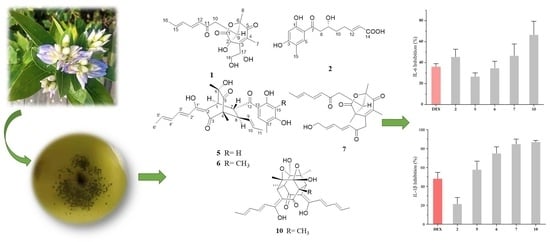
Mono- and Dimeric Sorbicillinoid Inhibitors Targeting IL-6 and IL-1β from the Mangrove-Derived Fungus Trichoderma reesei BGRg-3
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Identification
2.2. Anti-Inflammatory Activities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation
3.4. Extraction and Purification
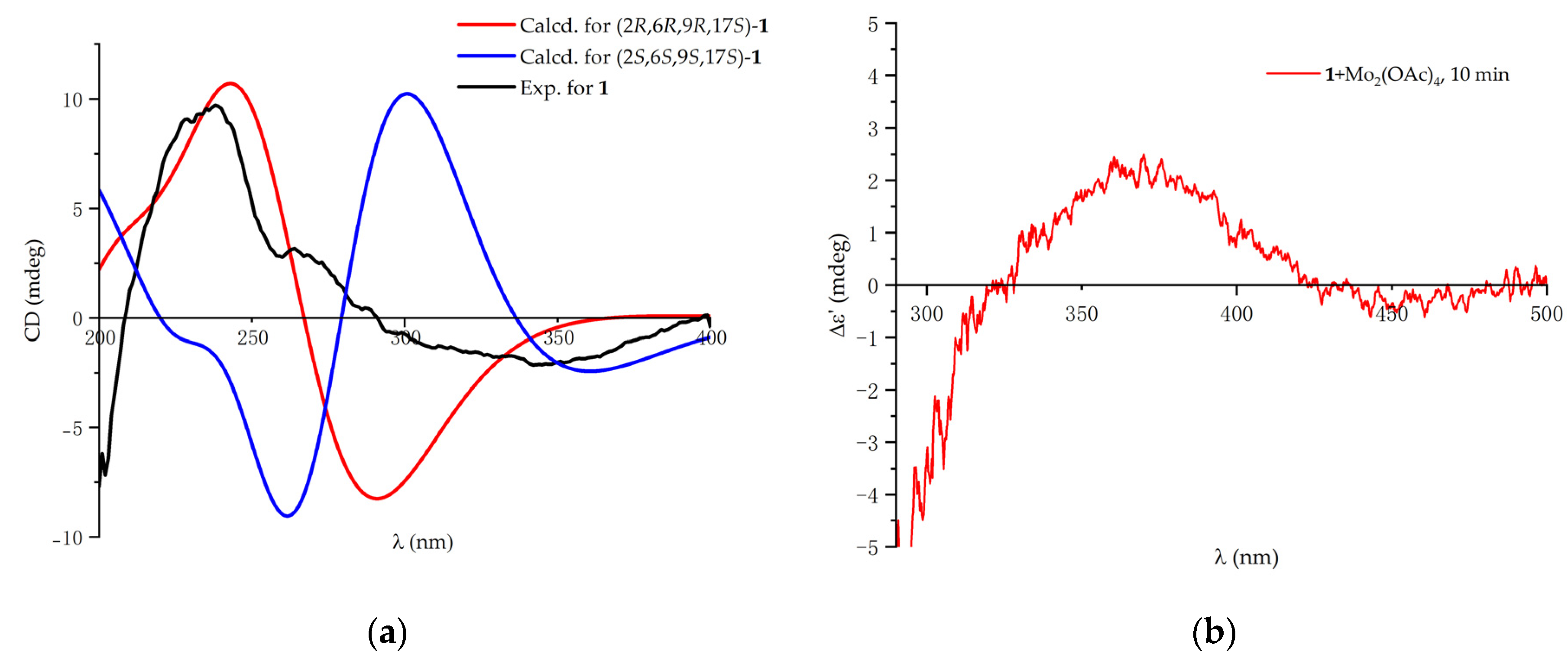
3.5. ECD Calculation
3.6. Absolute Configurations of the 17,18-Diol Moiety in 1
3.7. Anti-Inflammatory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Cai, R.; Liu, Z.; Cui, H.; She, Z. Secondary metabolites from mangrove-associated fungi: Source, chemistry and bioactivities. Nat. Prod. Rep. 2022, 39, 560–595. [Google Scholar] [CrossRef]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2023, 40, 275–325. [Google Scholar] [CrossRef]
- Harned, A.M.; Volp, K.A. The sorbicillinoid family of natural products: Isolation, biosynthesis, and synthetic studies. Nat. Prod. Rep. 2011, 28, 1790–1810. [Google Scholar] [CrossRef]
- Corral, P.; Esposito, F.P.; Tedesco, P.; Falco, A.; Tortorella, E.; Tartaglione, L.; Festa, C.; D’Auria, M.V.; Gnavi, G.; Varese, G.C.; et al. Identification of a sorbicillinoid-producing Aspergillus strain with antimicrobial activity against Staphylococcus aureus: A new polyextremophilic marine fungus from Barents sea. Mar. Biotechnol. 2018, 20, 502–511. [Google Scholar] [CrossRef]
