Antioxidant Activity, Stability in Aqueous Medium and Molecular Docking/Dynamics Study of 6-Amino- and N-Methyl-6-amino-L-ascorbic Acid
Abstract
1. Introduction
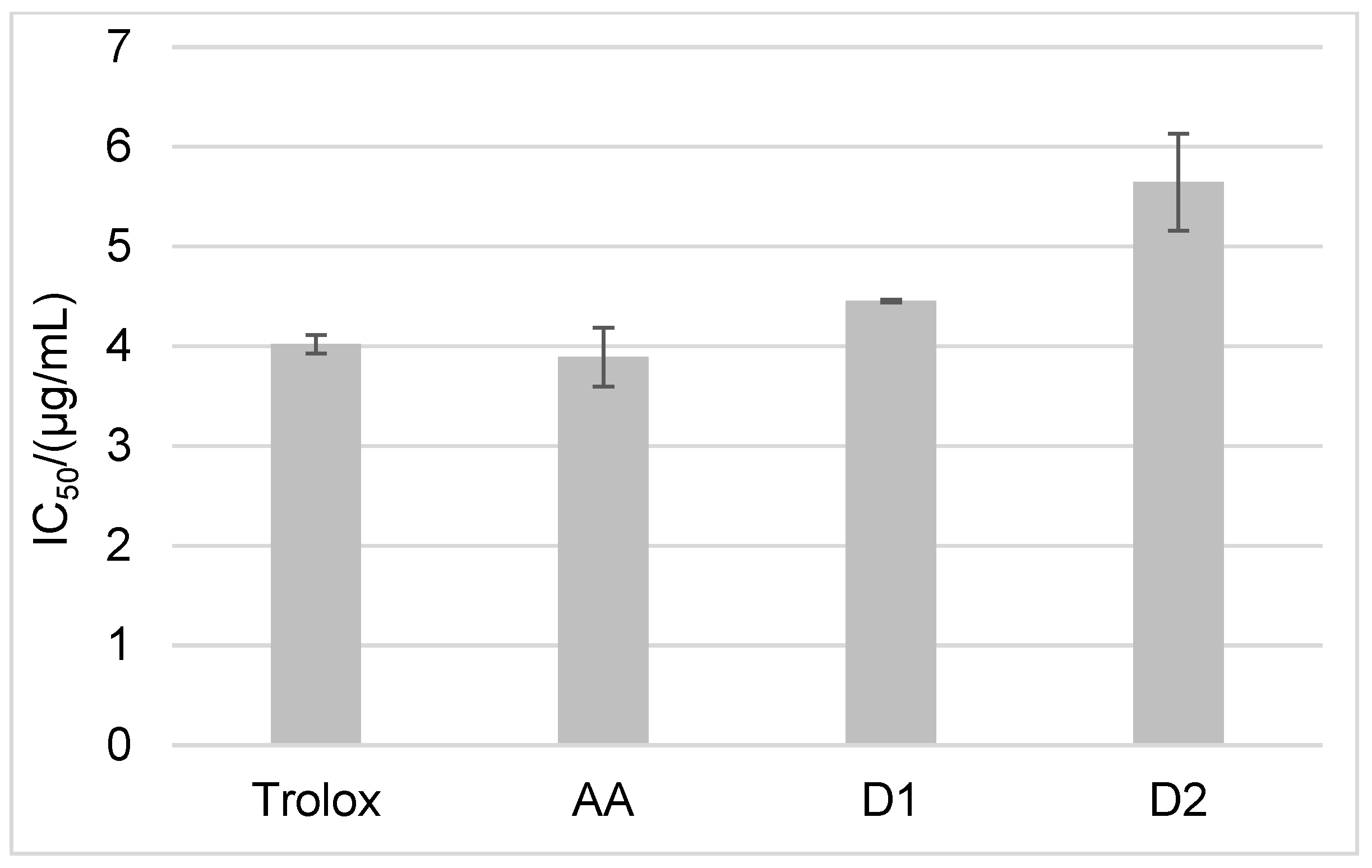
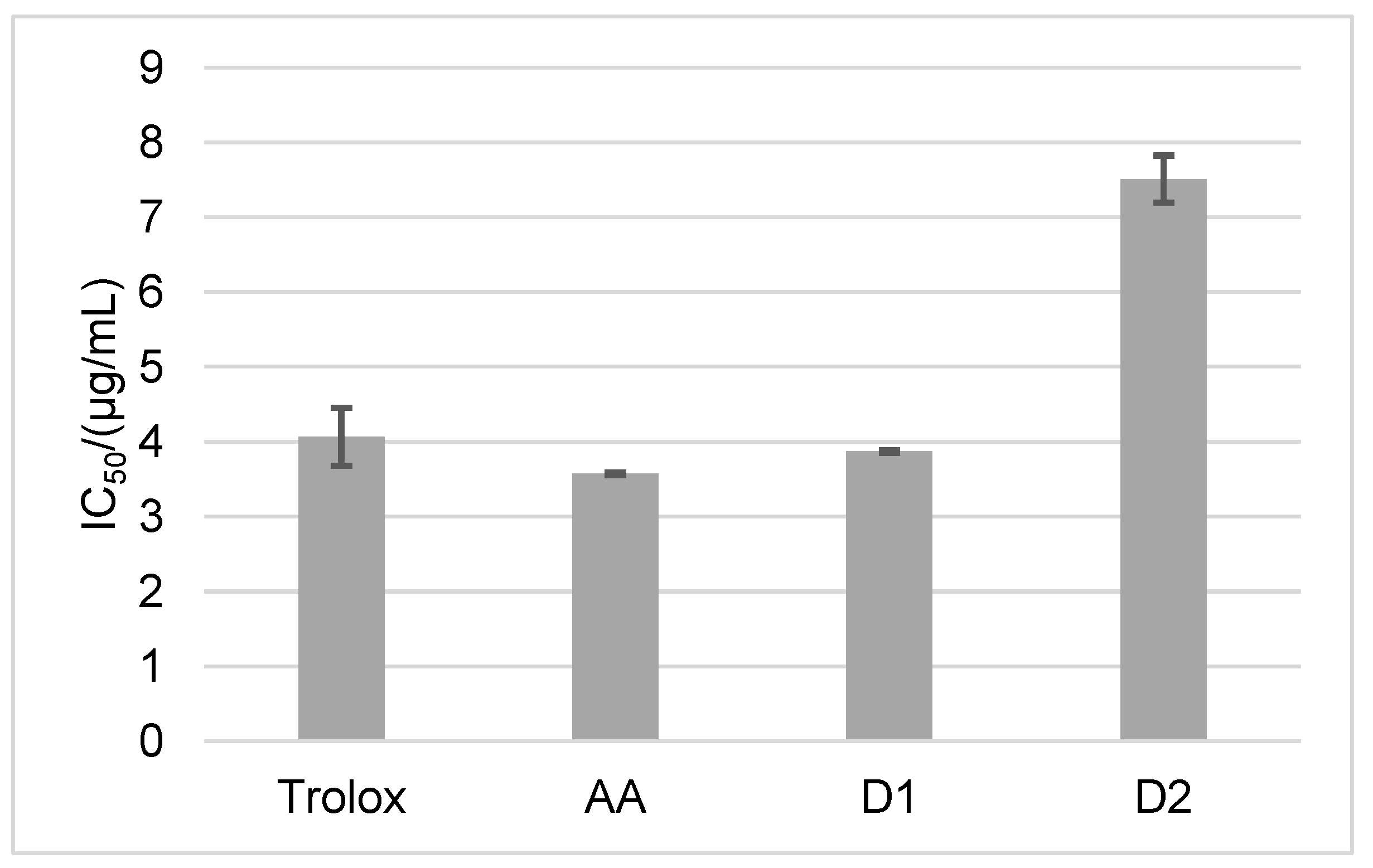
2. Results
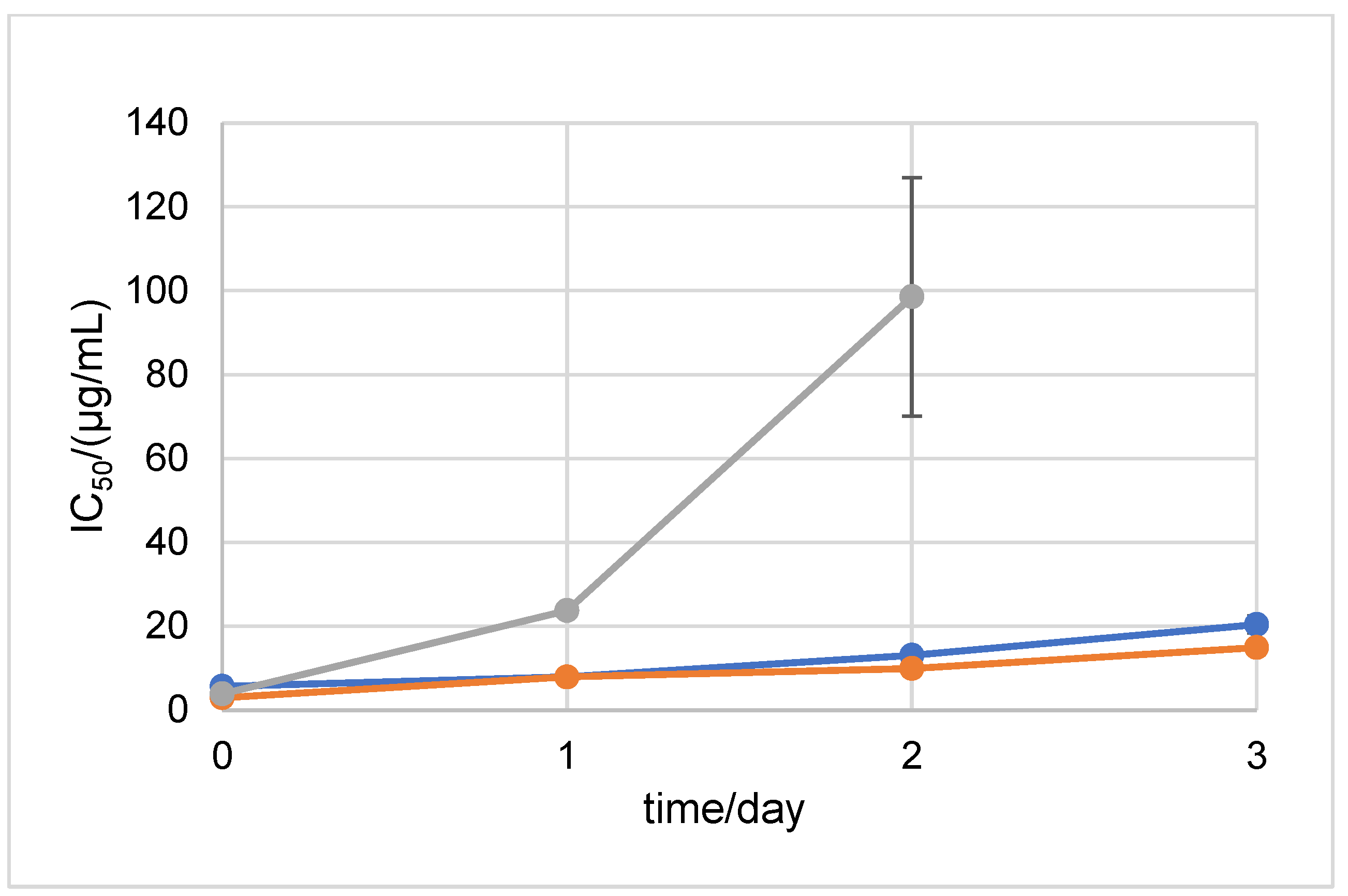
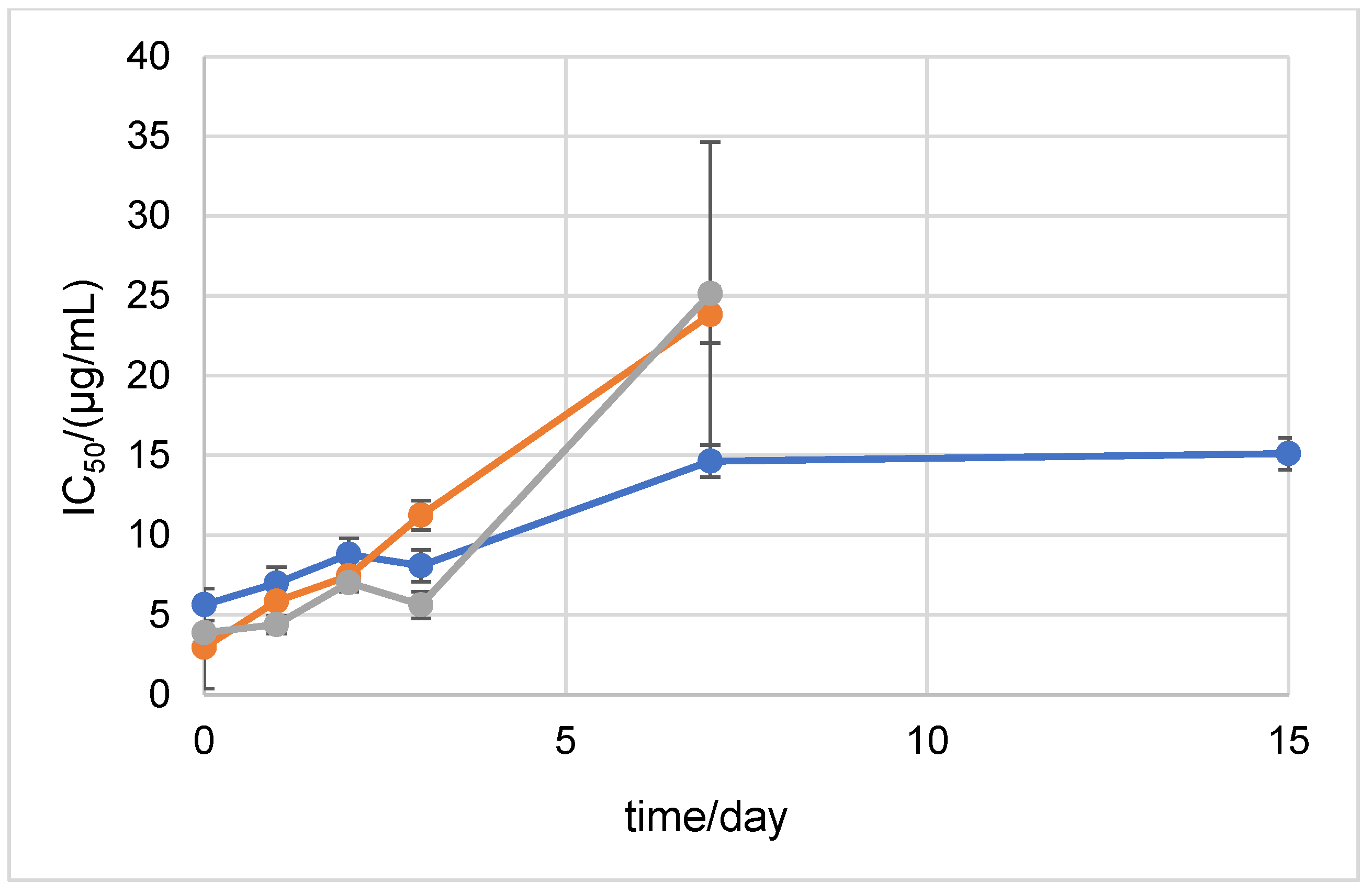
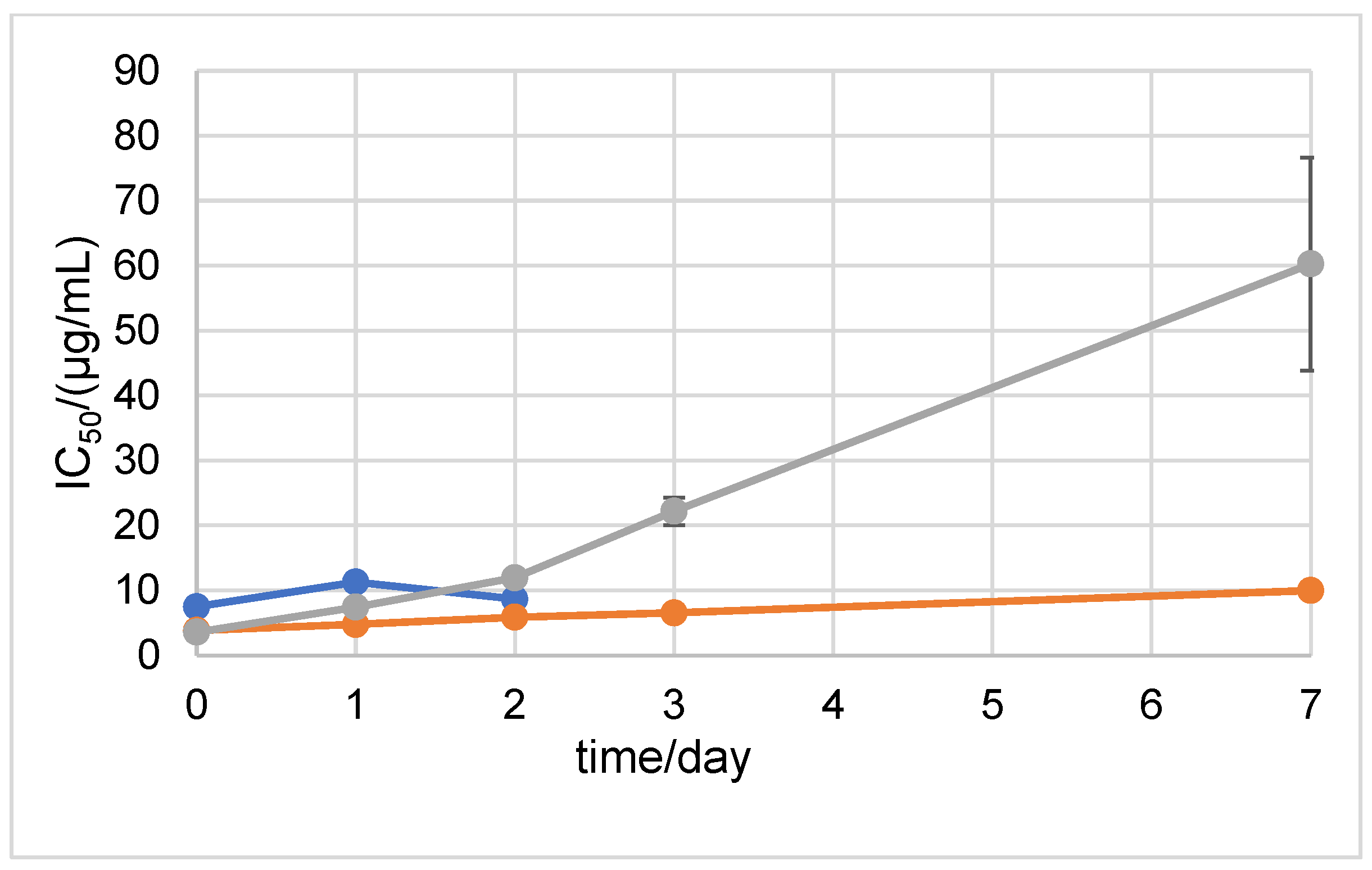
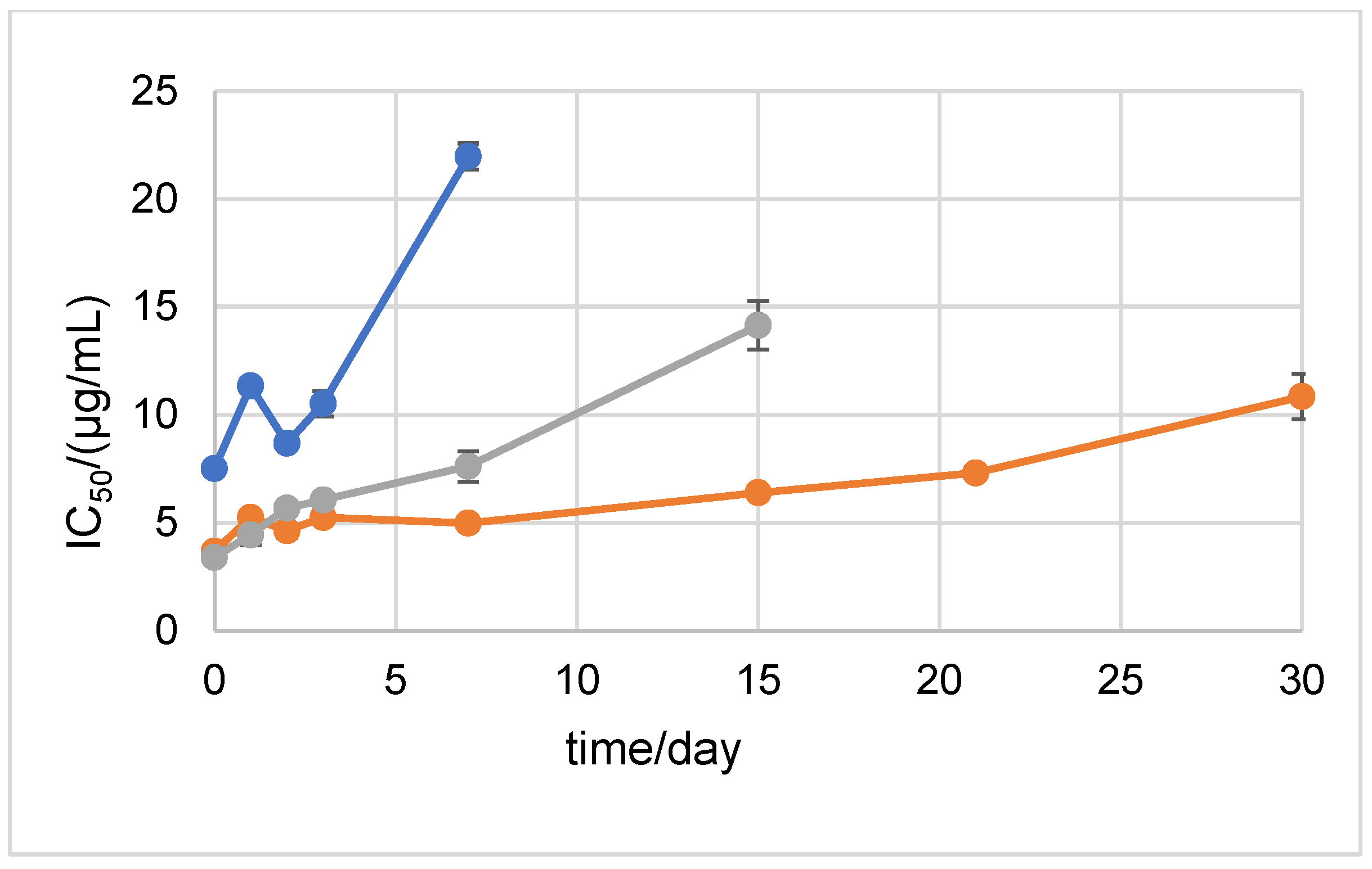
2.1. Stability Measured by Spectrophotometric Methods
2.2. Stability Measured by MS/MS
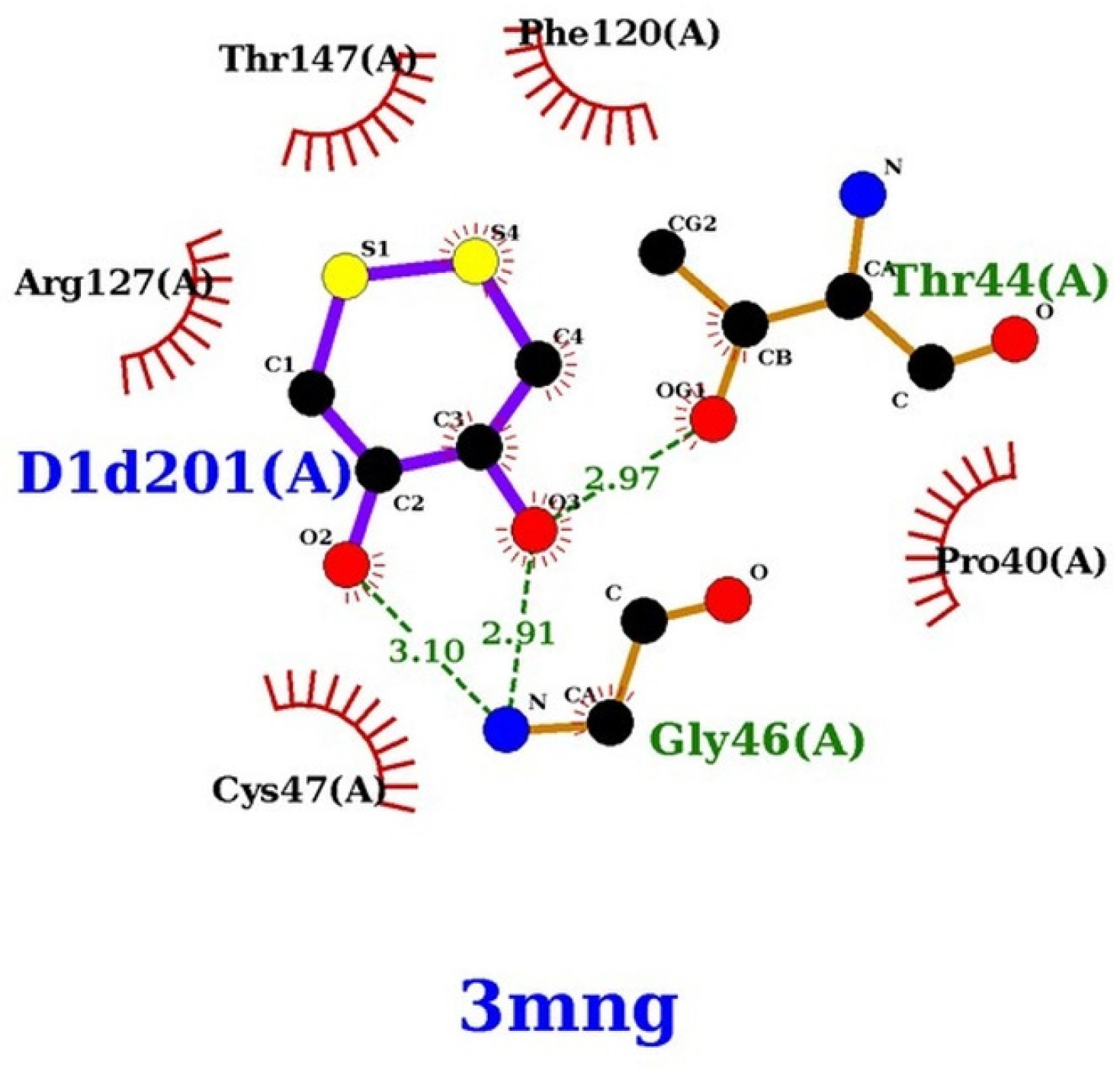
2.3. Docking Control Study of the DTT against Wild-Type Human Peroxiredoxin
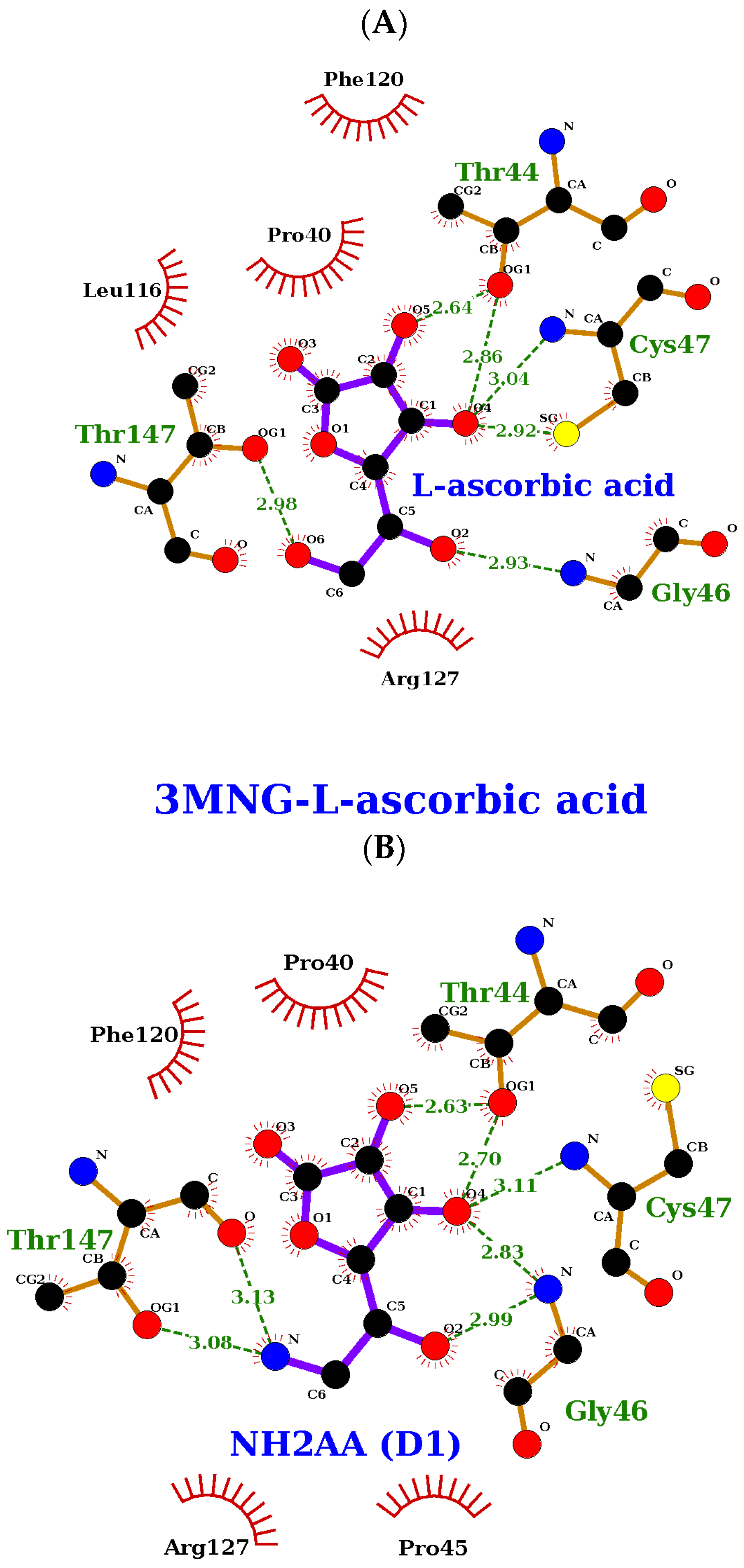
2.4. Molecular Docking Study of the Compound Library
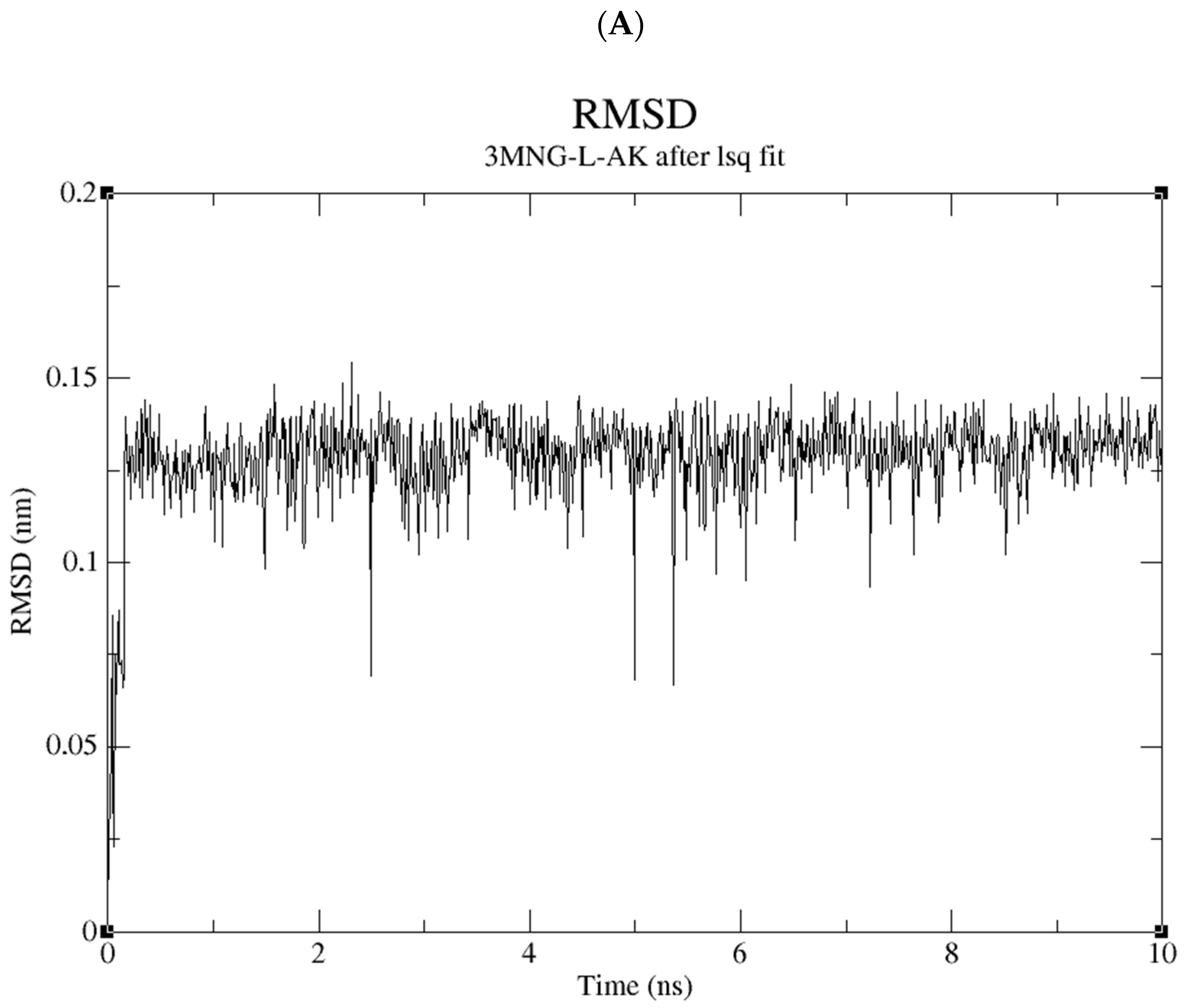
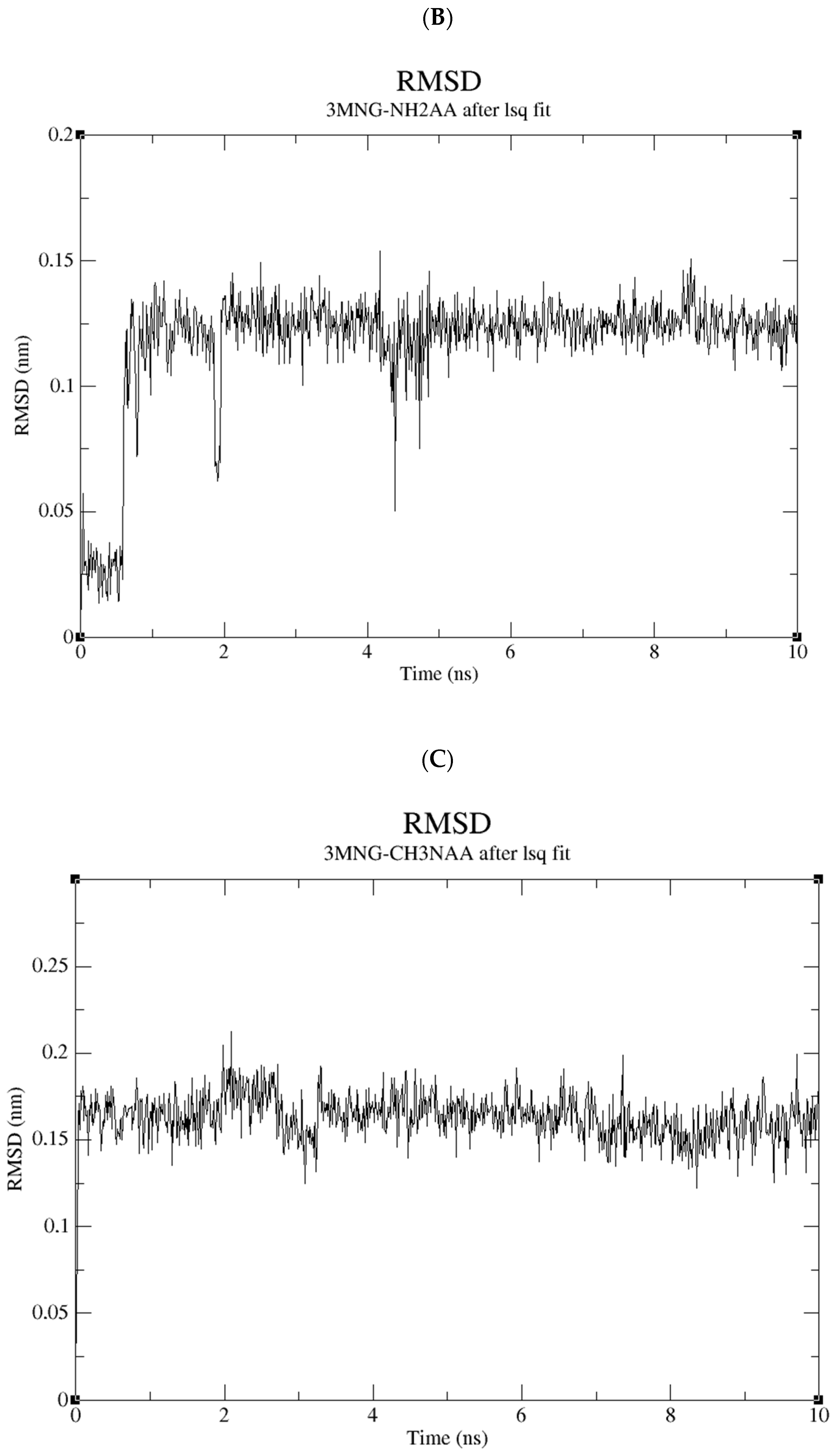
2.5. Molecular Dynamics Study of the Compound Library
3. Discussion
Possible Applications
4. Materials and Methods
4.1. Reagents
4.2. NMR
4.3. Sample Preparation for Measurements of Antioxidant Stability
4.3.1. ABTS
4.3.2. DPPH
4.4. MS/MS Stability Analysis
4.5. Molecular Docking
4.5.1. Protein Preparation
4.5.2. Cavity Search
4.5.3. (4S,5S)-1,2-Dithiane-4,5-diol (DTT) Optimization and Docking
4.5.4. Customized Small Ligand Library Preparation and High-Throughput Virtual Screening
4.6. Molecular Dynamics Simulation Studies
4.7. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Koo, N.; Min, D.B. Reactive Oxygen Species, Aging, and Antioxidative Nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Tu, Y.J.; Njus, D.; Schlegel, H.B. A Theoretical Study of Ascorbic Acid Oxidation and HOO/O2− Radical Scavenging. Org. Biomol. Chem. 2017, 15, 4417–4431. [Google Scholar] [CrossRef]
- Niki, E. Action of Ascorbic Acid as a Scavenger of Active and Stable Oxygen Radicals. Am. J. Clin. Nutr. 1991, 54, 1119S–1124S. [Google Scholar] [CrossRef]
- González, M.J.; Miranda-Massari, J.R.; Mora, E.M.; Guzmán, A.; Riordan, N.H.; Riordan, H.D.; Casciari, J.J.; Jackson, J.A.; Román-Franco, A. Orthomolecular Oncology Review: Ascorbic Acid and Cancer 25 Years Later. Integr. Cancer Ther. 2005, 4, 32–44. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C Pharmacokinetics in Healthy Volunteers: Evidence for a Recommended Dietary Allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Sun, H.; Wang, Y.; Riordan, H.D.; Hewitt, S.M.; Katz, A.; Wesley, R.A.; Levine, M. Vitamin C Pharmacokinetics: Implications for Oral and Intravenous Use. Ann. Intern. Med. 2004, 140, 533–537. [Google Scholar] [CrossRef]
