The Applicability of Chromatographic Retention Modeling on Chiral Stationary Phases in Reverse-Phase Mode: A Case Study for Ezetimibe and Its Impurities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results of the Preliminary Experiments
2.2. The Separation Model for the Chiralcel OD Column
2.3. The Separation Model for the Chiral CD-Ph Column
2.4. Applicability of the Methods and Practical Relevance
3. Materials and Methods
3.1. Chemicals and Samples
3.1.1. Chemicals
3.1.2. Artificial Samples for Retention Modeling
3.2. Equipment and Software
3.3. Chromatographic Columns
3.4. Preliminary Experiments and the Experimental Design Framework
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Tome, T.; Žigart, N.; Časar, Z.; Obreza, A. Development and Optimization of Liquid Chromatography Analytical Methods by Using AQbD Principles: Overview and Recent Advances. Org. Process Res. Dev. 2019, 23, 1784–1802. [Google Scholar] [CrossRef]
- Tome, T.; Casar, Z.; Obreza, A. Development of a Unified Reversed-Phase HPLC Method for Efficient Determination of EP and USP Process-Related Impurities in Celecoxib Using Analytical Quality by Design Principles. Molecules 2020, 25, 809. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.; Alhabib, N.A.; Alshora, D.; Bekhit, M.M.S.; Taha, E.; Mahdi, W.A.; Harthi, A.M. Application of Quality by Design Approach in the Optimization and Development of the UPLC Analytical Method for Determination of Fusidic Acid in Pharmaceutical Products. Separations 2023, 10, 318. [Google Scholar] [CrossRef]
- Ferencz, E.; Kovács, B.; Boda, F.; Foroughbakhshfasaei, M.; Kelemen, É.K.; Tóth, G.; Szabó, Z.I. Simultaneous Determination of Chiral and Achiral Impurities of Ivabradine on a Cellulose Tris(3-Chloro-4-Methylphenylcarbamate) Chiral Column Using Polar Organic Mode. J. Pharm. Biomed. Anal. 2020, 177, 112851. [Google Scholar] [CrossRef] [PubMed]
- Kormány, R.; Molnár, I.; Rieger, H.-J. Exploring Better Column Selectivity Choices in Ultra-High Performance Liquid Chromatography Using Quality by Design Principles. J. Pharm. Biomed. Anal. 2013, 80, 79–88. [Google Scholar] [CrossRef]
- Kormány, R.; Molnár, I.; Fekete, J. Renewal of an Old European Pharmacopoeia Method for Terazosin Using Modeling with Mass Spectrometric Peak Tracking. J. Pharm. Biomed. Anal. 2017, 135, 8–15. [Google Scholar] [CrossRef]
- Kormány, R.; Tamás, K.; Guillarme, D.; Fekete, S. A Workflow for Column Interchangeability in Liquid Chromatography Using Modeling Software and Quality-by-Design Principles. J. Pharm. Biomed. Anal. 2017, 146, 220–225. [Google Scholar] [CrossRef]
- Ferencz, E.; Kelemen, É.K.; Obreja, M.; Sipos, E.; Vida, S.; Urkon, M.; Szabó, Z.I. Computer-Assisted UHPLC Method Development and Optimization for the Determination of Albendazole and Its Related Substances. J. Pharm. Biomed. Anal. 2021, 203, 114203. [Google Scholar] [CrossRef]
- Ferencz, E.; Zöldhegyi, A.; Kelemen, É.K.; Obreja, M.; Urkon, M.; Sipos, E.; Tóth, G.; Molnár, I.; Szabó, Z.I. Analytical Quality by Design-Compliant Retention Modeling for Exploring Column Interchangeabilities in Separating Ezetimibe and Its Related Substances. J. Chromatogr. A 2022, 1682, 463494. [Google Scholar] [CrossRef]
- International Council for Harmonisation. ICH Q2(R2)—Validation of Analytical Procedures—Draft; ICH: Geneva, Switzerland, 2022. [Google Scholar]
- Internationa Council for Harmonisation. ICH Q14—Analytical Procedure Development—Draft; ICH: Geneva, Switzerland, 2022. [Google Scholar]
- Terzić, J.; Popović, I.; Stajić, A.; Tumpa, A.; Jančić-Stojanovic, B. Application of Analytical Quality by Design Concept for Bilastine and Its Degradation Impurities Determination by Hydrophilic Interaction Liquid Chromatographic Method. J. Pharm. Biomed. Anal. 2016, 125, 385–393. [Google Scholar] [CrossRef]
- Tumpa, A.; Stajić, A.; Jančić-Stojanović, B.; Medenica, M. Quality by Design in the Development of Hydrophilic Interaction Liquid Chromatography Method with Gradient Elution for the Analysis of Olanzapine. J. Pharm. Biomed. Anal. 2017, 134, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Dobričić, V.; Vukadinović, D.; Jančić-Stojanović, B.; Vladimirov, S.; Čudina, O. AQbD-Oriented Development of a New LC Method for Simultaneous Determination of Telmisartan and Its Impurities. Chromatographia 2017, 80, 1199–1209. [Google Scholar] [CrossRef]
- Wingert, N.R.; Ellwanger, J.B.; Bueno, L.M.; Gobetti, C.; Garcia, C.V.; Steppe, M.; Schapoval, E.E.S. Application of Quality by Design to Optimize a Stability-Indicating LC Method for the Determination of Ticagrelor and Its Impurities. Eur. J. Pharm. Sci. 2018, 118, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, B.; Tefas, L.; Vlase, L.; Tomuţă, I.; Porfire, A. A Quality by Design (QbD) Approach to the Development of a Gradient High-Performance Liquid Chromatography for the Simultaneous Assay of Curcuminoids and Doxorubicin from Long-Circulating Liposomes. J. Pharm. Biomed. Anal. 2018, 158, 395–404. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alshehri, S.; Shakeel, F.; Alqarni, M.H.; Aljarba, T.M.; Alam, P. Simultaneous Estimation of Escitalopram and Clonazepam in Tablet Dosage Forms Using HPLC-DAD Method and Optimization of Chromatographic Conditions by Box-Behnken Design. Molecules 2022, 27, 4209. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Muddassir, M.; Alarifi, A.; Ansari, M.T. Box-Behnken Assisted Validation and Optimization of an RP-HPLC Method for Simultaneous Determination of Domperidone and Lansoprazole. Separations 2021, 8, 5. [Google Scholar] [CrossRef]