- Ding, W.; Wang, F.; Li, Q.; Xue, Y.; Dong, Z.; Tian, D.; Chen, M.; Zhang, Y.; Hong, K.; Tang, J. Isolation and Characterization of Anti-Inflammatory Sorbicillinoids from the Mangrove-Derived Fungus Penicillium sp. DM815. Chem. Biodivers. 2021, 18, e2100229. [Google Scholar] [CrossRef]
- Rehman, S.U.; Yang, L.; Zhang, Y.; Wu, J.; Shi, T.; Haider, W.; Shao, C.; Wang, C. Sorbicillinoid derivatives from sponge-derived fungus Trichoderma reesei (HN-2016-018). Front. Microbiol. 2020, 11, 1334. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Che, Q.; Zhang, G.; Zhu, T.; Gu, Q.; Li, D. Sorbicillasins A–B and scirpyrone K from a deep-sea-derived fungus, Phialocephala sp. FL30r. Mar. Drugs 2018, 16, 245. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zhang, X.; Xue, M.; Zhao, Z.; Zhang, H.; Xu, D.; Lai, D.; Zhou, L. Recent advances in sorbicillinoids from fungi and their bioactivities (Covering 2016–2021). J. Fungi 2022, 8, 62. [Google Scholar] [CrossRef]
- Xie, C.; Zhang, D.; Lin, T.; He, Z.; Yan, Q.; Cai, Q.; Zhang, X.; Yang, X.; Chen, H. Antiproliferative sorbicillinoids from the deep-sea-derived Penicillium allii-sativi. Front. Microbiol. 2021, 11, 636948. [Google Scholar] [CrossRef]
- Meng, J.; Cheng, W.; Heydari, H.; Wang, B.; Zhu, K.; Konuklugil, B.; Lin, W. Sorbicillinoid-based metabolites from a sponge-derived fungus Trichoderma saturnisporum. Mar. Drugs 2018, 16, 226. [Google Scholar] [CrossRef]
- Deng, J.; Dai, H.; Wang, Y.; Chen, H.; Tan, Z.; Mei, W. Isolation and identification of the fungus Aspergillus sp. HNWSW-20 from Chinese agarwood and its secondary metabolites. Chin. J. Trop. Crops 2018, 39, 1618–1624. [Google Scholar]