- Ojha, R.; Prasad, R.; Manzoor, N.; Khan, L.A. Vitamin C Modulates Oxidative Stress Related Enzyme Activities in Candida Albicans. Turk. J. Biochem. 2010, 35, 35–40. [Google Scholar]
- Buettner, G.R.; Jurkiewicz, B.A. Ascorbate Free Radical as a Marker of Oxidative Stress: An EPR Study. Free Radic. Biol. Med. 1993, 14, 49–55. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic Acid: Metabolism and Functions of a Multi-Facetted Molecule. Curr. Opin. Plant Biol. 2000, 3, 229–235. [Google Scholar] [CrossRef]
- Yin, X.; Chen, K.; Cheng, H.; Chen, X.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C. Biosynthesis, Recycling and Degradation in Mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.D.; Walker, P.G.; Pont, S.D.A.; Marquis, N.; Vivera, S.; Gordon, S.L.; Brennan, R.M.; Viola, R. L-Ascorbic Acid Accumulation in Fruit of Ribes Nigrum Occurs by in Situ Biosynthesis via the l-Galactose Pathway. Funct. Plant Biol. 2007, 34, 1080–1091. [Google Scholar] [CrossRef]
- Green, M.A.; Fry, S.C. Vitamin C Degradation in Plant Cells via Enzymatic Hydrolysis of 4-O-Oxalyl-l-Threonate. Nature 2004, 433, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, J.C. Spontaneous Hydrolysis and Dehydration of Dehydroascorbic Acid in Aqueous Solution. Anal. Biochem. 1998, 260, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Connors, K.A.; Connors, K.A.; Amidon, G.L.; Stella, V.J. Chemical Stability of Pharmaceuticals: A Handbook for Pharmacists; John Wiley & Sons: New York, NY, USA, 1986; ISBN 978-0-471-87955-8. [Google Scholar]
- Finholt, P.; Paulssen, R.B.; Higuchi, T. Rate of Anaerobic Degradation of Ascorbic Acid in Aqueous Solution. J. Pharm. Sci. 1963, 52, 948–954. [Google Scholar] [CrossRef]
- Stamford, N.P.J. Stability, Transdermal Penetration, and Cutaneous Effects of Ascorbic Acid and Its Derivatives. J. Cosmet. Dermatol. 2012, 11, 310–317. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, Y.C.; Gao, M.Y.; Fang, Y.P. In Vitro Evaluation of Permeation Ability and In Vivo Whitening of Ascorbic Acid 2-Glucoside in Microemulsion. Int. J. Pharm. Sci. Res. 2016, 3, IJPSR-114. [Google Scholar]
