Novel Combination of Choline with Withania somnifera (L.) Dunal, and Bacopa monnieri (L.) Wetts Reduced Oxidative Stress in Microglia Cells, Promoting Neuroprotection
Abstract
:1. Introduction
2. Results
2.1. CRH 100 nM Reduced BV2 Cell Viability, Induced Morphological Changes after 24 h of Stimulation
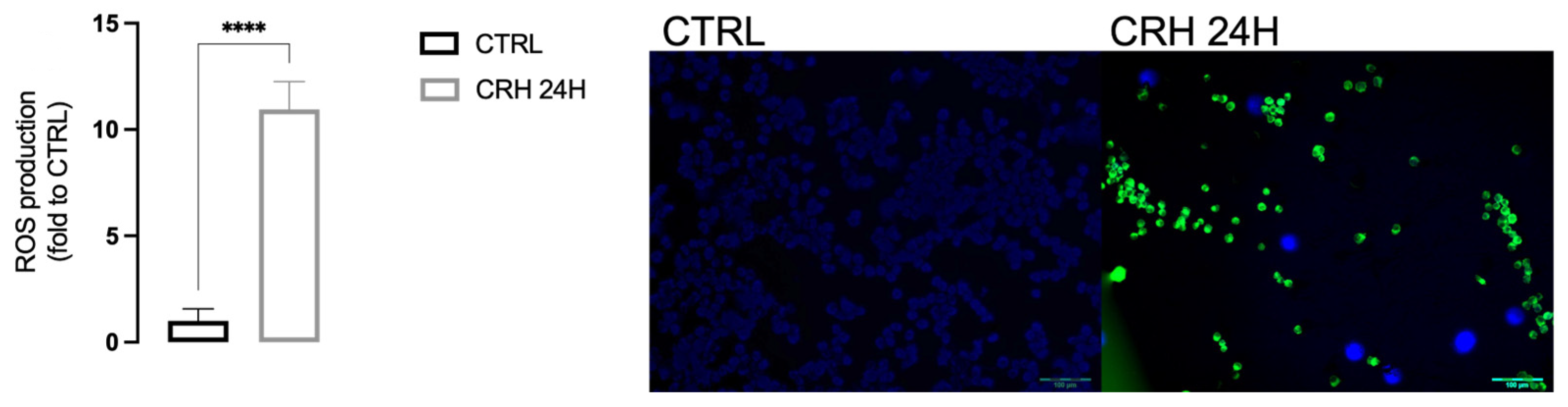
2.2. CRH Induced Oxidative Stress in BV2
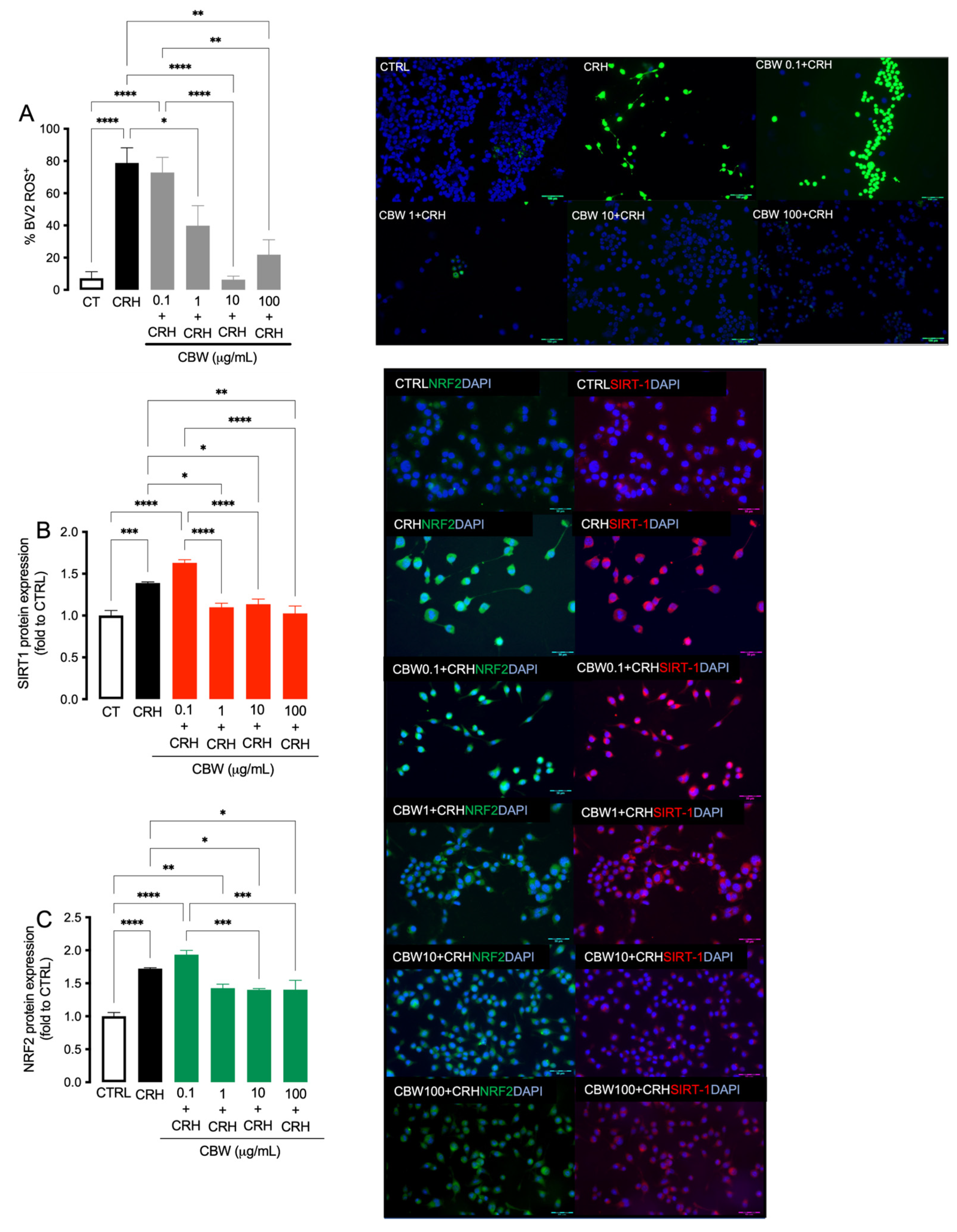
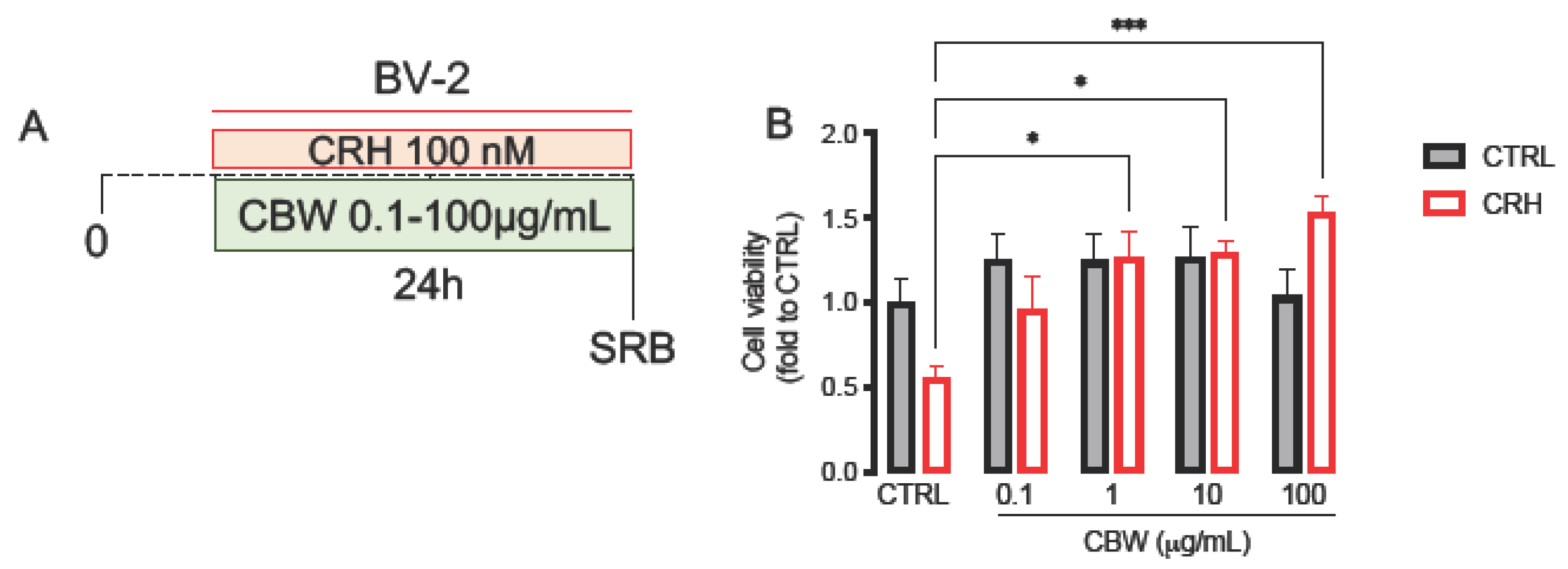
2.3. Evaluation of the Protective Effect of the Combination CBW in the Oxidative Stress Model on BV2 Cells
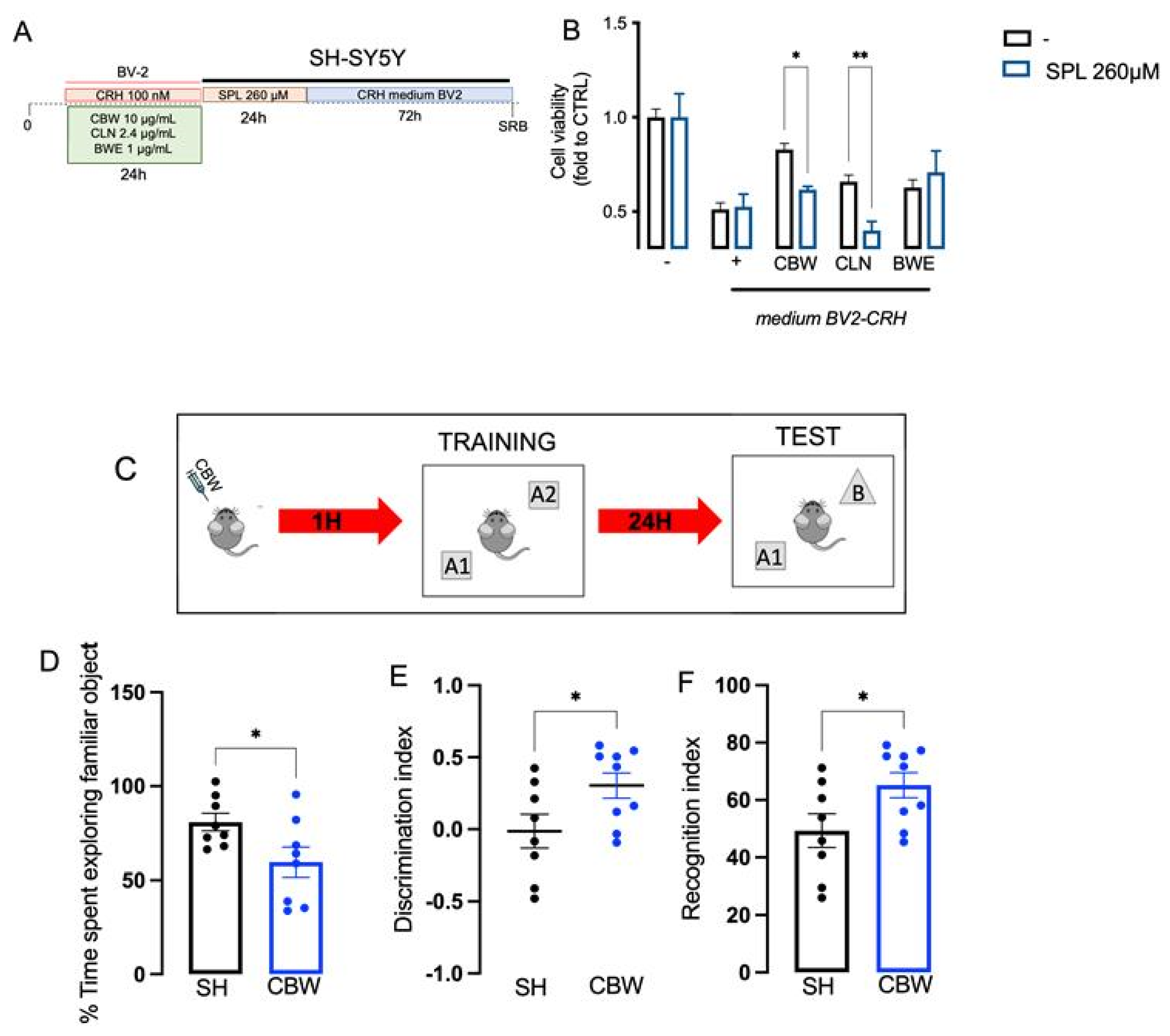
2.4. Evaluation of the Antioxidant Effect of CBW Association in the CRH-Stimulated BV2
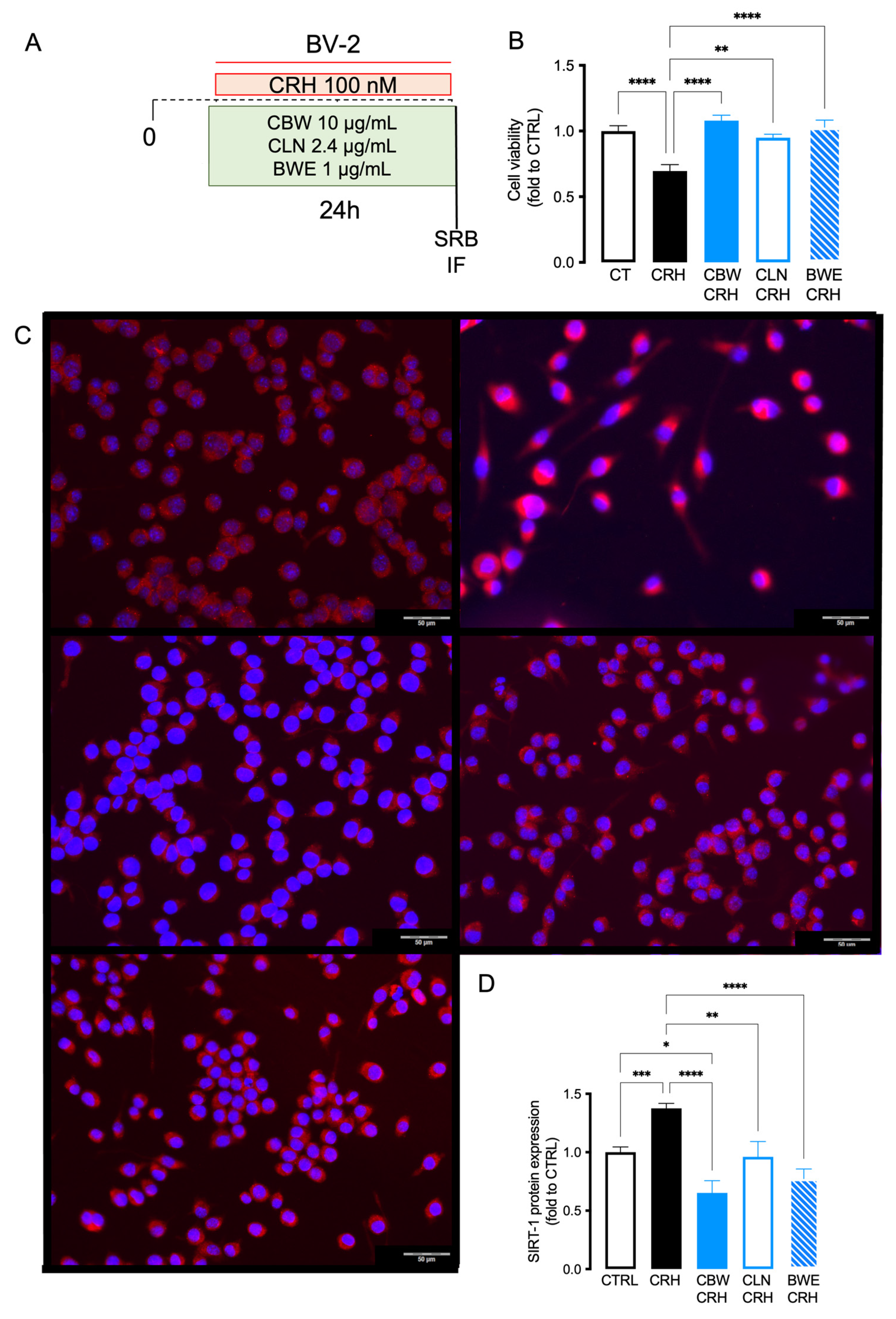
2.5. Comparison of the Effect between CBW and Its Principal Constituent (CLN and BW) in Reducing Oxidative Stress Produced by CRH on BV2 Cells
2.6. Implication of the Cholinergic System in the Neuroprotective Effect of CBW in the In Vitro Model of Stress and Effect on Memory Function In Vivo
3. Discussion
4. Materials and Methods
4.1. Chemicals and Drug Administration
4.2. Cell Culture
4.3. In Vitro Model of Neurotoxicity Induced by Oxidative-Stress-Correlated Microgliosis
4.4. Sulforhodamine B (SRB) Assay
4.5. Immunofluorescence Staining
4.6. Dosage of ROS Species Level
4.7. Animals
4.8. Evaluation of Mnemonic Functions with the NORT
