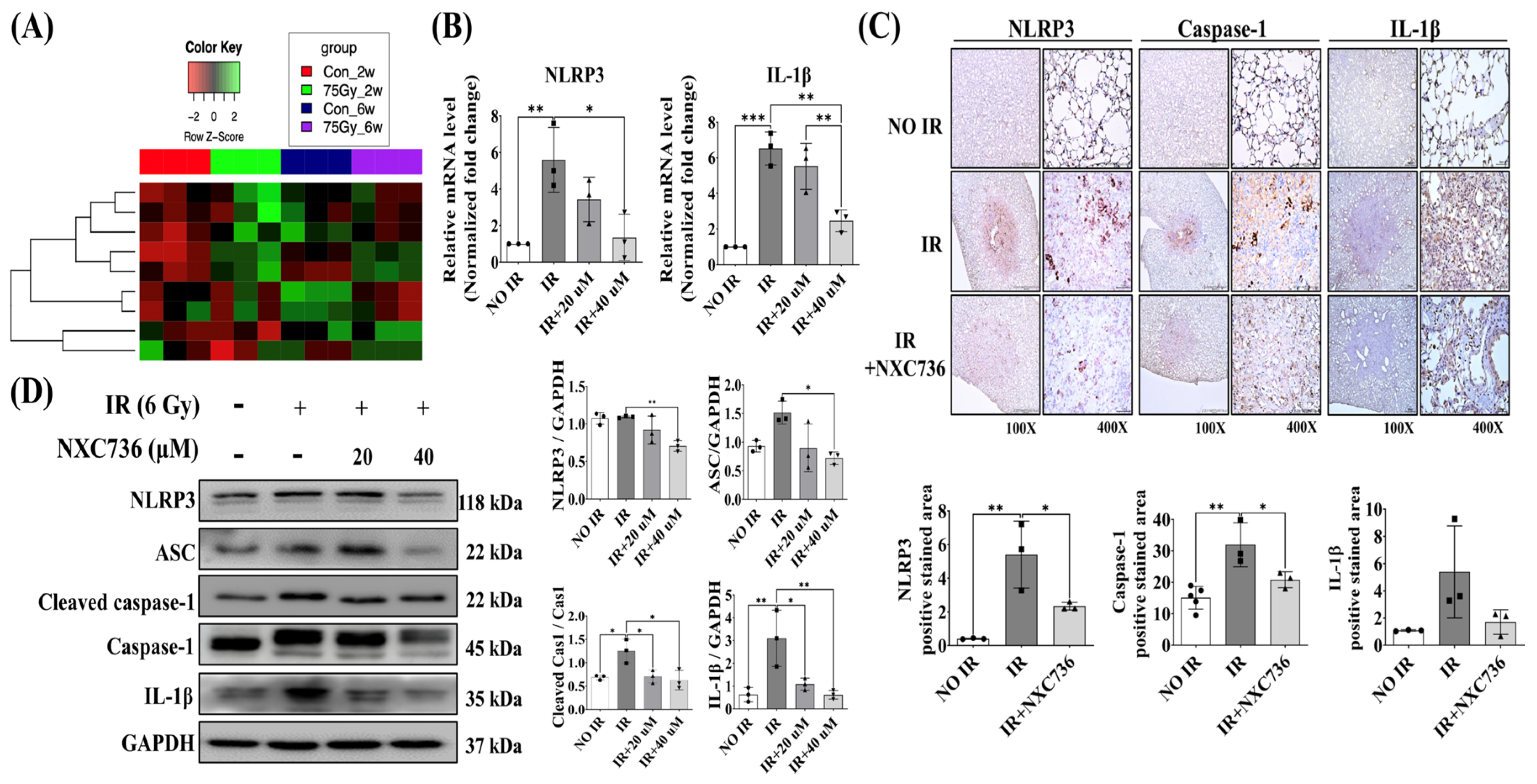
2.4. NXC736 Downregulated the NLRP3/IL-1β Signaling Pathway
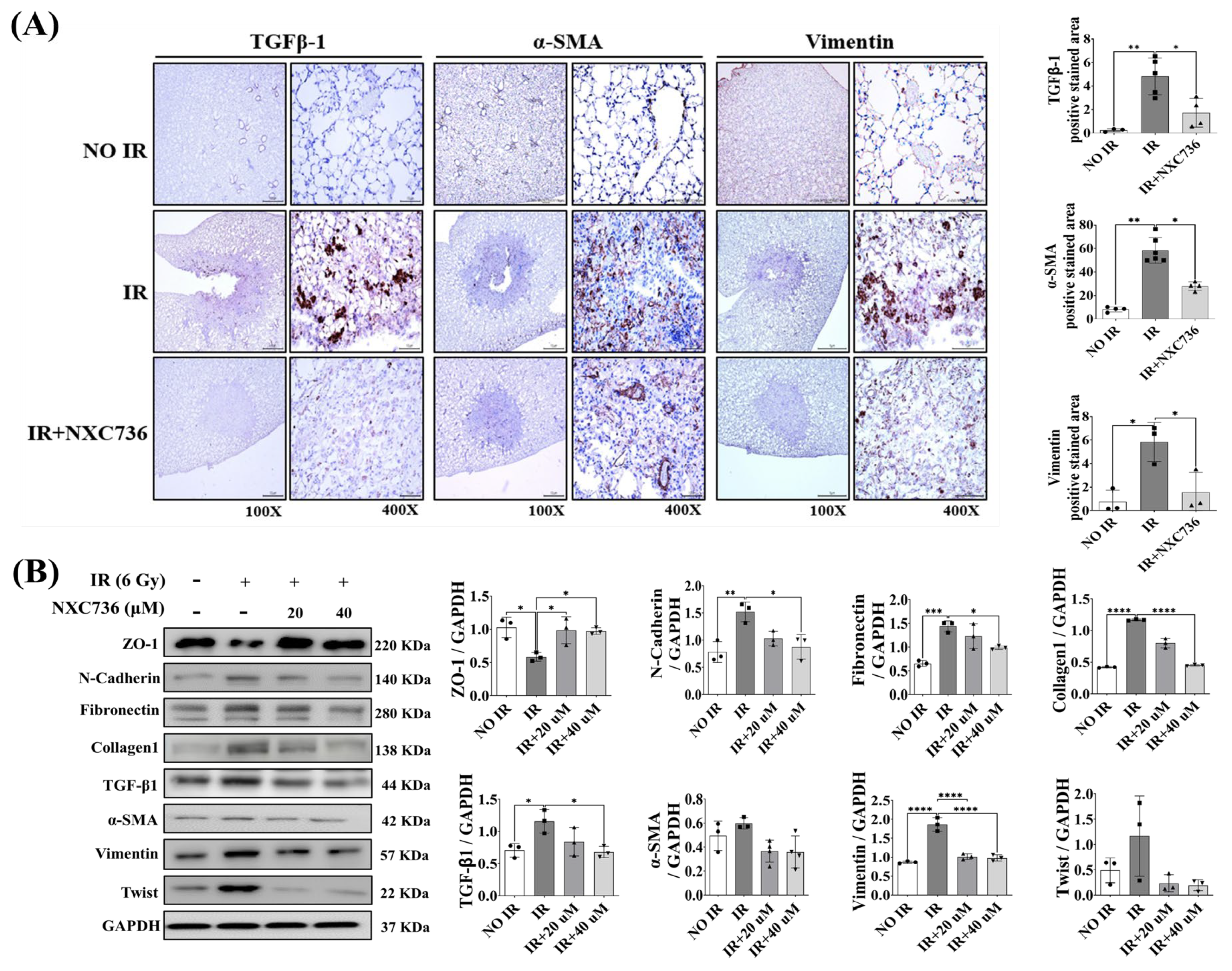
2.5. NXC736 Treatment Decreased the Expression of EMT-Related Marker
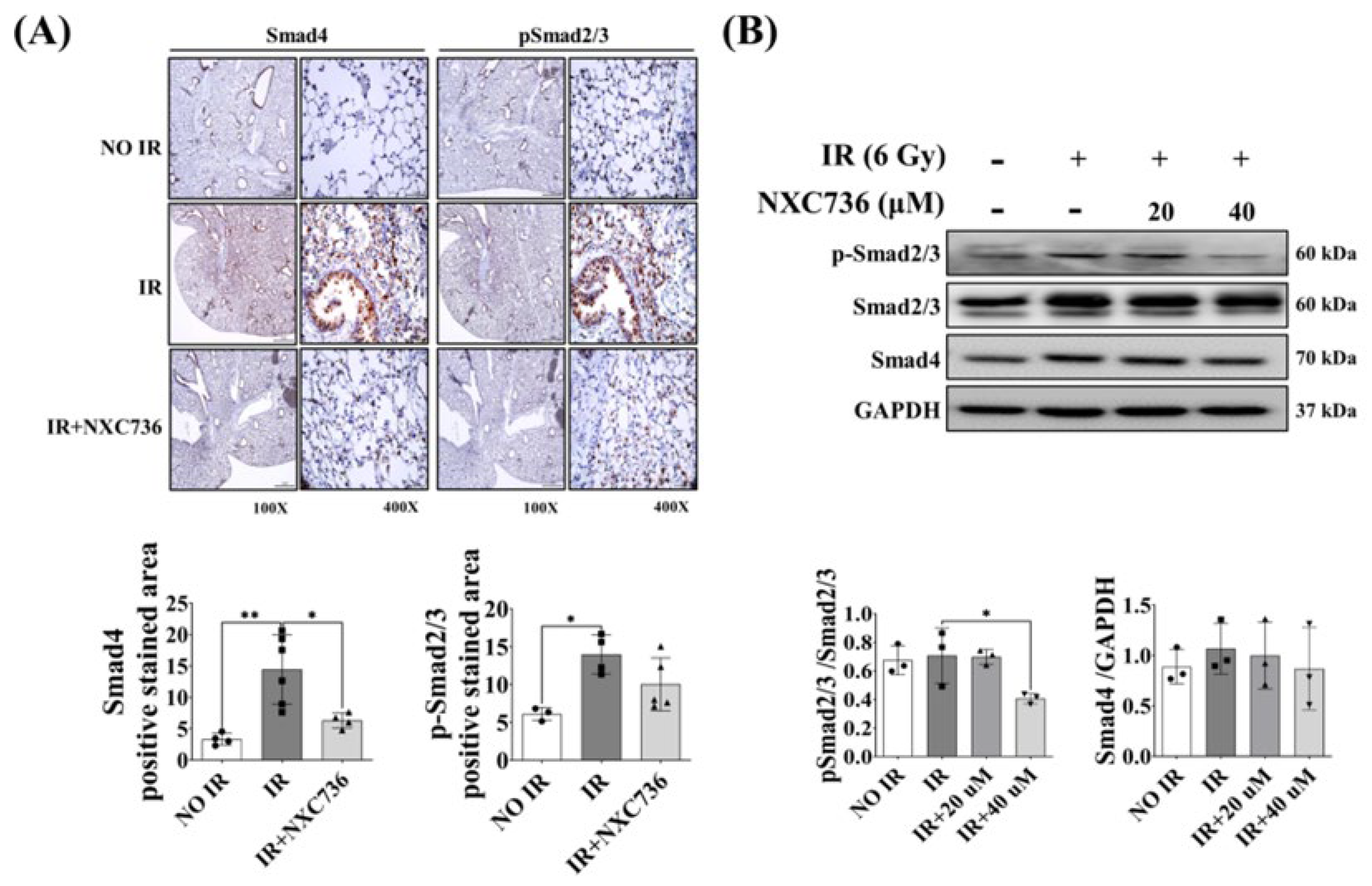
2.6. NXC736 Treatment Regulates the Smad Pathway Signaling
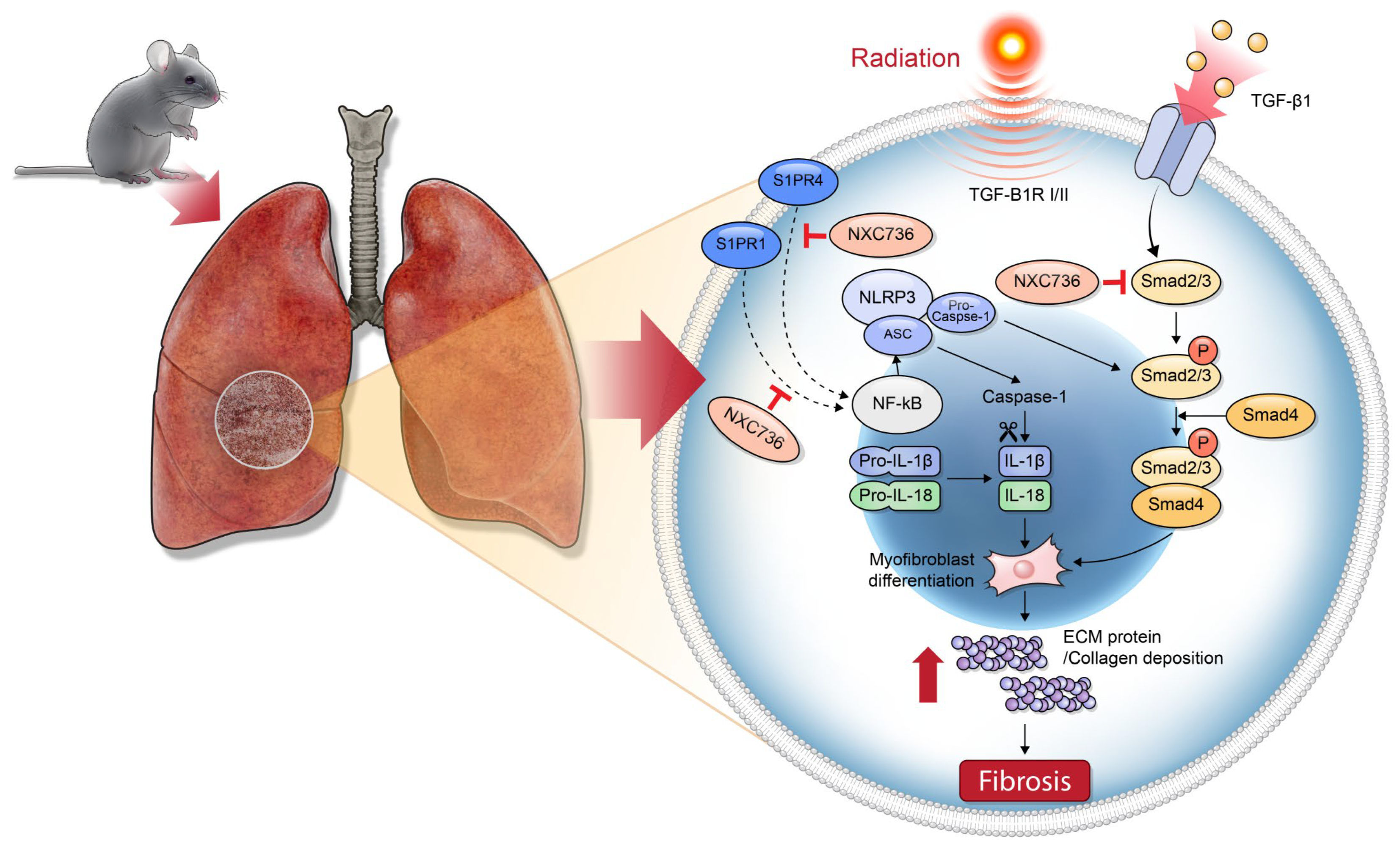
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Irradiation of Cells
4.3. Colony Formation Assay
4.4. Western Blot Analysis
4.5. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.6. Preparation of NXC736
4.7. Animal Experiments
4.8. Histological and Immunohistochemical Analysis
4.9. MRI
4.10. FlexiVent™ Analysis of the Lung
4.11. RNA Sequencing
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simone, C.B., II; Wildt, B.; Haas, A.R.; Pope, G.; Rengan, R.; Hahn, S.M. Stereotactic body radiation therapy for lung cancer. Chest 2013, 143, 1784–1790. [Google Scholar] [CrossRef]
- Mehta, V. Radiation pneumonitis and pulmonary fibrosis in non–small-cell lung cancer: Pulmonary function, prediction, and prevention. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Hernández, M.; Maldonado, F.; Lozano-Ruiz, F.; Muñoz-Montaño, W.; Nuñez-Baez, M.; Arrieta, O. Radiation-induced lung injury: Current evidence. BMC Pulm. Med. 2021, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.A.; Zahedipour, F.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Pulmonary fibrosis: Therapeutic and mechanistic insights into the role of phytochemicals. BioFactors 2021, 47, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Borthwick, L. The il-1 Cytokine Family and Its Role in Inflammation and Fibrosis in the Lung. Semin. Immunopathol. 2016, 38, 517–534. [Google Scholar] [CrossRef]