- Khalid, M.; Ahmad, S. Box–Behnken Design Used to Optimize the Simultaneous Quantification of Amitriptyline and Propranolol in Tablet Dosages by RP-HPLC-DAD Method and Their Stability Tests. Separations 2022, 9, 421. [Google Scholar] [CrossRef]
- Sha’at, M.; Spac, A.F.; Stoleriu, I.; Bujor, A.; Cretan, M.S.; Hartan, M.; Ochiuz, L. Implementation of QbD Approach to the Analytical Method Development and Validation for the Estimation of Metformin Hydrochloride in Tablet Dosage Forms by HPLC. Pharmaceutics 2022, 14, 1187. [Google Scholar] [CrossRef]
- Gurba-Bryśkiewicz, L.; Dawid, U.; Smuga, D.A.; Maruszak, W.; Delis, M.; Szymczak, K.; Stypik, B.; Moroz, A.; Błocka, A.; Mroczkiewicz, M.; et al. Implementation of QbD Approach to the Development of Chromatographic Methods for the Determination of Complete Impurity Profile of Substance on the Preclinical and Clinical Step of Drug Discovery Studies. Int. J. Mol. Sci. 2022, 23, 10720. [Google Scholar] [CrossRef]
- Guichard, N.; Fekete, S.; Guillarme, D.; Bonnabry, P.; Fleury-Souverain, S. Computer-Assisted UHPLC–MS Method Development and Optimization for the Determination of 24 Antineoplastic Drugs Used in Hospital Pharmacy. J. Pharm. Biomed. Anal. 2019, 164, 395–401. [Google Scholar] [CrossRef]
- Bobály, B.; D’Atri, V.; Beck, A.; Guillarme, D.; Fekete, S. Analysis of Recombinant Monoclonal Antibodies in Hydrophilic Interaction Chromatography: A Generic Method Development Approach. J. Pharm. Biomed. Anal. 2017, 145, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Fekete, S.; Sadat-Noorbakhsh, V.; Schelling, C.; Molnár, I.; Guillarme, D.; Rudaz, S.; Veuthey, J.L. Implementation of a Generic Liquid Chromatographic Method Development Workflow: Application to the Analysis of Phytocannabinoids and Cannabis Sativa Extracts. J. Pharm. Biomed. Anal. 2018, 155, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Kormány, R.; Molnár, I.; Fekete, J.; Guillarme, D.; Fekete, S. Robust UHPLC Separation Method Development for Multi-API Product Containing Amlodipine and Bisoprolol: The Impact of Column Selection. Chromatographia 2014, 77, 1119–1127. [Google Scholar] [CrossRef]
- Rácz, N.; Molnár, I.; Zöldhegyi, A.; Rieger, H.J.; Kormány, R. Simultaneous Optimization of Mobile Phase Composition and PH Using Retention Modeling and Experimental Design. J. Pharm. Biomed. Anal. 2018, 160, 336–343. [Google Scholar] [CrossRef]
- Horvath, C.; Melander, W.; Molnar, I. Solvophobic Interactions in Liquid Chromatography with Nonpolar Stationary Phases. J. Chromatogr. 1976, 125, 129–156. [Google Scholar] [CrossRef]
- Molnár, I. Searching for Robust HPLC Methods—Csaba Horváth and the Solvophobic Theory. Chromatographia 2005, 62, s7–s17. [Google Scholar] [CrossRef]
- Glajch, J.L.; Snyder, L.R. Computer-Assisted Method Development for High-Performance Liquid Chromatography; Elsevier: Amsterdam, The Netherlands, 1990; ISBN 0444887482. [Google Scholar]
- Zöldhegyi, A.; Rieger, H.J.; Molnár, I.; Fekhretdinova, L. Automated UHPLC Separation of 10 Pharmaceutical Compounds Using Software-Modeling. J. Pharm. Biomed. Anal. 2018, 156, 379–388. [Google Scholar] [CrossRef]
- Kopp, J.; Zauner, F.B.; Pell, A.; Hausjell, J.; Humer, D.; Ebner, J.; Herwig, C.; Spadiut, O.; Slouka, C.; Pell, R. Development of a Generic Reversed-Phase Liquid Chromatography Method for Protein Quantification Using Analytical Quality-by-Design Principles. J. Pharm. Biomed. Anal. 2020, 188, 113412. [Google Scholar] [CrossRef]
- Schmidt, A.H.; Molnár, I. Using an Innovative Quality-by-Design Approach for Development of a Stability Indicating UHPLC Method for Ebastine in the API and Pharmaceutical Formulations. J. Pharm. Biomed. Anal. 2013, 78–79, 65–74. [Google Scholar] [CrossRef]
- Fekete, S.; Molnár, I.; Guillarme, D. Separation of Antibody Drug Conjugate Species by RPLC: A Generic Method Development Approach. J. Pharm. Biomed. Anal. 2017, 137, 60–69. [Google Scholar] [CrossRef]
- Farsang, E.; Lukács, D.; József, S.; Horváth, K. Rapid UHPLC Method Development for Omeprazole Analysis in a Quality-by-Design Framework and Transfer to HPLC Using Chromatographic Modeling. LCGC N. Am. 2014, 32, 126–148. [Google Scholar] [CrossRef]
- Farsang, E.; Murisier, A.; Horváth, K.; Beck, A.; Kormány, R.; Guillarme, D.; Fekete, S. Tuning Selectivity in Cation-Exchange Chromatography Applied for Monoclonal Antibody Separations, Part 1: Alternative Mobile Phases and Fine Tuning of the Separation. J. Pharm. Biomed. Anal. 2019, 168, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Duivelshof, B.; Zöldhegyi, A.; Guillarme, D.; Lauber, M.; Fekete, S. Expediting the Chromatographic Analysis of COVID-19 Antibody Therapeutics with Ultra-Short Columns, Retention Modeling and Automated Method Development. J. Pharm. Biomed. Anal. 2022, 221, 115039. [Google Scholar] [CrossRef] [PubMed]
- Molnar Institute for Applied Chromatography. Available online: www.molnar-institute.com (accessed on 3 September 2023).
