Abstract
Tetragonia tetragonoides (Pall.) Kuntze (Aizoaceae, 2n = 2x = 32), a vegetable used for both food and medicine, is a halophyte that is widely distributed in the coastal areas of the tropics and subtropics. Saline–alkaline soils and drought stress are two major abiotic stressors that significantly affect the distribution of tropical coastal plants. Abscisic acid-, stress-, and ripening-induced (ASR) proteins belong to a family of plant-specific, small, and hydrophilic proteins with important roles in plant development, growth, and abiotic stress responses. Here, we characterized the ASR gene family from T. tetragonoides, which contained 13 paralogous genes, and divided TtASRs into two subfamilies based on the phylogenetic tree. The TtASR genes were located on two chromosomes, and segmental duplication events were illustrated as the main duplication method. Additionally, the expression levels of TtASRs were induced by multiple abiotic stressors, indicating that this gene family could participate widely in the response to stress. Furthermore, several TtASR genes were cloned and functionally identified using a yeast expression system. Our results indicate that TtASRs play important roles in T. tetragonoides’ responses to saline–alkaline soils and drought stress. These findings not only increase our understanding of the role ASRs play in mediating halophyte adaptation to extreme environments but also improve our knowledge of plant ASR protein evolution.
1. Introduction
Tetragonia tetragonoides (Pall.) Kuntze (also called New Zealand spinach or French spinach) is a halophyte and vegetable that is widely distributed in the coastal areas of tropical and subtropical regions. Tetragonia tetragonoides is highly adapted to seawater and drought conditions and can therefore be planted near the beach or on islands and big reefs [1]. Moreover, T. tetragonoides and its extracts are used in folk medicine for gastrointestinal diseases, such as gastric cancer, gastritis, gastric ulcers, and acid excess, and they may have antioxidant, antidiabetic, and anti-inflammatory effects [1,2]. The natural habitats of T. tetragonoides plants are usually harsh for most other plants, especially traditional crops or vegetables. To cope with these unfavorable environmental conditions, T. tetragonoides has adopted various resistance strategies. Related research can provide valuable information to improve abiotic stress tolerance in crops. It is therefore important to understand in depth the mechanisms by which the genome or specific genes of T. tetragonoides have adapted to extreme environmental abiotic stress, such as drought, saline–alkaline, and extreme high-temperature stress, to complete their life cycle.
Plants have evolved a number of different methods to deal with inevitable abiotic stress. First, the morphology of plants has developed with specialization for environmental adaptation. The increased succulence and small and glistening liquid-filled blisters in leaves and young stems enhance the salt and drought tolerance of T. tetragonoides in its natural habitats, and its large root system and proper creeping architecture also ensure its anti-wind capability [3]. Most importantly, as a halophyte species, the physiological and biological processes of T. tetragonoides in vivo, which include ion compartmentalization and homeostasis, ion extrusion, and osmotic adjustment at cellular or molecular levels, might be the key factors that determine its specific salinity stress tolerance [3,4]. Currently, research on the molecular mechanism underlying the ecological adaptability of this species is limited, and only a few reports are available [3,4,5,6]. Highly saline–alkaline soils, drought, and strong UV light or sunshine are crucial abiotic stress factors limiting the distribution of tropical coastal plants. As a special-habitat plant species, T. tetragonoides has adopted some special molecular and physiological mechanisms for its growth and reproduction in unusual ecological environments.
Abscisic acid (ABA)-, stress-, and ripening-induced (ASR) proteins are plant-specific families with conserved C-terminal ABA/WDS domains that play important roles in pollen or fruit ripening, abiotic stress tolerance, and the ABA signaling pathway in plants. ASRs belong to small hydrophilic proteins and are often encoded by small gene families. Since the first ASR gene was identified in tomato (Solanum lycopersicum L.) [7], an increasing number of ASR genes have been identified and cloned from different dicotyledons and monocotyledons [8,9,10,11,12]. Interestingly, no ASR gene members exist in the Brassicaceae family, including the model plant Arabidopsis thaliana. Although the ASR family has been widely studied, there have been only several reports on the function of ASRs in special-habitat plants [13,14,15,16,17,18,19]. Many genes in wild relatives provide genetic resources for germplasm improvement. More importantly, ASR genes playing crucial roles in the response to high salinity and drought stress in plants are of significance in plant genetic engineering.
Wild plant species are inevitably challenged by many severe environmental factors. In particular, in tropical coastal plant species, continuous salinity–alkali stress and seasonal drought, heat, UV light, and flooding are significant abiotic stressors that restrict the distribution of plants and control the productivity and alteration of generations. As an extreme halophyte, T. tetragonoides is a leaf succulent and can accumulate saline ions; therefore, it can grow in saline coastal regions. Undoubtedly, adaptation to salinity–alkali and drought stress in T. tetragonoides plants is also coupled with physiological and molecular mechanisms. ASR genes encode plant-specific hydrophilic proteins, which function as molecular chaperones or protective molecules in the cytosol [20] or act as transcription factors regulating gene expression in the nucleus during stress responses [21]. The involvement of ASRs in plant drought and salt tolerance has been documented in several species [15,16,18,19,22,23,24,25,26,27,28,29]. A reference genome sequence of T. tetragonoides has recently been generated. Because of the remarkable saline–alkaline and drought tolerance of this species, the identification of stress-relevant genes is important, as it may have important applications in the genetic improvement of crop resistance. In the present study, we identified ASR genes in T. tetragonoides (TtASRs) and characterized the structure of TtASRs and their chromosomal locations. We also investigated the expression profiles of TtASR genes in various tissues in response to different abiotic stressors and performed promoter analyses. Additionally, some TtASR genes were functionally identified using heterogeneous transgenic assays. Our results suggest that TtASRs may act as a protective molecule to help T. tetragonoides plants adapt to extreme abiotic stress in tropical and subtropical coastal regions.
2. Results
2.1. Identification of the T. tetragonoides ASR Family
Thirteen ASR genes were identified in the T. tetragonoides genome using the InterProscan search and BLAST confirmation (Table 1; Supplementary File Table S1). These genes encoded proteins that had a conserved ABA_WDS domain on their C-terminus, and according to their genome locus, the genes were designated as TtASR1–13. Among these, TtASR5 was the only reported gene that was isolated from the cDNA library of T. tetragonoides in our previous study [6]. Based on the similarity of their sequences, two possible segmentally duplicated gene pairs, namely TtASR4/TtASR13 and TtASR5/TtASR12, were identified in T. tetragonoides.

Table 1.
Nomenclature and subcellular localization of TtASR protein identified from T. tetragonoides.
2.2. Features of ASR Proteins
After verification of the complete open reading frame (ORF) of the full-length cDNA of each TtASR, the predicted amino acid sequences of the TtASRs were calculated with different biological programs. Analysis of the physicochemical properties revealed TtASRs ranging from 105 to 277 amino acids. The molecular weight varied from 12.09 to 29.1 kDa, and the pI ranged from 5.12 to 9.47. Except for TtASR6, which was neutral, seven TtASR proteins (TtASR4, TtASR5, TtASR7, TtASR9, TtASR11, TtASR12, and TtASR13) were weakly acidic, and five TtASR proteins (TtASR1, TtASR2, TtASR3, TtASR8, and TtASR10) were weakly alkaline. The instability index (II) ranged from 16.86 to 47.17, and the aliphatic index (AI) ranged from 16.68 to 62.9 (only two TtASRs, TtASR5 and TtASR12, had AI values < 40). The IIs of most of the TtASRs had values lower than 40, indicating that these proteins were relatively stable in vivo. The TtASR proteins also consisted of very high percentages of lysine (K), histidine (H), glycine (G), glutamic acid (E), and alanine (A) compared to the other amino acids (Table 1). We also predicted the disordered amino acid content of all TtASRs since some reported plant ASRs are intrinsically disordered proteins (IDPs), including SbASR-1 (Salicornia brachiata) [13], SlASR (Suaeda liaotungensis) [14], TtASR1 (wheat) [20], HvASR1 (barley) [20], and PgASR3 (pearl millet) [17]. Both TtASR5 and TtASR12 were predominantly disordered since they contained about 80% disordered amino acids. The other TtASRs showed a relatively lower disordered amino acid content, ranging from 35.85% to 56.07%. In general, the subcellular localization of most TtASRs was in the nucleus, which was predicted by different programs (Table 1). This is consistent with their biological functions as transcription factors or molecular chaperones.
2.3. Evolutionary Characterization and Chromosomal Locations of TtASRs
To investigate the phylogenetic relationships of TtASRs, we performed phylogenetic analysis of the TtASRs and other plant ASR proteins and established unrooted phylogenetic trees (Figure 1). All 13 TtASRs were distributed in the two larger subgroups (Figure 1A), and the chosen plant ASR proteins were classified into two subfamilies (Figure 1B). In general, TtASRs with adjacent gene localization were not evolutionarily close, such as TtASR4/TtASR5/TtASR6 and TtASR11/TtASR12/TtASR13 (Table 1), suggesting that they were not similar genes, and their functions may be highly diverse.
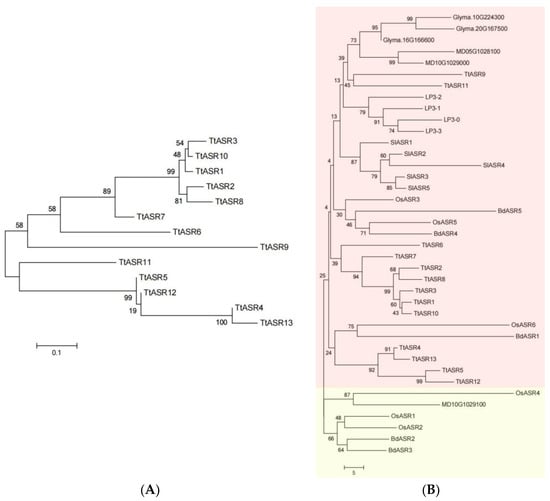
Figure 1.
(A) Phylogenetic relationships of the 13 TtASRs from T. tetragonoides. (B) Phylogenetic relationships of TtASRs and other plant ASRs. The different colors show different subgroups. The phylogenetic tree is constructed using MEGA 6.0 software, with ClustalW alignment, the neighbor-joining (NJ) method, the bootstrap method, and 1000 repetitions.