- Campos, P.M.B.G.M.; Gianeti, M.D.; Camargo, F.B.; Gaspar, L.R. Application of Tetra-Isopalmitoyl Ascorbic Acid in Cosmetic Formulations: Stability Studies and in Vivo Efficacy. Eur. J. Pharm. Biopharm. 2012, 82, 580–586. [Google Scholar] [CrossRef]
- Frei, B.; Lawson, S. Vitamin C and Cancer Revisited. Proc. Natl. Acad. Sci. USA 2008, 105, 11037–11038. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Liu, X.; Xu, C.; Wang, X. L-Ascorbic Acid-2-Glucoside Inhibits Helicobacter Pylori-Induced Apoptosis through Mitochondrial Pathway in Gastric Epithelial Cells. Biomed. Pharmacother. 2018, 97, 75–81. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Sil, B.C.; Moore, D.J.; Lucas, R.A.; Lane, M.E. 3-O-Ethyl-l-Ascorbic Acid: Characterisation and Investigation of Single Solvent Systems for Delivery to the Skin. Int. J. Pharm. X 2019, 1, 10025. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, C.; Li, J. Comparison of Vitamin c and Its Derivative Antioxidant Activity: Evaluated by Using Density Functional Theory. ACS Omega 2020, 5, 25467–25475. [Google Scholar] [CrossRef] [PubMed]
- Padma, R.; Parvathy, N.G.; Renjith, V.; Rahate, K.P. Quantitative Estimation of Tannins, Phenols and Antioxidant Activity of Methanolic Extract of Imperata Cylindrica. Int. J. Res. Pharm. Sci. 2013, 4, 73–77. [Google Scholar]
- Tan, P.H.; Sagoo, P.; Chan, C.; Yates, J.B.; Campbell, J.; Beutelspacher, S.C.; Foxwell, B.M.J.; Lombardi, G.; George, A.J.T. Inhibition of NF-κB and Oxidative Pathways in Human Dendritic Cells by Antioxidative Vitamins Generates Regulatory T Cells. J. Immunol. 2005, 174, 7633–7644. [Google Scholar] [CrossRef] [PubMed]
- Saffi, J.; Sonego, L.; Varela, Q.D.; Salvador, M. Antioxidant Activity of L-Ascorbic Acid in Wild-Type and Superoxide Dismutase Deficient Strains of Saccharomyces Cerevisiae. Redox Rep. 2006, 11, 179–184. [Google Scholar] [CrossRef] [PubMed]
- May, J.M.; Qu, Z.C.; Meredith, M.E. Mechanisms of Ascorbic Acid Stimulation of Norepinephrine Synthesis in Neuronal Cells. Biochem. Biophys. Res. Commun. 2012, 426, 148–152. [Google Scholar] [CrossRef]