- Kolb, M.; Margetts, P.J.; Anthony, D.C.; Pitossi, F.; Gauldie, J. Transient expression of il-1β induces acute lung injury and chronic repair leading to pulmonary fibrosis. J. Clin. Investig. 2001, 107, 1529–1536. [Google Scholar] [CrossRef]
- Dong, S.-H.; Liu, Y.-W.; Wei, F.; Tan, H.-Z.; Han, Z.-D. Asiatic acid ameliorates pulmonary fibrosis induced by bleomycin (blm) via suppressing pro-fibrotic and inflammatory signaling pathways. Biomed. Pharmacother. 2017, 89, 1297–1309. [Google Scholar] [CrossRef]
- Buckley, S.T.; Medina, C.; Ehrhardt, C. Differential susceptibility to epithelial-mesenchymal transition (emt) of alveolar, bronchial and intestinal epithelial cells in vitro and the effect of angiotensin ii receptor inhibition. Cell Tissue Res. 2010, 342, 39–51. [Google Scholar] [CrossRef]
- Budi, E.H.; Schaub, J.R.; Decaris, M.; Turner, S.; Derynck, R. Tgf-β as a driver of fibrosis: Physiological roles and therapeutic opportunities. J. Pathol. 2021, 254, 358–373. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.; Lei, W.; Hou, Y.; Zhang, Y.; Tang, R.; Yang, Z.; Tian, Y.; Zhu, Y.; Wang, C. The nlrp3 inflammasome in fibrosis and aging: The known unknowns. Ageing Res. Rev. 2022, 79, 101638. [Google Scholar] [CrossRef]
- Tian, R.; Zhu, Y.; Yao, J.; Meng, X.; Wang, J.; Xie, H.; Wang, R. Nlrp3 participates in the regulation of emt in bleomycin-induced pulmonary fibrosis. Exp. Cell Res. 2017, 357, 328–334. [Google Scholar] [CrossRef]
- Hosseinian, N.; Cho, Y.; Lockey, R.F.; Kolliputi, N. The role of the nlrp3 inflammasome in pulmonary diseases. Ther. Adv. Respir. Dis. 2015, 9, 188–197. [Google Scholar] [CrossRef]
