Design, Synthesis and Antitumor Activity of 1H-indazole-3-amine Derivatives
Abstract
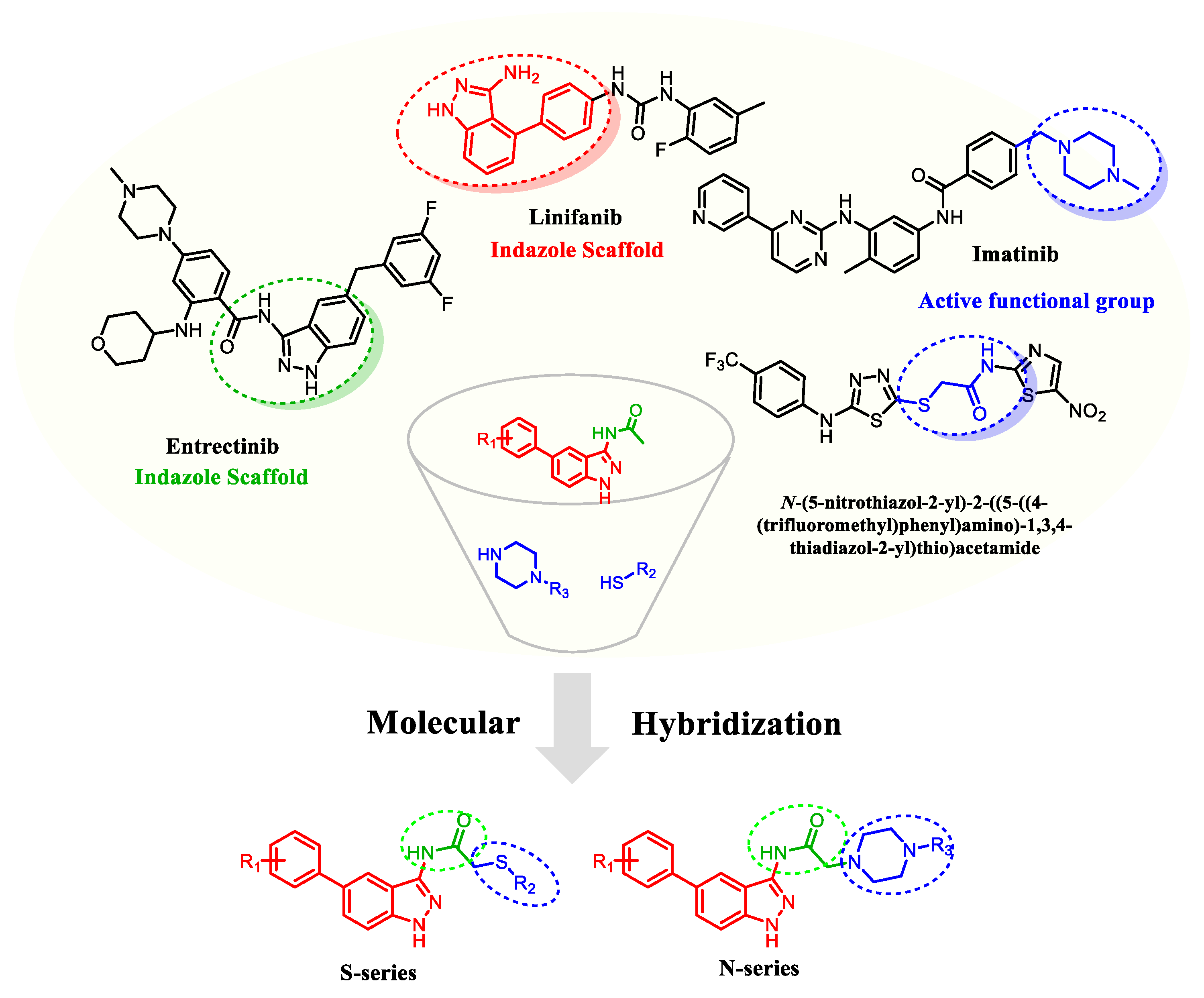
1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Anti-Proliferative Activity Analysis
2.3. Cell Apoptosis Analysis
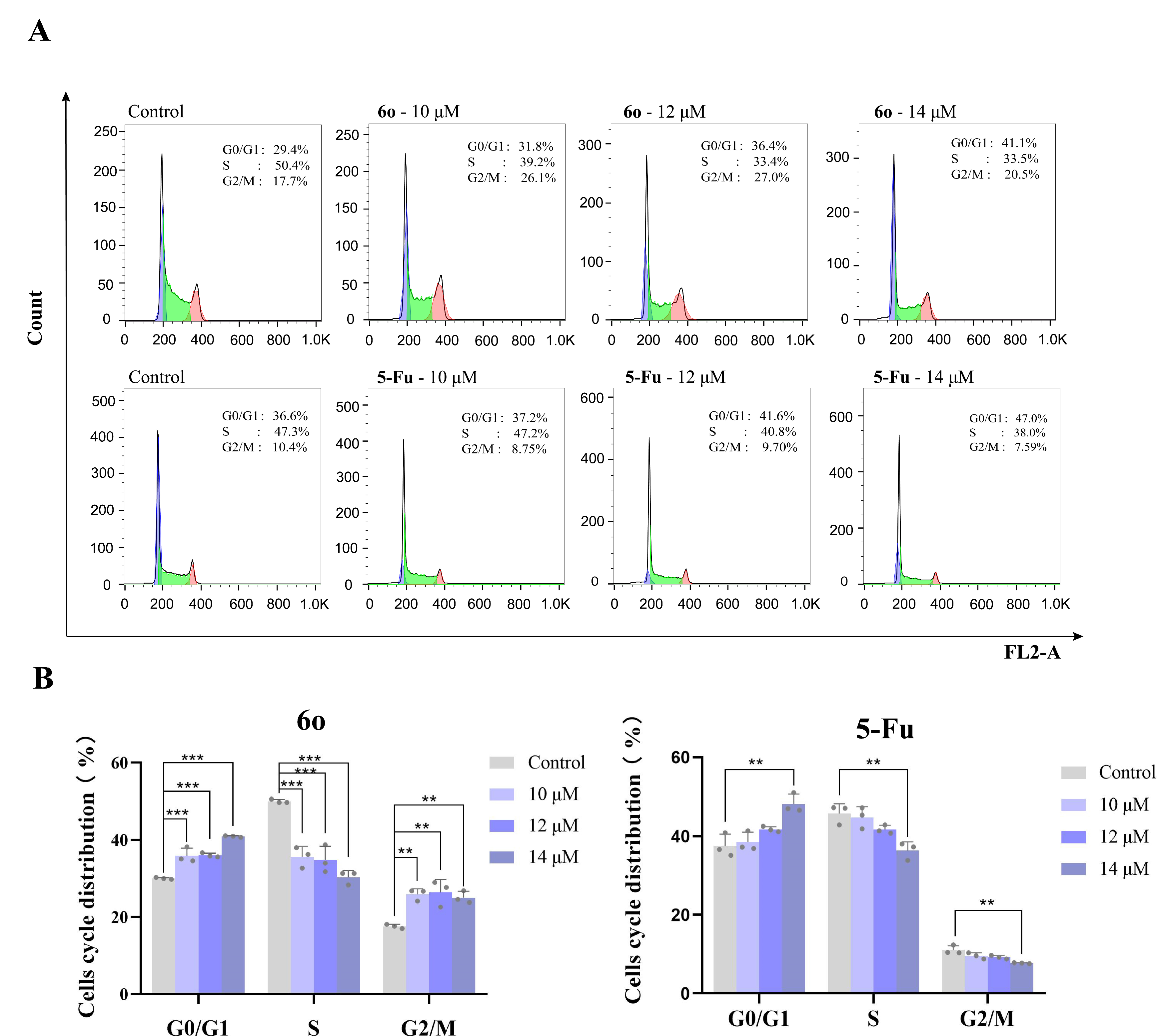
2.4. Cell Cycle Analysis
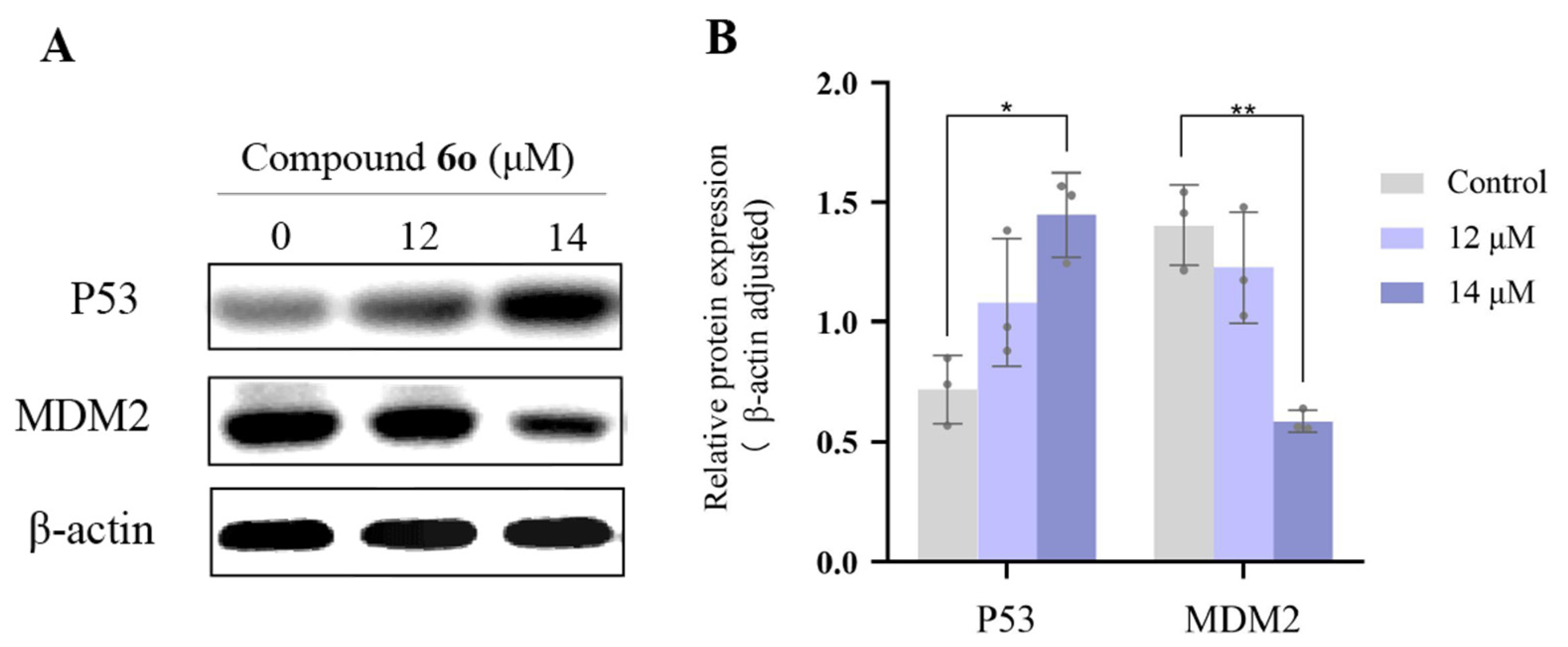
2.5. The Effect of Compound 6o on the Expression of P53 and MDM2 Protein
3. Materials and Methods
3.1. Chemistry and Instruments
3.2. Pharmacology
3.2.1. Cell Culture and Treatment
3.2.2. MTT Assay
3.2.3. Cell Apoptosis Detection Assay
3.2.4. Cell Cycle Analysis
3.2.5. Western Blotting Assay
3.2.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.Q. Cancer statistics: Updated cancer burden in China Preface. Chin. J. Cancer Res. 2015, 27, 1. [Google Scholar]
- Kerru, N.; Singh, P.; Koorbanally, N.; Raj, R.; Kumar, V. Recent advances (2015–2016) in anticancer hybrids. Eur. J. Med. Chem. 2017, 142, 179–212. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.C.; Xu, B. Transition metal-involving synthesis and utilization of N-containing heterocycles: Exploration of nitrogen sources. Chem. Rec. 2016, 16, 1701–1714. [Google Scholar] [CrossRef]
- Gaikwad, D.D.; Chapolikar, A.D.; Devkate, C.G.; Warad, K.D.; Tayade, A.P.; Pawar, R.P.; Domb, A.J. Synthesis of indazole motifs and their medicinal importance: An overview. Eur. J. Med. Chem. 2015, 90, 707–731. [Google Scholar] [CrossRef] [PubMed]
- Denya, I.; Malan, S.F.; Joubert, J. Indazole derivatives and their therapeutic applications: A patent review (2013–2017). Expert. Opin. Ther. Pat. 2018, 28, 441–453. [Google Scholar] [CrossRef]