- Klingelhoeffer, C.; Kämmerer, U.; Koospal, M.; Mühling, B.; Schneider, M.; Kapp, M.; Kübler, A.; Germer, C.T.; Otto, C. Natural Resistance to Ascorbic Acid Induced Oxidative Stress Is Mainly Mediated by Catalase Activity in Human Cancer Cells and Catalase-Silencing Sensitizes to Oxidative Stress. BMC Complement. Altern. Med. 2012, 12, 61. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of Vitamin C in the Function of the Vascular Endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Washio, K.; Inagaki, M.; Tsuji, M.; Morio, Y.; Akiyama, S.; Gotoh, H.; Gotoh, T.; Gotoh, Y.; Oguchi, K. Oral Vitamin C Supplementation in Hemodialysis Patients and Its Effect on the Plasma Level of Oxidized Ascorbic Acid and Cu/Zn Superoxide Dismutase, an Oxidative Stress Marker. Nephron Clin. Pract. 2008, 109, c49–c54. [Google Scholar] [CrossRef]
- Tamari, Y.; Nawata, H.; Inoue, E.; Yoshimura, A.; Yoshii, H.; Kashino, G.; Seki, M.; Enomoto, T.; Watanabe, M.; Tano, K. Protective Roles of Ascorbic Acid in Oxidative Stress Induced by Depletion of Superoxide Dismutase in Vertebrate Cells. Free Radic. Res. 2013, 47, 1–7. [Google Scholar] [CrossRef]
- Adikwu, E.; Deo, O. Hepatoprotective Effect of Vitamin C (Ascorbic Acid). Pharmacol. Pharm. 2013, 4, 84–92. [Google Scholar] [CrossRef]
- Koc, M.; Imik, H.; Odabasoglu, F. Gastroprotective and Anti-Oxidative Properties of Ascorbic Acid on Indomethacin-Induced Gastric Injuries in Rats. Biol. Trace Elem. Res. 2008, 126, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Kodrík, D.; Kłudkiewicz, B.; Sehnal, F. Glutathione-Ascorbic Acid Redox Cycle and Thioredoxin Reductase Activity in the Digestive Tract of Leptinotarsa Decemlineata (Say). Insect Biochem. Mol. Biol. 2009, 39, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Yang, J.; Ji, S.; Li, T.; Gao, S.; Sun, C.; Xu, H. Cancer Therapy by Targeting Thioredoxin Reductase Based on Selenium-Containing Dynamic Covalent Bond. CCS Chem. 2020, 2, 225–235. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Zheng, Q.-S.; Li, G.; Guo, D.-A.; Wang, Z.-R. Mechanism of Ascorbic Acid-Induced Reversion Against Malignant Phenotype in Human Gastric Cancer Cells. Biomed. Environ. Sci. 2006, 19, 385–391. [Google Scholar] [PubMed]
- Han, S.S.; Kim, K.; Hahm, E.R.; Lee, S.J.; Surh, Y.J.; Park, H.K.; Kim, W.S.; Jung, C.W.; Lee, M.H.; Park, K.; et al. L-Ascorbic Acid Represses Constitutive Activation of NF-KappaB and COX-2 Expression in Human Acute Myeloid Leukemia, HL-60. J. Cell. Biochem. 2004, 93, 257–270. [Google Scholar] [CrossRef]