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kivimäki, M.; Steptoe, A. Effects of Stress on the Development and Progression of Cardiovascular Disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, C.I.T. Stress Mechanisms and Metabolic Complications. Horm. Metab. Res. 2007, 39, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of Stress throughout the Lifespan on the Brain, Behaviour and Cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef]
- Marin, M.-F.; Lord, C.; Andrews, J.; Juster, R.-P.; Sindi, S.; Arsenault-Lapierre, G.; Fiocco, A.J.; Lupien, S.J. Chronic Stress, Cognitive Functioning and Mental Health. Neurobiol. Learn. Mem. 2011, 96, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Maier, S.F. Bi-Directional Immune–Brain Communication: Implications for Understanding Stress, Pain, and Cognition. Brain Behav. Immun. 2003, 17, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.G.; Fonken, L.K.; Watkins, L.R.; Maier, S.F. Microglia: Neuroimmune-Sensors of Stress. Semin. Cell Dev. Biol. 2019, 94, 176–185. [Google Scholar] [CrossRef]
- Bradley, S.J.; Bourgognon, J.M.; Sanger, H.E.; Verity, N.; Mogg, A.J.; White, D.J.; Butcher, A.J.; Moreno, J.A.; Molloy, C.; Macedo-Hatch, T.; et al. M1 Muscarinic Allosteric Modulators Slow Prion Neurodegeneration and Restore Memory Loss. J. Clin. Investig. 2017, 127, 487–499. [Google Scholar] [CrossRef]
- Maurer, S.V.; Williams, C.L. The Cholinergic System Modulates Memory and Hippocampal Plasticity via Its Interactions with Non-Neuronal Cells. Front. Immunol. 2017, 8, 1489. [Google Scholar] [CrossRef]
- Ben Achour, S.; Pascual, O. Glia: The Many Ways to Modulate Synaptic Plasticity. Neurochem. Int. 2010, 57, 440–445. [Google Scholar] [CrossRef]