- Hong, C.H.; Ko, M.S.; Kim, J.H.; Cho, H.; Lee, C.-H.; Yoon, J.E.; Yun, J.-Y.; Baek, I.-J.; Jang, J.E.; Lee, S.E. Sphingosine 1-phosphate receptor 4 promotes nonalcoholic steatohepatitis by activating nlrp3 inflammasome. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 925–947. [Google Scholar] [CrossRef] [PubMed]
- Vargas, W.S.; Perumal, J.S. Fingolimod and cardiac risk: Latest findings and clinical implications. Ther. Adv. Drug Saf. 2013, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, J.-M.; Lee, S.R.; Kim, H.-J.; Lee, J.H.; Choi, H.L.; Lee, Y.-J.; Lee, Y.-S.; Cho, J. Efferocytosis and enhanced fpr2 expression following apoptotic cell instillation attenuate radiation-induced lung inflammation and fibrosis. Biochem. Biophys. Res. Commun. 2022, 601, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.Y.; Lee, C.G.; Shim, H.S.; Lee, E.J.; Song, K.H.; Choi, B.W.; Story, M.D. Time, dose, and volume responses in a mouse pulmonary injury model following ablative irradiation. Lung 2016, 194, 81–90. [Google Scholar] [CrossRef]
- Jin, H.; Yoo, Y.; Kim, Y.; Kim, Y.; Cho, J.; Lee, Y.S. Radiation-induced lung fibrosis: Preclinical animal models and therapeutic strategies. Cancers 2020, 12, 1561. [Google Scholar] [CrossRef]
- Hodzic, J.; Dingjan, I.; Maas, M.J.; van der Meulen-Muileman, I.H.; de Menezes, R.X.; Heukelom, S.; Verheij, M.; Gerritsen, W.R.; Geldof, A.A.; van Triest, B. A cell-based high-throughput screening assay for radiation susceptibility using automated cell counting. Radiat. Oncol. 