- Cárcamo, J.M.; Pedraza, A.; Bórquez-Ojeda, O.; Golde, D.W. Vitamin C Suppresses TNF Alpha-Induced NFκB Activation by Inhibiting IκB Alpha Phosphorylation. Biochemistry 2002, 41, 12995–13002. [Google Scholar] [CrossRef]
- Bowie, A.G.; O’Neill, L.A.J. Vitamin C Inhibits NF-κB Activation by TNF via the Activation of P38 Mitogen-Activated Protein Kinase. J. Immunol. 2000, 165, 7180–7188. [Google Scholar] [CrossRef]
- Peng, Y.; Kwok, K.H.H.; Yang, P.H.; Ng, S.S.M.; Liu, J.; Wong, O.G.; He, M.L.; Kung, H.F.; Lin, M.C.M. Ascorbic Acid Inhibits ROS Production, NF-κB Activation and Prevents Ethanol-Induced Growth Retardation and Microencephaly. Neuropharmacology 2005, 48, 426–434. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFκB System. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Kong, E.H.; Ma, S.Y.; Jeong, J.Y.; Kim, K.H. Effects of L-Ascorbic Acid on the Production of pro-Inflammatory and Anti-Inflammatory Cytokines in C57BL/6 Mouse Splenocytes. Kosin Med. J. 2015, 30, 41–49. [Google Scholar] [CrossRef]
- Schwager, J.; Schulze, J. Influence of Ascorbic Acid on the Response to Mitogens and Interleukin Production of Porcine Lymphocytes. Int. J. Vitam. Nutr. Res. 1997, 67, 10–16. [Google Scholar] [PubMed]
- Schwager, J.; Schulze, J. Modulation of Interleukin Production by Ascorbic Acid. Vet. Immunol. Immunopathol. 1998, 64, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Han, S.S.; Park, C.H.; Hahm, E.R.; Lee, S.J.; Park, H.K.; Lee, S.H.; Kim, W.S.; Jung, C.W.; Park, K.; et al. L-Ascorbic Acid Induces Apoptosis in Acute Myeloid Leukemia Cells via Hydrogen Peroxide-Mediated Mechanisms. Int. J. Biochem. Cell Biol. 2004, 36, 2180–2195. [Google Scholar] [CrossRef]
- Chen, X.Y.; Chen, Y.; Qu, C.J.; Pan, Z.H.; Qin, Y.; Zhang, X.; Liu, W.J.; Li, D.F.; Zheng, Q. Vitamin C Induces Human Melanoma A375 Cell Apoptosis via Bax- and Bcl-2-Mediated Mitochondrial Pathways. Oncol. Lett. 2019, 18, 3880–3886. [Google Scholar] [CrossRef] [PubMed]
- Packer, L.; Weber, S.U.; Rimbach, G. Molecular Aspects of Alpha-Tocotrienol Antioxidant Action and Cell Signalling. J. Nutr. 2001, 131, 369S–373S. [Google Scholar] [CrossRef]
- Beyer, R.E. The Role of Ascorbate in Antioxidant Protection of Biomembranes: Interaction with Vitamin E and Coenzyme Q. J. Bioenerg. Biomembr. 1994, 26, 349–358. [Google Scholar] [CrossRef]