- Abe, N.; Murata, T.; Hirota, A. Novel oxidized sorbicillin dimers with 1,1-diphenyl-2-picrylhydrazyl-radical scavenging activity from a fungus. Biosci. Biotechnol. Biochem. 1998, 62, 2120–2126. [Google Scholar] [CrossRef]
- Andrade, R.; Ayer, W.A.; Trifonov, L.S. The metabolites of Trichoderma longibrachiatum. Part II The structures of trichodermolide and sorbiquinol. Can. J. Chem. 1996, 74, 371–379. [Google Scholar] [CrossRef]
- Li, J.; Chen, T.; Yu, J.; Jia, H.; Chen, C.; Long, Y. New Sorbicillinoids from the Mangrove Endophytic Fungus Trichoderma reesei SCNU-F0042. Mar. Drugs 2023, 21, 442. [Google Scholar] [CrossRef]
- Abe, N.; Murata, T.; Hirota, A. Novel DPPH radical scavengers, bisorbicillinol and demethyltrichodimerol, from a fungus. Biosci. Biotechnol. Biochem. 1998, 62, 661–666. [Google Scholar] [CrossRef]
- McMullin, D.R.; Renaud, J.B.; Barasubiye, T.; Sumarah, M.W.; Miller, J.D. Metabolites of Trichoderma species isolated from damp building materials. Can. J. Microbiol. 2017, 63, 621–632. [Google Scholar] [CrossRef]
- Di Bari, L.; Pescitelli, G.; Pratelli, C.; Pini, D.; Salvadori, P. Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4. 1. Snatzke’s method revisited. J. Org. Chem. 2001, 66, 4819–4825. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, L.; Yang, X.; Liu, D.; Guo, H.; Dai, H.; Song, F.; Zhang, L.; Guo, L.; Li, S.; et al. Ophiobolins P–T, five new cytotoxic and antibacterial sesterterpenes from the endolichenic fungus Ulocladium sp. Fitoterapia 2013, 90, 220–227. [Google Scholar] [CrossRef]