2015, 10, 55. [Google Scholar] [CrossRef][Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. Nf-κb signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Lu, J.; Zhong, Y.; Lin, Z.; Lin, X.; Chen, Z.; Wu, X.; Wang, N.; Zhang, H.; Huang, S.; Zhu, Y. Baicalin alleviates radiation-induced epithelial-mesenchymal transition of primary type ii alveolar epithelial cells via tgf-β and erk/gsk3β signaling pathways. Biomed. Pharmacother. 2017, 95, 1219–1224. [Google Scholar] [CrossRef]
- Young, M.M.; Kester, M.; Wang, H.G. Sphingolipids: Regulators of crosstalk between apoptosis and autophagy. J. Lipid Res. 2013, 54, 5–19. [Google Scholar] [CrossRef]
- Tanaka, S.; Zheng, S.; Kharel, Y.; Fritzemeier, R.G.; Huang, T.; Foster, D.; Okusa, M.D. Sphingosine 1-phosphate signaling in perivascular cells enhances inflammation and fibrosis in the kidney. Sci. Transl. Med. 2022, 14, 2681. [Google Scholar] [CrossRef]
- Hou, L.; Zhang, Z.; Yang, L.; Chang, N.; Zhao, X.; Zhou, X.; Li, L. NLRP3 inflammasome priming and activation in cholestatic liver injury via the sphingosine 1-phosphate/S1P receptor 2/Gα (12/13)/MAPK signaling pathway. J. Mol. Med. 2021, 99, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; He, X.; Zeng, M. The role of S1P and the related signaling pathway in the development of tissue fibrosis. Front. Pharmacol. 2019, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Biancatelli, R.M.C.; Solopov, P.A.; Catravas, J.D. The inflammasome nlr family pyrin domain-containing protein 3 (nlrp3) as a novel therapeutic target for idiopathic pulmonary fibrosis. Am. J. Pathol. 2022, 192, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhou, D.; Deng, H.; Chen, Y.; Wang, J.; Zhou, X.; Jie, X.; Xu, Y.; Wu, Z.; Wang, G. Activation of nlrp3 inflammasome in lung epithelial cells triggers radiation-induced lung injury. Respir. Res. 2023, 24, 25. [Google Scholar] [CrossRef]