- Papp, L.A.; Hancu, G.; Szabó, Z.I. Simultaneous Determination of Enantiomeric and Organic Impurities of Vildagliptin on a Cellulose Tris(3-Chloro-4-Methylphenylcarbamate) Column under Revered-Phase Conditions. J. Pharm. Biomed. Anal. 2023, 234, 115495. [Google Scholar] [CrossRef]
- Olegário dos Santos, N.; Ribeiro Wingert, N.; Steppe, M. Quality by Design Approach for Enantiomeric Evaluation by RP-HPLC Method of Rivaroxaban and Its Chiral Impurity. Microchem. J. 2023, 192, 108911. [Google Scholar] [CrossRef]
- Szabó, Z.I.; Bartalis-Fábián, Á.; Tóth, G. Simultaneous Determination of Escitalopram Impurities Including the R-Enantiomer on a Cellulose Tris(3,5-Dimethylphenylcarbamate)-Based Chiral Column in Reversed-Phase Mode. Molecules 2022, 27, 9022. [Google Scholar] [CrossRef]
- Monograph <1220>: Analytical Procedures Life Cycle. In United States Pharmacopeia—National Formulary; USP-NF: North Bethesda, MD, USA, 2022; Issue 1.
- Shaya, F.T.; Sing, K.; Milam, R.; Husain, F.; del Aguila, M.A.; Patel, M.Y. Lipid-Lowering Efficacy of Ezetimibe in Patients with Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analyses. Am. J. Cardiovasc. Drugs 2020, 20, 239–248. [Google Scholar] [CrossRef]
- Sasikala, C.H.V.A.; Padi, P.R.; Sunkara, V.; Ramayya, P.; Dubey, P.K.; Uppala, V.B.R.; Praveen, C. An Improved and Scalable Process for the Synthesis of Ezetimibe: An Antihypercholesterolemia Drug. Org. Process Res. Dev. 2009, 13, 907–910. [Google Scholar] [CrossRef]
- Śnieżek, M.; Stecko, S.; Panfil, I.; Furman, B.; Chmielewski, M. Total Synthesis of Ezetimibe, a Cholesterol Absorption Inhibitor. J. Org. Chem. 2013, 78, 7048–7057. [Google Scholar] [CrossRef]
- Matarashvili, I.; Kobidze, G.; Chelidze, A.; Dolidze, G.; Beridze, N.; Jibuti, G.; Farkas, T.; Chankvetadze, B. The Effect of Temperature on the Separation of Enantiomers with Coated and Covalently Immobilized Polysaccharide-Based Chiral Stationary Phases. J. Chromatogr. A 2019, 1599, 172–179. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Murray, P.G. An Instance of Temperature-dependent Elution Order of Enantiomers from a Chiral Brush-type HPLC Column. J. High Resolut. Chromatogr. 1993, 16, 285–288. [Google Scholar] [CrossRef]
- Balmér, K.; Lagerström, P.O.; Persson, B.A.; Schill, G. Reversed Retention Order and Other Stereoselective Effects in the Separation of Amino Alcohols on Chiralcel OD. J. Chromatogr. A 1992, 592, 331–337. [Google Scholar] [CrossRef]
