Imidazo-Pyrazole-Loaded Palmitic Acid and Polystyrene-Based Nanoparticles: Synthesis, Characterization and Antiproliferative Activity on Chemo-Resistant Human Neuroblastoma Cells
Abstract
:1. Introduction
2. Results and Discussion
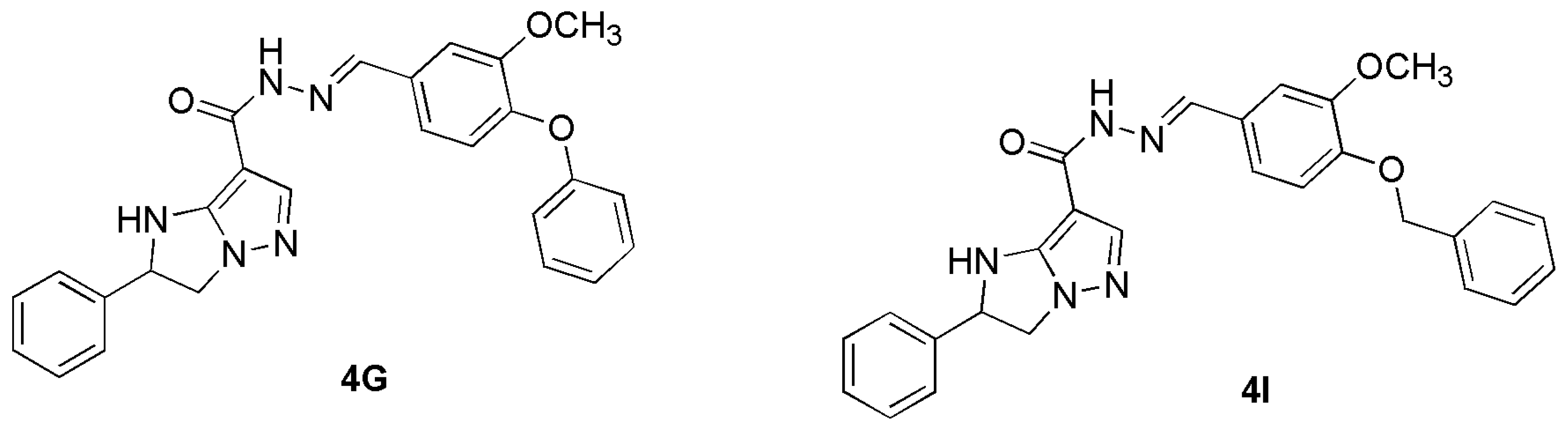
2.1. The Reasons of Our Choice
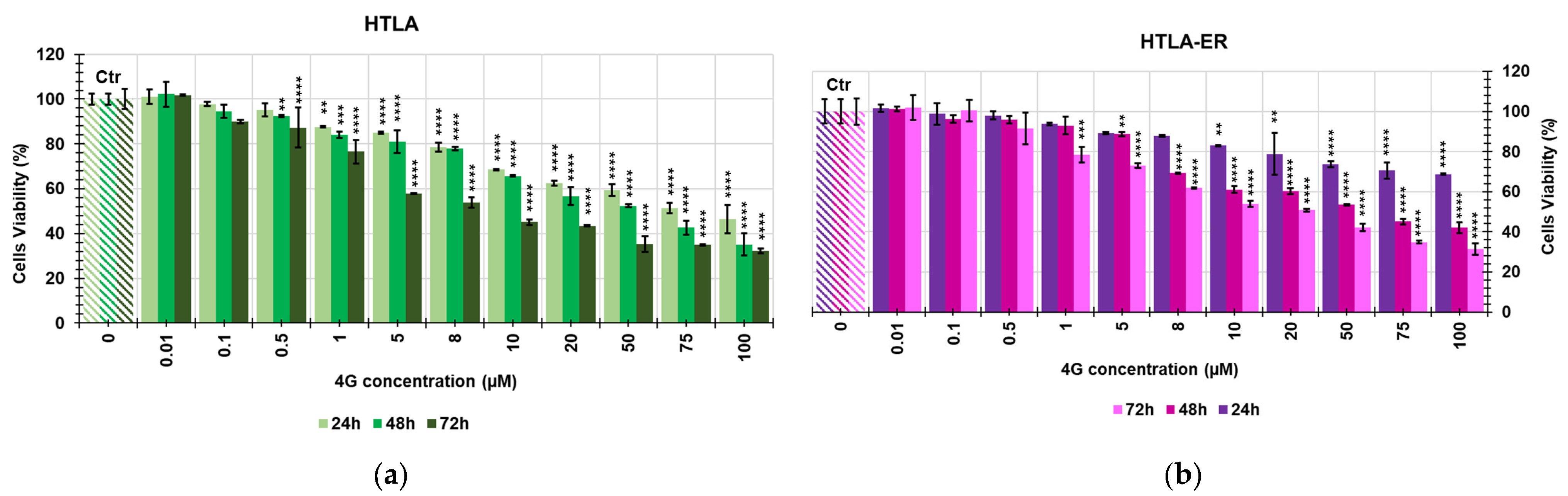
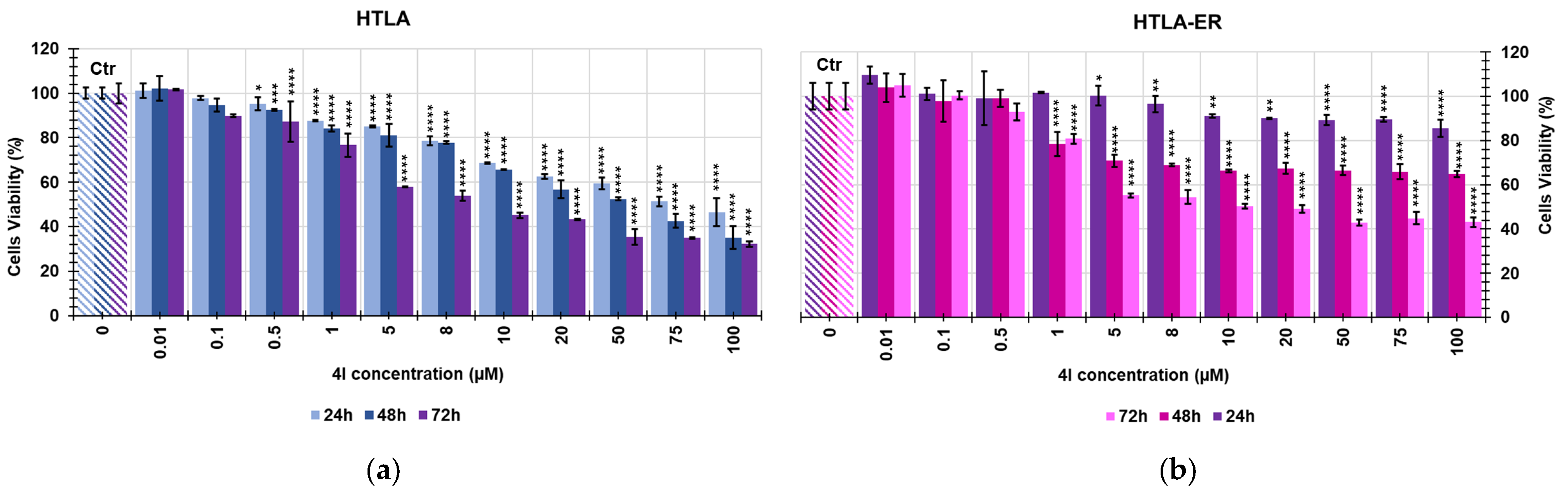
2.2. Screening of the Cytotoxic Effects of 4G and 4I on NB Cells
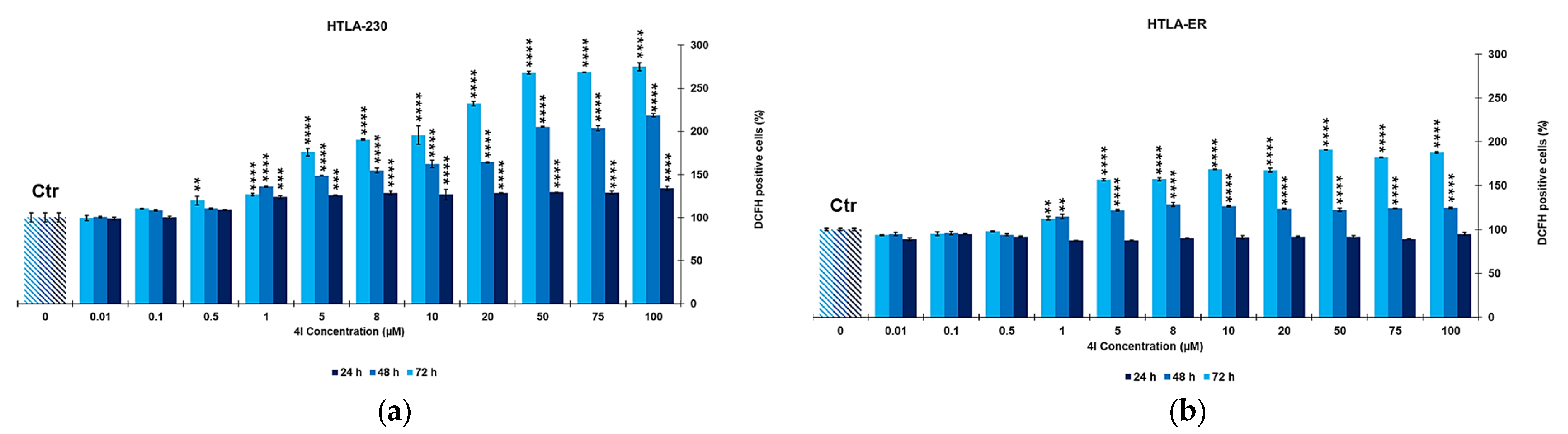
2.3. Dose Dependent Reactive Oxygen Species (ROS) Production in HTLA-230 and HTLA-ER Cells
