Inhibitory Mechanism of IL-6 Production by Orento in Oral Squamous Cell Carcinoma Cell Line CAL27 Stimulated by Pathogen-Associated Molecular Patterns from Periodontopathogenic Porphyromonas gingivalis
Abstract
1. Introduction
2. Results
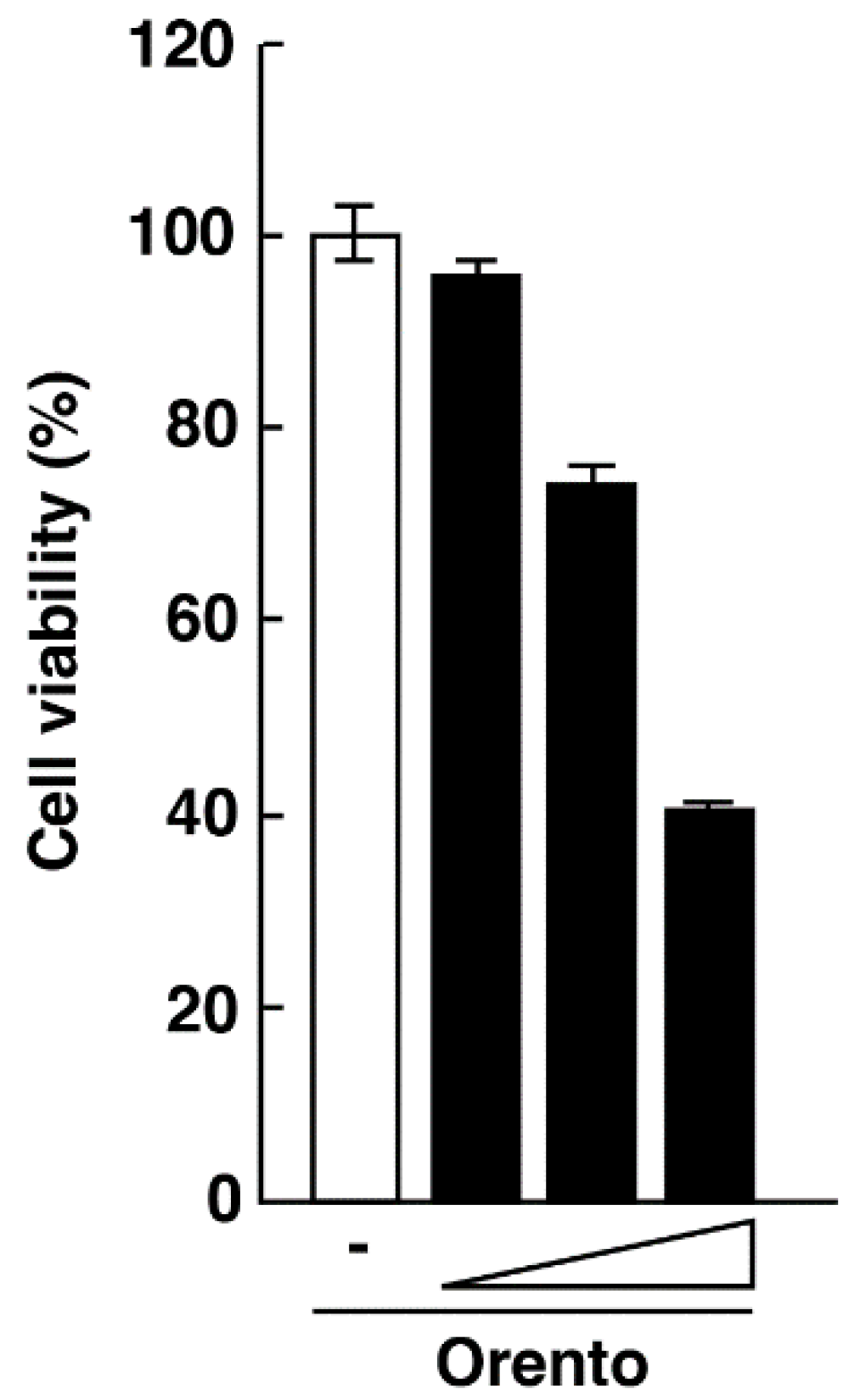
2.1. Effects of Orento on Cell Survival
2.2. Effects of Orento on PAMP-Stimulated IL-6 Production and Activation of Its Promoter in CAL27 Cells
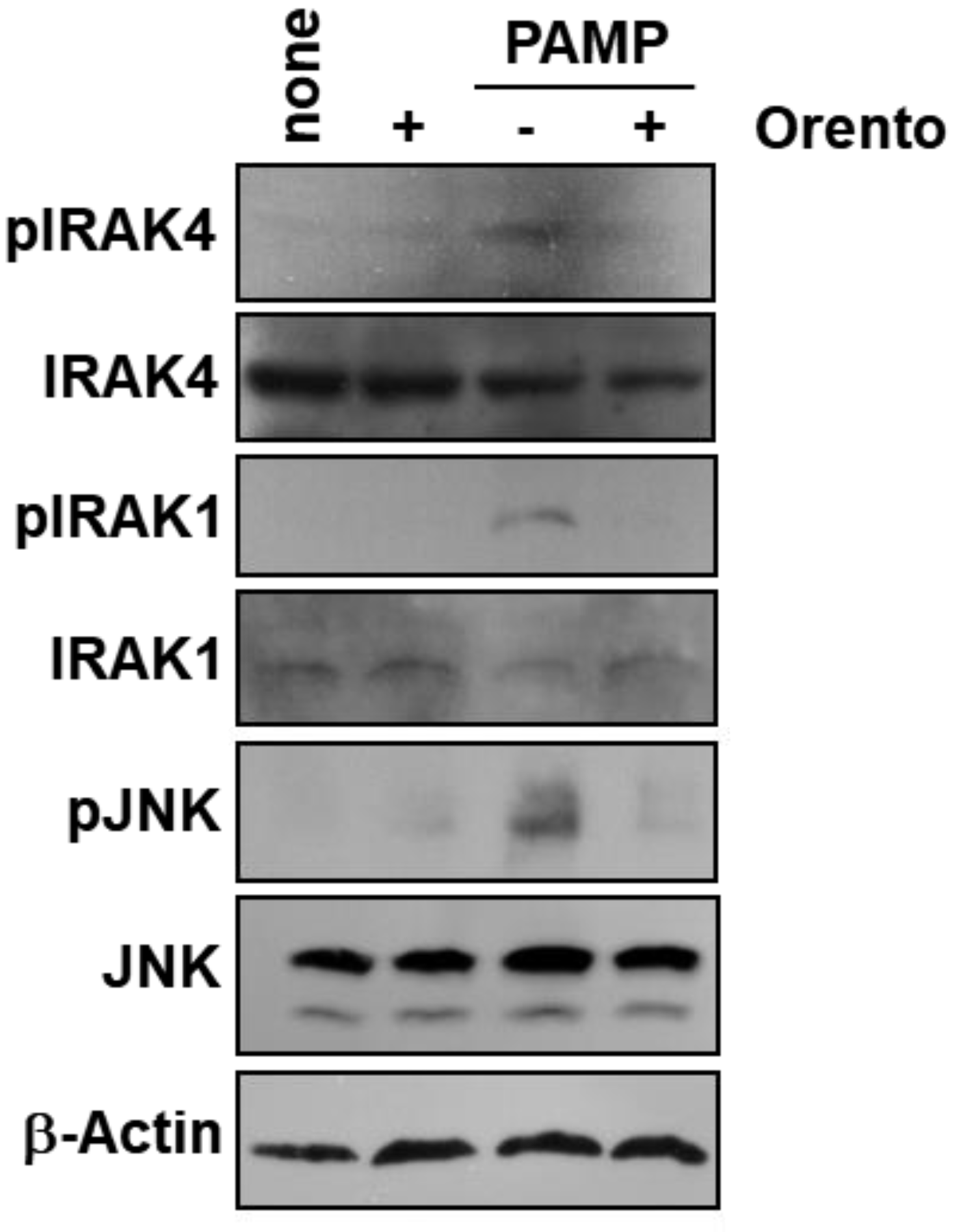
2.3. Effect of Orento on Protein Phosphorylation involved in PAMP-Mediated Signal Transduction Cascades
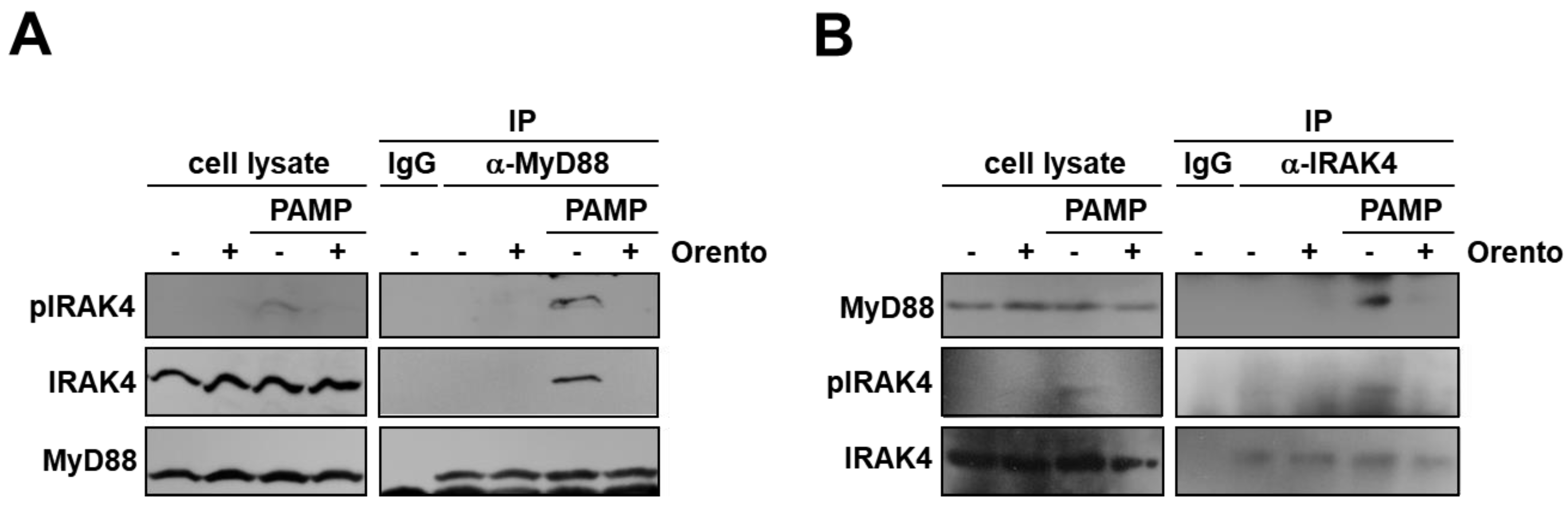
2.4. Inhibitory Effect of Orento on Binding between MyD88 and IRAK4
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents
4.3. Plasmid Construction
4.4. MTT Assay
4.5. Enzyme-Linked Immunosorbent Assays (ELISAs)
4.6. Transfection and Luciferase Assay
4.7. Western Blotting and Immunoprecipitation
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.-L.; Kaneko, A. Introduction to Kampo medicine for dental treatment—Oral pharmacotherapy that utilizes the advantages of Western and Kampo medicines. Jpn. Dent. Sci. Rev. 2018, 54, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Tsumura & Co. Tsumura Orento Extract Granules for Ethical Use, 4th ed.; Tsumura & Co.: Tokyo, Japan, 2007. [Google Scholar]
- Momota, Y.; Takano, H.; Azuma, M. Effectiveness of Kampo Medicines Against Intractable Stomatitis: A Mini-Review. Int. J. Med. Dent. Sci. 2019, 8, 1709–1714. [Google Scholar] [CrossRef]