- Nemati, M.H.; Shahir, M.H.; Harakinezhad, M.T.; Lotfalhian, H. Cold-Induced Ascites in Broilers: Effects of Vitamin C and Coenzyme Q10. Braz. J. Poult. Sci. 2017, 19, 537–544. [Google Scholar] [CrossRef]
- Shargorodsky, M.; Debby, O.; Matas, Z.; Zimlichman, R. Effect of Long-Term Treatment with Antioxidants (Vitamin C, Vitamin E, Coenzyme Q10 and Selenium) on Arterial Compliance, Humoral Factors and Inflammatory Markers in Patients with Multiple Cardiovascular Risk Factors. Nutr. Metab. 2010, 7, 55. [Google Scholar] [CrossRef]
- Gęgotek, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Synergistic Cytoprotective Effects of Rutin and Ascorbic Acid on the Proteomic Profile of 3D-Cultured Keratinocytes Exposed to UVA or UVB Radiation. Nutrients 2019, 11, 2672. [Google Scholar] [CrossRef]
- Li, W.; Wu, J.X.; Tu, Y.Y. Synergistic Effects of Tea Polyphenols and Ascorbic Acid on Human Lung Adenocarcinoma SPC-A-1 Cells. J. Zhejiang Univ. Sci. B 2010, 11, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Okonogi, S. Comparison and Combination Effects on Antioxidant Power of Curcumin with Gallic Acid, Ascorbic Acid, and Xanthone. Drug Discov. Ther. 2015, 9, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, G.; Horta, B.B.; Pimenta, D.C.; Augusto, O.; Netto, L.E.S. Reduction of 1-Cys Peroxiredoxins by Ascorbate Changes the Thiol-Specific Antioxidant Paradigm, Revealing Another Function of Vitamin C. Proc. Natl. Acad. Sci. USA 2007, 104, 4886–4891. [Google Scholar] [CrossRef] [PubMed]
- Shyamlal, B.R.K.; Yadav, L.; Tiwari, M.K.; Mathur, M.; Prikhodko, J.I.; Mashevskaya, I.V.; Yadav, D.K.; Chaudhary, S. Synthesis, Bioevaluation, Structure-Activity Relationship and Docking Studies of Natural Product Inspired (Z)-3-Benzylideneisobenzofuran-1(3H)-Ones as Highly Potent Antioxidants and Antiplatelet Agents. Sci. Rep. 2020, 10, 2307. [Google Scholar] [CrossRef]
- Šušković, B. Preparation of 6-Amino-6-Deoxy Derivatives of Ascorbic and Iso-Ascorbic Acid. Croat. Chem. Acta 1989, 62, 537–544. [Google Scholar] [CrossRef]
- Kralj, M.; Kojić-Prodić, B.; Banić, Z.; Grdiša, M.; Vela, V.; Šušković, E.; Pavelić, K. Synthesis, Structural Characterization and Cytotoxic Effect of 6-Amino-6-Deoxy-l-Ascorbic Acid Derivatives. Eur. J. Med. Chem. 1996, 31, 23–35. [Google Scholar] [CrossRef]