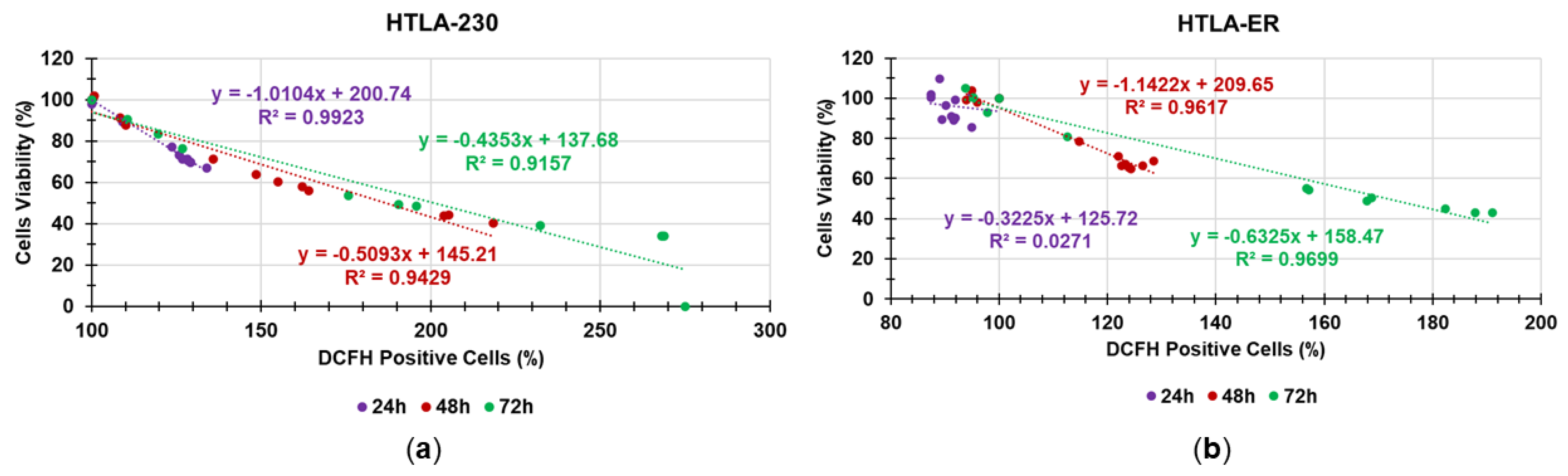
Correlation between ROS Production and Cytotoxic Effects of 4I
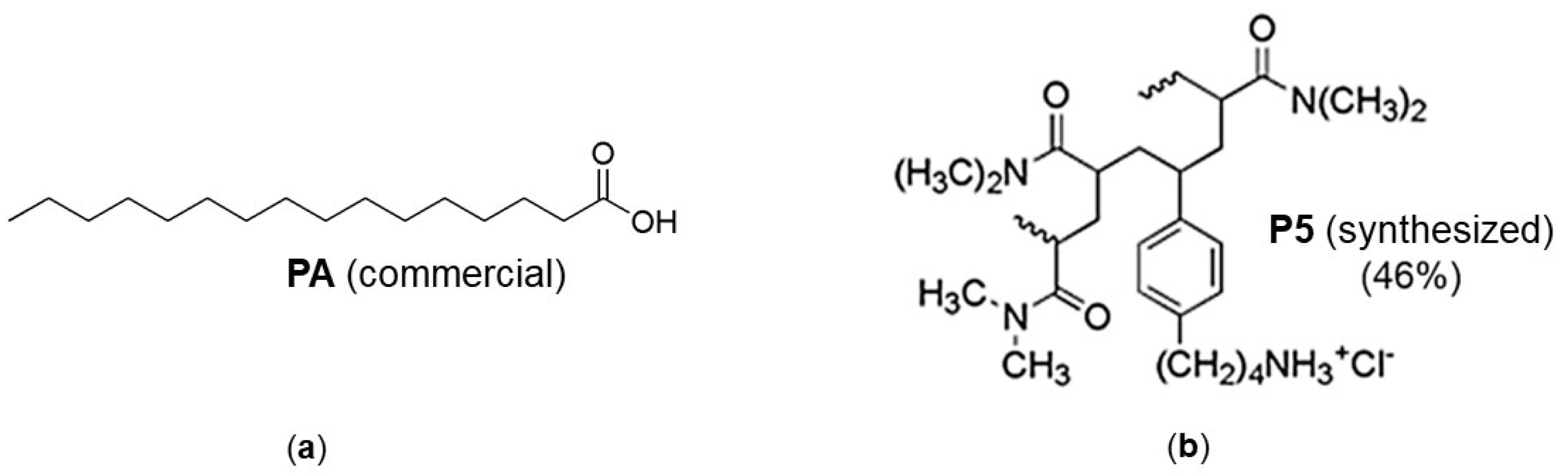
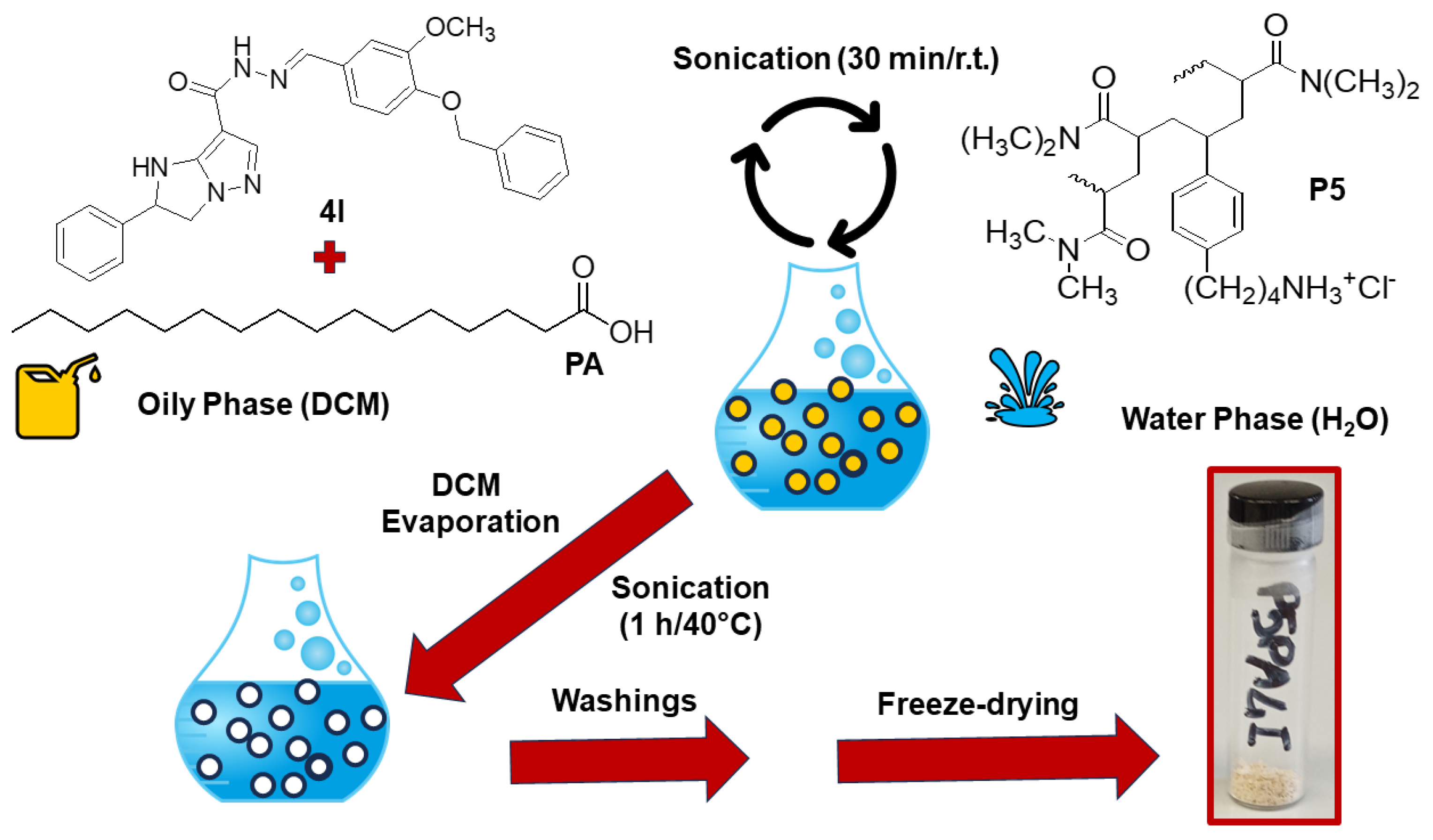
2.4. Preparation of P5PA and P5PA-4I Nanoparticles (NPs)
2.5. Particles Characterization
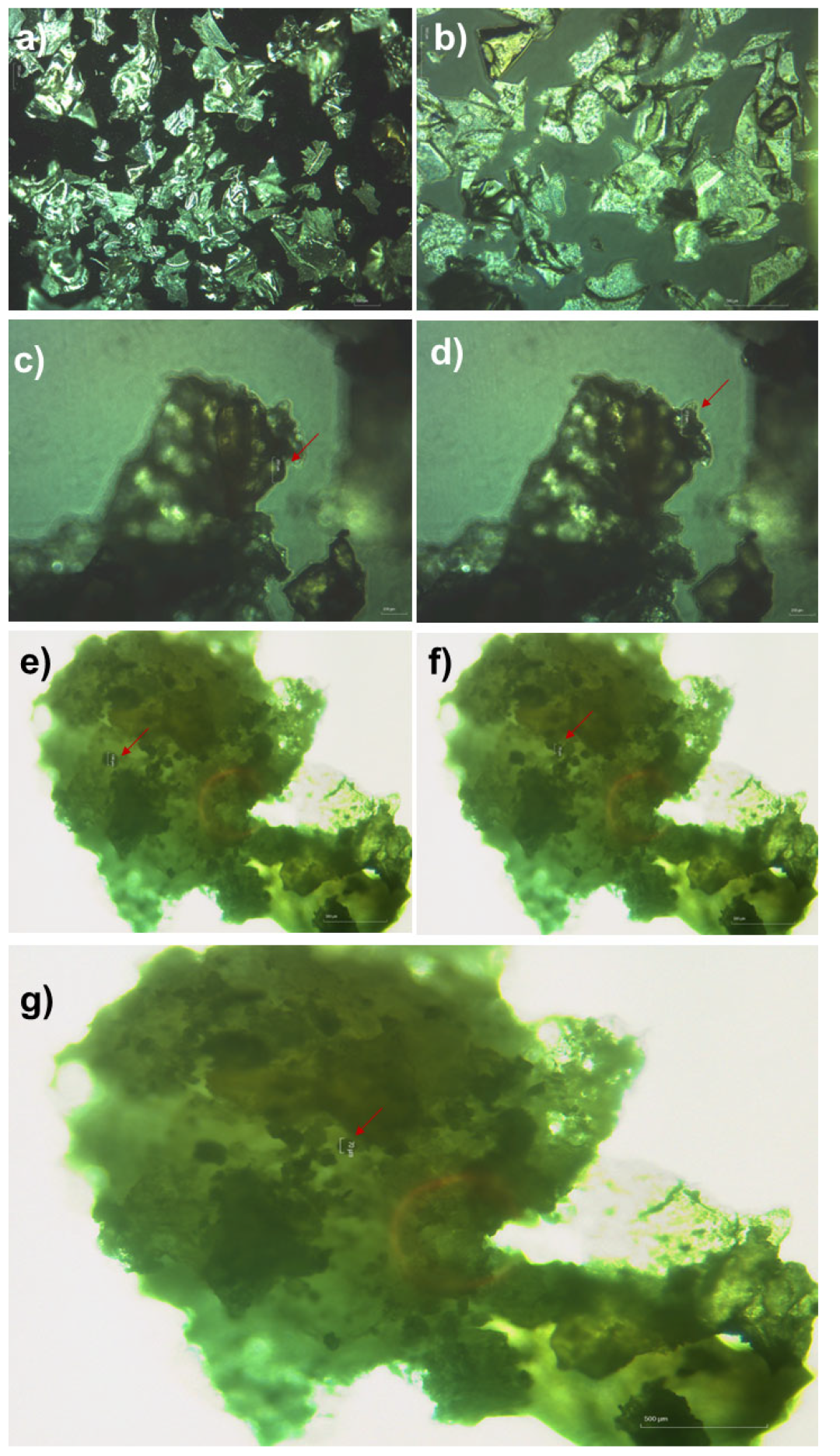
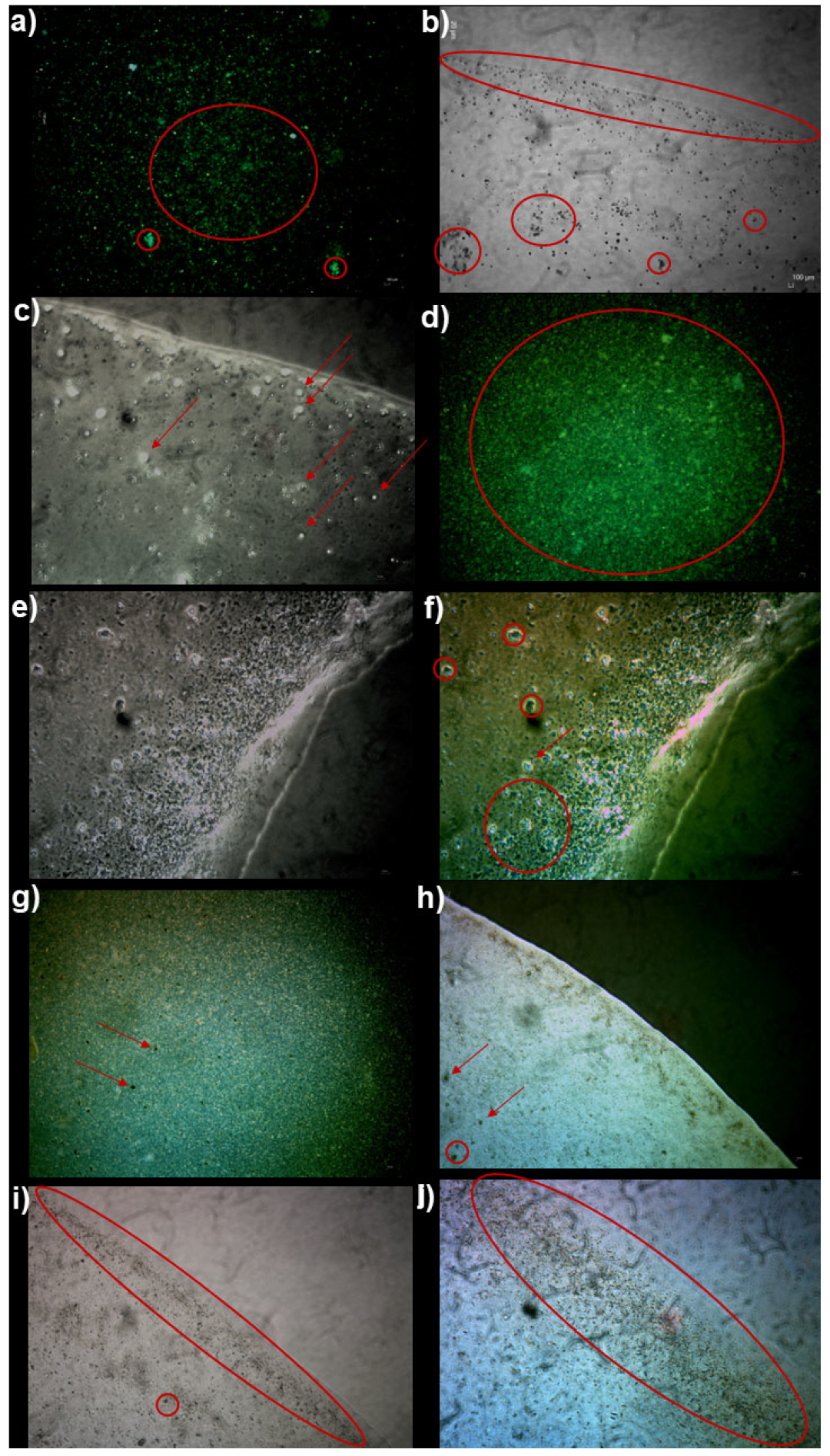
2.5.1. Morphology of P5PA and P5PA-4I by Optical Microscopy
2.5.2. Particle Size, Zeta Potential (ζ-p) and Polydispersity Index (PDI) of P5PA and P5PA-4 by DLS Analyses
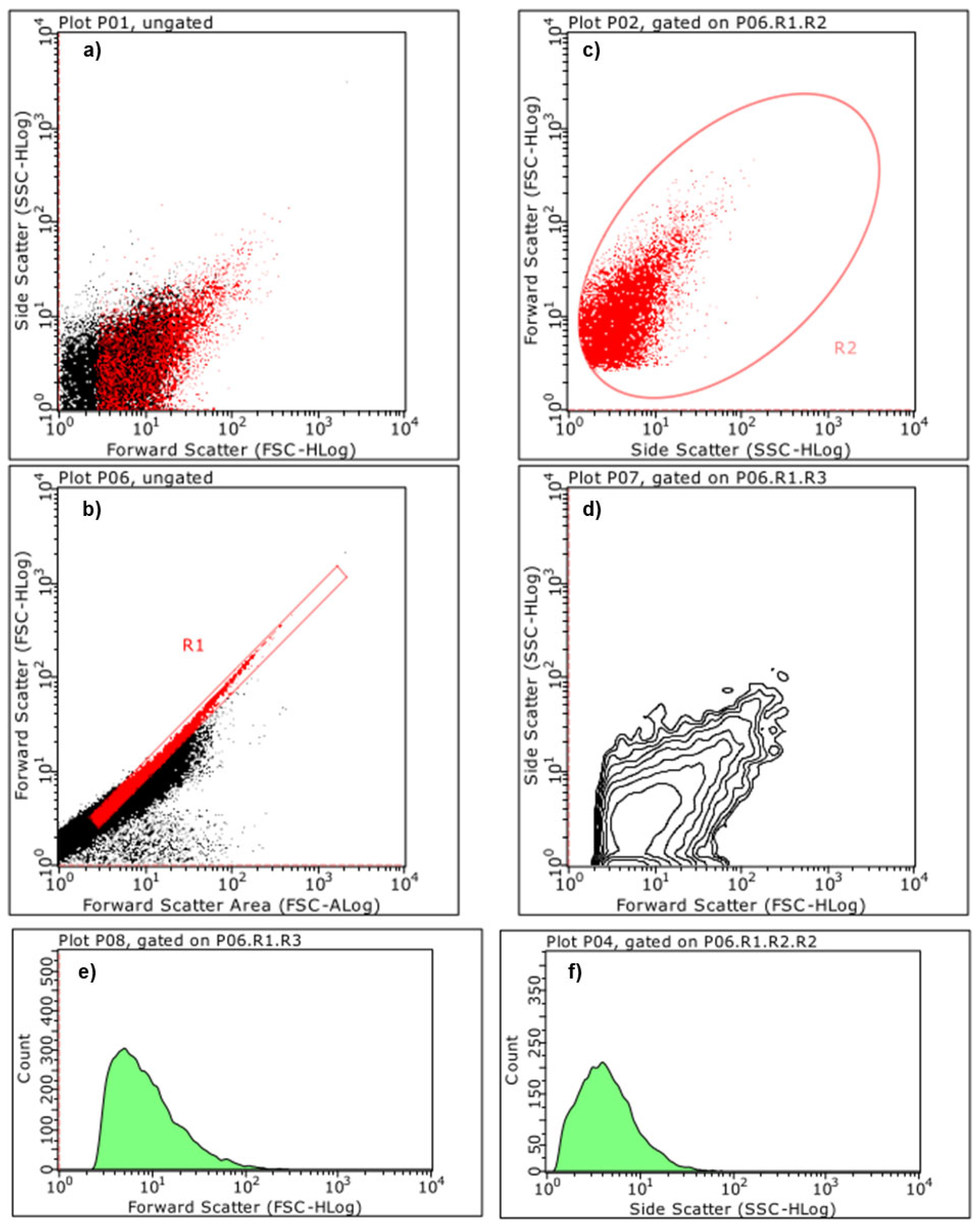