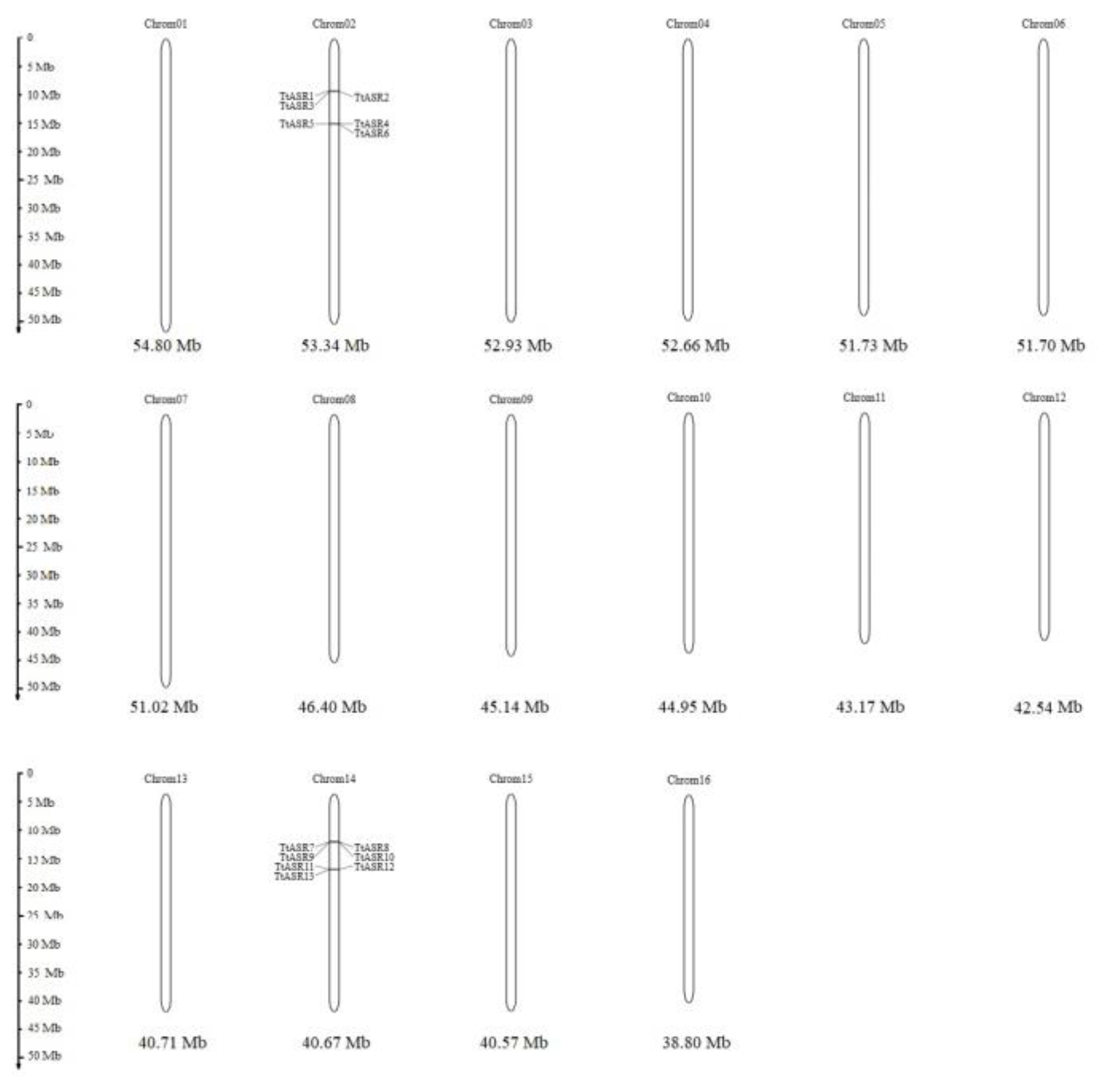
The evolutionary relationships among the TtASRs were also reflected by their chromosome maps (Figure 2). Interestingly, all 13 TtASRs were concentrated in the two syntenic blocks (Table 1). The encoding proteins of two gene pairs, TtASR4/TtASR13 and TtASR5/TtASR12, were highly homologous but distributed on different chromosomes.
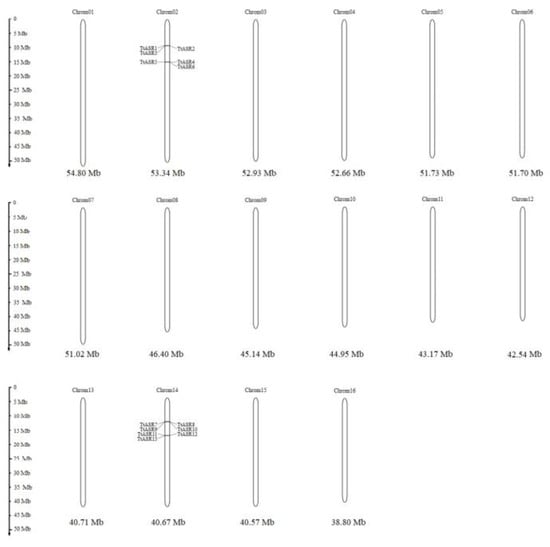
Figure 2.
Locations of the 13 TtASRs on 16 chromosomes of T. tetragonoides.
2.4. Gene Structures and Protein Motif Compositions
To better understand the structural features of TtASRs, gene structure analyses were performed using the GSDS tool. The results reveal that each TtASR member had two exons, ranging from 147 to 642 bp in length, and one intron ranging from 210 to 1641 bp in length (Figure 3A). The gene structures of all TtASRs were generally considered conservative.

Figure 3.
The genes’ structure and protein motif compositions of the TtASRs in T. tetragonoides. (A) The exon–intron organization of the TtASRs was constructed using GSDS 2.0; (B) the conserved motifs of each TtASR were identified using the MEME web server. Different motifs are represented by differently colored boxes.
Protein motifs are critical for biochemical functions. The conserved motif analyses were executed with the MEME tool with motifs 1–10 (Figure 3B). All TtASR proteins contained conserved ABA/WDS domains (motifs 1, 2, and 4) and a small N-terminal consensus containing a His-rich sequence (motif 3). Motif 8 was present in the N-terminal of TtASR11 and TtASR12, and motif 10 was present only in the C-terminus of TtASR9 and in the near N-terminus middle part of TtASR12. The other four motifs (5, 6, 7, and 9) were specific to TtASR5 and TtASR12, which were composed mainly of Gly-rich sequences.
2.5. Cis-Acting Regulatory Elements
The promoter sequences in the 2 kb region upstream of all TtASRs’ 5′-untranslated regions (5′-UTRs) were analyzed. Based on their predicted functions, the 17 identified cis-acting elements were classified into three groups, namely hormone response, stress response, and transcript factor (TF) binding sites (Figure 4, Table S3). The hormone response elements included salicylic acid (SA) response (TCA) and abscisic acid (ABA) response (including ABRE, ABRE3a, and ABRE4). The stress response elements included low-temperature-responsive (LTR) and defense- and stress-responsive (TC-rich repeat) stress response elements (STREs). The TF binding sites included MYB (MYB core element, involved in flavonoid biosynthesis, etc.), the MYB binding site involved in light responsiveness (MYB binding site) or other stress responses (MYB recognition site, MYB-like sequence), MYB binding site involved in drought-inducibility (MBS), MYBHv1 binding site (CCAAT-box), and MYC (dehydration responsive) or Myc (other abiotic stress responsive). The analysis revealed that the promoter regions of the TtASR genes’ promoter regions contained a large number of possible cis-acting elements, with a total of 231 elements identified. Moreover, most of the TtASR genes contained TF binding sites, such as MYB binding site elements and Myc-binding elements. Additionally, the ABA response elements (ABRE, ABRE3a, and ABRE4) were also detected in most TtASR promoters. These findings suggest that the TtASR genes may participate in many complex regulatory pathways involved in plant environmental stress responses.
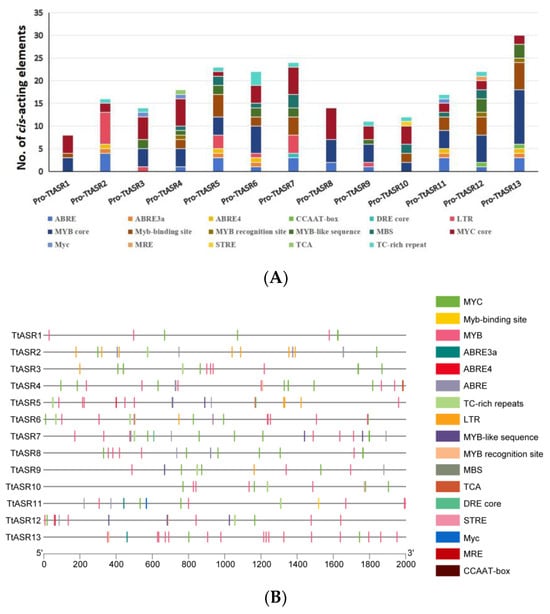
Figure 4.
Numbers and distribution of the cis-acting elements in the TtASRs’ promoter regions. (A) Summaries of the seventeen cis-acting elements in the TtASRs’ promoter regions; (B) distribution of the seventeen cis-acting elements in the TtASRs’ promoter regions. The elements are represented by different symbols. The scale bar represents 200 bp. The detailed information of all cis-acting elements in the TtASRs’ promoter regions is listed in Table S3.