- Reddy, G.S.; Mohanty, S.; Kumar, J.; Rao, B.V. Synthesis and Evaluation of Anticancer Activity of Indazole Derivatives. Russ. J. Gen. Chem. 2018, 88, 2394–2399. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Luo, C.; Yang, P.; Li, P.; Wu, C.L. Indazole scaffold: A generalist for marketed and clinical drugs. Med. Chem. Res. 2020, 30, 501–518. [Google Scholar] [CrossRef]
- Lopez, V.F.; Castillo, R.; Yepez, M.L.; Medina, F.J. Benzotriazoles and indazoles are scaffolds with biological activity against Entamoeba histolytica. J. Biomol. Screen. 2011, 16, 862–868. [Google Scholar] [CrossRef]
- Al-Salama, Z.T.; Keam, S.J. Entrectinib: First Global Approval. Drugs 2019, 79, 1477–1483. [Google Scholar] [CrossRef]
- Harris, P.A.; Boloor, A.; Cheung, M.; Kumar, R.; Crosby, R.M.; Davis-Ward, R.G.; Epperly, A.H.; Hinkle, K.W.; Hunter, R.N.; Johnson, J.H.; et al. Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-benzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J. Med. Chem. 2008, 51, 4632–4640. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kakutani, S.; Sato, Y.; Hanashi, A.; Kinoshita, Y.; Ishikawa, A. Drug review: Pazopanib. Jpn. J. Clin. Oncol. 2018, 48, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Niraparib: First Global Approval. Drugs. 2017, 77, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Jiang, F.; Cole, T.B.; Hradil, V.P.; Reuter, D.; Chakravartty, A.; Albert, D.H.; Davidsen, S.K.; Cox, B.F.; Mc Keegan, E.M.; et al. A novel multi-targeted tyrosine kinase inhibitor, linifanib (ABT-869), produces functional and structural changes in tumor vasculature in an orthotopic rat glioma model. Cancer. Chemother. Pharmacol. 2012, 69, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.; Lynskey, D.; Matakidou, A.; Fife, K.; Eisen, T. A novel strategy for axitinib dosing in the treatment of metastatic renal cell carcinoma. J. Clin. Oncol. 2017, 35, 464. [Google Scholar] [CrossRef]