- Fulde, K.; Frahm, A.W. Temperature-Induced Inversion of Elution Order in the Enantioseparation of Sotalol on a Cellobiohydrolase I-Based Stationary Phase. J. Chromatogr. A 1999, 858, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Aranyi, A.; Ilisz, I.; Pataj, Z.; Szatmári, I.; Fülöp, F.; Péter, A. High-Performance Liquid Chromatographic Enantioseparation of 1-(Phenylethylamino)- or 1-(Naphthylethylamino)Methyl-2-Naphthol Analogs and a Temperature-Induced Inversion of the Elution Sequence on Polysaccharide-Based Chiral Stationary Phases. J. Chromatogr. A 2011, 1218, 4869–4876. [Google Scholar] [CrossRef] [PubMed]
- Chankvetadze, L.; Ghibradze, N.; Karchkhadze, M.; Peng, L.; Farkas, T.; Chankvetadze, B. Enantiomer Elution Order Reversal of Fluorenylmethoxycarbonyl-Isoleucine in High-Performance Liquid Chromatography by Changing the Mobile Phase Temperature and Composition. J. Chromatogr. A 2011, 1218, 6554–6560. [Google Scholar] [CrossRef] [PubMed]
- Foroughbakhshfasaei, M.; Dobó, M.; Boda, F.; Szabó, Z.I.; Tóth, G. Comparative Chiral Separation of Thalidomide Class of Drugs Using Polysaccharide-Type Stationary Phases with Emphasis on Elution Order and Hysteresis in Polar Organic Mode. Molecules 2021, 27, 111. [Google Scholar] [CrossRef] [PubMed]
- Szabó, Z.I.; Foroughbakhshfasaei, M.; Noszál, B.; Tóth, G. Enantioseparation of Racecadotril Using Polysaccharide-Type Chiral Stationary Phases in Polar Organic Mode. Chirality 2018, 30, 95–105. [Google Scholar] [CrossRef]
- Matarashvili, I.; Chankvetadze, L.; Tsintsadze, T.; Farkas, T.; Chankvetadze, B. HPLC Separation of Enantiomers of Some Chiral Carboxylic Acid Derivatives Using Polysaccharide-Based Chiral Columns and Polar Organic Mobile Phases. Chromatographia 2015, 78, 473–479. [Google Scholar] [CrossRef]
- Matarashvili, I.; Shvangiradze, I.; Chankvetadze, L.; Sidamonidze, S.; Takaishvili, N.; Farkas, T.; Chankvetadze, B. High-Performance Liquid Chromatographic Separations of Stereoisomers of Chiral Basic Agrochemicals with Polysaccharide-Based Chiral Columns and Polar Organic Mobile Phases. J. Sep. Sci. 2015, 38, 4173–4179. [Google Scholar] [CrossRef]
- Gegenava, M.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. Enantioseparation of Selected Chiral Sulfoxides in High-Performance Liquid Chromatography with Polysaccharide-Based Chiral Selectors in Polar Organic Mobile Phases with Emphasis on Enantiomer Elution Order. J. Sep. Sci. 2014, 37, 1083–1088. [Google Scholar] [CrossRef]