- Gamage, R.; Wagnon, I.; Rossetti, I.; Childs, R.; Niedermayer, G.; Chesworth, R.; Gyengesi, E. Cholinergic Modulation of Glial Function during Aging and Chronic Neuroinflammation. Front. Cell Neurosci. 2020, 14, 577912. [Google Scholar] [CrossRef]
- Velazquez, R.; Ferreira, E.; Knowles, S.; Fux, C.; Rodin, A.; Winslow, W.; Oddo, S. Lifelong Choline Supplementation Ameliorates Alzheimer’s Disease Pathology and Associated Cognitive Deficits by Attenuating Microglia Activation. Aging Cell 2019, 18, e13037. [Google Scholar] [CrossRef] [PubMed]
- Esmaealzadeh, N.; Iranpanah, A.; Sarris, J.; Rahimi, R. A Literature Review of the Studies Concerning Selected Plant-Derived Adaptogens and Their General Function in Body with a Focus on Animal Studies. Phytomedicine 2022, 105, 154354. [Google Scholar] [CrossRef] [PubMed]
- Panossian, A.G.; Efferth, T.; Shikov, A.N.; Pozharitskaya, O.N.; Kuchta, K.; Mukherjee, P.K.; Banerjee, S.; Heinrich, M.; Wu, W.; Guo, D.A.; et al. Evolution of the Adaptogenic Concept from Traditional Use to Medical Systems: Pharmacology of Stress- and Aging-Related Diseases. Med. Res. Rev. 2021, 41, 630–703. [Google Scholar] [CrossRef] [PubMed]
- Todorova, V.; Ivanov, K.; Delattre, C.; Nalbantova, V.; Karcheva-Bahchevanska, D.; Ivanova, S. Plant Adaptogens—History and Future Perspectives. Nutrients 2021, 13, 2861. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Biagi, M.; Dalia, P.; Corsi, L. Rhodiola rosea L. Modulates Inflammatory Processes in a CRH-Activated BV2 Cell Model. Phytomedicine 2020, 68, 153143. [Google Scholar] [CrossRef]