- Zhang, P.; Deng, Y.; Lin, X.; Chen, B.; Li, J.; Liu, H.; Chen, S.; Liu, L. Anti-inflammatory mono- and dimeric sorbicillinoids from the marine-derived fungus Trichoderma reesei 4670. J. Nat. Prod. 2019, 82, 947–957. [Google Scholar] [CrossRef]
- Spartan’ 14; Wavefunction Inc.: Irvine, CA, USA, 2013.
- Frisch, M.J.T.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Bruhn, T.H.Y.; Schaumlöffel, A.; Bringmann, G. SpecDis, version 1.53; University of Wuerzburg: Wurzburg, Germany, 2011.
- Kahlert, L.; Bassiony, E.F.; Cox, R.J.; Skellam, E.J. Diels–Alder Reactions During the Biosynthesis of Sorbicillinoids. Angew. Chem. Int. Edit. 2020, 59, 5816–5822. [Google Scholar] [CrossRef]
- Ren, S.; Zeng, Y.; Wang, Q.; Lin, Q.; Yin, X.; Chen, S.; Wang, M.; Liu, L.; Gao, Z. Major Facilitator Superfamily Transporter Participates in the Formation of Dimeric Sorbicillinoids Pigments. J. Agric. Food Chem. 2023, 71, 12216–12224. [Google Scholar] [CrossRef]

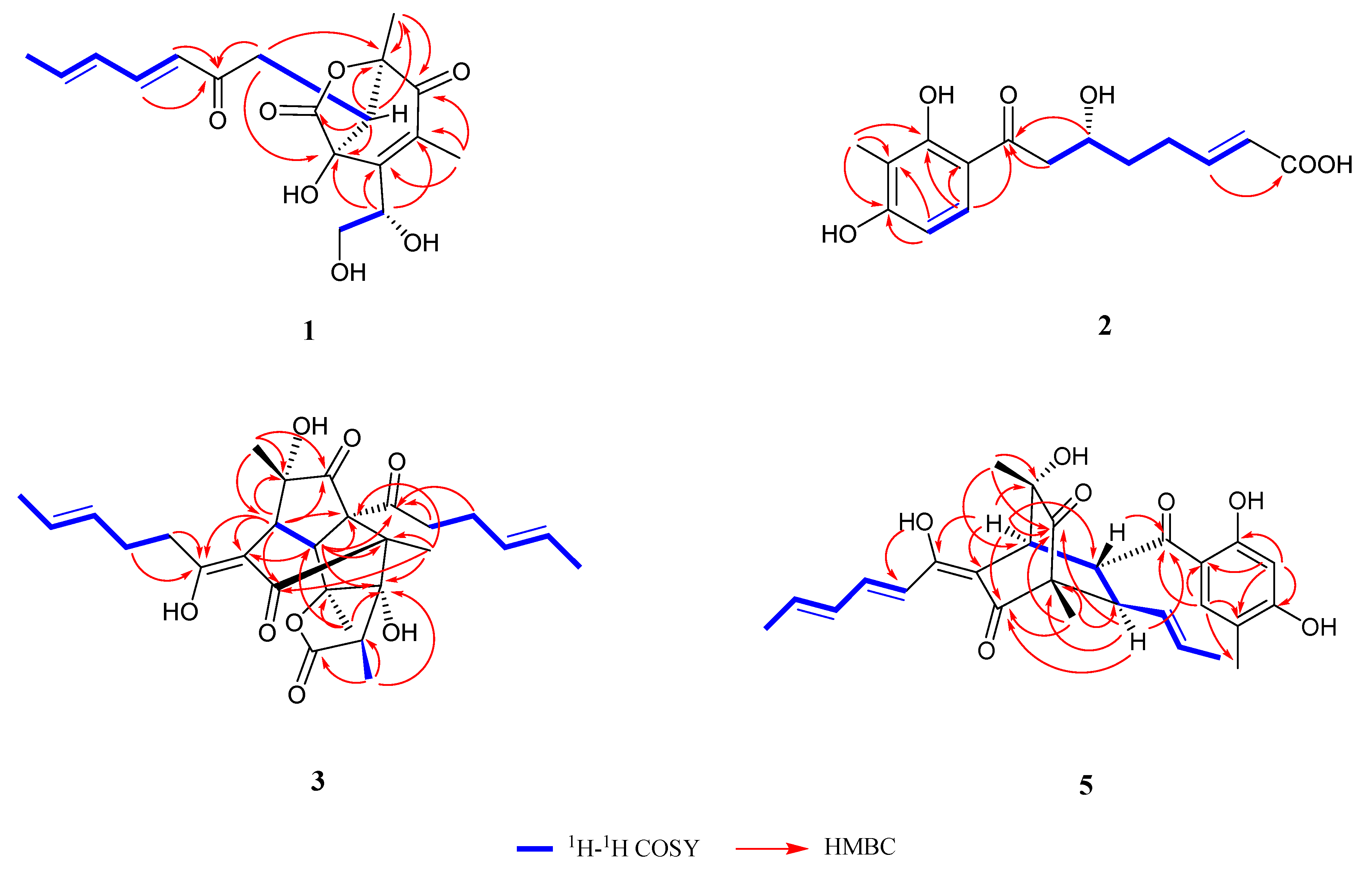
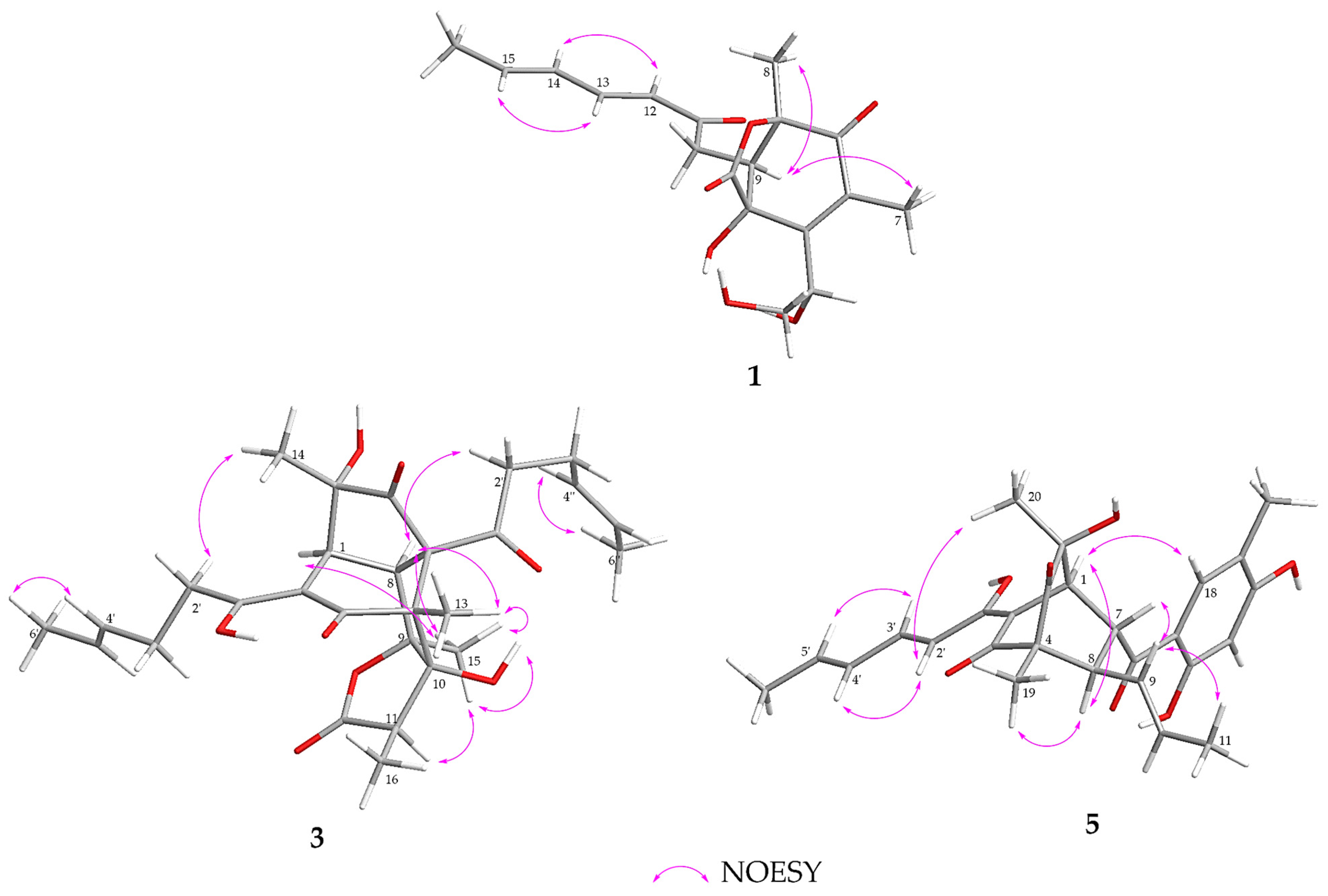

| Position | 1 (CD3OD) | 2 (CD3OD) | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 175.4 (C) | 164.0 (C) | ||
| 2 | 69.4 (C) | 114.2 (C) | ||
| 3 | 151.2 (C) | 164.4 (C) | ||
| 4 | 132.2 (C) | 108.0 (CH) | 6.4 (1H, d, J = 8.9) | |
| 5 | 202.9 (C) | 130.8 (CH) | 7.62 (1H, d, J = 8.9) | |
| 6 | 76.4 (C) | 112.3 (C) | ||
| 7 | 11.0 (CH3) | 1.85 (3H, s) | 204.7 (C) | |
| 8 | 24.2 (CH3) | 1.42 (3H, s) | 46.2 (CH2) | 3.13 (1H, dd, J = 15.6, 8.0)3.01 (1H, dd, J = 15.6, 4.4) |