- Mskhiladze, A.; Karchkhadze, M.; Dadianidze, A.; Fanali, S.; Farkas, T.; Chankvetadze, B. Enantioseparation of Chiral Antimycotic Drugs by HPLC with Polysaccharide-Based Chiral Columns and Polar Organic Mobile Phases with Emphasis on Enantiomer Elution Order. Chromatographia 2013, 76, 1449–1458. [Google Scholar] [CrossRef]
- Mosiashvili, L.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. On the Effect of Basic and Acidic Additives on the Separation of the Enantiomers of Some Basic Drugs with Polysaccharide-Based Chiral Selectors and Polar Organic Mobile Phases. J. Chromatogr. A 2013, 1317, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Jibuti, G.; Mskhiladze, A.; Takaishvili, N.; Karchkhadze, M.; Chankvetadze, L.; Farkas, T.; Chankvetadze, B. HPLC Separation of Dihydropyridine Derivatives Enantiomers with Emphasis on Elution Order Using Polysaccharide-Based Chiral Columns. J. Sep. Sci. 2012, 35, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Lämmerhofer, M.; Di Eugenio, P.; Molnar, I.; Lindner, W. Computerized Optimization of the High-Performance Liquid Chromatographic Enantioseparation of a Mixture of 4-Dinitrophenyl Amino Acids on a Quinine Carbamate-Type Chiral Stationary Phase Using Drylab. J. Chromatogr. B Biomed. Sci. Appl. 1997, 689, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Molnar, I. Computerized Design of Separation Strategies by Reversed-Phase Liquid Chromatography: Development of DryLab Software. J. Chromatogr. A 2002, 965, 175–194. [Google Scholar] [CrossRef]
- Wagdy, H.A.; Hanafi, R.S.; El-Nashar, R.M.; Aboul-Enein, H.Y. Predictability of Enantiomeric Chromatographic Behavior on Various Chiral Stationary Phases Using Typical Reversed Phase Modeling Software. Chirality 2013, 25, 506–513. [Google Scholar] [CrossRef]
- Wagdy, H.A.; Hanafi, R.S.; El-Nashar, R.M.; Aboul-Enein, H.Y. Enantiomeric Separation of Underivatized Amino Acids: Predictability of Chiral Recognition on Ristocetin a Chiral Stationary Phase. Chirality 2014, 26, 132–135. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, Y.; Galella, E.; Tomasella, F.P.; Fish, W.P. Separation of Atropisomers by Chiral Liquid Chromatography and Thermodynamic Analysis of Separation Mechanism. J. Pharm. Anal. 2017, 7, 156–162. [Google Scholar] [CrossRef]