- Luo, H.; Liu, X.; Liu, H.; Wang, Y.; Xu, K.; Li, J.; Liu, M.; Guo, J.; Qin, X. Act001 ameliorates ionizing radiation-induced lung injury by inhibiting nlrp3 inflammasome pathway. Biomed. Pharmacother. 2023, 163, 114808. [Google Scholar] [CrossRef]
- Kim, J.-Y.; An, Y.-M.; Yoo, B.R.; Kim, J.-M.; Han, S.Y.; Na, Y.; Lee, Y.-S.; Cho, J. Hsp27 inhibitor attenuates radiation-induced pulmonary inflammation. Sci. Rep. 2018, 8, 4189. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, Y.; Hu, S.G.; Zhang, Q.B.; Yu, J.; Zhang, Y.M. T-lymphocyte kv1. 3 channel activation triggers the nlrp3 inflammasome signaling pathway in hypertensive patients. Exp. Ther. Med. 2017, 14, 147–154. [Google Scholar] [CrossRef]
- Trachalaki, A.; Tsitoura, E.; Mastrodimou, S.; Invernizzi, R. Enhanced il-1b release following nlrp3 and aim2 inflammasome stimulation linked to mtros is in airway macrophages in pulmonary fibrosis. Front. Immunol. 2022, 12, 661811. [Google Scholar] [CrossRef]
- Su, J.; Morgani, S.M.; David, C.J.; Wang, Q.; Er, E.E.; Huang, Y.-H.; Basnet, H.; Zou, Y.; Shu, W.; Soni, R.K. Tgf-β orchestrates fibrogenic and developmental emts via the ras effector rreb1. Nature 2020, 577, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Jolly, M.K.; Ward, C.; Eapen, M.S.; Myers, S.; Hallgren, O.; Levine, H.; Sohal, S.S. Epithelial–mesenchymal transition, a spectrum of states: Role in lung development, homeostasis, and disease. Dev. Dyn. 2018, 247, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The nf-κb