| 9 | 45.2 (CH) | 3.06 (1H, t, J = 5.2) | 68.8 (CH) | 4.2 (1H, tt, J = 8.4, 4.4) |
| 10 | 35.2 (CH2) | 3.61 (1H, dd, J = 17.3, 5.2)2.86 (1H, dd, J = 17.3, 5.2) | 36.8 (CH2) | 1.69 (2H, m) |
| 11 | 201.3 (C) | 29.3 (CH2) | 2.36 (2H, m) | |
| 12 | 129.0 (CH) | 6.18 (1H, d, J = 15.6) | 148.8 (CH) | 6.92 (1H, dt, J = 15.4, 6.9) |
| 13 | 144.4 (CH) | 7.25 (1H, dd, J = 15.6, 9.6) | 124.3 (CH) | 5.87 (1H, d, J = 15.4) |
| 14 | 131.7 (CH) | 6.28 (overlap) | 171.8 (C) | |
| 15 | 141.4 (CH) | 6.28 (overlap) | 7.6 (CH3) | 2.04 (3H, s) |
| 16 | 18.8 (CH3) | 1.86 (3H, d, J = 5.0) | ||
| 17 | 81.8 (CH) | 5.38 (1H, dd, J = 3.1, 3.1) | ||
| 18 | 62.4 (CH2) | 3.98 (1H, dd, J = 12.3, 3.1)3.88 (1H, dd, J = 12.3, 3.1) | ||
| Position | 3 (CDCl3) | 4 (CDCl3) | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 44.2 (CH) | 3.25 (1H, d, J = 5.1) | 44.4 (CH) | 3.38 (1H, d, J = 5.4) |
| 2 | 108.0 (C) | 107.3 (C) | ||
| 3 | 185.3 (C) | 194.5 (C) | ||
| 4 | 65.3 (C) | 68.0 (C) | ||
| 5 | 73.5 (C) | 73.5 (C) | ||
| 6 | 210.2 (C) | 210.3 (C) | ||
| 7 | 84.2 (C) | 84.1 (C) | ||
| 8 | 56.5 (CH) | 3.62 (1H, d, J = 5.1) | 56.9 (CH) | 3.73 (1H, d, J = 5.4) |
| 9 | 94.2 (C) | 93.8 (C) | ||
| 10 | 88.5 (C) | 88.3 (C) | ||
| 11 | 41.2 (CH) | 2.68 (1H, q, J = 7.7) | 41.1 (CH) | 2.66 (1H, q, J = 8.0) |
| 12 | 178.5 (C) | 177.9 (C) | ||
| 13 | 10.0 (CH3) | 1.24 (3H, s) | 10.3 (CH3) | 1.36 (3H, s) |
| 14 | 17.4 (CH3) | 1.23 (3H, s) | 18.1 (CH3) | 1.23 (3H, s) |
| 15 | 24.2 (CH3) | 1.32 (3H, s) | 24.3 (CH3) | 1.39 (3H, s) |