- Ezetimibe Monograph. United States Pharmacopeia—National Formulary; USP-NF: North Bethesda, MD, USA, 2022; Issue 1. [Google Scholar]
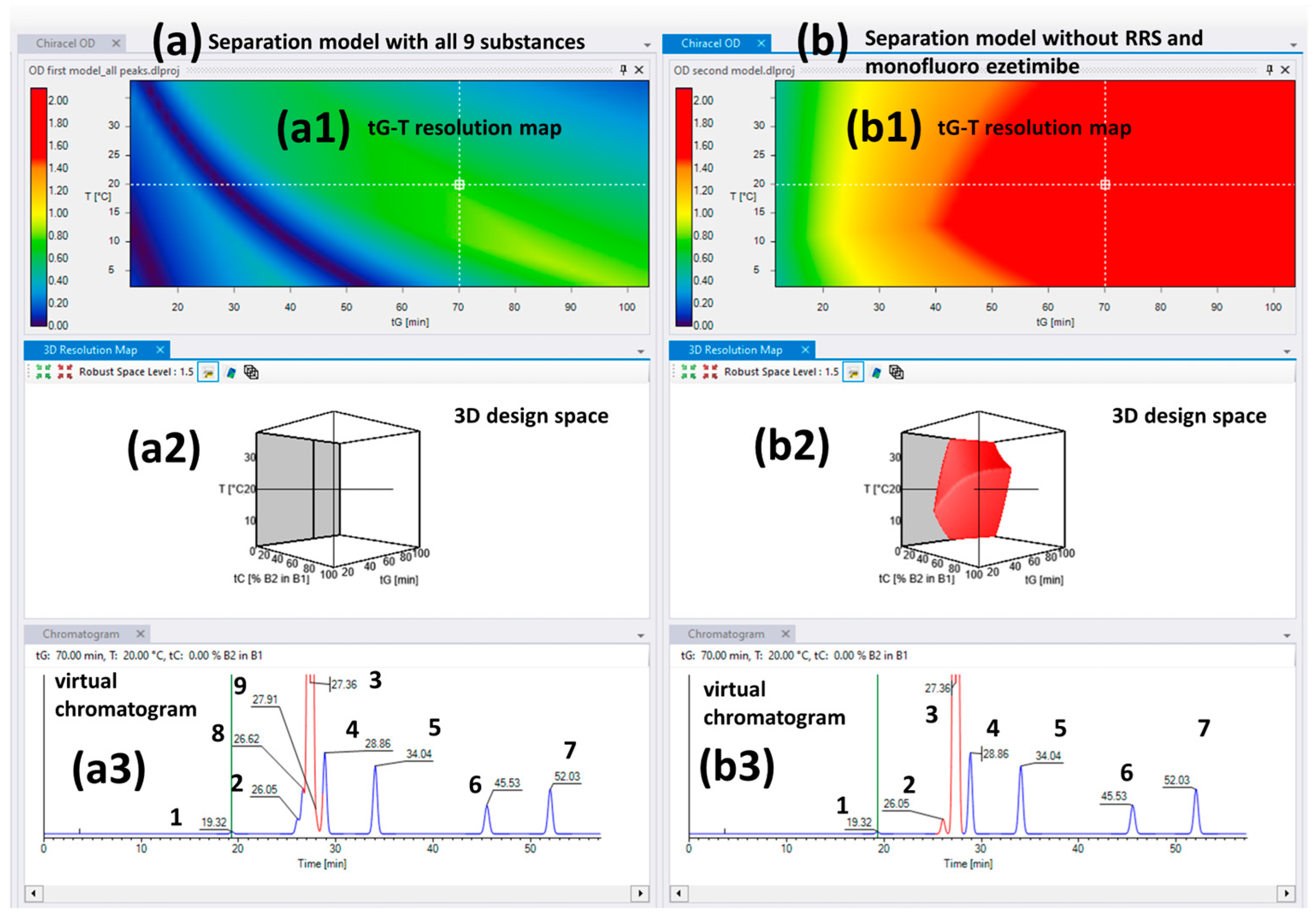
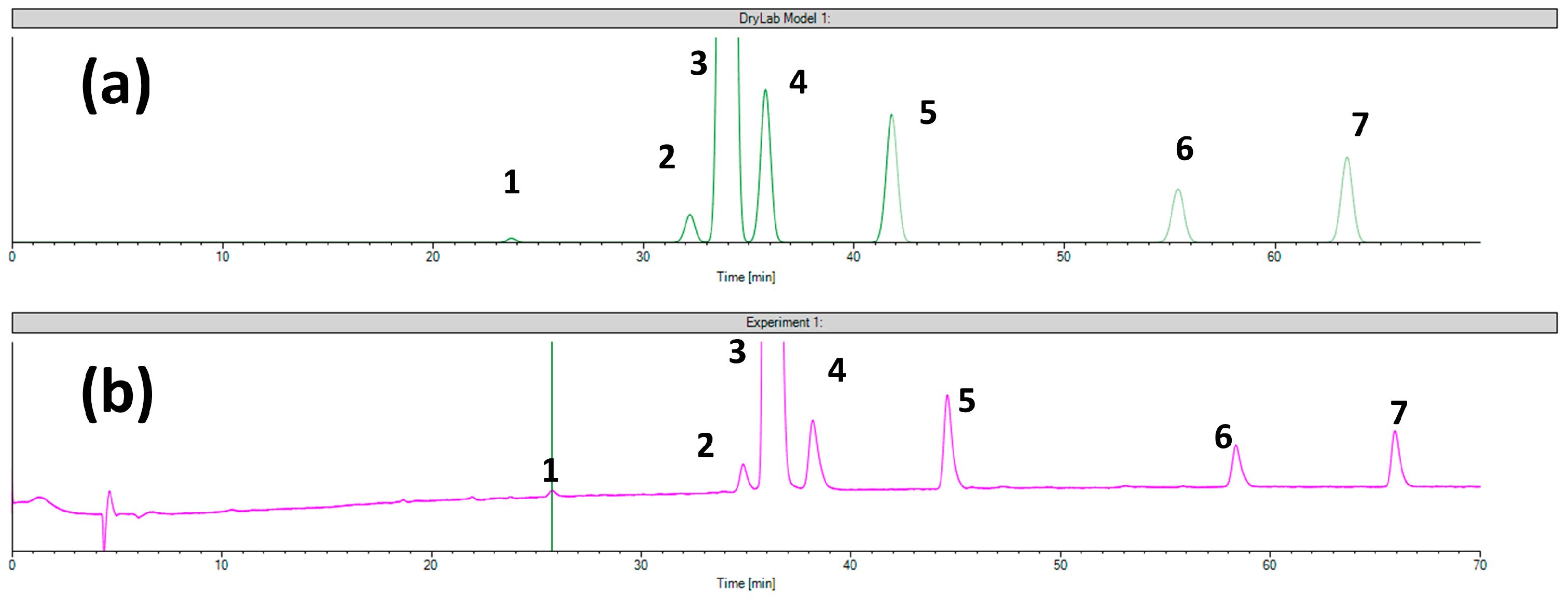
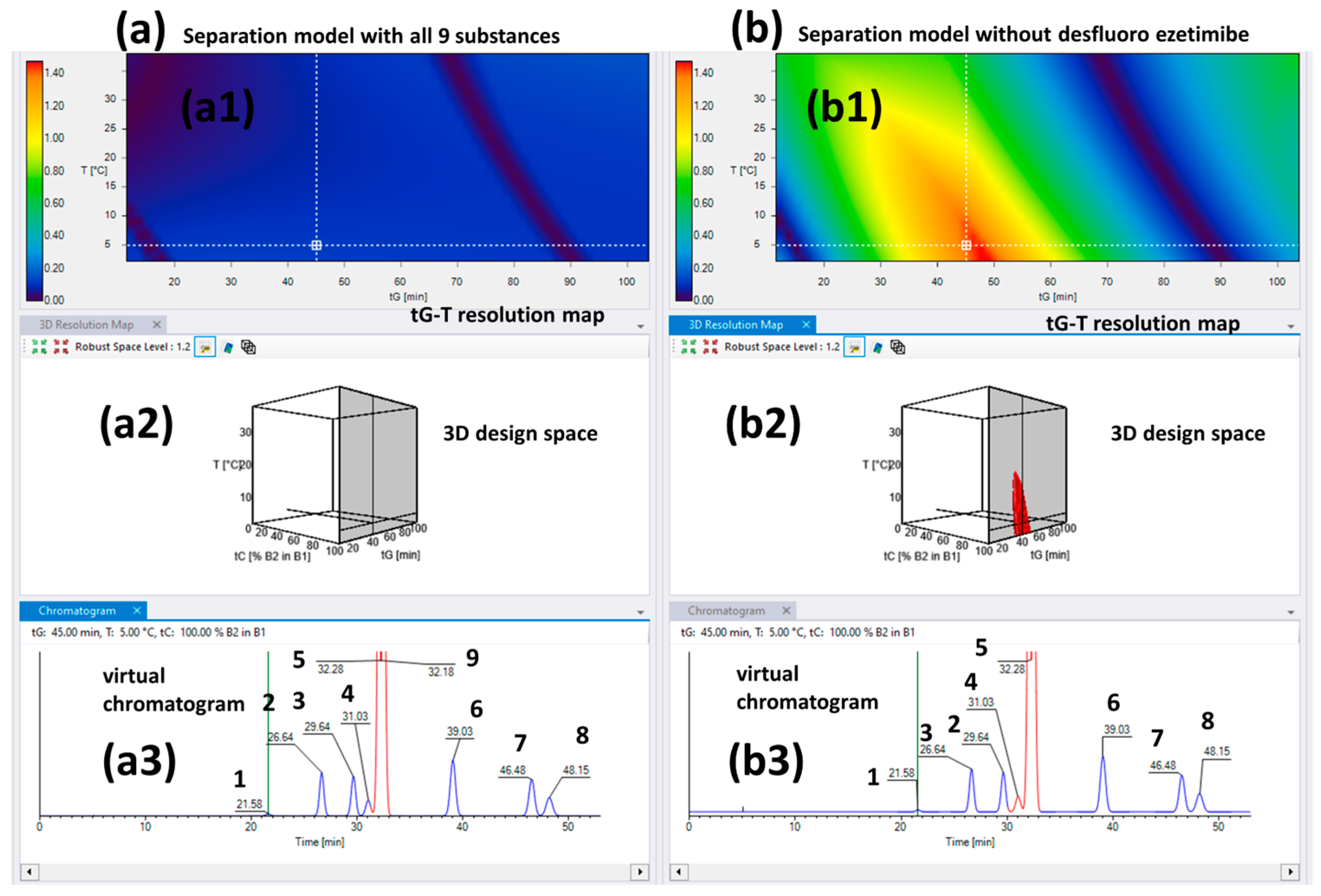
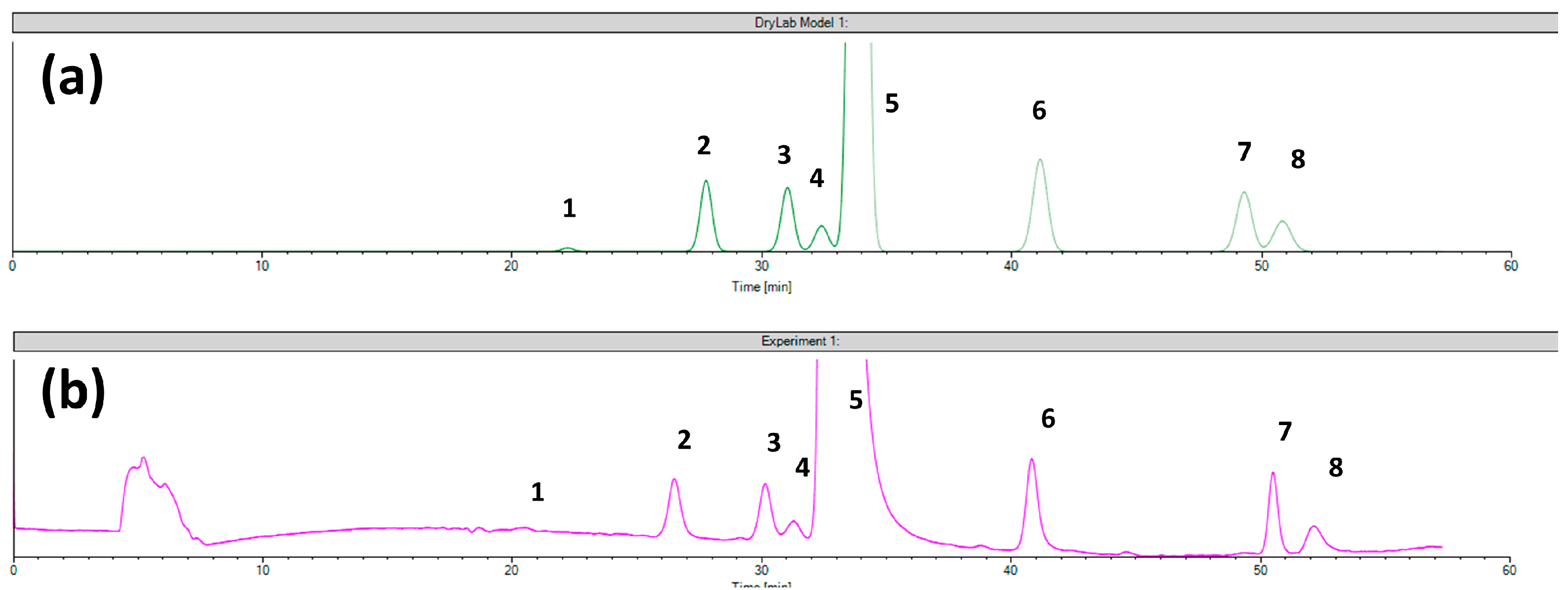
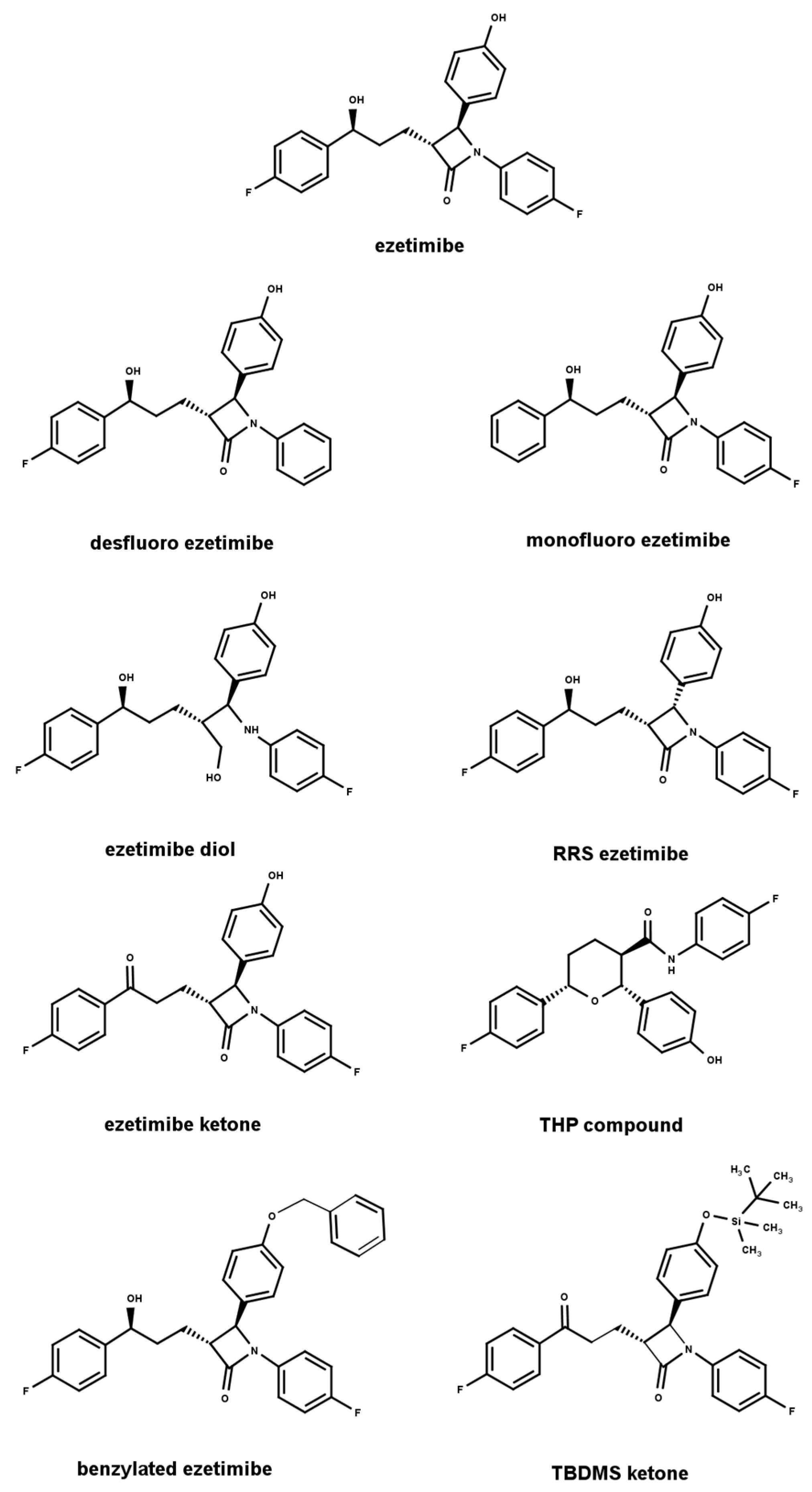
| Column | Chiralcel OD Column | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Setpoint No. | Setpoint 1 | Setpoint 2 | Setpoint 3 | Setpoint 4 | |||||
| Conditions | T = 15 °C Flow: 0.7 mL/min Gradient: | T = 20 °C Flow: 0.7 mL/min Gradient: | T = 30 °C Flow: 0.7 mL/min Gradient: | T = 20 °C Flow: 0.7 mL/min Gradient: | |||||
| Time (min.) | Org. (%) | Time (min.) | Org. (%) | Time (min.) | Org. (%) | Time (min.) | Org. (%) | ||
| 0 | 26 | 0 | 26 | 0 | 34 | 0 | 50 | ||
| 70 | 86 | 40 | 86 | 24 | 53 | 50 | 100 | ||
| 45 | 86 | 40 | 95 | 60 | 100 | ||||
| 45 | 95 | ||||||||
| tC: 100% ACN | tC: 100% ACN | tC: 100% ACN | tC: 100% MeOH | ||||||
| Rt (min.) | Drylab | Exp. | Drylab | Exp. | Drylab | Exp. | Drylab | Exp. | |
| Compounds | ezetimibe diol | 23.72 | 25.73 | 20.19 | 20.12 | 17.50 | 17.57 | 27.29 | 25.00 |
| desfluoro ezetimibe | 32.20 | 34.86 | 25.72 | 25.48 | - | - | - | - | |
| ezetimibe API | 33.97 | 36.16 | 26.70 | 26.25 | 25.70 | 27.81 | 36.61 | 35.91 | |
| THP compound | 35.79 | 38.17 | 27.80 | 27.33 | 27.55 | 30.06 | - | - | |
| monofluoro ezetimibe | - | - | - | - | - | - | 38.28 | 37.26 | |
| ezetimibe ketone | 41.79 | 44.59 | 31.80 | 31.40 | 32.27 | 35.40 | 45.92 | 46.41 | |
| TBDMS ketone | 56.38 | 58.33 | 40.10 | 39.57 | 39.38 | 41.58 | 50.29 | 52.44 | |
| benzylated ezetimibe | 63.41 | 65.94 | 44.31 | 43.62 | 42.26 | 43.60 | 52.23 | 55.50 | |
| Rs,crit. | 1.60 | 1.63 | 1.23 | 1.29 | 2.35 | 2.56 | 1.52 | 1.56 | |
| Average of retention time errors (%) | 5.88 | 1.27 | 5.59 | 4.15 | |||||
| Column | Chiral CD-Ph Column | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Setpoint No. | Setpoint 1 | Setpoint 2 | Setpoint 3 | Setpoint 4 | |||||
| Conditions | T = 5 °C Flow: 0.5 mL/min Gradient: | T = 5 °C Flow: 0.5 mL/min Gradient: | T = 5 °C Flow: 0.7 mL/min Gradient: | T = 5 °C Flow: 0.5 mL/min Gradient: | |||||
| Time (min.) | Org. (%) | Time (min.) | Org. (%) | Time (min.) | Org. (%) | Time (min.) | Org. (%) | ||