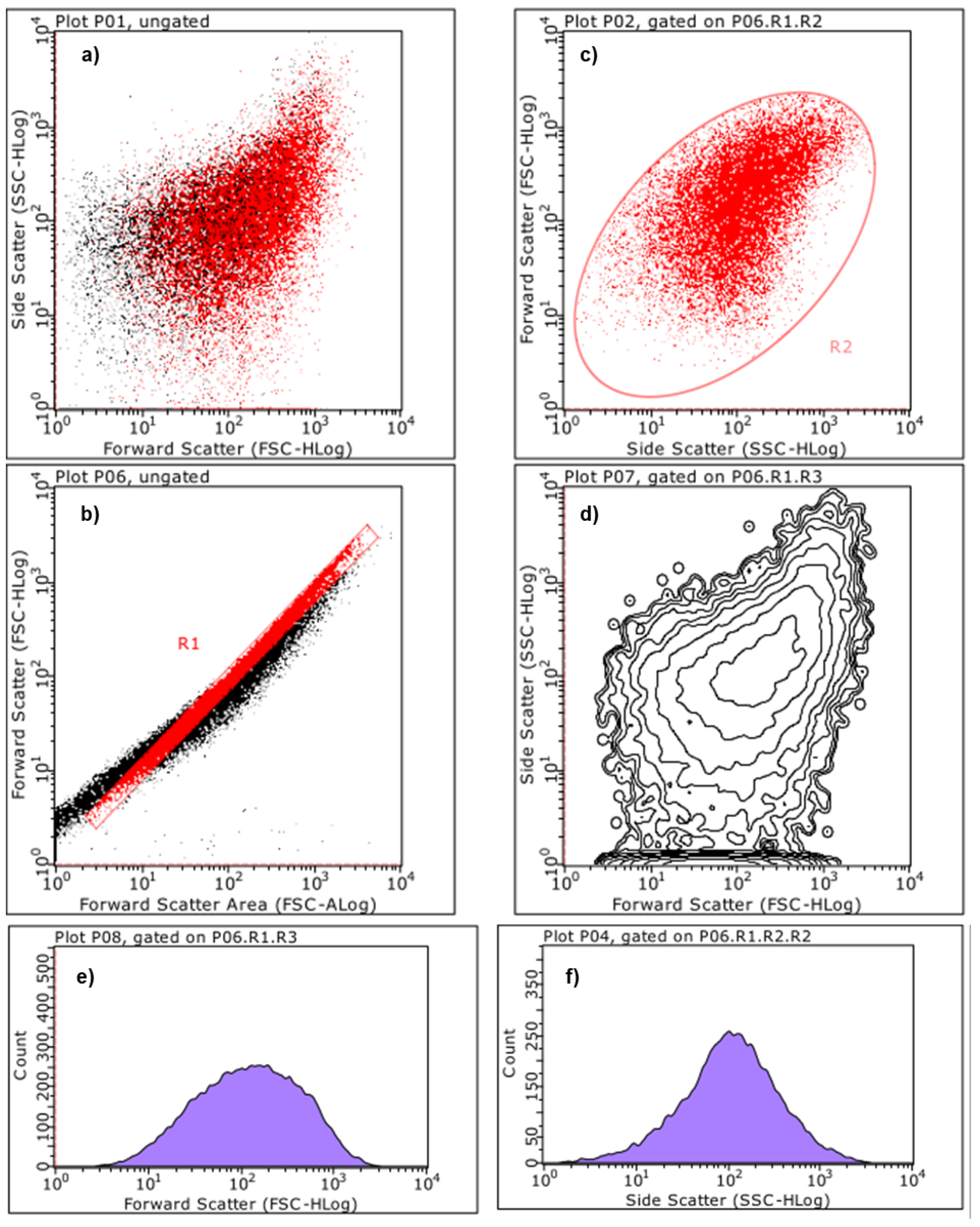
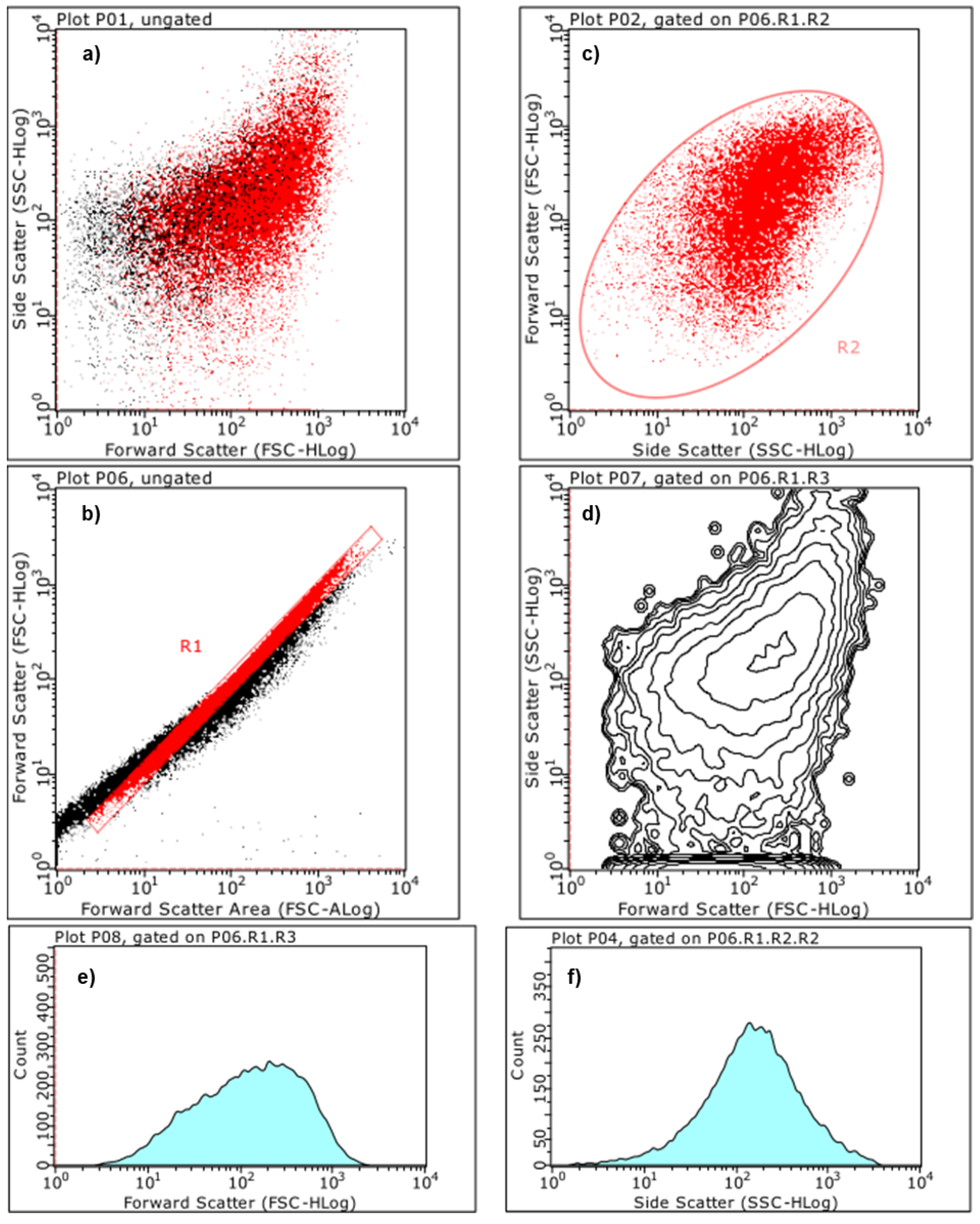
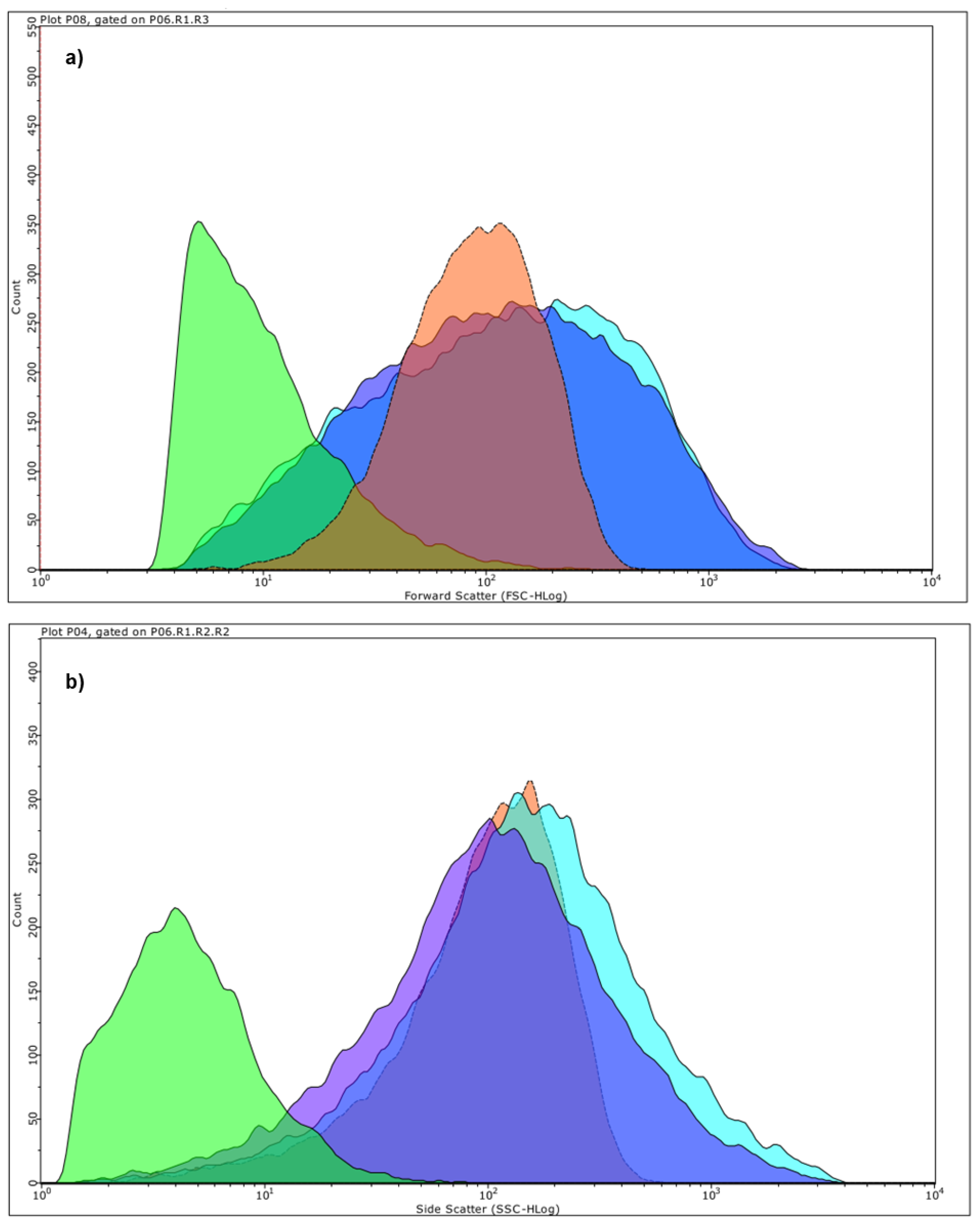
2.5.3. Estimation of Particles Size and Complexity Distributions by Cytofluorimetric Analyses
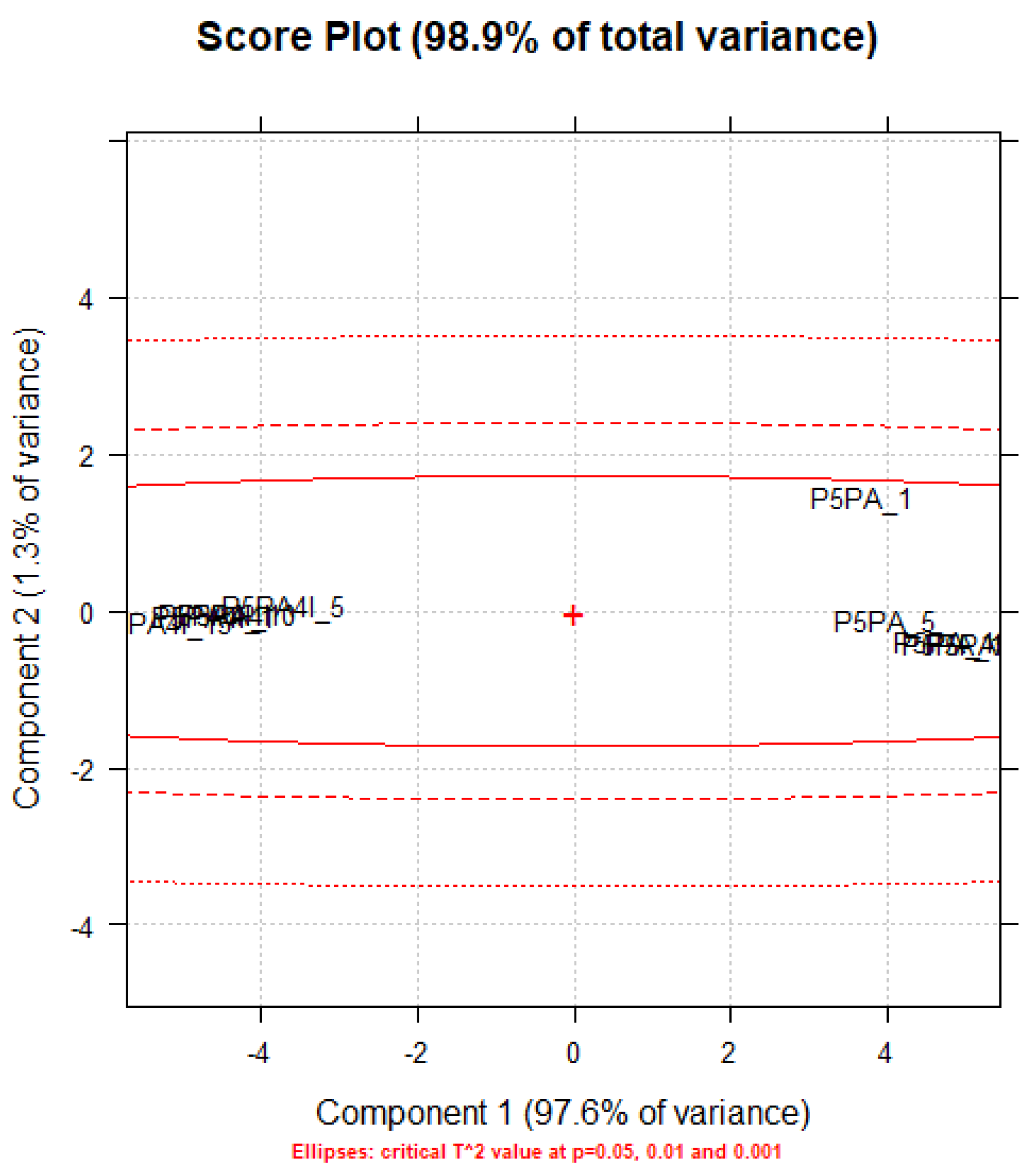
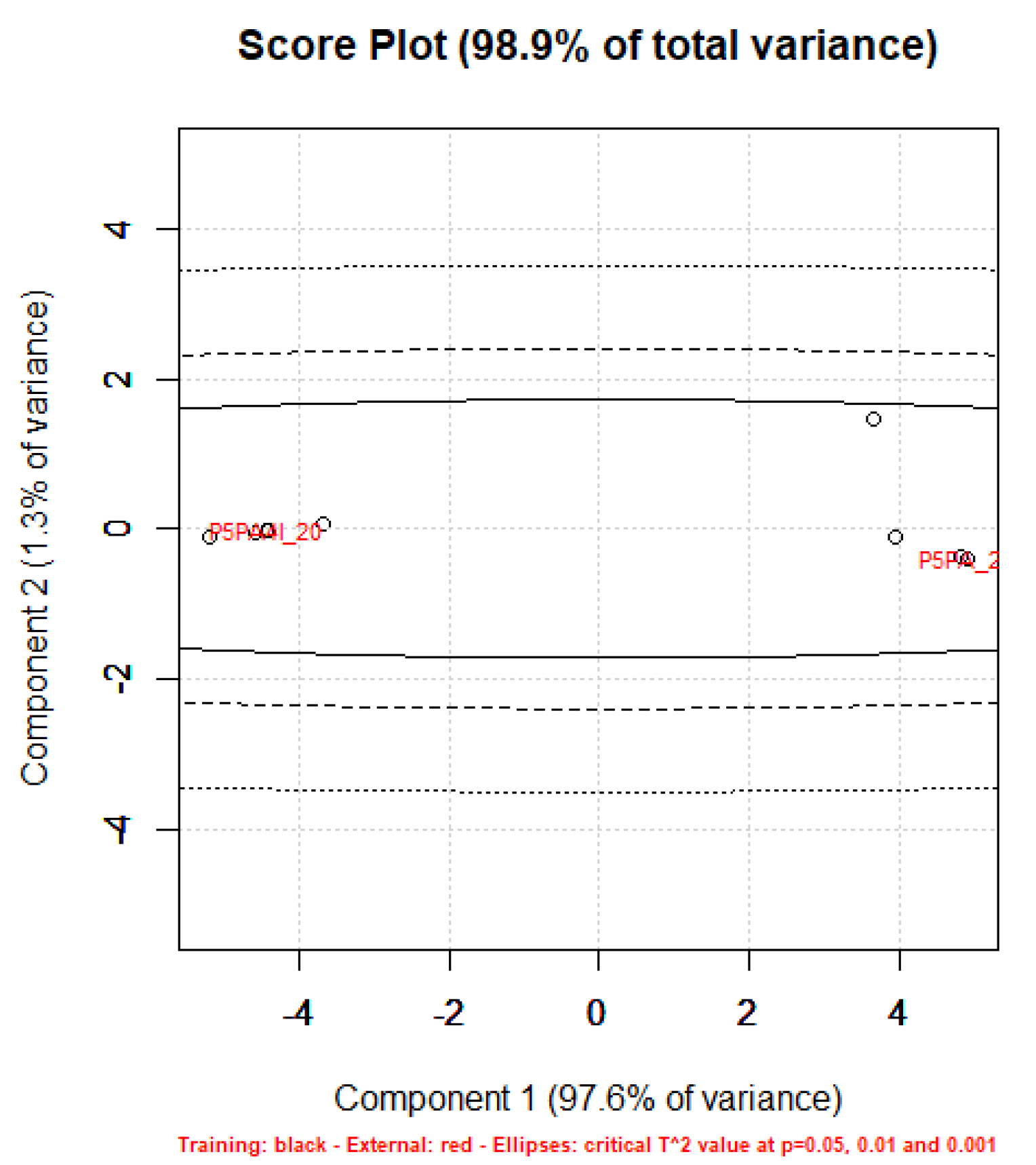
Principal Component Analysis (PCA) on Statistics by Flow Cytometry
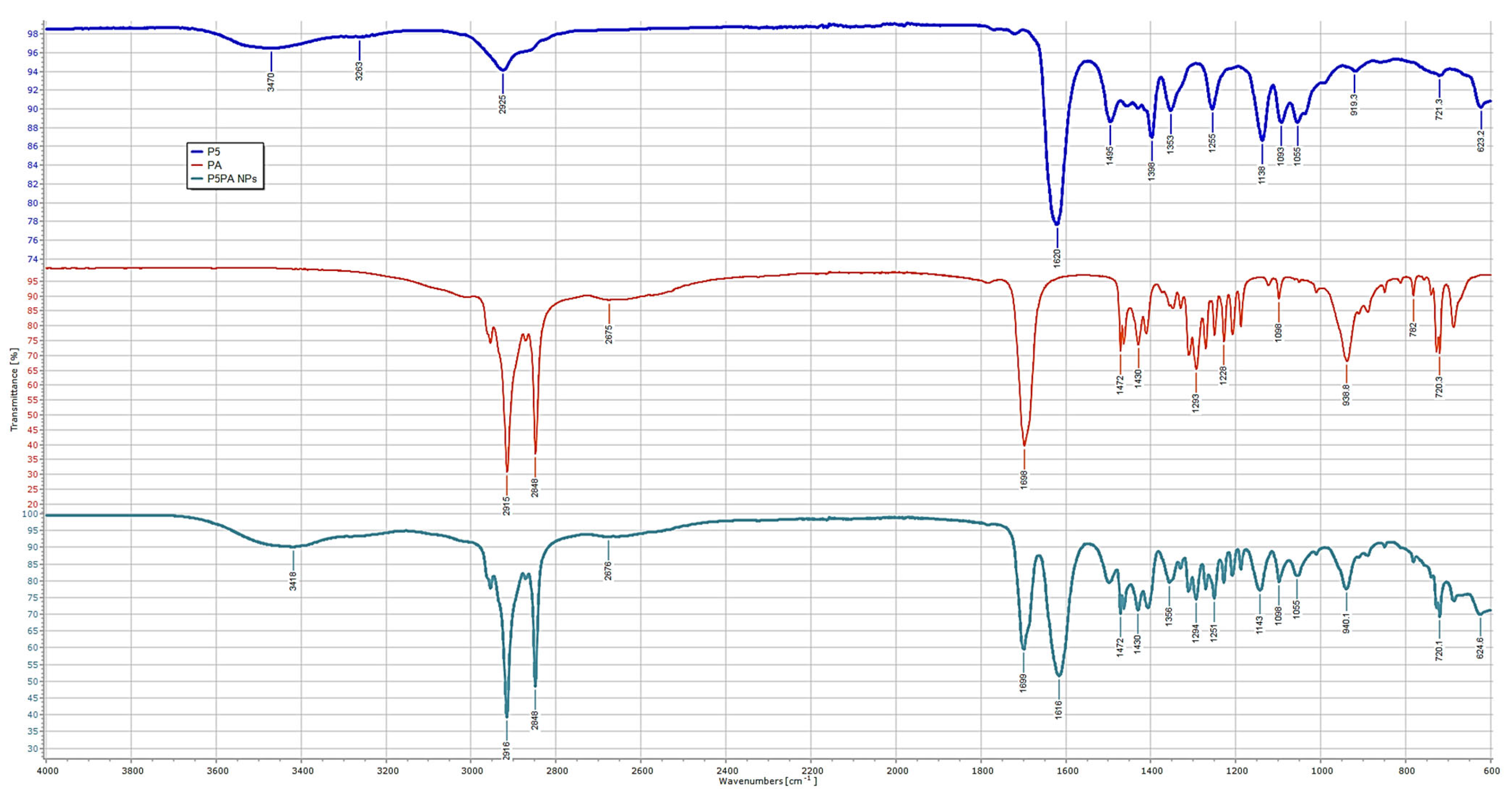
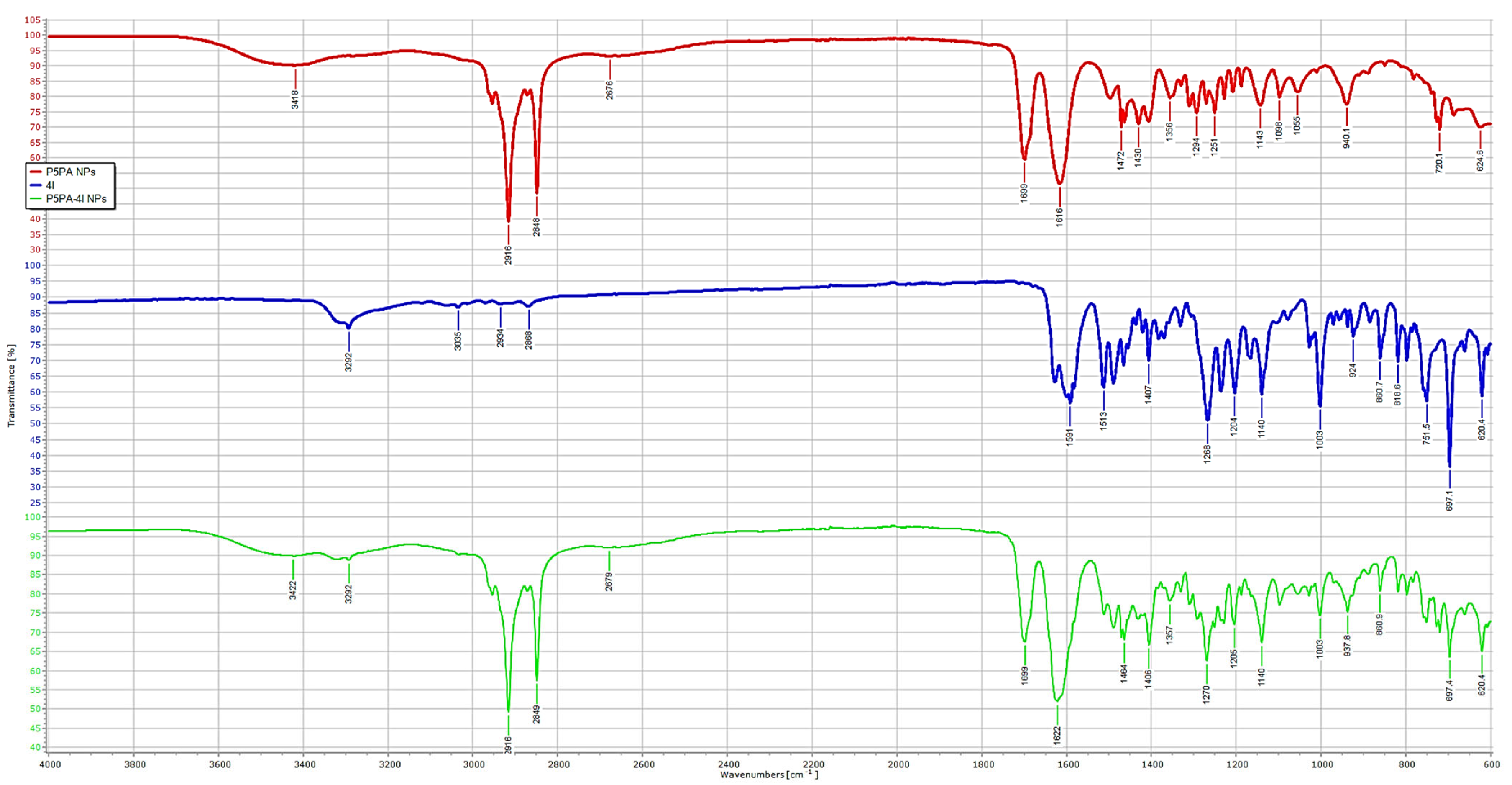
2.6. Chemometric Assisted ATR-FTIR Analyses
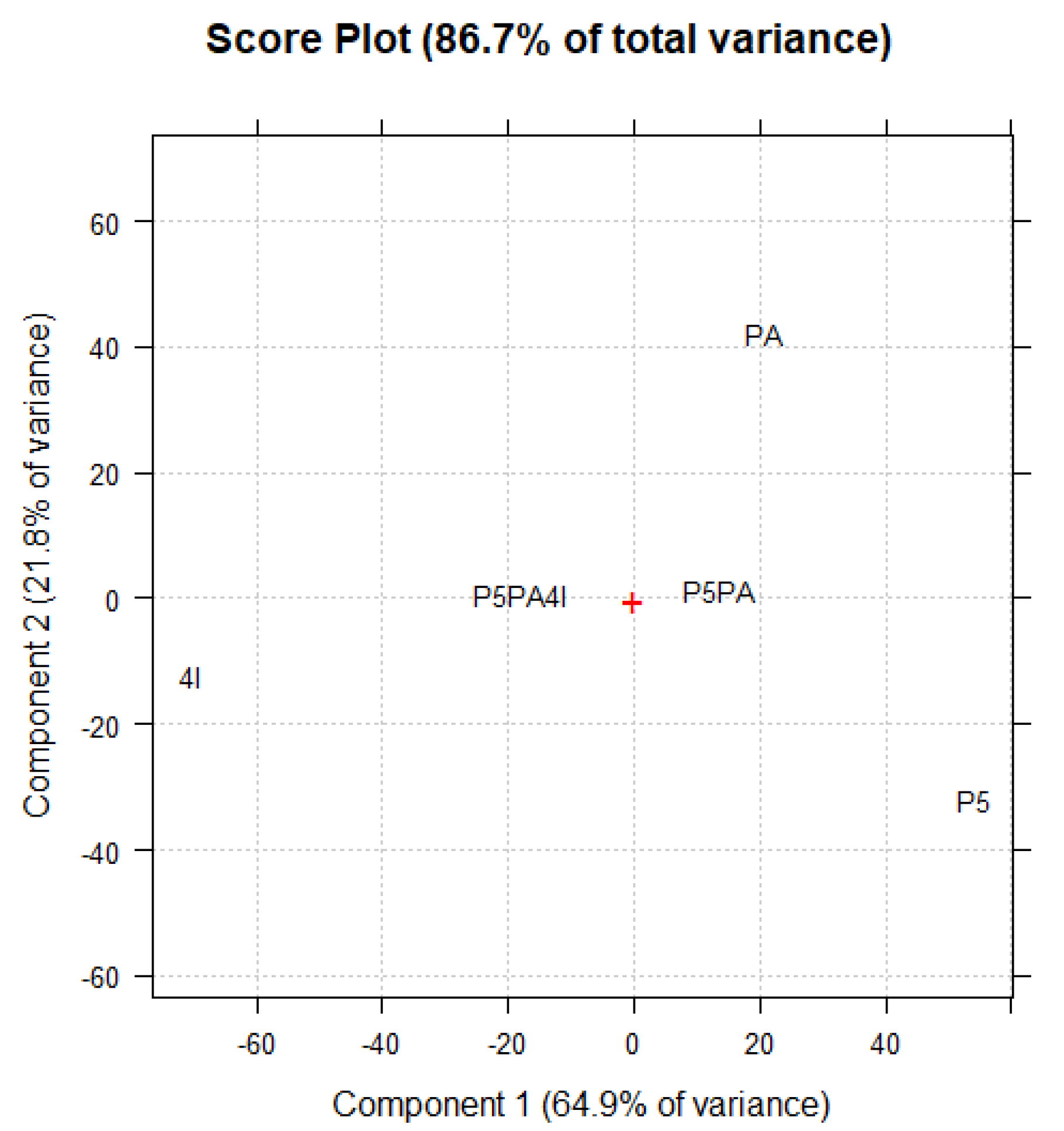
Principal Components Analysis (PCA) on the ATR-FTIR Data