2.6. Expression Profiles of TtASRs in Different Tissues and Plants in Response to Different Stressors
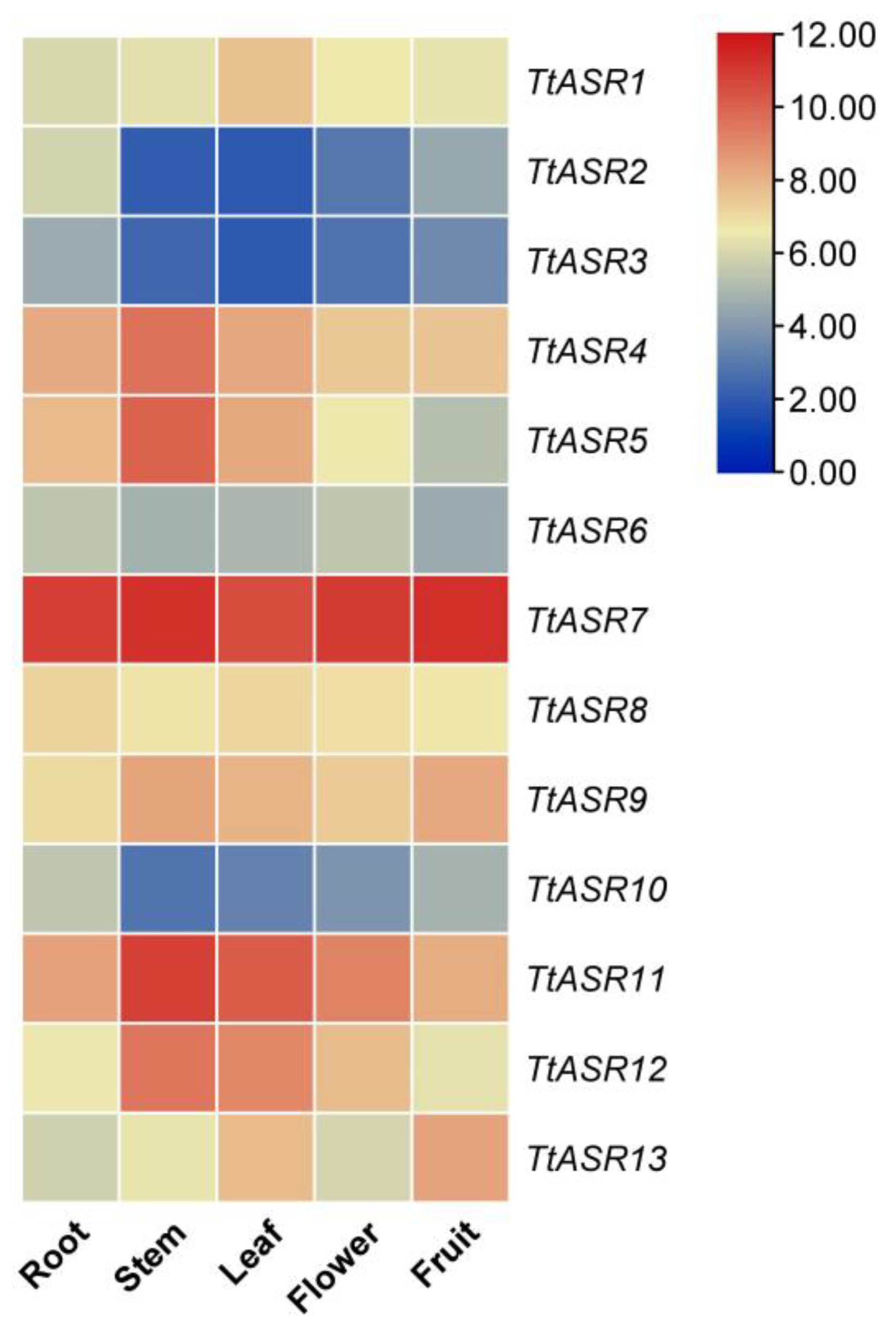
Tissue-specific expression profiles of TtASRs were analyzed via RNA-Seq in the root, stem, leaf, flower bud, and young fruit of T. tetragonoides plants. Overall, four TtASRs, namely TtASR4, TtASR7, TtASR11, and TtASR12, were highly expressed in all five tissues, and TtASR7 almost presented a constitutively high expression pattern in all five tissues (Figure 5). TtASR2, TtASR3, TtASR6, and TtASR10 showed obviously lower expression levels in all five tissues compared to the other TtASRs. This expression profile indicates that different TtASR members play specific roles in the regulation of T. tetragonoides development by regulating its expression levels.
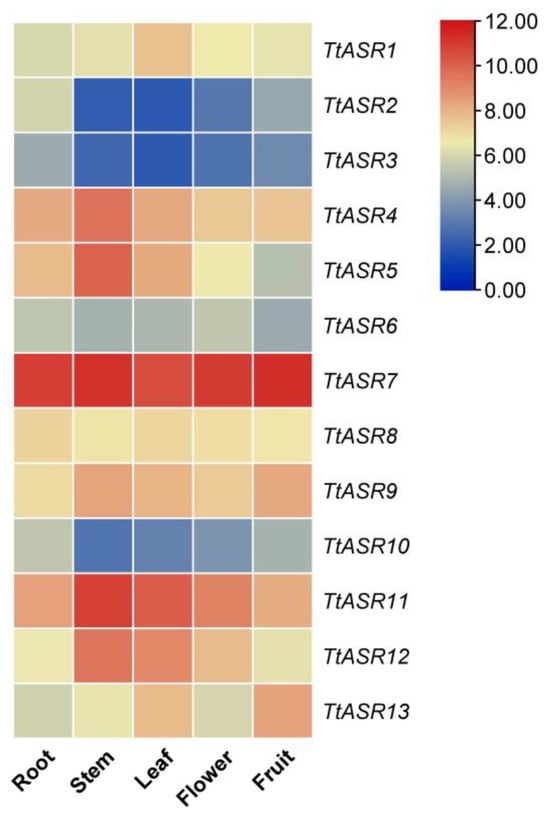
Figure 5.
Heatmaps showing the expression levels of the TtASRs in the root, stem, leaf, flower bud, and young fruit of T. tetragonoides plants. The expression level of each gene is shown in FPKM (log2). Red denotes high expression levels, and blue denotes low expression levels.
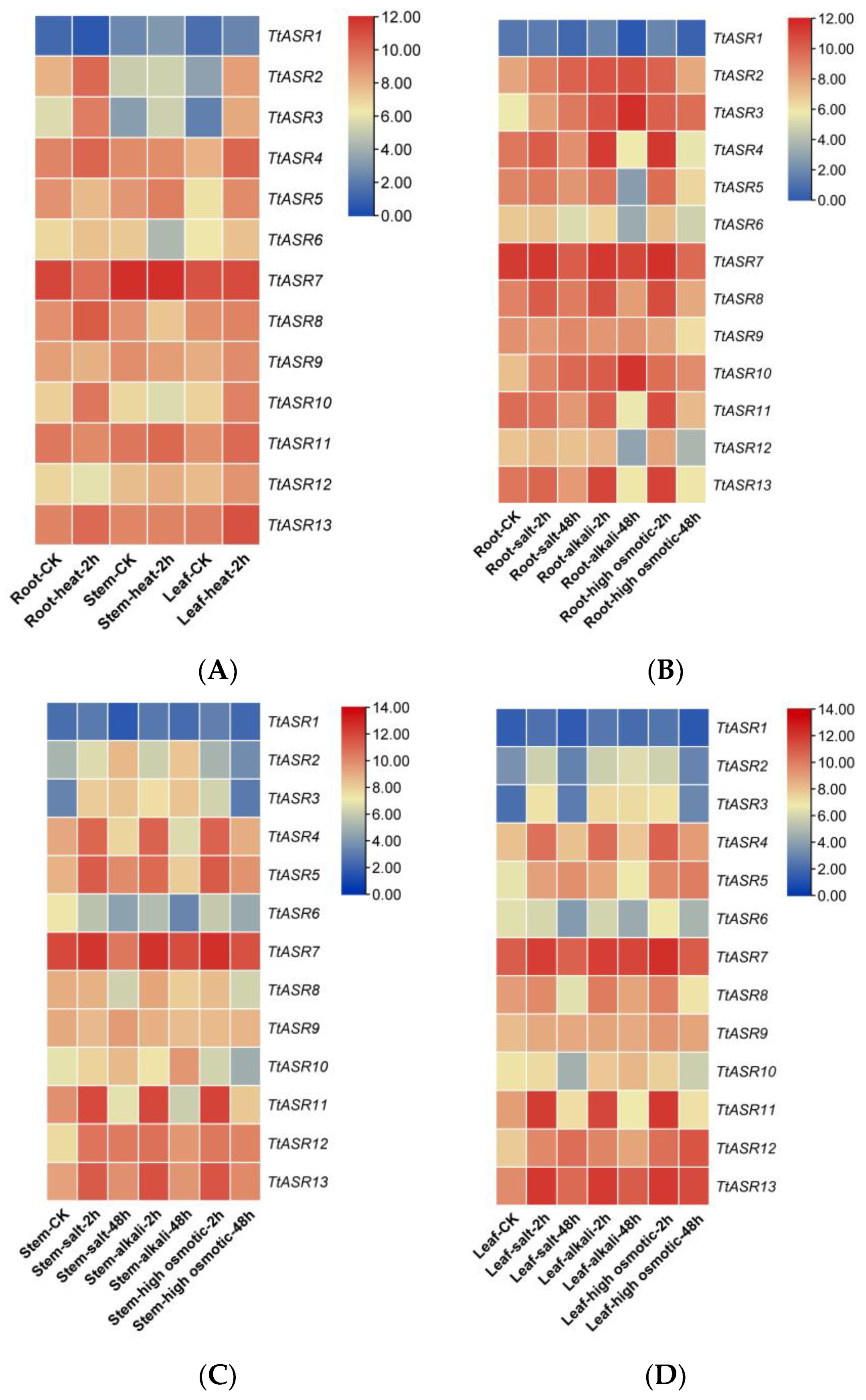
We also performed gene expression analysis in different T. tetragonoides tissues under various stress challenges, including heat (45 °C), salt (600 mM NaCl), alkalis (150 mM NaHCO3, pH 8.2), and high osmotic stress (drought simulated with 300 mM mannitol), mainly based on the natural habitat of T. tetragonoides. Heat stress for T. tetragonoides plants was only continued for 2 h because longer treatment (45 °C for 2 h) led to obvious wilting. The other three stress challenges lasted 2 days (48 h). The RNA-Seq results show that under the heat (physical) challenge, the expression changes in TtASRs were more distinct in the roots and leaves than in the stems. TtASR2, TtASR3, TtASR4, TtASR5, TtASR10, and TtASR13 presented induced expression changes in at least two tissues. When challenged by salt, alkali, or drought (chemical) stress, the expression changes in TtASRs seemed to be more distinct in the aerial parts of the T. tetragonoides plants (leaves and stems) than in the underground parts (roots), indicating that these challenges caused injury to root tissues and inhibited the activities of cells, especially in the root samples under 48 h stress treatments (Figure 6). Overall, the expression of TtASR3, TtASR4, TtASR5, TtASR11, TtASR12, and TtASR13 clearly induced transcriptional patterns in at least two tissues under these stress challenges (Figure 6).