- Spartinou, A.; Nyktari, V.; Papaioannou, A. Granisetron: A review of pharmacokinetics and clinical experience in chemotherapy induced-nausea and vomiting. Expert. Opin. Drug. Metab. Toxicol. 2017, 13, 1289–1297. [Google Scholar] [CrossRef]
- Ai-Bogami, A.S. Mechanochemical synthesis of cyclohexenones and indazoles as potential antimicrobial agents. Res. Chem. Intermed. 2016, 42, 5457–5477. [Google Scholar] [CrossRef]
- Wang, S.; Shi, J.T.; Wang, X.R.; Mu, H.X.; Wang, X.T.; Xu, K.Y.; Wang, Q.S.; Chen, S.W. 1H-Indazoles derivatives targeting PI3K/AKT/mTOR pathway: Synthesis, anti-tumor effect and molecular mechanism. Bioorg. Chem. 2023, 133, 106412. [Google Scholar] [CrossRef]
- Deng, X.; Okram, B.; Ding, Q.; Zhang, J.; Choi, Y.; Adrian, F.J.; Wojciechowski, A.; Zhang, G.; Che, J.; Bursulaya, B.; et al. Expanding the diversity of allosteric bcr-abl inhibitors. J. Med. Chem. 2010, 53, 6934–6946. [Google Scholar] [CrossRef]
- Porter, N.J.; Shen, S.; Barinka, C.; Kozikowski, A.P.; Christianson, D.W. Molecular Basis for the Selective Inhibition of Histone Deacetylase 6 by a Mercaptoacetamide Inhibitor. Med. Chem. Lett. 2018, 9, 1301–1305. [Google Scholar] [CrossRef]
- Konsoula, Z.; Cao, H.; Velena, A.; Jung, M. Pharmacokinetics-pharmacodynamics and antitumor activity of mercaptoacetamide-based histone deacetylase inhibitors. Mol. Cancer. Ther. 2009, 8, 2844–2851. [Google Scholar] [CrossRef]