2.7. Determination of the Exact Amount of 4I Loaded in P5PA-4I NPs
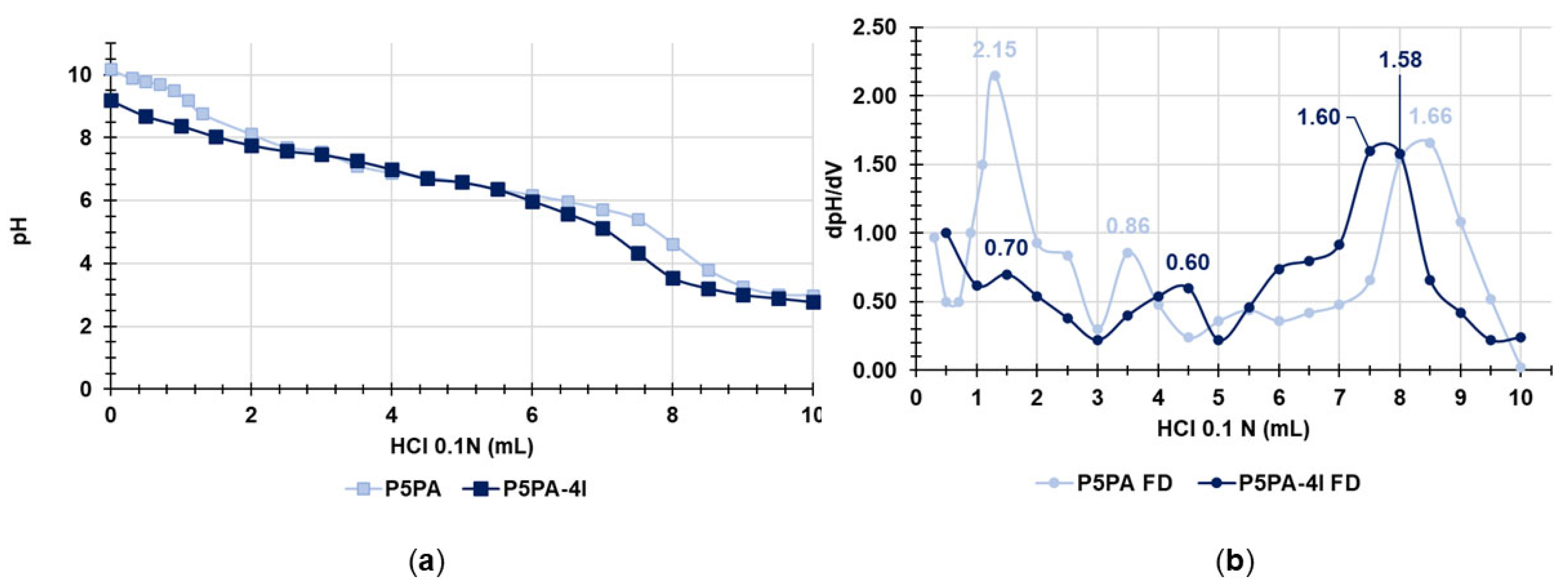
2.8. Potentiometric Titrations of P5PA and of P5PA-4I NPs
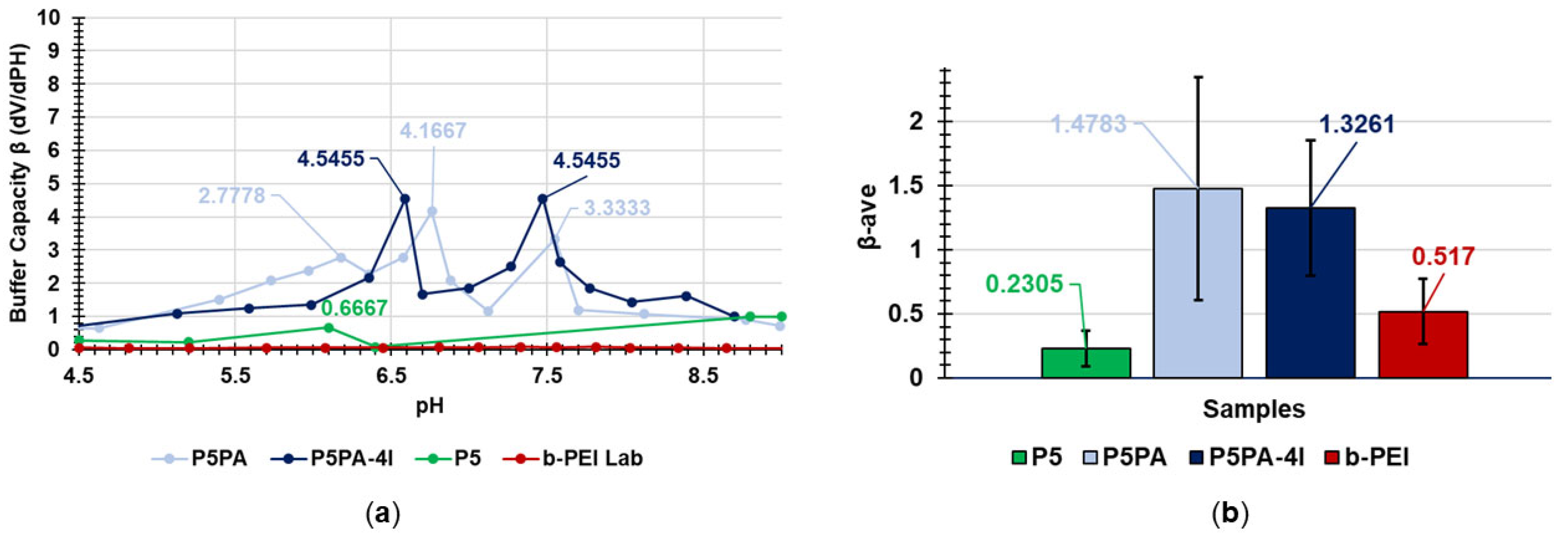
2.8.1. Buffer Capacity
2.8.2. Evaluation of the Chemical Stability of P5PA and P5PA-4I NPs Dispersions over Time
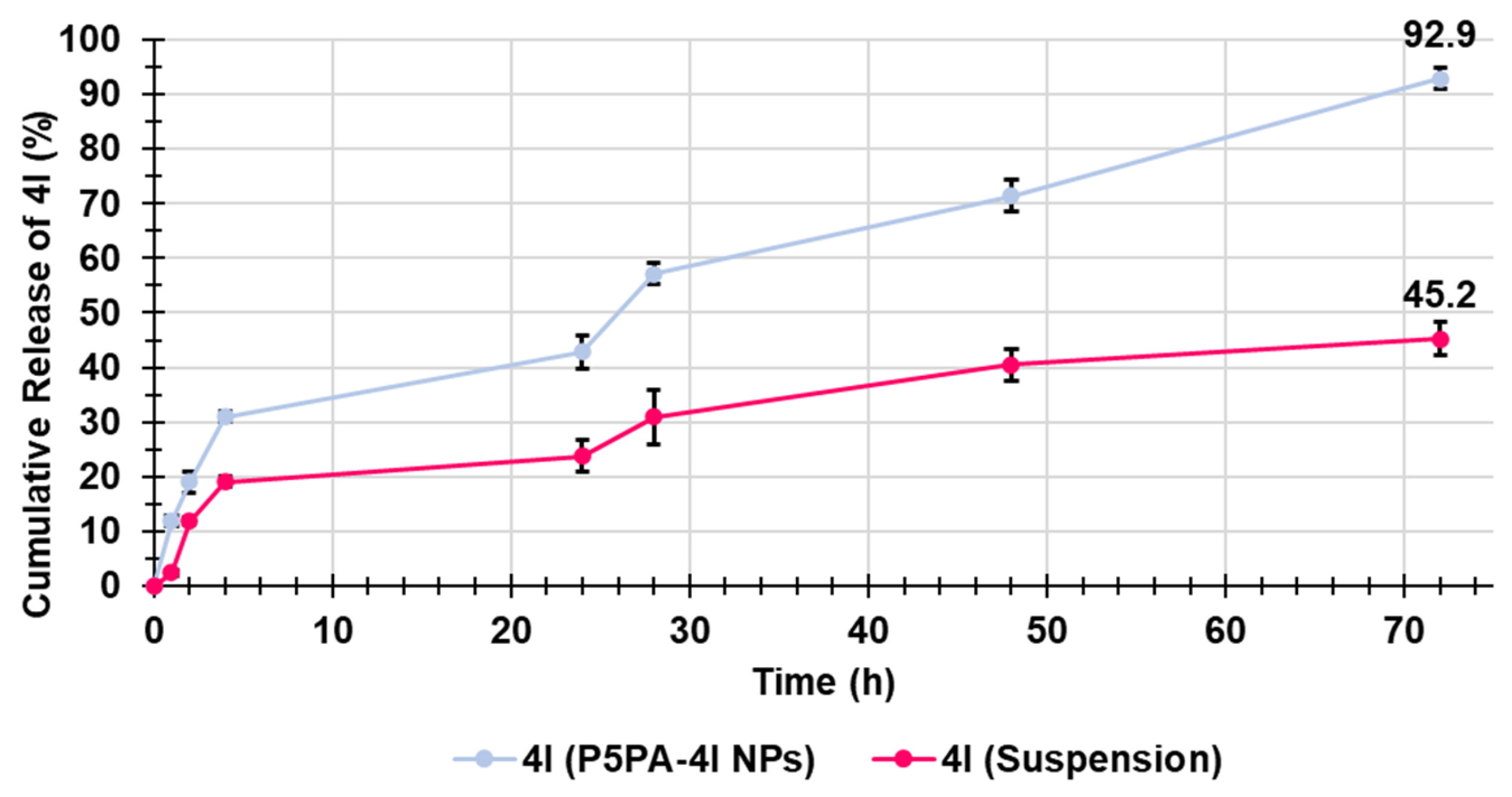
2.9. Evaluation of 4I In Vitro Release Profile
Kinetic Studies
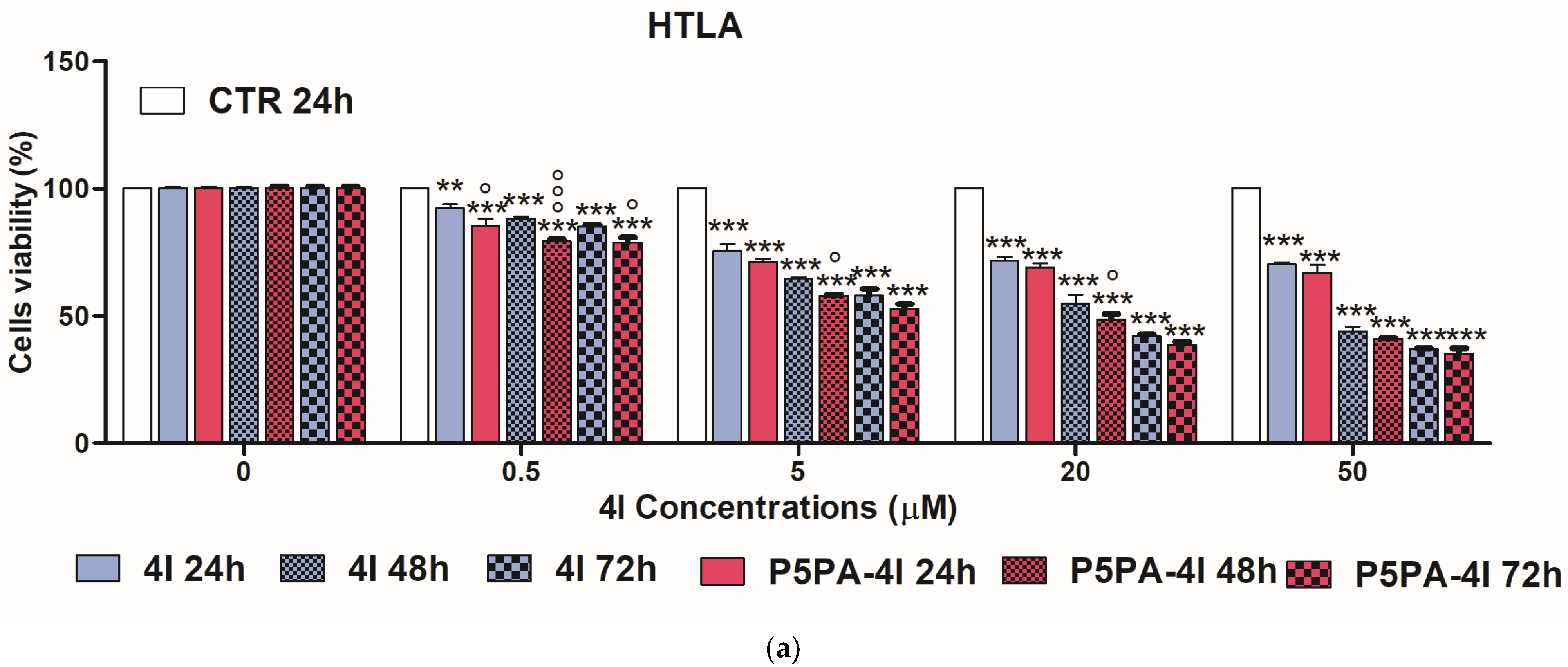
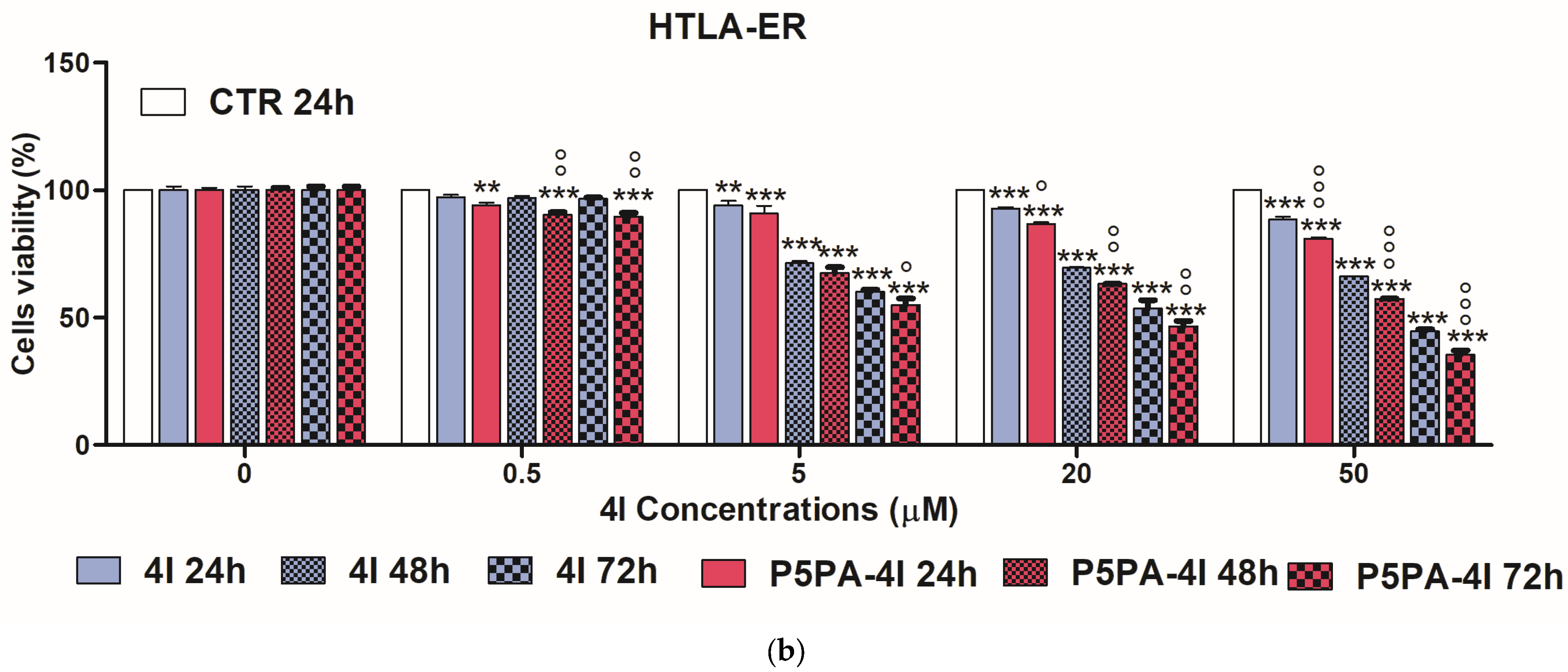
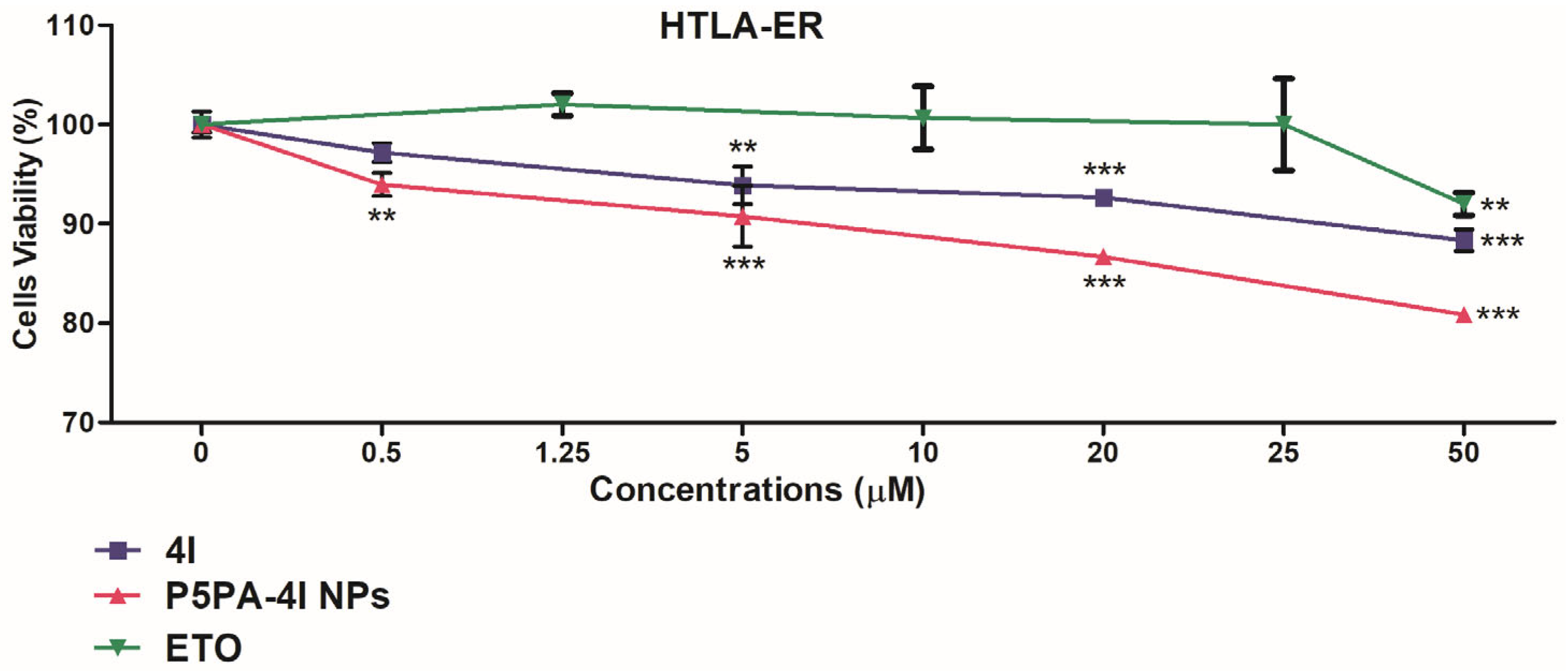