| 16 | 13.8 (CH3) | 1.19 (3H, d, J = 7.7) | 13.7 (CH3) | 1.21 (3H, d, J = 8.0) |
| 1′ | 195.2 (C) | 177.3 (C) | ||
| 2′ | 36.4 (CH2) | 2.50, 2.32 (2H, m) | 119.0 (CH) | 6.14 (1H, d, J = 14.8) |
| 3′ | 26.8 (CH2) | 2.28 (2H, m) | 144.6 (CH) | 7.39 (1H, dd, J = 14.8, 10.4) |
| 4′ | 129.4 (CH) | 5.37–5.52 (m) | 131.0 (CH) | 6.1–6.5 (m) |
| 5′ | 126.5 (CH) | 5.37–5.52 (m) | 141.5 (CH) | 6.1–6.5 (m) |
| 6′ | 18.0 (CH3) | 1.61 (3H, d, J = 6.3) | 18.9 (CH3) | 1.90 (3H, d, J = 5.2) |
| 1″ | 212.2 (C) | 199.4 (C) | ||
| 2″ | 40.9 (CH2) | 2.89, 2.55 (2H, m) | 123.8 (CH) | 6.46 (1H, d, J = 14.8) |
| 3″ | 26.2 (CH2) | 2.19 (2H, m) | 147.9 (CH) | 7.42 (1H, dd, J = 14.8, 11.2) |
| 4″ | 128.7 (CH) | 5.28 (1H, m) | 130.3 (CH) | 6.21 (1H, dd, J = 15.2, 11.2) |
| 5″ | 127.1 (CH) | 5.37–5.52 (m) | 145.0 (CH) | 6.36 (1H, dq, J = 15.2, 7.6) |
| 6″ | 18.0 (CH3) | 1.59 (3H, d, J = 6.4) | 19.0 (CH3) | 1.89 (3H, d, J = 7.6) |
| Position | 5 (CDCl3) | 6 (CDCl3) | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 47.1 (CH) | 3.31 (1H, d, J = 1.8) | 47.0 (CH) | 3.32 (1H, d, J = 1.5) |
| 2 | 106.7 (C) | 106.8 (C) | ||
| 3 | 198.0 (C) | 198.0 (C) | ||
| 4 | 63.2 (C) | 63.2 (C) | ||
| 5 | 211.5 (C) | 211.5 (C) | ||
| 6 | 75.9 (C) | 75.7 (C) | ||
| 7 | 46.7 (CH) | 4.27 (1H, dd, J = 6.5, 1.8) | 46.7 (CH) | 4.28 (1H, dd, J = 6.5, 1.5) |
| 8 | 46.4 (CH) | 3.23 (1H, dd, J = 10.0, 6.5) | 46.5 (CH) | 3.26 (1H, dd, J = 10.0, 6.5) |
| 9 | 128.2 (CH) | 5.02 (1H, ddq, J = 15.0, 10.0, 1.8) | 128.6 (CH) | 5.02 (1H, ddq, J = 15.0, 10.0, 1.5) |