- Segretti, M.C.; Vallerini, G.P.; Brochier, C.; Langley, B.; Wang, L.; Hancock, W.W.; Kozikowski, A.P. Thiol-Based Potent and Selective HDAC6 Inhibitors Promote Tubulin Acetylation and T-Regulatory Cell Suppressive Function. ACS Med. Chem. Lett. 2015, 6, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Shallal, H.M.; Russu, W.A. Discovery, synthesis, and investigation of the antitumor activity of novel piperazinyl pyrimidine derivatives. Eur. J. Med. Chem. 2011, 46, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- Rossari, F.; Minutolo, F.; Orciuolo, E. Past, present, and future of Bcr-Abl inhibitors: From chemical development to clinical efficacy. J. Hematol. Oncol. 2018, 11, 84–97. [Google Scholar] [CrossRef]
- Wu, C.; Yang, P.; Wang, H.; Zhang, S.; Wang, J.; Zhai, H.; Yang, Y.; Cao, Y. Synthesis and Antitumor Activity of Novel 5- and 6-Substituted Indazole Derivatives. Chin. J. Org. Chem. 2022, 42, 590. [Google Scholar]
- Hu, H.; Wang, X.; Chan, G.K.; Chang, J.H.; Do, S.; Drummond, J.; Ebens, A.; Lee, W.; Ly, J.; Lyssikatos, J.P.; et al. Discovery of 3,5-substituted 6-azaindazoles as potent pan-Pim inhibitors. Bioorg. Med. Chem. Lett. 2015, 25, 5258–5264. [Google Scholar] [CrossRef]
- Wang, X.; Kolesnikov, A.; Tay, S.; Chan, G.; Chao, Q.; Do, S.; Drummond, J.; Ebens, A.J.; Liu, N.; Ly, J.; et al. Discovery of 5-Azaindazole (GNE-955) as a Potent Pan-Pim Inhibitor with Optimized Bioavailability. J. Med. Chem. 2017, 60, 4458–4473. [Google Scholar] [CrossRef] [PubMed]
- El-Damasy, A.K.; Jin, H.; Seo, S.H.; Bang, E.K.; Keum, G. Design, synthesis, and biological evaluations of novel 3-amino-4-ethynyl indazole derivatives as Bcr-Abl kinase inhibitors with potent cellular antileukemic activity. Eur. J. Med. Chem. 2020, 207, 112710. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liang, L.; Sun, Y.; Si, R.; Zhang, Q.; Wang, J.; Fu, J.; Zhang, J.; Zhang, J. Discovery of novel Bcr-Abl (T315I) inhibitors with flexible linker. Part 1: Confirmation optimization of phenyl-1H-indazol-3-amine as hinge binding moiety. Eur. J. Med. Chem. 2019, 178, 232–242. [Google Scholar] [CrossRef]