2.10. Cytotoxic Effects of 4I, P5PA and of P5PA-4I on NB Cells
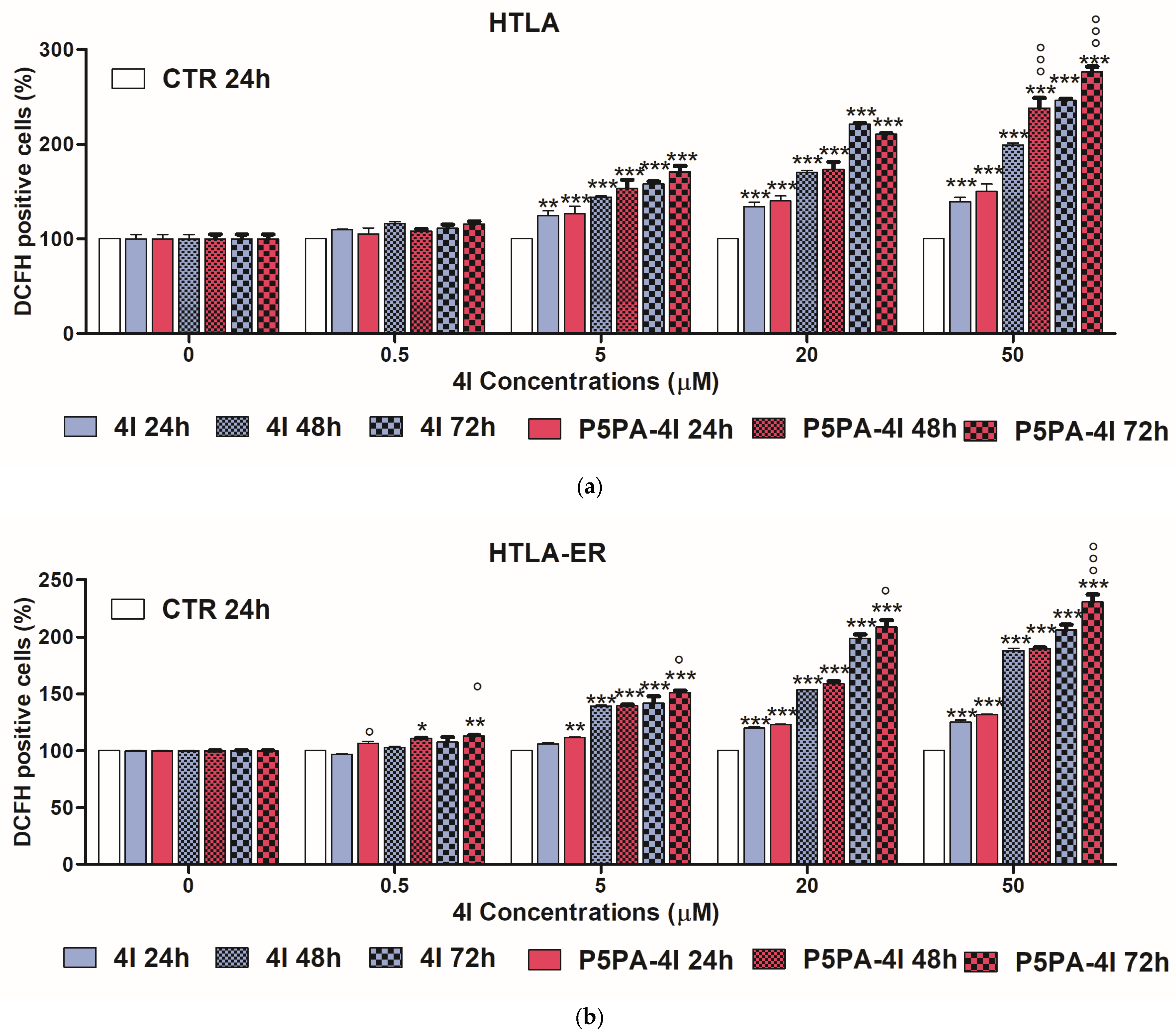
2.11. Effects of 4I and P5PA-4I on ROS Production in NB Cells
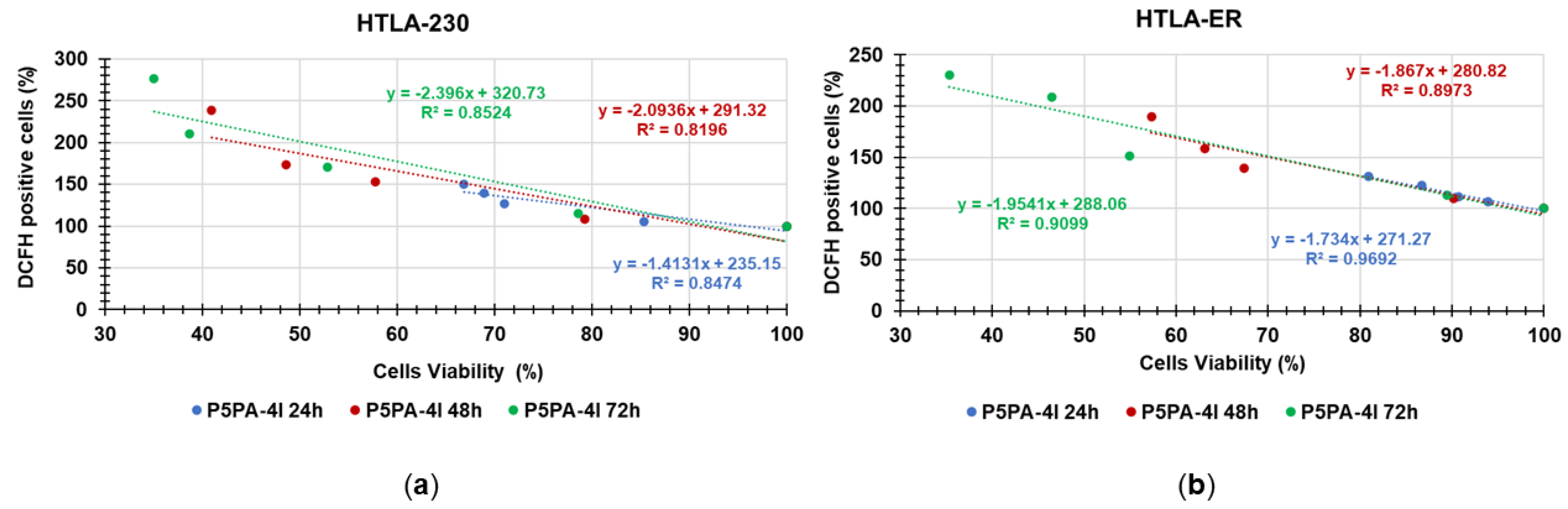
Correlation between H2O2 Production and Cytotoxicity of P5PA-4I NPs
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Preparation of P5PA and P5PA-4I Nanoparticles (NPs)
3.3. Particles Characterization
3.3.1. Morphology of P5, P5PA and P5PA-4I NPs
3.3.2. Dynamic Light Scattering (DLS) Analysis
3.3.3. Cytofluorimetric Analyses
3.4. Chemometric Assisted ATR-FTIR Spectroscopy
3.5. Content of 4I in P5PA-4I, Drug Loading (DL%) and Entrapment Efficiency (EE%)
3.6. Potentiometric Titration of R4HG and R4HG-4I
3.7. Evaluation of 4I In Vitro Release Behaviors
3.8. Biological Experiments
3.8.1. Cell Culture Conditions
3.8.2. Cell Treatments
3.8.3. Cell Viability
3.8.4. Evaluation of Hydrogen Peroxide (H2O2) Production
3.9. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NIH. National Cancer Institute. Neuroblastoma Treatment (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/types/neuroblastoma/hp/neuroblastoma-treatment-pdq (accessed on 1 August 2023).