- Yang, Y.; Hahm, E.; Kim, Y.; Kang, J.; Lee, W.; Han, I.; Myung, P.; Kang, H.; Park, H.; Cho, D. Regulation of IL-18 Expression by CRH in Mouse Microglial Cells. Immunol. Lett. 2005, 98, 291–296. [Google Scholar] [CrossRef]
- Gradus, J.L. Posttraumatic Stress Disorder and Death from Suicide. Curr. Psychiatry Rep. 2018, 20, 98. [Google Scholar] [CrossRef]
- Bisht, K.; Sharma, K.; Tremblay, M.È. Chronic Stress as a Risk Factor for Alzheimer’s Disease: Roles of Microglia-Mediated Synaptic Remodeling, Inflammation, and Oxidative Stress. Neurobiol. Stress 2018, 9, 9–21. [Google Scholar] [CrossRef]
- Bolton, J.L.; Short, A.K.; Othy, S.; Kooiker, C.L.; Shao, M.; Gunn, B.G.; Beck, J.; Bai, X.; Law, S.M.; Savage, J.C.; et al. Early Stress-Induced Impaired Microglial Pruning of Excitatory Synapses on Immature CRH-Expressing Neurons Provokes Aberrant Adult Stress Responses. Cell Rep. 2022, 38, 110600. [Google Scholar] [CrossRef]
- Kato, T.A.; Hayakawa, K.; Monji, A.; Kanba, S. Missing and Possible Link between Neuroendocrine Factors, Neuropsychiatric Disorders and Microglia. Front. Integr. Neurosci. 2013, 7, 53. [Google Scholar] [CrossRef]
- Kritas, S.K.; Cerullp, A.S.; Caraffa, A.; Antinolfi, P.; Pantalones, A.; Rosati, M.; Tep, M.; Spezialp, A.; Sagginf, R.; Conti, P. Corticotropin-releasing hormone, microglia and mental disorders. Int. J. Immunopathol. Pharmacol. 2014, 27, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Sugama, S.; Conti, B. Interleukin-18 and Stress. Brain Res. Rev. 2008, 58, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Schramm, E.; Waisman, A. Microglia as Central Protagonists in the Chronic Stress Response. Neurol.—Neuroimmunol. Neuroinflamm. 2022, 9, e200023. [Google Scholar] [CrossRef] [PubMed]