- Migliorini, A.; Oliviero, C.; Gasperi, T.; Loreto, M.A. The Suzuki Reaction Applied to the Synthesis of Novel Pyrrolyl and Thiophenyl Indazoles. Molecules 2012, 17, 4508–4521. [Google Scholar] [CrossRef]
- Hagmann, W.K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Vulpetti, A.; Dalvit, C. Fluorine local environment: From screening to drug design. Drug. Discov. Today. 2012, 17, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.R.; Jones, R.N.; Baud, M.G.; Wilcken, R.; Boeckler, F.M.; Fersht, A.R.; Joerger, A.C.; Spencer, J. Harnessing Fluorine-Sulfur Contacts and Multipolar Interactions for the Design of p53 Mutant Y220C Rescue Drugs. ACS Chem. Biol. 2016, 11, 2265–2274. [Google Scholar] [CrossRef]
- Liu, J.; Peng, X.; Dai, Y.; Zhang, W.; Ren, S.; Ai, J.; Geng, M.; Li, Y. Design, synthesis and biological evaluation of novel FGFR inhibitors bearing an indazole scaffold. Org. Biomol. Chem. 2015, 13, 7643–7676. [Google Scholar] [CrossRef] [PubMed]
- Fridman, J.S.; Lowe, S.W. Control of apoptosis by p53. Oncogene 2003, 22, 9030–9040. [Google Scholar] [CrossRef] [PubMed]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer. 2002, 2, 594–604. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.H.; Wang, G.; Yang, Y.S.; Yuan, Y.; Ouyang, L. The past, present and future of potential small-molecule drugs targeting p53-MDM2/MDMX for cancer therapy. Eur. J. Med. Chem. 2019, 176, 92–104. [Google Scholar] [CrossRef]
- Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef]