- Nwizu, N.; Wactawski-Wende, J.; Genco, R.J. Periodontal disease and cancer: Epidemiologic studies and possible mechanisms. Periodontology 2020, 83, 213–233. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, D.; Inquimbert, C.; Ottolenghi, L.; Carrouel, F. Periodontal Pathogens as Risk Factors of Cardiovascular Diseases, Diabetes, Rheumatoid Arthritis, Cancer, and Chronic Obstructive Pulmonary Disease—Is There Cause for Consideration? Microorganisms 2019, 7, 424. [Google Scholar] [CrossRef]
- Costalonga, M.; Herzberg, M.C. The oral microbiome and the immunobiology of periodontal disease and caries. Immunol. Lett. 2014, 162, 22–38. [Google Scholar] [CrossRef]
- Kerr, J.E.; Abramian, J.R.; Dao, D.-H.V.; Rigney, T.W.; Fritz, J.; Pham, T.; Gay, I.; Parthasarathy, K.; Wang, B.-Y.; Zhang, W.; et al. Genetic Exchange of Fimbrial Alleles Exemplifies the Adaptive Virulence Strategy of Porphyromonas gingivalis. PLoS ONE 2014, 9, e91696. [Google Scholar] [CrossRef][Green Version]
- Katz, J.N.; Onate, M.D.; Pauley, K.M.; Bhattacharyya, I.; Cha, S. Presence of Porphyromonas gingivalis in gingival squamous cell carcinoma. Int. J. Oral Sci. 2011, 3, 209–215. [Google Scholar] [CrossRef]
- Oh, H.; Masuno, K.; Okusa, N.; Makita, Y.; Fujiwara, S.-I.; Imamura, Y. Effect of Orento, a Traditional Japanese Medicine, on IL-6, IL-8 Secretion, Type 1 Collagen Production and Alkaline Phosphatase Secretion in the Human Osteosarcoma Cell Line Saos-2. Medicines 2020, 7, 61. [Google Scholar] [CrossRef]
- Sharma, N.; Akhade, A.S.; Ismaeel, S.; Qadri, A. Serum-borne lipids amplify TLR-activated inflammatory responses. J. Leukoc. Biol. 2020, 109, 821–831. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2020, 33, 127–148. [Google Scholar] [CrossRef]
- Yao, Q.-W.; Zhou, D.-S.; Peng, H.-J.; Ji, P.; Liu, D.-S. Association of periodontal disease with oral cancer: A meta-analysis. Tumor Biol. 2014, 35, 7073–7077. [Google Scholar] [CrossRef] [PubMed]