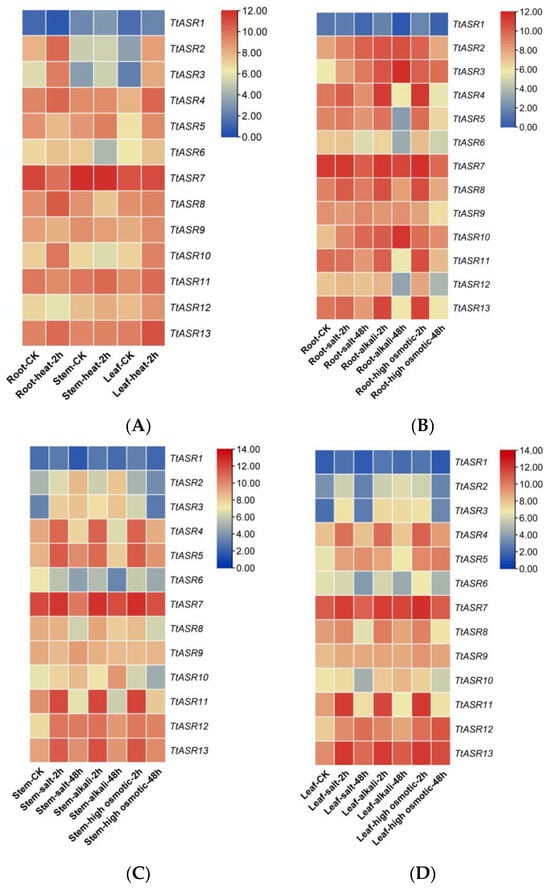
Figure 6.
Heatmaps showing (A) the expression patterns of the TtASRs under heat; (B–D) the expression patterns of the TtASRs under different high-salinity (600 mM NaCl), high-alkali (150 mM NaHCO3), and high-osmotic-stress (300 mM mannitol) challenges, where the figures are separated according to the different T. tetragonoides tissues, including roots (B), stems (C), and leaves (D). The expression level of each gene is shown in FPKM (log2). Red denotes high expression levels, and blue denotes low expression levels.
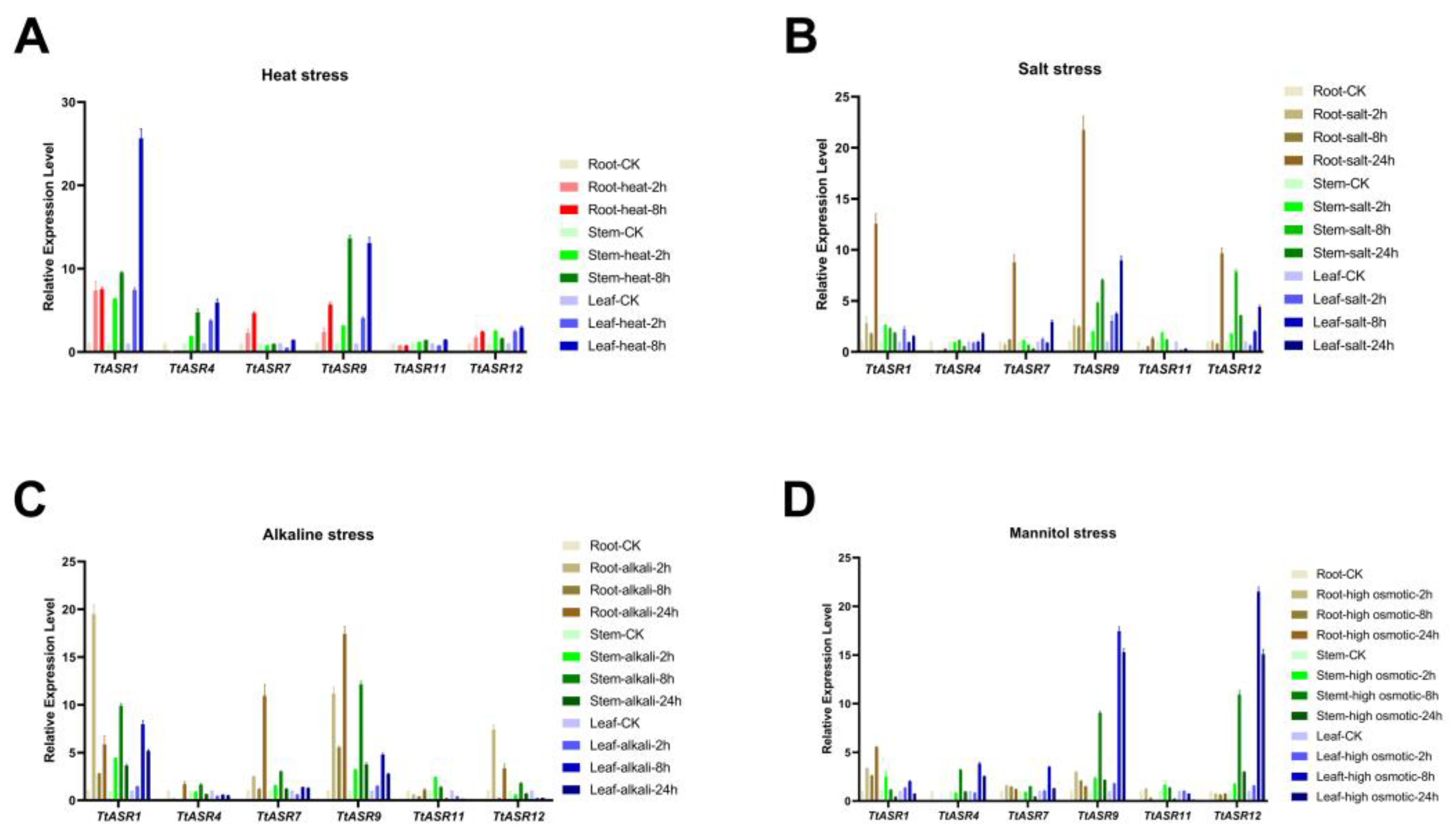
The expression profiles of stress-responsive TtASRs were also validated via qRT-PCR. Based on the previous RNA-Seq results, several candidate genes, including TtASR1, TtASR4, TtASR7, TtASR9, TtASR11, and TtASR12, were chosen, and the treatment time points were refined to 0, 2, 8, and 24 h. In the root sample, salt/alkalis caused obvious induced expression of TtASR1, TtASR7, TtASR9, and TtASR12, while heat upregulated the expression of TtASR7 and TtASR9. In the stem sample, TtASR1, TtASR9, and TtASR12 presented elevated expressions under four stress challenges, and heat seemed to cause the most change. In the leaf sample, the expression changes in TtASR1, TtASR4, TtASR9, and TtASR12 were the most significant (Figure 7).
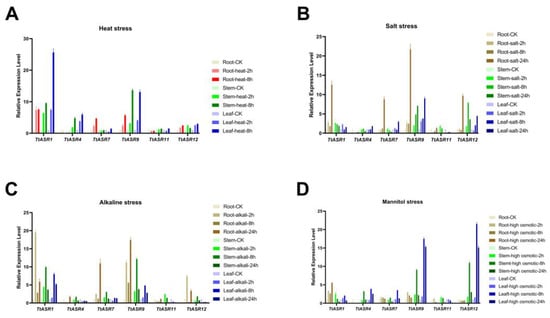
Figure 7.
Quantitative RT-PCR detection of the expression levels of the selected TtASRs (TtASR1, TtASR4, TtASR7, TtASR9, TtASR11, and TtASR12) when responding to different stresses, including heat, high salinity, high alkaline, and high osmotic stress in T. tetragonoides seedling plants. (A) Parameters: 45 °C heat; (B) 600 mM NaCl; (C) 150 mM NaHCO3; (D) 300 mM mannitol. Relative expression values were calculated using the 2−ΔCt method with housekeeping gene TtACT as a reference gene.
2.7. Abiotic Stress Tolerance of Yeast Heterologously Expressing TtASRs
We performed the functional identification of several candidate TtASRs with a yeast heterologous expression system. In brief, six TtASRs, namely TtASR1, TtASR4, TtASR7, TtASR9, TtASR11, and TtASR12, were cloned and functionally verified under different challenges. Following the standard gene cloning and construction procedures for generating the expression vectors TtASRs-pYES2, the target genes were transformed into different yeast strains, including the WT and several mutants (ycf1∆ sensitive to Cd, smf1∆ sensitive to Cd, cot1∆ sensitive to Co, cup2∆ sensitive to Cu, pmr1∆ sensitive to Mn, and zrc1∆cot1∆ sensitive to Zn).
To gain insight into the functional significance of these six TtASRs in salt tolerance, we explored the effect of NaCl on the activities of yeast expressing different TtASRs and empty vector pYES2 (as a negative control). As shown in Figure 8, all six TtASRs improved the salt tolerance of yeast, and yeast expressing TtASR1, TtASR7, and TtASR9 seemed to survive well, even at a high NaCl concentration (1.25 M). Yeast-transformed pYES2 presented weak viability at both 1 and 1.25 M NaCl compared with yeast expressing six TtASRs.
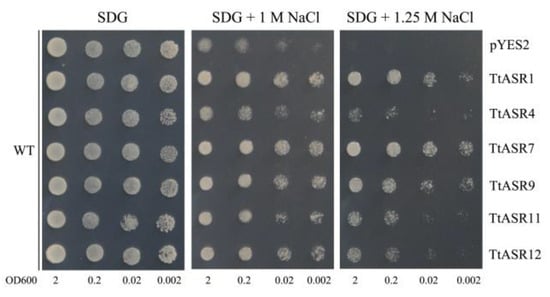
Figure 8.
The salt tolerance confirmations of the six candidate TtASRs’ expression in yeast. The WT strain transformed with empty vector pYES2 and six different TtASR-pYES2 recombinant vectors were spotted on SDG-Ura medium plates supplemented with different concentrations of NaCl (1 M and 1.25 M), 2 μL of each serial dilution (10-fold, from left to right in each panel) of yeast cultures was spotted in turn, and then the plates were incubated for 2 to 5 days at 30 °C.