- Sussams, R.; Schlotz, W.; Clough, Z.; Amin, J.; Simpson, S.; Abbott, A.; Beardmore, R.; Sharples, R.; Raybould, R.; Brookes, K.; et al. Psychological Stress, Cognitive Decline and the Development of Dementia in Amnestic Mild Cognitive Impairment. Sci. Rep. 2020, 10, 3618. [Google Scholar] [CrossRef]
- Cornell, J.; Salinas, S.; Huang, H.Y.; Zhou, M. Microglia Regulation of Synaptic Plasticity and Learning and Memory. Neural Regen. Res. 2022, 17, 705–716. [Google Scholar]
- Okada, T.; Muto, E.; Yamanaka, T.; Uchino, H.; Inazu, M. Functional Expression of Choline Transporters in Microglia and Their Regulation of Microglial M1/M2 Polarization. Int. J. Mol. Sci. 2022, 23, 8924. [Google Scholar] [CrossRef]
- Fourrier, C.; Remus-Borel, J.; Greenhalgh, A.D.; Guichardant, M.; Bernoud-Hubac, N.; Lagarde, M.; Joffre, C.; Layé, S. Docosahexaenoic Acid-Containing Choline Phospholipid Modulates LPS-Induced Neuroinflammation in vivo and in Microglia in vitro. J. Neuroinflamm. 2017, 14, 170. [Google Scholar] [CrossRef]
- Tayebati, S.K.; Martinelli, I.; Moruzzi, M.; Amenta, F.; Tomassoni, D. Choline and Choline Alphoscerate Do Not Modulate Inflammatory Processes in the Rat Brain. Nutrients 2017, 9, 1084. [Google Scholar] [CrossRef]
- Fatima, U.; Roy, S.; Ahmad, S.; Al-Keridis, L.A.; Alshammari, N.; Adnan, M.; Islam, A.; Hassan, M.I. Investigating Neuroprotective Roles of Bacopa Monnieri Extracts: Mechanistic Insights and Therapeutic Implications. Biomed. Pharmacother. 2022, 153, 113469. [Google Scholar] [CrossRef]