- Gare, J.; Kanoute, A.; Meda, N.; Viennot, S.; Bourgeois, D.; Carrouel, F. Periodontal Conditions and Pathogens Associated with Pre-Eclampsia: A Scoping Review. Int. J. Environ. Res. Public Health 2021, 18, 7194. [Google Scholar] [CrossRef] [PubMed]
- Martinon, P.; Fraticelli, L.; Giboreau, A.; Dussart, C.; Bourgeois, D.; Carrouel, F. Nutrition as a Key Modifiable Factor for Periodontitis and Main Chronic Diseases. J. Clin. Med. 2021, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Nativel, B.; Couret, D.; Giraud, P.; Meilhac, O.; D’Hellencourt, C.L.; Viranaïcken, W.; Da Silva, C.R. Porphyromonas gingivalis lipopolysaccharides act exclusively through TLR4 with a resilience between mouse and human. Sci. Rep. 2017, 7, 15789. [Google Scholar] [CrossRef] [PubMed]
- Palani, C.D.; Ramanathapuram, L.; Lam-Ubol, A.; Kurago, Z.B. Toll-like receptor 2 induces adenosine receptor A2a and promotes human squamous carcinoma cell growth via extracellular signal regulated kinases 1/2. Oncotarget 2018, 9, 6814–6829. [Google Scholar] [CrossRef]
- Snyder, M.L.; Snyder, G.A. Cobbling Together the Myddosome. Structure 2020, 28, 598–600. [Google Scholar] [CrossRef]
- Deliz-Aguirre, R.; Cao, F.; Gerpott, F.H.U.; Auevechanichkul, N.; Chupanova, M.; Mun, Y.; Ziska, E.; Taylor, M.J. MyD88 oligomer size functions as a physical threshold to trigger IL1R Myddosome signaling. J. Cell Biol. 2021, 220, e202012071. [Google Scholar] [CrossRef]
- Ferrao, R.; Zhou, H.; Shan, Y.; Liu, Q.; Li, Q.; Shaw, D.E.; Li, X.; Wu, H. IRAK4 Dimerization and trans -Autophosphorylation Are Induced by Myddosome Assembly. Mol. Cell 2014, 55, 891–903. [Google Scholar] [CrossRef]