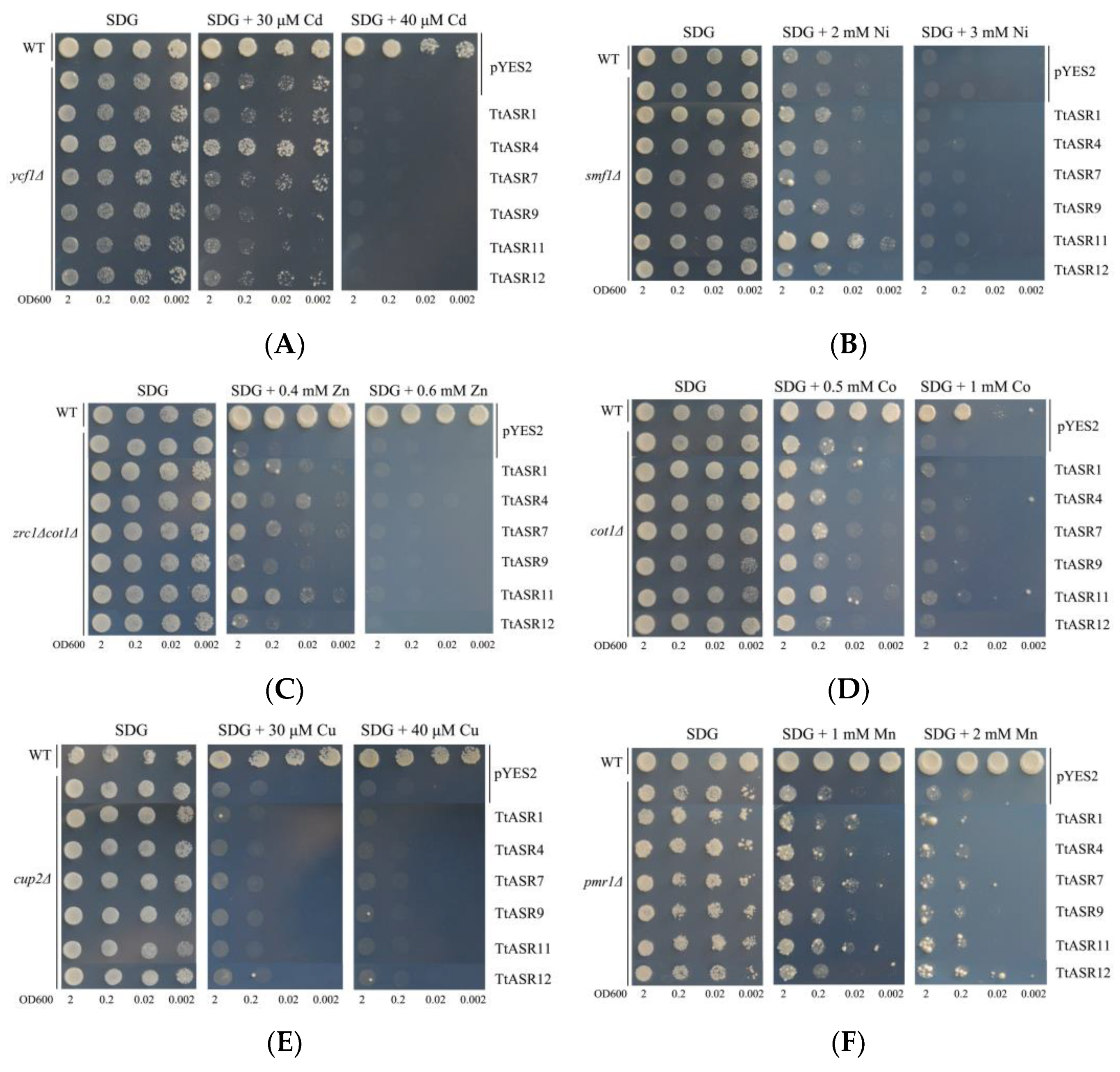
We also tested the heavy metal tolerance of yeast expressing six TtASRs. The ycf1∆ strain expressing TtASR4 grew well under low Cd concentration stress (30 μM), while even under a higher Cd stress (40 μM), the Cd tolerance was completely inhibited (Figure 9A). Similar results were also observed in smf1∆ and zrc1∆cot1∆ mutant strains (Figure 9B,C). TtASR1 and TtASR11 slightly elevated the Ni tolerance of smf1∆ (2 mM Ni), and TtASR1, TtASR4, TtASR7, and TtASR11 slightly elevated the Zn tolerance of zrc1∆cot1∆ (0.4 mM Zn). However, for the other three heavy-metal-sensitive yeast mutant strains, namely cot1∆, cup2∆, and pmr1∆, none of the six tested TtASRs exhibited signs of elevated Co, Cu, and Mn tolerance for yeast cell activities (Figure 9D–F).
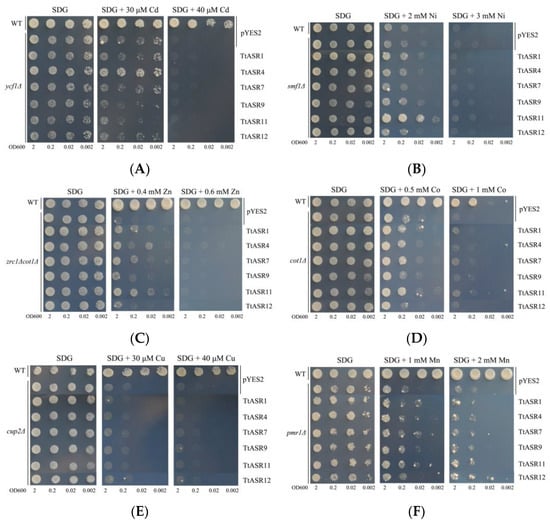
Figure 9.
The heavy metal tolerance confirmations of the six TtASRs’ expression in yeast. Yeast cultures were adjusted to OD600 = 2, and 2 μL of each serial dilution (10-fold, from left to right in each panel) was spotted on SDG-Ura medium plates supplemented with different concentrations of metal stressors. The plates were incubated for 2 to 5 days at 30 °C. (A) Cadmium stress tolerance confirmations in yeast mutant strain ycf1∆; (B) nickel stress tolerance confirmations in yeast mutant strain smf1∆; (C) zinc stress tolerance confirmations in yeast double-mutant strain zrc1∆cot1∆; (D) cobalt stress tolerance confirmations in yeast mutant strain cot1∆; (E) copper stress tolerance confirmations in yeast mutant strain cup2∆; (F) manganese stress tolerance confirmations in yeast mutant strain pmr1∆. The WT strain transformed with pYES2 was used as a positive control, and mutant strains transformed with pYES2 were used as negative controls.
3. Discussion
Tetragonia tetragonoides is an excellent wild vegetable resource that has the concomitant functions of both medicine and foodstuff and is also used as a useful landscape plant for the restoration of the ecological environment of some tropical coastal regions and coral islands. This plant species presents extreme saline–alkaline and drought resistance, and understanding its tolerance mechanism will open up opportunities to apply this knowledge to improve abiotic stress tolerance in crops for future genetic improvement. The ASR gene family is plant-specific, functioning in plant resistance to unfavorable stimuli, and it has been suggested that it plays multiple roles in plants during plant development and evolution [9]. Since the first isolation of SlASR from tomato (Solanum lycopersicum), ripe fruit, and water-stressed leaf cDNA libraries in 1993 [7], increasing evidence has suggested a protective function for ASR proteins against high-salinity or desiccation [30,31] and other stressors [32]. And plant ASRs have been considered as metal-responsive genes and are involved in plant detoxification of some heavy metals [33,34]. Plant ASRs are widely distributed in many species and have been reported to be involved in the response of plants to various abiotic stressors [9,35], leading to great interest in ASRs as promising genes for the genetic improvement of crop abiotic stress tolerance.
As stress-inducible genes, most ASRs have been cloned and reported in some crops [10,11,12,16,17,21,23,26,27,28,29,30,31,32,33,34] and several wild plants [13,14,15,22,24,25], mainly being involved in salt, drought, cold, and oxidative stress, disease resistance, ABA responses, and other developmental or metabolism processes. However, there is little information on ASR genes in halophytes, and only specific ASR members have been characterized in special-habitat plants, including halophytes [13,14,18,19,36]. Tetragonia tetragonoides is widely distributed in coastal sandy fields of the tropics and subtropics, possessing the typical characteristics of drought, salinity/alkali, and heat tolerance, disease resistance with medical nutrition, and high economic value [37]. Inevitably, there is a strong or moderate association between typical stress-related genes (such as ASRs) and plant stress adaptability (such as drought and salinity/alkali tolerance). To further expand this knowledge, in the present study, we comparatively investigated the sequences and expression patterns of the TtASR gene family in wild T. tetragonoides, providing further insights into the possible roles of TtASRs in this special-habitat species adapted to tropical coastal regions.
The ASR family is a small and relatively conserved family across the plant kingdom, although it is missing from the Brassicaceae family. The availability of genome sequencing is a driving force for the investigation of these functional genes. Several ASR families from different plant species have been systematically characterized, such as in monocotyledon banana (Musaceae, at least four members) [8], maize (nine ZmASRs) [34], wheat (hexaploid, thirty-three TaASRs) [11,38], foxtail millet (six SiASRs) [24], Brachypodium distachyon (Poaceae, five BdASRs) [25], dicotyledon tomato (Solanaceae, five SlASRs) [30], common bean (Phaseolus vulgaris L., at least two PvASRs) [39], apple (Rosaceae, five Md-ASRs) [32], and gymnosperm loblolly pine (Pinus taeda L., four PtASRs) [36]. Except for hexaploid wheat, with 33 TaASR members, all the other above-reported diploid species possess no more than 10 ASR members. In T. tetragonoides, 13 TtASRs were characterized, and some gene pairs showed obvious expansion, such as TtASR4/TtASR13 and TtASR5/TtASR12. Accordingly, the genome size of T. tetragonoides is moderate, at about 400 Mb (16 pairs of chromosomes). Plants growing in tropical coastal regions are often subject to drought, high salt/alkali stress, and heat. The different rates of gene gain and loss can cause the copy number of homologous gene groups to vary among species, which provides a genetic basis for phenotypic adaptation, species diversification, and even genome size adjustment. In contrast, the obvious expansion of the number of TtASRs in the T. tetragonoides genome may be the result of the adaptive evolution in response to special habitats with multiple stress challenges, especially for high-salinity and water-deficient habitats.