- Oyeleke, M.B.; Owoyele, B.V. Saponins and Flavonoids from Bacopa Floribunda Plant Extract Exhibit Antioxidant and Anti-Inflammatory Effects on Amyloid Beta 1-42-Induced Alzheimer’s Disease in BALB/c Mice. J. Ethnopharmacol. 2022, 288, 114997. [Google Scholar] [CrossRef]
- Rastogi, M.; Ojha, R.P.; Devi, B.P.; Aggarwal, A.; Agrawal, A.; Dubey, G.P. Amelioration of Age Associated Neuroinflammation on Long Term Bacosides Treatment. Neurochem. Res. 2012, 37, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.; Stevens, J. Does Bacopa Monnieri Improve Memory Performance in Older Persons? Results of a Randomized, Placebo-Controlled, Double-Blind Trial. J. Altern. Complement. Med. 2010, 16, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Proćków, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A Comprehensive Review on Ethnopharmacology, Pharmacotherapeutics, Biomedicinal and Toxicological Aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef] [PubMed]
- Varodayan, F.P.; Patel, R.R.; Matzeu, A.; Wolfe, S.A.; Curley, D.E.; Khom, S.; Gandhi, P.J.; Rodriguez, L.; Bajo, M.; D’Ambrosio, S.; et al. The Amygdala Noradrenergic System Is Compromised with Alcohol Use Disorder. Biol. Psychiatry 2022, 91, 1008–1018. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.J.; Park, S.K.; Park, J.H.; Jeong, H.R.; Lee, S.; Lee, H.; Seol, E.; Hoe, H.S. Donepezil Regulates LPS and Aβ-Stimulated Neuroinflammation through MAPK/NLRP3 Inflammasome/STAT3 Signaling. Int. J. Mol. Sci. 2021, 22, 10637. [Google Scholar] [CrossRef]