- Shang, L.; Deng, D.; Buskermolen, J.K.; Roffel, S.; Janus, M.M.; Krom, B.P.; Crielaard, W.; Gibbs, S. Commensal and Pathogenic Biofilms Alter Toll-Like Receptor Signaling in Reconstructed Human Gingiva. Front. Cell Infect. Microbiol. 2019, 9, 282. [Google Scholar] [CrossRef]
- Akhtar, M.; Guo, S.; Guo, Y.-F.; Zahoor, A.; Shaukat, A.; Chen, Y.; Umar, T.; Deng, P.G.; Guo, M. Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Trop. 2020, 207, 105458. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Saliasi, I.; Llodra, J.C.; Bravo, M.; Tramini, P.; Dussart, C.; Viennot, S.; Carrouel, F. Effect of a Toothpaste/Mouthwash Containing Carica papaya Leaf Extract on Interdental Gingival Bleeding: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2018, 15, 2660. [Google Scholar] [CrossRef] [PubMed]
- Mo, C.; Wang, L.; Zhang, J.; Numazawa, S.; Tang, H.; Tang, X.; Han, X.J.; Li, J.; Yang, M.; Wang, Z.; et al. The Crosstalk Between Nrf2 and AMPK Signal Pathways Is Important for the Anti-Inflammatory Effect of Berberine in LPS-Stimulated Macrophages and Endotoxin-Shocked Mice. Antioxid. Redox Signal. 2014, 20, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-F.; Zhao, T.-T.; Zhang, H.-J.; Huang, X.-R.; Zhang, W.-K.; Zhang, L.; Yan, M.-H.; Dong, X.; Wang, H.; Wen, Y.-M.; et al. Renoprotective effect of berberine on type 2 diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 662–670. [Google Scholar] [CrossRef]
- Imamura, Y.; Wang, P.-L.; Masuno, K.; Sogawa, N. Salivary protein histatin 3 regulates cell proliferation by enhancing p27 Kip1 and heat shock cognate protein 70 ubiquitination. Biochem. Biophys. Res. Commun. 2016, 470, 269–274. [Google Scholar] [CrossRef]