| 10 | 130.8 (CH) | 5.44 (1H, dq, J = 15.0, 6.5) | 130.6 (CH) | 5.45 (1H, dq, J = 15.0, 7.0) |
| 11 | 17.9 (CH3) | 1.61 (3H, dd, J = 6.5, 1.8) | 17.8 (CH3) | 1.60 (3H, dd, J = 6.5, 1.5) |
| 12 | 202.0 (C) | 202.1 (C) | ||
| 13 | 112.8 (C) | 112.0 (C) | ||
| 14 | 164.2 (C) | 161.9 (C) | ||
| 15 | 103.5 (CH) | 6.34 (1H,s) | 110.7 (C) | |
| 16 | 161.2 (C) | 159.0 (C) | ||
| 17 | 116.4 (C) | 115.0 (C) | ||
| 18 | 132.4 (CH) | 7.70 (1H, s) | 129.0 (CH) | 7.59 (1H, s) |
| 19 | 10.1 (CH3) | 1.16 (3H, s) | 10.0 (CH3) | 1.16 (3H, s) |
| 20 | 24.5 (CH3) | 1.21 (3H, s) | 24.3 (CH3) | 1.20 (3H, s) |
| 21 | 15.6 (CH3) | 2.25 (3H,s) | 16.0 (CH3) | 2.26 (3H,s) |
| 22 | - | - | 7.5 (CH3) | 2.12 (3H,s) |
| 1′ | 169.0 (C) | 168.8 (C) | ||
| 2′ | 117.2 (CH) | 5.57 (1H, d, J = 14.9) | 117.2 (CH) | 5.53 (1H, d, J = 15.0) |
| 3′ | 142.8 (CH) | 7.19 (1H,dd, J = 14.9, 10.7) | 142.4 (CH) | 7.17 (1H,dd, J = 15.0, 10.5) |
| 4′ | 130.9 (CH) | 5.97 (1H, m) | 130.8 (CH) | 5.93 (1H, ddq, J = 15.0, 10.0, 1.5) |
| 5′ | 139.7 (CH) | 6.08 (1H, dq, J = 14.9, 6.9) | 139.4 (CH) | 6.08 (1H, dq, J = 15.0, 7.0) |
| 6′ | 18.9 (CH3) | 1.82 (3H, d, J = 6.9) | 18.9 (CH3) | 1.82 (3H, dd, J = 7.0, 1.5) |
| Raw 264.7 | IL-6 Inhibition (%) 1 | IL-1β Inhibition (%) 1 | Viability of Cell (%) 1 |
|---|---|---|---|
| Compound | 25 μM | ||
| 1 | NA | NA | 79.85 ± 2.07 |
| 2 | 45.55 ± 9.22 | 21.63 ± 5.78 | 96.40 ± 6.26 |
| 3 | NA | NA | 98.39 ± 4.72 |
| 4 | NA | NA | 97.88 ± 2.84 |
| 5 | 26.76 ± 3.44 | 57.77 ±14.38 | 106.09 ± 0.99 |
| 6 | 34.86 ± 5.55 | 75.08 ± 6.80 | 86.72 ± 2.83 |