In our study, phylogenetic analysis divided TtASR proteins into two major groups with distinctive evolutionary relationships (Figure 1A). We also identified, via conserved motif analysis, 10 motifs in these proteins (Figure 3B), which presented highly conservative features, especially in the ABA/WDS domain (Figure 3B). Clearly, the gene structure of TtASRs is also relatively conservative, and all TtASRs have two exons and one intron. The conservation in the sequence and structure of TtASRs indicates that the main functions were relatively conserved and presented little diversity. Therefore, to a certain extent, this has reflected the important biological functions of TtASRs as stress regulators, which are also relatively conserved or maybe consolidated.
However, the biological functions of plant ASRs can be regulated in multiple ways, mainly through transcriptional control, based on their strength or absence. Previous studies have shown that plant ASRs may be involved in the response to various stressors and related signaling molecules at the transcript level [9,35]. The cis-acting regulatory elements in promoter regions are the key factors that initiate transcriptional responses to various signals and stimuli. Our analysis of the TtASR promoter regions indicated that most TtASR promoters contain rich cis-acting elements in response to phytohormones and light. ABREs (including ABRE3a and ABRE4), as major cis-acting elements in ABA-responsive gene expression, were the most abundant cis-acting elements. The ABA signaling pathway is central to stress responses in plants. Moreover, most TtASRs had multiple stress-responsive elements, such as the MYB transcription factor-regulating site (including the MYB core, MYB binding site, MYB recognition site, MYB-like sequence, MRE for light responsiveness, and MBS involved in drought stress), MYC transcription factor-regulating site (including the MYC core and Myc), and TC-rich repeats (involved in defense and stress responsiveness) (Figure 4). The frequent occurrence of these stress-related TtASR promoters further suggests a close relationship between the TtASR family and the plant stress response. This also implies the essential roles of TtASR family genes in drought and salt/alkali adaptability.
To gain further insights into the function of TtASRs in developmental regulation and stress tolerance in the growth cycle of T. tetragonoides, the expression profiles of TtASRs were investigated using RNA-Seq (Figure 5 and Figure 6) combined with qRT-PCR (Figure 7). In general, tissue- and development-specific ASR accumulation in plants is universal, especially when this kind of protein is named “ripening-induced”. ASRs also belong to numerous desiccation-tolerant proteins identified in vivo. The relative expression levels of the tomato ASR genes presented obvious differences in vegetative and reproductive tissues, as well as being differentially responsive to ABA and abiotic stress [30]. For example, the transcriptional profile of rice ASR genes in different tissues seemed to be specific to certain tissues and organs [31]. The six SiASRs in foxtail millet were expressed in both roots and leaves, although their expression profiles were predominantly differentiated [24]. In our study, the TtASR transcripts were localized more in mature stems and leaves than in young roots, flowers, and fruits (Figure 5), indicating their regulatory roles in development, as well as their possible protective roles in stress and water deprivation sensing. Water deprivation can be caused by multiple factors in plants; however, for tropical coastal-growing T. tetragonoides, these factors are nothing more than direct sunlight (oxidative stress), dramatic temperature fluctuations (heat), and osmotic pressure (ion stress or drought).
To further clarify the function of TtASRs under abiotic stress conditions, TtASRs were ectopically expressed in the yeast system (Figure 8 and Figure 9). Our results for yeast growth under different challenges indicate that these TtASRs exercise their protective roles in yeast cells, both under high salinity and under heavy metal toxicity. Many studies have demonstrated that ASR proteins play important roles in halophytes or drought-enduring plant responses to multiple abiotic stressors, especially salt and drought tolerance [9,11,13,14,15,16,17,35,40]. Most of this research was carried out with a plant heterologous transgenic system, such as in Arabidopsis [14] or tobacco [13], while some research was performed with bacteria [15] or yeast [6,27,34], both of which have shorter growth cycles and more obvious phenotypes under challenges. To quickly assess TtASRs’ roles in stress tolerance, a yeast heterologous expression system was used. Heterologous expression of all six TtASRs could improve the salt tolerance of yeast cells (Figure 8), while the extent to which it improved differed among different members. This difference may also reflect the specificity and adjustability of the TtASRs’ functions.
Plant ASRs can also act as metalloproteins, which may be due to their possible N-terminal metal-binding domain, which is composed of a stretch of at least six His residues [33,34,41], or other typical amino acid stretches, such as “PEHAHKHK” [20,42]. To date, there have only been a few reports on plant ASRs related to metal stress [33,34,42,43]. In the present study, the possible metal tolerance of six TtASRs was evaluated with a series of metal-sensitive yeast mutant strains (Figure 9), including cadmium-sensitive yeast mutant ycf1Δ, cobalt-sensitive cot1Δ, copper-sensitive cup2Δ, manganese-sensitive pmr1Δ, nickel-sensitive smf1Δ, and zinc-sensitive double-mutant zrc1Δcot1Δ. Interestingly, the metal chelation or tolerance mediated by different TtASRs was also member-specific (Figure 9), which was still somewhat similar to the results of ZmASRs [34]. Compared with other plant-specialized metal detoxification proteins, such as metallothionein [44] or phytochelatin synthase [45], the plant ASRs’ detoxification capability was perhaps slightly inferior. Many halophytes also accumulate metals and are planted for their phytoremediation potential [46,47,48]. Being a native tropical coastal species, T. tetragonoides can survive under a mixture of salt and toxic metal ions, whereas most glycophytes cannot, thus giving them the potential ability to perform phytoremediation, particularly in heavy-metal-polluted soils in offshore areas. It is important to explore the possible biochemical mechanisms that enable T. tetragonoides to thrive under toxic levels of salinity and heavy metals. Undoubtedly, the tolerance of TtASRs to high salinity and multiple metals gives this species the potential for compound ion toxicity. More research is needed to investigate the effects of these and other related economic uses of T. tetragonoides.
4. Materials and Methods
4.1. Identification of TtASR Genes in T. tetragonoides
The T. tetragonoides genome was completely sequenced, and proteins were noted with InterProscan (https://www.ebi.ac.uk/interpro/search/sequence/ (accessed on 1 March 2023)) to assess the conserved domains and motifs (e < 1 × 10−5). The ABA_WDS domain (Pfam No. PF02496 or InterPro No. IPR003496) was searched as a model, and the protein sequences containing this domain were screened using HMM3.0 software. The domains were then confirmed using the NCBI CDD program (https://www.ncbi.nlm.nih.gov/structure/cdd (accessed on 1 March 2023)).
4.2. Multiple Sequence Alignment and Phylogenetic Analysis of TtASR Family Proteins
Multiple sequence alignments of all ASR proteins (Solanum lycopersicum [30], Oryza sativa [31], Brachypodium distachyon [25], Glycine max (G.max Wm82.a2.v1; https://phytozome-next.jgi.doe.gov/ (accessed on 1 March 2023), Malus × domestica [32], and Pinus taeda [36]) were performed using ClustalW software by MEGA (version 6.06), and a phylogenetic tree was constructed using the neighbor-joining (NJ) phylogenetic method in MEGA 6.0 with 1000 bootstrap replicates. TtASRs were mapped on T. tetragonoides chromosomes according to the positional information of the TtASR genes in the T. tetragonoides genome database and were displayed using MapInspect software (https://mybiosoftware.com/mapinspect-compare-display-linkage-maps.html (accessed on 1 March 2023)).
4.3. Analysis of Gene Structures and Conserved Protein Motifs
The exon–intron structures of the TtASR genes were analyzed using the web server GSDS (Gene Structure Display Server, http://gsds.cbi.pku.edu.cn/ (accessed on 1 March 2023)) based on the comparison of cDNA sequences with their genomic DNA sequences. The subcellular localization of each TtASR protein was predicted using the online tool WoLF_PSORT (https://www.genscript.com/wolf-psort.html (accessed on 1 March 2023)) and the Plant-mPLoc server (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi (accessed on 1 March 2023)). The 3D structures of the TtASRs were analyzed using the Phyre2 program (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index (accessed on 1 March 2023)). The motifs of TtASR proteins were predicted using MEME (http://meme-suite.org/index.html (accessed on 1 March 2023)), with the maximum number of motifs set to 10 and the optimum motif width set to 20–50 residues. The identified TtASR protein sequences were used to compute the MW and pI of proteins using Expasy tools (https://web.expasy.org/protparam/ (accessed on 1 March 2023)).
4.4. Promoter Sequence Profiling of TtASRs
Putative TtASR promoter sequences (2000 bp upstream of ATG) were retrieved from the T. tetragonoides genome database (Table S1). Sequences were then uploaded to the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/ (accessed on 1 March 2023)) for cis-acting regulatory element analysis. The cis-acting elements were classified as hormone specific (gibberellin-responsive elements, MeJA-responsive elements, salicylic acid-responsive elements, abscisic acid response elements, and ethylene response elements), abiotic stress responding (low-temperature response elements, elicitor response elements, defense and stress response elements, stress response elements, and pathogen- or wounding- inducible elements), and transcript factor binding sites (MYB and MYC binding sites). The elements were summarized, and TtASRs’ promoters were visualized using TBtools 2.003 [49].
4.5. Plant Materials and Stress Treatments
Tetragonia tetragonoides plants growing in the South China Botanical Garden (SCBG, 23°18′76″ N, 113°37′02″ E) were used in this study. To analyze the tissue-specific transcriptional patterns of the identified TtASRs, roots, stems, leaves, flowers, and fruits were gathered from T. tetragonoides plants grown in the SCBG. To analyze the expression patterns of the TtASRs responding to various stressors, 60-day-old T. tetragonoides seedlings were exposed to different stressors. In brief, for heat stress, seedlings were moved to a 45 °C illumination incubator. For the high-osmotic-stress treatment, seedlings were removed from their pots, carefully washed with water to remove soil from the roots, and transferred to a 300 mM mannitol solution. For high salt stress, the seedlings were soaked in a 600 mM NaCl solution. For high alkali stress, the seedlings were soaked in a 150 mM NaHCO3 solution (pH 8.2). The seedling roots, stems, and young leaves from the T. tetragonoides plants were collected at 0, 2, and 48 h during the previously described stress treatments, with the 0 time point used as the control. All samples were immediately frozen in liquid nitrogen and stored at −80 °C for subsequent gene expression analysis. Three independent biological replicates were used.