| 0 | 60 | 0 | 50 | 0 | 50 | 0 | 50 | ||
| 50 | 100 | 30 | 100 | 30 | 100 | 40 | 100 | ||
| 55 | 100 | 42 | 100 | 40 | 100 | ||||
| tC: 100% MeOH | tC: 100% MeOH | tC: 100% MeOH | tC: 50% MeOH in ACN | ||||||
| Rt (min.) | Drylab | Exp. | Drylab | Exp. | Drylab | Exp. | Drylab | Exp. | |
| Compounds | ezetimibe diol | 22.22 | 20.48 | 23.02 | 23.11 | 19.05 | 18.32 | 18.97 | 18.06 |
| THP compound | 27.76 | 26.46 | 26.19 | 26.59 | 22.37 | 21.89 | - | - | |
| RRS ezetimibe | 31.02 | 30.10 | 28.07 | 28.51 | 24.17 | 23.79 | - | - | |
| monofluoro ezetimibe | 32.38 | 31.22 | - | - | - | - | - | - | |
| ezetimibe API | 33.85 | 33.16 | 29.79 | 30.00 | 25.74 | 25.20 | 23.96 | 23.60 | |
| ezetimibe ketone | 41.13 | 40.78 | 34.06 | 34.19 | 29.70 | 29.63 | 28.55 | 28.35 | |
| TBDMS ketone | 49.30 | 50.45 | 38.73 | 37.85 | 33.93 | 34.68 | 37.13 | 37.62 | |
| benzylated ezetimibe | 50.83 | 52.08 | 41.10 | 39.11 | 35.15 | 35.34 | 34.76 | 35.07 | |
| Rs,crit. | 1.36 * 1.42 ** | 1.33 * 1.44 ** | 1.99 | 1.75 | 2.09 | 1.80 | - | - | |
| Average of retention time errors (%) | 3.47 | 1.70 | 1.84 | 1.89 | |||||
| Column | Chiralcel OD Column | ||||
|---|---|---|---|---|---|
| Setpoint No. | Setpoint 1 | Setpoint 1 | Setpoint 1 | ||
| Measurement | After development | 2 years after development | 2 years after development | ||
| Sample | Artificial mixture without placebo | 1st injection of artificial sample containing placebo components | 20th injection of artificial sample containing placebo components | ||
| Rt (min.) | Drylab | Exp. | Exp. | Exp. | |
| Compounds | ezetimibe diol | 23.72 | 25.73 | 25.57 | 25.65 |
| desfluoro ezetimibe | 32.20 | 34.86 | 34.78 | 34.87 | |
| ezetimibe API | 33.97 | 36.16 | 35.99 | 36.06 | |
| THP compound | 35.79 | 38.17 | 37.91 | 37.98 | |
| ezetimibe ketone | 41.79 | 44.59 | 44.55 | 44.61 | |
| TBDMS ketone | 56.38 | 58.33 | 58.30 | 58.36 | |
| benzylated ezetimibe | 63.41 | 65.94 | 65.75 | 65.81 | |
| Rs,crit.1 | 1.60 | 1.63 | 2.02 | 1.99 | |
| Rs,crit.2 | 1.69 | 2.26 | 2.77 | 2.77 | |
| Average of retention time errors (%) | 5.88 | 5.55 | 5.73 | ||
| Mean ± SD (20 injections) | Rtezetimibe | 36.02 ± 0.20 | |||
| Rs,crit.1 | 1.99 ± 0.02 | ||||
| Rs,crit.2 | 2.71 ± 0.07 | ||||
| Column | Chiral CD-Ph Column | ||||
|---|---|---|---|---|---|
| Setpoint no. | Setpoint 3 | Setpoint 3 | Setpoint 3 | ||