| 7 | 46.68 ± 11.80 | 84.80 ± 4.49 | 92.05 ± 1.86 |
| 8 | NA | NA | 55.44 ± 4.23 |
| 9 | NA | NA | 83.02 ± 0.59 |
| 10 | 66.64 ± 11.36 | 87.05 ± 1.94 | 117.24 ± 4.72 |
| 11 | NA | NA | 83.96 ± 0.70 |
| 12 | NA | NA | 84.94 ± 1.81 |
| DEX 2 | 36.19 ± 3.75 | 48.19 ± 9.50 | NT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, T.; Sun, B.; Tan, Q.; Ouyang, H.; Wang, B.; Yu, H.; She, Z. Mono- and Dimeric Sorbicillinoid Inhibitors Targeting IL-6 and IL-1β from the Mangrove-Derived Fungus Trichoderma reesei BGRg-3. Int. J. Mol. Sci. 2023, 24, 16096. https://doi.org/10.3390/ijms242216096
Liu Y, Chen T, Sun B, Tan Q, Ouyang H, Wang B, Yu H, She Z. Mono- and Dimeric Sorbicillinoid Inhibitors Targeting IL-6 and IL-1β from the Mangrove-Derived Fungus Trichoderma reesei BGRg-3. International Journal of Molecular Sciences. 2023; 24(22):16096. https://doi.org/10.3390/ijms242216096
Chicago/Turabian StyleLiu, Yufeng, Tao Chen, Bing Sun, Qi Tan, Hui Ouyang, Bo Wang, Huijuan Yu, and Zhigang She. 2023. "Mono- and Dimeric Sorbicillinoid Inhibitors Targeting IL-6 and IL-1β from the Mangrove-Derived Fungus Trichoderma reesei BGRg-3" International Journal of Molecular Sciences 24, no. 22: 16096. https://doi.org/10.3390/ijms242216096
APA StyleLiu, Y., Chen, T., Sun, B., Tan, Q., Ouyang, H., Wang, B., Yu, H., & She, Z. (2023). Mono- and Dimeric Sorbicillinoid Inhibitors Targeting IL-6 and IL-1β from the Mangrove-Derived Fungus Trichoderma reesei BGRg-3. International Journal of Molecular Sciences, 24(22), 16096. https://doi.org/10.3390/ijms242216096