4.6. Expression Analysis of TtASRs
A transcriptome database was constructed for T. tetragonoides using Illumina HiSeq X sequencing technology. The quality of the RNA sequencing (RNA-Seq) datasets created from five tissues (roots, stems, young leaves, flowers, and young seeds collected from T. tetragonoides growing in SCBG) was examined using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 1 March 2023)), which produced 40 Gb clean reads. Clean reads were mapped to the T. tetragonoides reference genome using Tophat v.2.0.10 (http://tophat.cbcb.umd.edu/ (accessed on 1 March 2023)). Gene expression levels were calculated as fragments per kilobase of transcript per million mapped reads (FPKM) according to the length of the gene, and the read counts were mapped to the gene, as follows: FPKM = total exon fragments/[mapped reads (millions) × exon length (kb)]. The expression levels (log2) of TtASRs were visualized as clustered heatmaps using TBtools.
The transcript abundance of several TtASRs’ transcripts was also investigated using qRT-PCR. In brief, total RNA was extracted from T. tetragonoides seedling tissues under stress treatments and reverse-transcribed to cDNA. Total RNA samples were isolated using the EasyPure® Plant RNA Kit (TransGen Biotech, Beijing, China), the RNA was quantified using NanoDrop1000 (NanoDrop Technologies, Inc., Wilmington, DE, USA), and the integrity was evaluated on 0.8% agarose gel. The cDNA was synthesized using cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) according to the manufacturer’s instructions. Quantitative reverse transcription PCR (qRT-PCR) was conducted using the LightCycler480 system (Roche, Basel, Switzerland) and TransStart Tip Green qPCR SuperMix (TransGen Biotech, Beijing, China). All gene expression data obtained via qRT-PCR were normalized to the expression of TtACT (NCBI accession No.: MH33308). The primers used for qRT-PCR (TtACTRTF/TtACTRTR for the reference gene and other TtASR-specific primer pairs) are listed in Table S2.
4.7. In Vivo Stress Tolerance Assay for TtASR Overexpression in Yeast
The ORFs of several TtASRs were PCR-amplified from different cDNA sample templates of T. tetragonoides with gene-specific primer pairs (Table S2). The PCR fragments of the ORFs were then purified and cloned into the BamHI and EcoRI sites of pYES2 to yield recombinant plasmids of TtASRs-pYES2 and sequenced. The recombinant plasmid TtASRs-pYES2 and the empty vector pYES2 (a negative control) were transformed into wild-type (WT) Saccharomyces cerevisiae (BY47471; MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0) and several heavy-metal-sensitive mutant strains cot1Δ, cup2Δ, pmr1Δ, smf1Δ, and ycf1Δ and double-mutant strain zrc1Δcot1Δ (BY4741; MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0; zrc1::natMX cot1::kanMX4). The WT (Y00000) and mutant strains cot1Δ (Y01613), cup2Δ (Y04533), pmr1Δ (Y04534), smf1Δ (Y06272), and ycf1Δ (Y04069) were obtained from Euroscarf (http://www.euroscarf.de/index.php?name=News (accessed on 1 March 2023)). The double-mutant strain zrc1Δcot1Δ was provided by Yuan et al. [50]. Plasmids were introduced into yeast strains using a standard polyethylene glycol (PEG)–lithium acetate-based transformation protocol. The yeast spot assay for NaCl and heavy metal tolerance was performed as previously described [6].
4.8. Statistical Analysis
All experiments in this study were repeated three times independently, and the results are shown as the mean ± standard deviation (SD) (n ≥ 3). Pairwise differences between means were analyzed using Student’s t-tests in Microsoft Excel 2010.
5. Conclusions
In the present study, 13 TtASR genes were identified in the T. tetragonoides genome and divided into two clades. A phylogenetic tree was constructed to confirm the relationships between TtASRs and other plant ASR proteins. Gene duplication plays a pertinent role in generating gene families in the process of evolution. The expansion of the TtASR gene family mainly occurred through segmental duplication. The conservation and diversity of genes in this gene family were also illustrated with gene exon/intron structure analysis and protein motif composition. Many cis-acting elements involved in stress, development, and hormone response were identified in the promoter regions of TtASR members, suggesting that TtASRs may participate in the response to various abiotic stressors and in growth regulation, thereby mediating the plant’s adaptability to tropical coastal areas. RNA-Seq data and qRT-PCR analysis results demonstrate that TtASRs were widely expressed in different T. tetragonoides tissues, with the highest expression present in the stems and leaves; some TtASRs were obviously induced by stress challenges. This study provides important clues for future research on the TtASR family in T. tetragonoides and sheds light on the molecular regulatory mechanisms underlying stress responses and environmental adaptability.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms242115815/s1.
Author Contributions
Conceptualization, M.Z. and S.J.; methodology, H.L. and M.Z.; software, Z.W.; validation, Z.W. and Q.D.; formal analysis and investigation, H.L. and M.Z.; resources, L.C. and Z.H.; data curation, Z.W., S.J. and M.Z.; writing—original draft preparation, M.Z.; writing—review and editing, M.Z.; visualization, Z.W.; supervision, M.Z. and S.J.; project administration, M.Z. and S.J.; funding acquisition, S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Sciences Foundation of China (No. 32270380 and U1701246), the National Key R&D Program of China (2022YFC3103700), and the Guangdong Science and Technology Program (No. 2019B121201005). The funders had no roles in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors sincerely thank the gardeners and workers who have charge of the cultivation and management of T. tetragonoides plants in the South China Botanical Garden (Guangzhou City, China) and field selection, seedling selection, and seed collection from the coastal saline regions of China.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, K.Y.; Kim, S.H.; Yang, W.K.; Lee, G.J. Effect of Tetragonia tetragonoides (Pall.) Kuntze extract on andropause symptoms. Nutrients 2022, 14, 4572. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.A.; Choi, H.J.; Kang, J.S.; Choi, Y.H.; Joo, W.H. Antioxidant activities of the solvent extracts from Tetragonia tetragonioides. J. Life Sci. 2008, 18, 220–227. [Google Scholar] [CrossRef]
- Atzori, G.; Nissim, W.; Macchiavelli, T.; Vita, F.; Azzarello, E.; Pandolfi, C.; Masi, E.; Mancuso, S. Tetragonia tetragonioides (Pallas) Kuntz. as promising salt-tolerant crop in a saline agricultural context. Agric. Water Manag. 2020, 240, 106261. [Google Scholar] [CrossRef]
- Kaur, C.; Kanth, B.K.; Lee, K.Y.; Kumari, S.; Lee, G.J. De novo transcriptome analysis for exploration of genes responding to salinity in a halophyte New Zealand spinach (Tetragonia tetragonioides). Plant Biotechnol. Rep. 2022, 16, 741–755. [Google Scholar] [CrossRef]
- Nissim, W.G.; Masi, E.; Pandolfi, C.; Mancuso, S.; Atzori, G. The response of halophyte (Tetragonia tetragonioides (Pallas) Kuntz.) and glycophyte (Lactuca sativa L.) crops to diluted seawater and NaCl solutions: A comparison between two salinity stress types. Appl. Sci. 2021, 11, 6336. [Google Scholar] [CrossRef]
- Ye, Y.; Lin, R.; Su, H.; Chen, H.; Luo, M.; Yang, L.; Zhang, M. The functional identification of glycine-rich TtASR from Tetragonia tetragonoides (Pall.) Kuntze involving in plant abiotic stress tolerance. Plant Physiol. Biochem. 2019, 143, 212–223. [Google Scholar] [CrossRef]
- Iusem, N.D.; Bartholomew, D.M.; Hitz, W.D.; Scolnik, P.A. Tomato (Lycopersicon esculentum) transcript induced by water deficit and ripening. Plant Physiol. 1993, 102, 1353–1354. [Google Scholar] [CrossRef]
- Henry, I.M.; Carpentier, S.C.; Pampurova, S.; Van Hoylandt, A.; Panis, B.; Swennen, R.; Remy, S. Structure and regulation of the Asr gene family in banana. Planta 2011, 234, 785–798. [Google Scholar] [CrossRef]
- González, R.M.; Iusem, N.D. Twenty years of research on Asr (ABA-stress-ripening) genes and proteins. Planta 2014, 239, 941–949. [Google Scholar] [CrossRef]
- Jia, H.; Jiu, S.; Zhang, C.; Wang, C.; Tariq, P.; Liu, Z.; Wang, B.; Cui, L.; Fang, J. Abscisic acid and sucrose regulate tomato and strawberry fruit ripening through the abscisic acid-stress-ripening transcription factor. Plant Biotechnol. J. 2016, 14, 2045–2065. [Google Scholar] [CrossRef]
- Yacoubi, I.; Gadaleta, A.; Mathlouthi, N.; Hamdi, K.; Giancaspro, A. Abscisic acid-stress-ripening genes involved in plant response to high salinity and water deficit in durum and common wheat. Front. Plant Sci. 2022, 13, 789701. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, J.; Medici, A.; Gaillard, C.; Verbeke, J.; Gibon, Y.; Rolin, D.; Laloi, M.; Finkelstein, R.R.; Atanassova, R. Grape ASR regulates glucose transport, metabolism and signaling. Int. J. Mol. Sci. 2022, 23, 6194. [Google Scholar] [CrossRef] [PubMed]