family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Jeon, S.; Yoo, Y.J.; Jin, H.; Won, H.Y.; Yoon, K.; Na, Y.; Cho, J.; Lee, Y.S. The Hsp27-mediated IkBα-NFκB signaling axis promotes radiation-induced lung fibrosis. Clin. Cancer Res. 2019, 25, 5364–5375. [Google Scholar] [CrossRef] [PubMed]
- Wielpütz, M.O.; Puderbach, M.; Kopp-Schneider, A.; Stahl, M.; Fritzsching, E.; Sommerburg, O.; Ley, S.; Sumkauskaite, M.; Biederer, J.; Kauczor, H.-U. Magnetic resonance imaging detects changes in structure and perfusion, and response to therapy in early cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 2014, 189, 956–965. [Google Scholar] [CrossRef]
- Fain, S.; Schiebler, M.L.; McCormack, D.G.; Parraga, G. Imaging of lung function using hyperpolarized helium-3 magnetic resonance imaging: Review of current and emerging translational methods and applications. J. Magn. Reson. Imaging 2010, 32, 1398–1408. [Google Scholar] [CrossRef]
- Gadodia, A.; Seith, A.; Sharma, R.; Thakar, A. Mri and mr sialography of juvenile recurrent parotitis. Pediatr. Radiol. 2010, 40, 1405–1410. [Google Scholar] [CrossRef]
- Vanoirbeek, J.A.; Rinaldi, M.; De Vooght, V.; Haenen, S.; Bobic, S.; Gayan-Ramirez, G.; Hoet, P.H.; Verbeken, E.; Decramer, M.; Nemery, B. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am. J. Respir. Cell Mol. Biol. 2010, 42, 96–104. [Google Scholar] [CrossRef]
- Kim, H.; Park, S.-H.; Han, S.Y.; Lee, Y.-S.; Cho, J.; Kim, J.-M. Lxa4-fpr2 signaling regulates radiation-induced pulmonary fibrosis via crosstalk with tgf-β/smad signaling. Cell Death Dis. 2020, 11, 653. [Google Scholar] [CrossRef]