- Neuroblastoma. Available online: https://neuroblastoma.org/conoscere-la-malattia/neuroblastoma/ (accessed on 23 September 2023).
- American Cancer Society. Key Statistic about Neuroblastoma. Available online: https://www.cancer.org/cancer/types/neuroblastoma/about/key-statistics.html (accessed on 1 August 2023).
- Mahapatra, S.; Challagundla, K.B. Neuroblastoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448111/ (accessed on 1 August 2023).
- London, W.B.; Castleberry, R.P.; Matthay, K.K.; Look, A.T.; Seeger, R.C.; Shimada, H.; Thorner, P.; Brodeur, G.; Maris, J.M.; Reynolds, C.P.; et al. Evidence for an Age Cutoff Greater than 365 Days for Neuroblastoma Risk Group Stratification in the Children’s Oncology Group. JCO 2005, 23, 6459–6465. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, W.; Jin, M.; Sun, Q.; Zhang, Z.; Qin, H.; Su, Y.; Zhi, T.; Xie, Y.; Gao, Y.X.; et al. Biological Features and Clinical Outcome in Infant Neuroblastoma: A Multicenter Experience in Beijing. Eur. J. Pediatr. 2021, 180, 2055–2063. [Google Scholar] [CrossRef]
- Patient. Neuroblastoma. Available online: https://www.cancer.gov/types/neuroblastoma/patient/neuroblastoma-treatment-pdq (accessed on 1 August 2023).
- PDQ Pediatric Treatment Editorial Board. Neuroblastoma Treatment (PDQ®): Patient Version. In PDQ Cancer Information Summaries; National Cancer Institute (US): Bethesda, MD, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK65838/ (accessed on 1 August 2023).
- NIH. National Cancer Institute. Drugs Approved for Neuroblastoma. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/neuroblastoma (accessed on 1 August 2023).
- NIH. National Cancer Institute. BuMel. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/bumel (accessed on 1 August 2023).
- NIH. National Cancer Institute. CEM. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/cem (accessed on 1 August 2023).
- NIH. National Cancer Institute. Side Effects of Cancer Treatment. Available online: https://www.cancer.gov/about-cancer/treatment/side-effects (accessed on 1 August 2023).
- Zhou, X.; Wang, X.; Li, N.; Guo, Y.; Yang, X.; Lei, Y. Therapy Resistance in Neuroblastoma: Mechanisms and Reversal Strategies. Front. Pharmacol. 2023, 14, 1114295. [Google Scholar] [CrossRef] [PubMed]
- Almstedt, E.; Elgendy, R.; Hekmati, N.; Rosén, E.; Wärn, C.; Olsen, T.K.; Dyberg, C.; Doroszko, M.; Larsson, I.; Sundström, A.; et al. Integrative Discovery of Treatments for High-Risk Neuroblastoma. Nat. Commun. 2020, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Nolan, J.C.; Frawley, T.; Tighe, J.; Soh, H.; Curtin, C.; Piskareva, O. Preclinical Models for Neuroblastoma: Advances and Challenges. Cancer Lett. 2020, 474, 53–62. [Google Scholar] [CrossRef]
- Friedman, D.N.; Henderson, T.O. Late Effects and Survivorship Issues in Patients with Neuroblastoma. Children 2018, 5, 107. [Google Scholar] [CrossRef]
- Zuccari, G.; Zorzoli, A.; Marimpietri, D.; Brullo, C.; Alfei, S. Pyrazole-Enriched Cationic Nanoparticles Induced Early- and Late-Stage Apoptosis in Neuroblastoma Cells at Sub-Micromolar Concentrations. Pharmaceuticals 2023, 16, 393. [Google Scholar] [CrossRef]
- Moreno, L.; Barone, G.; DuBois, S.G.; Molenaar, J.; Fischer, M.; Schulte, J.; Eggert, A.; Schleiermacher, G.; Speleman, F.; Chesler, L.; et al. Accelerating Drug Development for Neuroblastoma: Summary of the Second Neuroblastoma Drug Development Strategy Forum from Innovative Therapies for Children with Cancer and International Society of Paediatric Oncology Europe Neuroblastoma. Eur. J. Cancer 2020, 136, 52–68. [Google Scholar] [CrossRef]
- Brullo, C.; Rapetti, F.; Alfei, S.; Maric, I.; Rizzelli, F.; Mapelli, M.; Rosano, C.; Viale, M.; Bruno, O. Discovery of New Antiproliferative Imidazopyrazole Acylhydrazones Able to Interact with Microtubule Systems. ChemMedChem 2020, 15, 961–969. [Google Scholar] [CrossRef]
- de Araujo Junior, R.F.; Eich, C.; Jorquera, C.; Schomann, T.; Baldazzi, F.; Chan, A.B.; Cruz, L.J. Correction to: Ceramide and Palmitic Acid Inhibit Macrophage-Mediated Epithelial–Mesenchymal Transition in Colorectal Cancer. Mol. Cell. Biochem. 2020, 469, 179–180. [Google Scholar] [CrossRef]
- He, Y.; Rezaei, S.; Júnior, R.F.; Cruz, L.J.; Eich, C. Multifunctional Role of Lipids in Modulating the Tumorigenic Properties of 4T1 Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 4240. [Google Scholar] [CrossRef]
- Lin, L.; Ding, Y.; Wang, Y.; Wang, Z.; Yin, X.; Yan, G.; Zhang, L.; Yang, P.; Shen, H. Functional Lipidomics: Palmitic Acid Impairs Hepatocellular Carcinoma Development by Modulating Membrane Fluidity and Glucose Metabolism. Hepatology 2017, 66, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Maly, I.V.; Hofmann, W.A. Effect of Palmitic Acid on Exosome-Mediated Secretion and Invasive Motility in Prostate Cancer Cells. Molecules 2020, 25, 2722. [Google Scholar] [CrossRef]
- Zafaryab, M.; Fakhri, K.; Khan, M.; Hajela, K.; Moshahid, M.; Rizvi, A.; Moshahid, A.; Rizvi, M. In Vitro Assessment of Cytotoxic and Apoptotic Potential of Palmitic Acid for Breast Cancer Treatment. Int. J. Life Sci. Res. 2019, 7, 166–174. [Google Scholar]
- Zhu, S.; Jiao, W.; Xu, Y.; Hou, L.; Li, H.; Shao, J.; Zhang, X.; Wang, R.; Kong, D. Palmitic Acid Inhibits Prostate Cancer Cell Proliferation and Metastasis by Suppressing the PI3K/Akt Pathway. Life Sci. 2021, 286, 120046. [Google Scholar] [CrossRef] [PubMed]
- Corn, K.C.; Windham, M.A.; Rafat, M. Lipids in the Tumor Microenvironment: From Cancer Progression to Treatment. Prog. Lipid Res. 2020, 80, 101055. [Google Scholar] [CrossRef]
- Li, G.; Liu, D.; Kimchi, E.T.; Kaifi, J.T.; Qi, X.; Manjunath, Y.; Liu, X.; Deering, T.; Avella, D.M.; Fox, T.; et al. Nanoliposome C6-Ceramide Increases the Anti-Tumor Immune Response and Slows Growth of Liver Tumors in Mice. Gastroenterology 2018, 154, 1024–1036.e9. [Google Scholar] [CrossRef]
- Urso, C.J.; Zhou, H. Role of CD36 in Palmitic Acid Lipotoxicity in Neuro-2a Neuroblastoma Cells. Biomolecules 2021, 11, 1567. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; de Araújo Júnior, R.F.; Cavalcante, R.S.; Yu, Z.; Schomann, T.; Gu, Z.; Eich, C.; Cruz, L.J. Effective Breast Cancer Therapy Based on Palmitic Acid-Loaded PLGA Nanoparticles. Biomater. Adv. 2023, 145, 213270. [Google Scholar] [CrossRef]
- González-González, A.; Sánchez-Arribas, N.; Santini, E.; Rodríguez-Villafuerte, J.L.; Carbone, C.; Ravera, F.; Ortega, F.; Liggieri, L.; Rubio, R.G.; Guzmán, E. Effects of Oil Phase on the Inversion of Pickering Emulsions Stabilized by Palmitic Acid Decorated Silica Nanoparticles. Colloids Interfaces 2022, 6, 27. [Google Scholar] [CrossRef]
- Alfei, S.; Piatti, G.; Caviglia, D.; Schito, A.M. Synthesis, Characterization, and Bactericidal Activity of a 4-Ammoniumbuthylstyrene-Based Random Copolymer. Polymers 2021, 13, 1140. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Valenti, G.E.; Domenicotti, C. Synthesis of Polystyrene-Based Cationic Nanomaterials with Pro-Oxidant Cytotoxic Activity on Etoposide-Resistant Neuroblastoma Cells. Nanomaterials 2021, 11, 977. [Google Scholar] [CrossRef]
- Zuccari, G.; Russo, E.; Villa, C.; Zorzoli, A.; Marimpietri, D.; Marchitto, L.; Alfei, S. Preparation and Characterization of Amorphous Solid Dispersions for the Solubilization of Fenretinide. Pharmaceuticals 2023, 16, 388. [Google Scholar] [CrossRef]
- Colla, R.; Izzotti, A.; De Ciucis, C.; Fenoglio, D.; Ravera, S.; Speciale, A.; Ricciarelli, R.; Furfaro, A.L.; Pulliero, A.; Passalacqua, M.; et al. Glutathione-mediated antioxidant response and aerobic metabolism: Two crucial factors involved in determining the multi-drug resistance of high-risk neuroblastoma. Oncotarget 2016, 7, 70715–70737. [Google Scholar] [CrossRef]
- Alfei, S.; Marengo, B.; Zuccari, G.; Turrini, F.; Domenicotti, C. Dendrimer Nanodevices and Gallic Acid as Novel Strategies to Fight Chemoresistance in Neuroblastoma Cells. Nanomaterials 2020, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Alfei, S.; Marengo, B.; Domenicotti, C. Polyester-Based Dendrimer Nanoparticles Combined with Etoposide Have an Improved Cytotoxic and Pro-Oxidant Effect on Human Neuroblastoma Cells. Antioxidants 2020, 9, 50. [Google Scholar] [CrossRef]
- Xue, X.; Kim, H. Detection of Total Reactive Oxygen Species in Adherent Cells by 2′,7′-Dichlorodihydrofluorescein Diacetate Staining. JoVE 2020, 160, e60682. [Google Scholar] [CrossRef]
- Nambiar, N.; Nagireddy, P.K.R.; Pedapati, R.; Kantevari, S.; Lopus, M. Tubulin- and ROS-Dependent Antiproliferative Mechanism of a Potent Analogue of Noscapine, N-Propargyl Noscapine. Life Sci. 2020, 258, 118238. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Giottonini, K.Y.; Rodríguez-Córdova, R.J.; Gutiérrez-Valenzuela, C.A.; Peñuñuri-Miranda, O.; Zavala-Rivera, P.; Guerrero-Germán, P.; Lucero-Acuña, A. PLGA Nanoparticle Preparations by Emulsification and Nanoprecipitation Techniques: Effects of Formulation Parameters. RSC Adv. 2020, 10, 4218–4231. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Fang, C.; Feng, J.; Tan, Y.; Wu, Q.; Zhou, X.; Li, J.; Gong, T. Palmitic Acid-Modified Human Serum Albumin Paclitaxel Nanoparticles Targeting Tumor and Macrophages against Breast Cancer. Eur. J. Pharm. Biopharm. 2023, 183, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jin, Z.; Ma, D.; Yin, T.H.; Zhao, K. Palmitic Acid- and Cysteine-Functionalized Nanoparticles Overcome Mucus and Epithelial Barrier for Oral Delivery of Drug. Bioeng. Transl. Med. 2023, 8, e10510. [Google Scholar] [CrossRef] [PubMed]
- Ashaolu, T.J. Nanoemulsions for health, food, and cosmetics: A review. Environ. Chem. Lett. 2021, 19, 3381–3395. [Google Scholar] [CrossRef]
- Yan, X.; Diao, M.; Li, C.; Lu, C.; Zhao, P.; Zhang, T. Formation and Properties of Starch-Palmitic Acid Complex Nanoparticles and Their Influence on Pickering Emulsions. Int. J. Biol. Macromol. 2022, 204, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Kuntworbe, N.; Al-Kassas, R. Design and In Vitro Haemolytic Evaluation of Cryptolepine Hydrochloride-Loaded Gelatine Nanoparticles as a Novel Approach for the Treatment of Malaria. AAPS PharmSciTech 2012, 13, 568–581. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.T.; Nguyen, D.C.; Han, M. Average Size and Zeta Potential of Nanobubbles in Different Reagent Solutions. J. Nanoparticle Res. 2019, 21, 173. [Google Scholar] [CrossRef]
- van Gaal, E.V.B.; Spierenburg, G.; Hennink, W.E.; Crommelin, D.J.A.; Mastrobattista, E. Flow cytometry for rapid size determination and sorting of nucleic acid containing nanoparticles in biological fluids. J. Control. Release 2010, 141, 328–338. [Google Scholar] [CrossRef]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 20, 5.1.1–5.1.11. [Google Scholar] [CrossRef]
- AAT Bioquest. What Is FSC and SSC in Flow Cytometry? Available online: https://www.aatbio.com/resources/faq-frequently-asked-questions/What-is-FSC-and-SSC-in-flow-cytometry (accessed on 23 August 2023).