- Jha, B.; Lal, S.; Tiwari, V.; Yadav, S.K.; Agarwal, P.K. The SbASR-1 gene cloned from an extreme halophyte Salicornia brachiata enhances salt tolerance in transgenic tobacco. Mar. Biotechnol. 2012, 14, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.X.; Yang, X.; Li, X.L.; Yu, X.D.; Li, Q.L. The SlASR gene cloned from the extreme halophyte Suaeda liaotungensis K. enhances abiotic stress tolerance in transgenic Arabidopsis thaliana. Gene 2014, 549, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Padaria, J.C.; Yadav, R.; Tarafdar, A.; Lone, S.A.; Kumar, K.; Sivalingam, P.N. Molecular cloning and characterization of drought stress responsive abscisic acid-stress-ripening (Asr1) gene from wild jujube, Ziziphus nummularia (Burm.f.) Wight & Arn. Mol. Biol. Rep. 2016, 43, 849–859. [Google Scholar]
- Sachdeva, S.; Bharadwaj, C.; Singh, R.K.; Jain, P.K.; Patil, B.S.; Roorkiwal, M.; Varshney, R. Characterization of ASR gene and its role in drought tolerance in chickpea (Cicer arietinum L.). PLoS ONE 2020, 15, e0234550. [Google Scholar] [CrossRef]
- Meena, R.P.; Vishwakarma, H.; Ghosh, G.; Gaikwad, K.; Chellapilla, T.S.; Singh, M.P.; Padaria, J.C. Novel ASR isolated from drought stress responsive SSH library in pearl millet confers multiple abiotic stress tolerance in PgASR3 transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 156, 7–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, H.; Zhou, T.; Zhu, Z.; Zhang, Y.; Zhao, X.; Wang, C. ThASR3 confers salt and osmotic stress tolerances in transgenic Tamarix and Arabidopsis. BMC Plant Biol. 2022, 22, 586. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.H.; Ren, W.; Gao, H.J.; Lü, X.P.; Zhao, Q.; Zhang, H.; Rensing, C.; Zhang, J.L. HaASR2 from Haloxylon ammodendron confers drought and salt tolerance in plants. Plant Sci. 2023, 328, 111572. [Google Scholar] [CrossRef]
- Hamdi, K.; Salladini, E.; O’Brien, D.P.; Brier, S.; Chenal, A.; Yacoubi, I.; Longhi, S. Structural disorder and induced folding within two cereals, ABA stress and ripening (ASR) proteins. Sci. Rep. 2017, 7, 15544. [Google Scholar] [CrossRef]
- Hu, W.; Huang, C.; Deng, X.; Zhou, S.; Chen, L.; Li, Y.; Wang, C.; Ma, Z.; Yuan, Q.; Wang, Y.; et al. TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ. 2013, 36, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chen, Y.C.; Jauh, G.Y.; Wang, C.S. A lily ASR protein involves abscisic acid signaling and confers drought and salt resistance in Arabidopsis. Plant Physiol. 2005, 139, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, W.; Wang, Y.; Feng, R.; Zhang, Y.; Liu, J.; Jia, C.; Miao, H.; Zhang, J.; Xu, B.; et al. The MaASR gene as a crucial component in multiple drought stress response pathways in Arabidopsis. Funct. Integr. Genom. 2015, 15, 247–260. [Google Scholar] [CrossRef]
- Feng, Z.J.; Xu, Z.S.; Sun, J.; Li, L.C.; Chen, M.; Yang, G.X.; He, G.Y.; Ma, Y.Z. Investigation of the ASR family in foxtail millet and the role of ASR1 in drought/oxidative stress tolerance. Plant Cell Rep. 2016, 35, 115–128. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Feng, J.; Yang, X.; Huang, Q.; Xiao, J.; Liu, Y.; Yang, G.; He, G. Identification of the ASR gene family from Brachypodium distachyon and functional characterization of BdASR1 in response to drought stress. Plant Cell Rep. 2016, 35, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Yin, Z.; Jiang, J.; Zhang, M.; Guo, X.; Ye, Z.; Zhao, Y.; Xiong, H.; Zhang, Z.; et al. OsASR5 enhances drought tolerance through a stomatal closure pathway associated with ABA and H2O2 signalling in rice. Plant Biotechnol. J. 2017, 15, 183–196. [Google Scholar] [CrossRef]
- Hamdi, K.; Brini, F.; Kharrat, N.; Masmoudi, K.; Yakoubi, I. Abscisic acid, Stress, and Ripening (TtASR1) gene as a functional marker for salt tolerance in durum wheat. BioMed Res. Int. 2020, 2020, 7876357. [Google Scholar] [CrossRef]
- Yoon, J.S.; Kim, J.Y.; Kim, D.Y.; Seo, Y.W. A novel wheat ASR gene, TaASR2D, enhances drought tolerance in Brachypodium distachyon. Plant Physiol. Biochem. 2021, 159, 400–414. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Jiang, Y.; Li, A.; Cheng, B.; Wu, J. OsASR6 enhances salt stress tolerance in rice. Int. J. Mol. Sci. 2022, 23, 9340. [Google Scholar] [CrossRef]
- Golan, I.; Dominguez, P.G.; Konrad, Z.; Shkolnik-Inbar, D.; Carrari, F.; Bar-Zvi, D. Tomato ABSCISIC ACID STRESS RIPENING (ASR) gene family revisited. PLoS ONE 2014, 9, e107117. [Google Scholar] [CrossRef]
- Pérez-Díaz, J.; Wu, T.M.; Pérez-Díaz, R.; Ruíz-Lara, S.; Hong, C.Y.; Casaretto, J.A. Organ- and stress-specific expression of the ASR genes in rice. Plant Cell Rep. 2014, 33, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Zhong, Y.; Li, Y.; Zheng, D.; Cheng, Z.M. Genome-wide identification and expression analysis of the apple ASR gene family in response to Alternaria alternata f. sp. mali. Genome 2016, 59, 866–878. [Google Scholar] [CrossRef]
- Li, R.H.; Liu, G.B.; Wang, H.; Zheng, Y.Z. Effects of Fe3+ and Zn2+ on the structural and thermodynamic properties of a soybean ASR protein. Biosci. Biotechnol. Biochem. 2013, 77, 475–481. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Q.; Yu, H.; Li, L.; Zhang, G.; Chen, X.; Jiang, M.; Tan, M. Comprehensive analysis of the cadmium tolerance of abscisic acid-, stress- and ripening-induced proteins (ASRs) in maize. Int. J. Mol. Sci. 2019, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, J.H.; Li, R.H.; Yang, H.Y.; Zheng, Y.J. Research progress on plant ASR protein. Chin. Bull. Life Sci. 2015, 27, 626–630. (In Chinese) [Google Scholar]
- Lecoy, J.; Ranade, S.S.; García-Gil, M.R. Analysis of the ASR and LP3 homologous gene families reveal positive selection acting on LP3-3 gene. Gene 2023, 850, 146935. [Google Scholar] [CrossRef]
- He, L.; Wang, W.Q.; Lin, G.H. Effects of salinity on the growth and photosynthetic characteristics of a coastal wetland plant species Tetragonia tetragonoides (Pall.) Kuntze. Chin. J. Ecol. 2012, 31, 3044–3049. (In Chinese) [Google Scholar]
- Li, H.; Guan, H.; Zhuo, Q.; Wang, Z.; Li, S.; Si, J.; Zhang, B.; Feng, B.; Kong, L.A.; Wang, F.; et al. Genome-wide characterization of the abscisic acid-, stress- and ripening-induced (ASR) gene family in wheat (Triticum aestivum L.). Biol. Res. 2020, 53, 23. [Google Scholar] [CrossRef]
- Cortés, A.J.; Chavarro, M.C.; Madriñán, S.; This, D.; Blair, M.W. Molecular ecology and selection in the drought-related Asr gene polymorphisms in wild and cultivated common bean (Phaseolus vulgaris L.). BMC Genet. 2012, 13, 58. [Google Scholar] [CrossRef]
- Fischer, I.; Camus-Kulandaivelu, L.; Allal, F.; Stephan, W. Adaptation to drought in two wild tomato species: The evolution of the Asr gene family. New Phytol. 2011, 190, 1032–1044. [Google Scholar] [CrossRef]
- Virlouvet, L.; Jacquemot, M.P.; Gerentes, D.; Corti, H.; Bouton, S.; Gilard, F.; Valot, B.; Trouverie, J.; Tcherkez, G.; Falque, M.; et al. The ZmASR1 protein influences branched-chain amino acid biosynthesis and maintains kernel yield in maize under water-limited conditions. Plant Physiol. 2011, 157, 917–936. [Google Scholar] [CrossRef] [PubMed]
- Rom, S.; Gilad, A.; Kalifa, Y.; Konrad, Z.; Karpasas, M.M.; Goldgur, Y.; Bar-Zvi, D. Mapping the DNA- and zinc-binding domains of ASR1 (abscisic acid stress ripening), an abiotic-stress regulated plant specific protein. Biochimie 2006, 88, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Arenhart, R.A.; Schunemann, M.; Bucker Neto, L.; Margis, R.; Wang, Z.Y.; Margis-Pinheiro, M. Rice ASR1 and ASR5 are complementary transcription factors regulating aluminium responsive genes. Plant Cell Environ. 2016, 39, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.; Pu, L.; Lin, R.; Mo, H.; Wang, Z.; Jian, S.; Zhang, M. Roles of Canavalia rosea metallothioneins in metal tolerance and extreme environmental adaptation to tropical coral reefs. J. Plant Physiol. 2022, 268, 153559. [Google Scholar] [CrossRef]
- Su, H.; Zou, T.; Lin, R.; Zheng, J.; Jian, S.; Zhang, M. Characterization of a phytochelatin synthase gene from Ipomoea pes-caprae involved in cadmium tolerance and accumulation in yeast and plants. Plant Physiol. Biochem. 2020, 155, 743–755. [Google Scholar] [CrossRef]
- Nikalje, G.C.; Suprasanna, P. Coping with metal toxicity-cues from halophytes. Front. Plant Sci. 2018, 9, 777. [Google Scholar] [CrossRef]
- Aziz, I.; Mujeeb, A. Halophytes for phytoremediation of hazardous metal(loid)s: A terse review on metal tolerance, bio-indication and hyperaccumulation. J. Hazard Mater. 2022, 424, 127309. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, R.; Rajput, V.D.; Singh, V.K. Halophytes for the sustainable remediation of heavy metal-contaminated sites: Recent developments and future perspectives. Chemosphere 2023, 313, 137524. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Zheng, S.; Dai, H.; Meng, Q.; Huang, R.; Tong, H.; Yuan, L. Identification and expression analysis of the ZRT, IRT-like protein (ZIP) gene family in Camellia sinensis (L.) O. Kuntze. Plant Physiol. Biochem. 2022, 172, 87–100. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).