- Ruiz-Carmona, S.; Alvarez-Garcia, D.; Foloppe, N.; Garmendia-Doval, A.B.; Juhos, S.; Schmidtke, P.; Barril, X.; Hubbard, R.E.; Morley, S.D. RDock: A Fast, Versatile and Open Source Program for Docking Ligands to Proteins and Nucleic Acids. PLoS Comput. Biol. 2014, 10, e1003571. [Google Scholar] [CrossRef]
- Miletic, V.; Nikolic, P.; Kinkela, D. Structure-Based Molecular Docking in the Identification of Novel Inhibitors Targeting SARS-CoV-2 Main Protease. In Proceedings of the 2021 44th International Convention on Information, Communication and Electronic Technology (MIPRO), Opatija, Croatia, 27 September–1 October 2021; pp. 406–411. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple Ligand-Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Ferri, M.; Gianotti, A.; Tassoni, A. Optimisation of Assay Conditions for the Determination of Antioxidant Capacity and Polyphenols in Cereal Food Components. J. Food Compos. Anal. 2013, 30, 94–101. [Google Scholar] [CrossRef]
- Padh, H. Cellular Functions of Ascorbic Acid. Biochem. Cell Biol. 2011, 68, 1166–1173. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Yosipovitch, G. Skin PH: From Basic Science to Basic Skin Care. Acta Derm. Venereol. 2013, 93, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Baspinar, Y.; Borchert, H.H. Penetration and Release Studies of Positively and Negatively Charged Nanoemulsions—Is There a Benefit of the Positive Charge? Int. J. Pharm. 2012, 430, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Landfester, K.; Musyanovych, A.; Guy, R.H. Disposition of Charged Nanoparticles after Their Topical Application to the Skin. Ski. Pharmacol. Physiol. 2010, 23, 117–123. [Google Scholar] [CrossRef] [PubMed]
- EMBL-EBI. PDB 3mng Structure Summary Protein Data Bank in Europe (PDBe). Available online: https://www.ebi.ac.uk/pdbe/entry/pdb/3MNG (accessed on 31 October 2022).
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved Side-Chain Torsion Potentials for the Amber Ff99SB Protein Force Field. Proteins Struct. Funct. Bioinform. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Sousa Da Silva, A.W.; Vranken, W.F. ACPYPE—AnteChamber PYthon Parser InterfacE. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Bixon, M.; Lifson, S. Potential Functions and Conformations in Cycloalkanes. Tetrahedron 1967, 23, 769–784. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N⋅log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1998, 98, 10089–10092. [Google Scholar] [CrossRef]