- Zhang, W.; Tian, Y.; Hu, X.; He, S.; Niu, Q.; Chen, C.; Zhu, S.; Yan, X. Light-Scattering Sizing of Single Submicron Particles by High-Sensitivity Flow Cytometry. Anal. Chem. 2018, 90, 12768–12775. [Google Scholar] [CrossRef]
- Alfei, S.; Oliveri, P.; Malegori, C. Assessment of the Efficiency of a Nanospherical Gallic Acid Dendrimer for Long-Term Preservation of Essential Oils: An Integrated Chemometric-Assisted FTIR Study. Chem. Sel. 2019, 4, 8891–8901. [Google Scholar] [CrossRef]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and Characterization of Nanoemulsion Encapsulating Curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Rosso, A.; Lollo, G.; Chevalier, Y.; Troung, N.; Bordes, C.; Bourgeois, S.; Maniti, O.; Granjon, T.; Dugas, P.-Y.; Urbaniak, S.; et al. Development and Structural Characterization of a Novel Nanoemulsion for Oral Drug Delivery. Colloids Surf. A Physicochem. Eng. Asp. 2020, 593, 124614. [Google Scholar] [CrossRef]
- Friedrich, R.B.; Dimer, F.A.; Guterres, S.S.; Beck, R.C.R.; Pohlmann, A.R. Nanoencapsulation of Tacrolimus in Lipid-Core Nanocapsules Showed Similar Immunosuppressive Activity After Oral and Intraperitoneal Administrations. J. Biomed. Nanotechnol. 2014, 10, 1599–1609. [Google Scholar] [CrossRef]
- Zu, G.; Meijer, M.; Mergel, O.; Zhang, H.; van Rijn, P. 3D-Printable Hierarchical Nanogel-GelMA Composite Hydrogel System. Polymers 2021, 13, 2508. [Google Scholar] [CrossRef] [PubMed]
- Palmitic Acid. Available online: https://en.wikipedia.org/wiki/Palmitic_acid (accessed on 1 August 2023).
- Alfei, S.; Castellaro, S. Synthesis and characterization of polyester-based dendrimers containing peripheral arginine or mixed amino acids as potential vectors for gene and drug delivery. Macromolecular Research 2017, 25, 1172. [Google Scholar] [CrossRef]
- Von Seel, F. Grundlagen Der Analytischen Chemie, 5th ed.; Geier, G., Ed.; Verlag Chemie: Weinheim, Germany, 1970; Volume 82, p. 962. [Google Scholar]
- Aravindan, L.; Bicknell, K.A.; Brooks, G.; Khutoryanskiya, V.V.; Williams, A.C. Effect of acyl chain length on transfection efficiency and toxicity of polyethylenimine. Int. J. Pharm. 2009, 378, 201–210. [Google Scholar] [CrossRef]
- Richard, I.; Thibault, M.M.; Crescenzo, G.D.; Buschmann, M.D.; Lavertu, M. Ionization Behavior of Chitosan and Chitosan-DNA Polyplexes Indicate That Chitosan Has a Similar Capability to Induce a Proton-Sponge Effect as PEI. Biomacromolecules 2013, 14, 1732–1740. [Google Scholar] [CrossRef]
- Mircioiu, C.; Voicu, V.; Anuta, V.; Tudose, A.; Celia, C.; Paolino, D.; Fresta, M.; Sandulovici, R.; Mircioiu, I. Mathematical Modeling of Release Kinetics from Supramolecular Drug Delivery Systems. Pharmaceutics 2019, 11, 140. [Google Scholar] [CrossRef]
- Bruschi, M.L. (Ed.) Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Sawston, UK, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Khan, K.U.; Akhtar, N.; Minhas, M.U. Poloxamer-407-Co-Poly (2-Acrylamido-2-Methylpropane Sulfonic Acid) Cross-Linked Nanogels for Solubility Enhancement of Olanzapine: Synthesis, Characterization, and Toxicity Evaluation. AAPS PharmSciTech 2020, 21, 141. [Google Scholar] [CrossRef]
- Shoukat, H.; Pervaiz, F.; Khan, M.; Rehman, S.; Akram, F.; Abid, U.; Noreen, S.; Nadeem, M.; Qaiser, R.; Ahmad, R.; et al. Development of β-cyclodextrin/polyvinypyrrolidone-co-poly (2-acrylamide-2-methylpropane sulphonic acid) hybrid nanogels as nano-drug delivery carriers to enhance the solubility of Rosuvastatin: An in vitro and in vivo evaluation. PLoS ONE 2022, 17, e0263026. [Google Scholar] [CrossRef]


| Standard Chemotherapeutics [9] | Maintenance Therapeutics [8] | Targeted Therapeutics [7] | Immunotherapy [8] |
|---|---|---|---|
| Cyclophosphamide | Isotretinoin Dinutuximab GM-CSF IL-2 | Pembrolizumab *** Dinutuximab *** Crizotinib (TKI) AZD1775 (TKI) Lorlatinib (TKI) Vorinostat (HDI) Eflornithine (ODI) | CAR T-cell |
| Danyelza (Naxitamab-gqgk) | |||
| Dinutuximab | |||
| Doxorubicin Hydrochloride | |||
| Naxitamab-gqgk | |||
| Unituxin (Dinutuximab) | |||
| Vincristine Sulfate | |||
| BuMeI * | |||
| CEM ** |
| Compound | Cells | IC50 48 h (µM) | IC50 72 h (µM) |
|---|---|---|---|
| 4G | HTLA-230 | 37.39 | 12.31 |
| HTLA-ER | 41.45 | 20.49 | |
| 4I | HTLA-230 | 24.95 | 10.78 |
| HTLA-ER | 91.38 | 18.17 |
| Entry | (mg; mmol) | (% wt/wt) | DCM (mL) | H2O (mL) | P5PA-4I (mg) | P5PA (mg) | MECs (%; mg; mmol) |
|---|---|---|---|---|---|---|---|
| P5 * | 216.0; 0.0424 | 51.7 | N.E.D. | 5 | 402.0 | N.E.D. | 51.7; 207.8; 0.0407 |
| PA ** | 115.1; 0.4489 | 27.5 | 12 | N.E.D. | 27.5; 110.6; 0.4314 | ||
| 4I *** | 86.9; 0.1859 | 20.8 | 20.8; 83.6; 0.1788 | ||||
| P5 * | 238.2; 0.0467 | 66.7 | N.E.D. | 5 | N.E.D. | 337.5 | 66.7; 225.0; 0.0441 |
| PA ** | 119.1; 0.4372 | 33.3 | 12 | N.E.D. | N.E.D. | 33.3; 112.5; 0.4387 |
| Entry | Z-AVE, nm | ζ-p, mV | PDI |
|---|---|---|---|
| P5PA | 516.4 ± 50.2 | +6.97 ± 2.53 | 0.391 |
| P5PA-4I | 541.5 ± 7.1 | +8.53 ± 2.98 | 0.195 |
| P5 | 334.0 ± 27.0 | +57.6 ± 1.70 | 1.012 |
| Entry | Mean FSC * | Median FSC * | Mean SSC * | Median SSC * | Diameter (nm) | Complexity |
|---|---|---|---|---|---|---|
| P5 | 4.45 | 4.14 | 10.57 | 9.40 | 317 ± 11 | 9.99 ± 0.83 |
| P5PA | 91.88 | 96.53 | 129.92 | 139.27 | 988 ± 8 | 134.60 ± 6.61 |
| P5PA-4I | 149.90 | 156.59 | 145.61 | 167.73 | 1094 ± 6 | 156.67 ± 15.64 |
| H2O ** | 15.32 | 16.02 | 14.29 | 13.11 | 598 ± 7 | 13.70 ± 0.83 |
| Beads *** | 91.55 | 107.87 | 98.14 | 103.92 | 1000 ± 0 | 101.03 ± 4.09 |
| Abs | 4I (mg/mL) AD | 4I (mg/mL) BD | 4I (mg) in 50.0 * mg | 4I (mg) in 402.0 ** mg | DL (%) | EE (%) |
|---|---|---|---|---|---|---|
| 0.9949 | 0.101 | 1.01 | 10.43 ± 0.25 *** | 83.9 ± 2.01 | 20.9 | 96.6 |
| 1.0322 | 0.105 | 1.05 | ||||
| 1.0508 | 0.107 | 1.07 |
| Entry | Weight (mg) | HCl 0.1 N * (mL) | HCl 0.1 N ** (mL) | PA (mmol) | Nbasic (mmol) | mmolesPA/g | mmoles Nbasic/g |
|---|---|---|---|---|---|---|---|
| P5PA | 60.6 | 2.2 ± 0.02 | 5.0 ± 0.02 | 0.22 ± 0.002 | 0.50 ± 0.002 | 3.6 ± 0.03 | 8.3 ± 0.03 |
| P5PA-4I | 60.8 | 3.0 ± 0.02 | 3.5 ± 0.02 | 0.30 ± 0.002 | 0.35 ± 0.002 | 4.9 ± 0.03 | 5.8 ± 0.03 |
| P5 | 8.0 | 1.0 ± 0.02 | N.E.D. | N.E.D. | 0.10± 0.002 | N.E.D. | 12.5± 0.03 |
| Entry | β1 | β2 | βAVE |
|---|---|---|---|
| P5PA | 2.7778 | 4.1667 | 1.4783 ± 0.8685 |
| P5PA-4I | 4.5455 | 4.5455 | 1.3261 ± 0.5276 |
| P5 | 0.6667 | N.E.D. | 0.2305 ± 0.1354 |
| b-PEI | 0.0760 | 0.0826 | 0.5170 ± 0.2541 |
| Kinetic Model | R2 of P5PA-4I | R2 of 4I-Suspension |
|---|---|---|
| Zero-order | 0.9285 | 0.8600 |
| First-order | 0.9395 | 0.9080 |
| Hixson–Crowel | 0.9285 | 0.8600 |
| Higuchi | 0.9734 | 0.9488 |
| Korsmeyer–Peppas | 0.9614 | 0.8086 |
| Compound | Cells | IC50 24 h (µM) | IC50 48 h (µM) | IC50 72 h (µM) |
|---|---|---|---|---|
| 4I | HTLA-230 | 76.51 ± 6.33 | 23.75 ± 4.93 | 13.06 ± 1.53 |
| HTLA-ER | 337.3 ± 55.03 | 61.87 ± 0.58 | 22.02 ± 2.07 | |
| P5PA-4I | HTLA-230 | 62.59 ± 5.40 | 16.47 ± 1.26 | 8.62 ± 1.44 |
| HTLA-ER | 178.3 ± 12.58 | 40.70 ± 1.70 | 13.55 ± 1.27 | |
| ETO | HTLA-ER | 591.7 ± 45.30 | N.R. | N.R. |
| Sample | mg/mL | Events | Ps/mL | Total Volume (µL) | Acquisition Time (s) | FSC Gain | SSC |
|---|---|---|---|---|---|---|---|
| H2O | N.E.D. | 33,602 | N.E.D. | 10.48965 | 44.44768 | 28.10003 | 2.828427 |
| BEADS | N.E.D. | 33,602 | 1,570,000 | 7.557068 | 32.02148 | 28.10003 | 2.828427 |
| P5 | 4.1 | 33,602 | 1,660,000 | 6.074351 | 25.73878 | 28.10003 | 2.828427 |
| P5PA | 5.3 | 33,602 | 1,770,000 | 11.07857 | 46.9431 | 28.10003 | 2.828427 |
| P5PA4I | 5.1 | 33,602 | 1,840,000 | 11.03581 | 46.7619 | 28.10003 | 2.828427 |
| Entry | Concentrations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4I * | µM | 0 | 0.01 | 0.1 | 0.5 | 1 | 5 | 8 | 10 | 20 | 50 | 75 | 100 |
| µg/mL | 0 | 0.005 | 0.05 | 0.25 | 0.5 | 2.5 | 4.0 | 5.0 | 10.0 | 25.0 | 37.5 | 50.0 | |
| P5PA4I NPs ** | µg/mL | 0 | 0.024 | 0.24 | 1.2 | 2.4 | 12 | 19.2 | 24 | 48 | 120 | 180 | 240 |
| P5PA NPs *** | µg/mL | 0 | 0.019 | 0.19 | 0.95 | 1.9 | 9.5 | 15.2 | 19 | 38 | 95 | 142.5 | 190 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenti, G.E.; Marengo, B.; Milanese, M.; Zuccari, G.; Brullo, C.; Domenicotti, C.; Alfei, S. Imidazo-Pyrazole-Loaded Palmitic Acid and Polystyrene-Based Nanoparticles: Synthesis, Characterization and Antiproliferative Activity on Chemo-Resistant Human Neuroblastoma Cells. Int. J. Mol. Sci. 2023, 24, 15027. https://doi.org/10.3390/ijms241915027
Valenti GE, Marengo B, Milanese M, Zuccari G, Brullo C, Domenicotti C, Alfei S. Imidazo-Pyrazole-Loaded Palmitic Acid and Polystyrene-Based Nanoparticles: Synthesis, Characterization and Antiproliferative Activity on Chemo-Resistant Human Neuroblastoma Cells. International Journal of Molecular Sciences. 2023; 24(19):15027. https://doi.org/10.3390/ijms241915027
Chicago/Turabian StyleValenti, Giulia Elda, Barbara Marengo, Marco Milanese, Guendalina Zuccari, Chiara Brullo, Cinzia Domenicotti, and Silvana Alfei. 2023. "Imidazo-Pyrazole-Loaded Palmitic Acid and Polystyrene-Based Nanoparticles: Synthesis, Characterization and Antiproliferative Activity on Chemo-Resistant Human Neuroblastoma Cells" International Journal of Molecular Sciences 24, no. 19: 15027. https://doi.org/10.3390/ijms241915027
APA StyleValenti, G. E., Marengo, B., Milanese, M., Zuccari, G., Brullo, C., Domenicotti, C., & Alfei, S. (2023). Imidazo-Pyrazole-Loaded Palmitic Acid and Polystyrene-Based Nanoparticles: Synthesis, Characterization and Antiproliferative Activity on Chemo-Resistant Human Neuroblastoma Cells. International Journal of Molecular Sciences, 24(19), 15027. https://doi.org/10.3390/ijms241915027