- Warnes, G.; Bolker, B.; Bonebakker, L.; Gentleman, R.; Liaw, W.; Lumley, T.; Maechler, M.; Magnusson, A.; Moeller, S.; Schwartz, M. Gplots: Various R Programming Tools for Plotting Data. R Package Version 3.0.1. The Comprehensive RArchive Network. 2016. Available online: https://cran.r-project.org/web/packages/gplots/index.html (accessed on 15 October 2023).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Park, S.; Cui, R.; Lee, H.; Choi, H.; Farh, M.E.-A.; Jo, H.I.; Lee, J.H.; Song, H.J.; Lee, Y.-J.; et al. NXC736 Attenuates Radiation-Induced Lung Fibrosis via Regulating NLRP3/IL-1β Signaling Pathway. Int. J. Mol. Sci. 2023, 24, 16265. https://doi.org/10.3390/ijms242216265
Kim SY, Park S, Cui R, Lee H, Choi H, Farh ME-A, Jo HI, Lee JH, Song HJ, Lee Y-J, et al. NXC736 Attenuates Radiation-Induced Lung Fibrosis via Regulating NLRP3/IL-1β Signaling Pathway. International Journal of Molecular Sciences. 2023; 24(22):16265. https://doi.org/10.3390/ijms242216265
Chicago/Turabian StyleKim, Sang Yeon, Sunjoo Park, Ronglan Cui, Hajeong Lee, Hojung Choi, Mohamed El-Agamy Farh, Hai In Jo, Jae Hee Lee, Hyo Jeong Song, Yoon-Jin Lee, and et al. 2023. "NXC736 Attenuates Radiation-Induced Lung Fibrosis via Regulating NLRP3/IL-1β Signaling Pathway" International Journal of Molecular Sciences 24, no. 22: 16265. https://doi.org/10.3390/ijms242216265
APA StyleKim, S. Y., Park, S., Cui, R., Lee, H., Choi, H., Farh, M. E.-A., Jo, H. I., Lee, J. H., Song, H. J., Lee, Y.-J., Lee, Y.-S., Lee, B. Y., & Cho, J. (2023). NXC736 Attenuates Radiation-Induced Lung Fibrosis via Regulating NLRP3/IL-1β Signaling Pathway. International Journal of Molecular Sciences, 24(22), 16265. https://doi.org/10.3390/ijms242216265