- Imamura, Y.; Wang, P.-L. Salivary histatin 3 inhibits heat shock cognate protein 70-mediated inflammatory cytokine production through toll-like receptors in human gingival fibroblasts. J. Inflamm. 2014, 11, 4. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, Y.; Makita, Y.; Masuno, K.; Oh, H. Inhibitory Mechanism of IL-6 Production by Orento in Oral Squamous Cell Carcinoma Cell Line CAL27 Stimulated by Pathogen-Associated Molecular Patterns from Periodontopathogenic Porphyromonas gingivalis. Int. J. Mol. Sci. 2023, 24, 697. https://doi.org/10.3390/ijms24010697
Imamura Y, Makita Y, Masuno K, Oh H. Inhibitory Mechanism of IL-6 Production by Orento in Oral Squamous Cell Carcinoma Cell Line CAL27 Stimulated by Pathogen-Associated Molecular Patterns from Periodontopathogenic Porphyromonas gingivalis. International Journal of Molecular Sciences. 2023; 24(1):697. https://doi.org/10.3390/ijms24010697
Chicago/Turabian StyleImamura, Yasuhiro, Yoshimasa Makita, Kazuya Masuno, and Hourei Oh. 2023. "Inhibitory Mechanism of IL-6 Production by Orento in Oral Squamous Cell Carcinoma Cell Line CAL27 Stimulated by Pathogen-Associated Molecular Patterns from Periodontopathogenic Porphyromonas gingivalis" International Journal of Molecular Sciences 24, no. 1: 697. https://doi.org/10.3390/ijms24010697
APA StyleImamura, Y., Makita, Y., Masuno, K., & Oh, H. (2023). Inhibitory Mechanism of IL-6 Production by Orento in Oral Squamous Cell Carcinoma Cell Line CAL27 Stimulated by Pathogen-Associated Molecular Patterns from Periodontopathogenic Porphyromonas gingivalis. International Journal of Molecular Sciences, 24(1), 697. https://doi.org/10.3390/ijms24010697