| Ligand | Best Score | Aromatic Rings | Hb | Hb | Rotatable Bonds | MW | Bonds | Atoms |
|---|---|---|---|---|---|---|---|---|
| Acceptors | Donors | |||||||
| DTT | −26.335 | 0 | 2 | 2 | 0 | 152.228 | 10 | 10 |
| AA | −26.641 | 0 | 6 | 4 | 2 | 176.130 | 16 | 16 |
| D1 | −25.576 | 0 | 5 | 6 | 2 | 176.153 | 18 | 18 |
| D2 | −26.521 | 0 | 5 | 5 | 3 | 190.180 | 18 | 18 |
| Constituent | Mode | m/z Precursor Ion | frag/V | m/z Fragment Ions | CE/V |
|---|---|---|---|---|---|
| AA | - | 174.8 | 90 | 130.3 | 6 |
| 114.5 | 6 | ||||
| 86.4 | 18 | ||||
| D1 | + | 175.9 | 80 | 157.8 | 4 |
| 140.8 | 10 | ||||
| 56.1 | 14 | ||||
| D2 | + | 189.9 | 110 | 171.8 | 6 |
| 140.7 | 10 | ||||
| 70.1 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saftić Martinović, L.; Birkic, N.; Miletić, V.; Antolović, R.; Štanfel, D.; Wittine, K. Antioxidant Activity, Stability in Aqueous Medium and Molecular Docking/Dynamics Study of 6-Amino- and N-Methyl-6-amino-L-ascorbic Acid. Int. J. Mol. Sci. 2023, 24, 1410. https://doi.org/10.3390/ijms24021410
Saftić Martinović L, Birkic N, Miletić V, Antolović R, Štanfel D, Wittine K. Antioxidant Activity, Stability in Aqueous Medium and Molecular Docking/Dynamics Study of 6-Amino- and N-Methyl-6-amino-L-ascorbic Acid. International Journal of Molecular Sciences. 2023; 24(2):1410. https://doi.org/10.3390/ijms24021410
Chicago/Turabian StyleSaftić Martinović, Lara, Nada Birkic, Vedran Miletić, Roberto Antolović, Danijela Štanfel, and Karlo Wittine. 2023. "Antioxidant Activity, Stability in Aqueous Medium and Molecular Docking/Dynamics Study of 6-Amino- and N-Methyl-6-amino-L-ascorbic Acid" International Journal of Molecular Sciences 24, no. 2: 1410. https://doi.org/10.3390/ijms24021410
APA StyleSaftić Martinović, L., Birkic, N., Miletić, V., Antolović, R., Štanfel, D., & Wittine, K. (2023). Antioxidant Activity, Stability in Aqueous Medium and Molecular Docking/Dynamics Study of 6-Amino- and N-Methyl-6-amino-L-ascorbic Acid. International Journal of Molecular Sciences, 24(2), 1410. https://doi.org/10.3390/ijms24021410