- Remenapp, A.; Coyle, K.; Orange, T.; Lynch, T.; Hooper, D.; Hooper, S.; Conway, K.; Hausenblas, H.A. Efficacy of Withania somnifera Supplementation on Adult’s Cognition and Mood. J. Ayurveda Integr. Med. 2022, 13, 100510. [Google Scholar] [CrossRef]
- Xing, D.; Yoo, C.; Gonzalez, D.; Jenkins, V.; Nottingham, K.; Dickerson, B.; Leonard, M.; Ko, J.; Faries, M.; Kephart, W.; et al. Effects of Acute Ashwagandha Ingestion on Cognitive Function. Int. J. Environ. Res. Public Health 2022, 19, 11852. [Google Scholar] [CrossRef]
- Pravina, K.; Ravindra, K.R.; Goudar, K.S.; Vinod, D.R.; Joshua, A.J.; Wasim, P.; Venkateshwarlu, K.; Saxena, V.S.; Amit, A. Safety Evaluation of BacoMindTM in Healthy Volunteers: A Phase I Study. Phytomedicine 2007, 14, 301–308. [Google Scholar] [CrossRef]
- Borgonetti, V.; Benatti, C.; Governa, P.; Isoldi, G.; Pellati, F.; Alboni, S.; Tascedda, F.; Montopoli, M.; Galeotti, N.; Manetti, F.; et al. Non-Psychotropic Cannabis sativa L. Phytocomplex Modulates Microglial Inflammatory Response through CB2 Receptors-, Endocannabinoids-, and NF-ΚB-Mediated Signaling. Phytother. Res. 2022, 36, 2246–2263. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Biagi, M.; Galeotti, N. Novel Therapeutic Approach for the Management of Mood Disorders: In Vivo and In Vitro Effect of a Combination of L-Theanine, Melissa officinalis L. and Magnolia Officinalis Rehder & E.H. Wilson. Nutrients 2020, 12, 1803. [Google Scholar] [CrossRef]
- Borgonetti, V.; Galeotti, N. Microglia Senescence Is Related to Neuropathic Pain–Associated Comorbidities in the Spared Nerve Injury Model. Pain 2022, 164, 1106. [Google Scholar] [CrossRef] [PubMed]