| Measurement | After Development | 2 years after development | 2 years after development | ||
| Sample | Artificial mixture without placebo | 1st injection of artificial sample containing placebo components | 20th injection of artificial sample containing placebo components | ||
| Rt (min.) | Drylab | Exp. | Exp. | Exp. | |
| Compounds | ezetimibe diol | 19.05 | 18.32 | 19.27 | 19.31 |
| THP compound | 22.37 | 21.89 | 22.72 | 22.81 | |
| RRS ezetimibe | 24.17 | 23.79 | 24.52 | 24.61 | |
| ezetimibe API | 25.74 | 25.20 | 25.99 | 26.09 | |
| ezetimibe ketone | 29.70 | 29.63 | 29.88 | 29.95 | |
| TBDMS ketone | 33.93 | 34.68 | 34.27 | 34.36 | |
| benzylated ezetimibe | 35.15 | 35.34 | 35.38 | 35.48 | |
| Rs,crit. | 2.09 | 1.80 | 1.62 | 1.63 | |
| Average of retention time errors (%) | 1.84 | 1.04 | 1.35 | ||
| Mean ± SD (20 injection) | Rtezetimibe | 26.03 ± 0.16 | |||
| Rs,crit. | 1.63 ± 0.06 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferencz, E.; Kelemen, É.-K.; Obreja, M.; Tóth, G.; Urkon, M.; Zöldhegyi, A.; Sipos, E.; Szabó, Z.-I. The Applicability of Chromatographic Retention Modeling on Chiral Stationary Phases in Reverse-Phase Mode: A Case Study for Ezetimibe and Its Impurities. Int. J. Mol. Sci. 2023, 24, 16097. https://doi.org/10.3390/ijms242216097
Ferencz E, Kelemen É-K, Obreja M, Tóth G, Urkon M, Zöldhegyi A, Sipos E, Szabó Z-I. The Applicability of Chromatographic Retention Modeling on Chiral Stationary Phases in Reverse-Phase Mode: A Case Study for Ezetimibe and Its Impurities. International Journal of Molecular Sciences. 2023; 24(22):16097. https://doi.org/10.3390/ijms242216097
Chicago/Turabian StyleFerencz, Elek, Éva-Katalin Kelemen, Mona Obreja, Gergő Tóth, Melinda Urkon, Arnold Zöldhegyi, Emese Sipos, and Zoltán-István Szabó. 2023. "The Applicability of Chromatographic Retention Modeling on Chiral Stationary Phases in Reverse-Phase Mode: A Case Study for Ezetimibe and Its Impurities" International Journal of Molecular Sciences 24, no. 22: 16097. https://doi.org/10.3390/ijms242216097
APA StyleFerencz, E., Kelemen, É.-K., Obreja, M., Tóth, G., Urkon, M., Zöldhegyi, A., Sipos, E., & Szabó, Z.-I. (2023). The Applicability of Chromatographic Retention Modeling on Chiral Stationary Phases in Reverse-Phase Mode: A Case Study for Ezetimibe and Its Impurities. International Journal of Molecular Sciences, 24(22), 16097. https://doi.org/10.3390/ijms242216097