- Borgonetti, V.; Les, F.; López, V.; Galeotti, N. Attenuation of Anxiety-like Behavior by Helichrysum stoechas (L.) Moench Methanolic Extract through up-Regulation of Erk Signaling Pathways in Noradrenergic Neurons. Pharmaceuticals 2020, 13, 472. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borgonetti, V.; Galeotti, N. Novel Combination of Choline with Withania somnifera (L.) Dunal, and Bacopa monnieri (L.) Wetts Reduced Oxidative Stress in Microglia Cells, Promoting Neuroprotection. Int. J. Mol. Sci. 2023, 24, 14038. https://doi.org/10.3390/ijms241814038
Borgonetti V, Galeotti N. Novel Combination of Choline with Withania somnifera (L.) Dunal, and Bacopa monnieri (L.) Wetts Reduced Oxidative Stress in Microglia Cells, Promoting Neuroprotection. International Journal of Molecular Sciences. 2023; 24(18):14038. https://doi.org/10.3390/ijms241814038
Chicago/Turabian StyleBorgonetti, Vittoria, and Nicoletta Galeotti. 2023. "Novel Combination of Choline with Withania somnifera (L.) Dunal, and Bacopa monnieri (L.) Wetts Reduced Oxidative Stress in Microglia Cells, Promoting Neuroprotection" International Journal of Molecular Sciences 24, no. 18: 14038. https://doi.org/10.3390/ijms241814038
APA StyleBorgonetti, V., & Galeotti, N. (2023). Novel Combination of Choline with Withania somnifera (L.) Dunal, and Bacopa monnieri (L.) Wetts Reduced Oxidative Stress in Microglia Cells, Promoting Neuroprotection. International Journal of Molecular Sciences, 24(18), 14038. https://doi.org/10.3390/ijms241814038