- Greenblatt, M.S.; Bennett, W.P.; Hollstein, M.; Harris, C.C. Mutations in the p53 tumor suppressor gene: Clues to cancer etiology and molecular pathogenesis. Cancer. Res. 1994, 54, 4855–4878. [Google Scholar]
- Fakharzadeh, S.S.; Trusko, S.P.; George, D.L. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991, 10, 1565–1569. [Google Scholar] [CrossRef]
- Oliner, J.D.; Kinzler, K.W.; Meltzer, P.S.; George, D.L.; Vogelstein, B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature 1992, 358, 80–83. [Google Scholar] [CrossRef]
- Jones, S.N.; Hancock, A.R.; Vogel, H.; Donehower, L.A.; Bradley, A. Overexpression of Mdm2 in mice reveals a p53-independent role for Mdm2 in tumorigenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 15608–15612. [Google Scholar] [CrossRef] [PubMed]



| Compounds | IC50 ± SD a (μM) | |||
|---|---|---|---|---|
| K562 | A549 | PC-3 | Hep-G2 | |
| 5a | 9.32 ± 0.59 | 4.66 ± 0.45 | 15.48 ± 1.33 | 12.67 ± 1.31 |
| 5b | 6.97 ± 0.99 | 5.25 ± 0.57 | 38.43 ± 24.12 | >50 |
| 5c | 9.33 ± 0.64 | 5.63 ± 0.59 | 60.74 ± 2.91 | 4.76 ± 0.63 |
| 5d | 27.23 ± 0.66 | 16.64 ± 3.22 | 19.67 ± 2.38 | 45.90 ± 2.24 |
| 5e | >50 | 7.23 ± 0.40 | 12.83 ± 3.09 | 8.20 ± 0.91 |
| 5f | >50 | 4.48 ± 0.24 | 12.46 ± 4.55 | >50 |
| 5g | 10.31 ± 1.14 | 6.40 ± 0.26 | 18.62 ± 5.73 | 11.87 ± 0.72 |
| 5h | 14.35 ± 2.13 | 9.26 ± 0.37 | 13.02 ± 7.53 | 11.28 ± 0.75 |
| 5i | 6.27 ± 1.22 | 4.43 ± 0.15 | 6.57 ± 1.10 | 5.50 ± 0.17 |
| 5j | 8.49 ± 1.87 | 3.99 ± 0.16 | 14.72 ± 4.55 | 5.36 ± 0.25 |
| 5k | 8.86 ± 0.66 | 7.78 ± 0.18 | 12.62 ± 2.17 | 3.32 ± 0.43 |
| 5l | 8.19 ± 1.43 | 14.06 ± 1.71 | >50 | 14.92 ± 0.77 |
| 5m | 13.13 ± 0.46 | 20.21 ± 3.14 | >50 | 35.64 ± 4.21 |
| 5n | 7.25 ± 1.07 | 20.00 ± 1.19 | >50 | 6.57 ± 0.13 |
| 5o | 8.09 ± 1.27 | 6.64 ± 0.47 | 12.51 ± 2.10 | 8.30 ± 0.56 |
| 5p | 15.42 ± 0.15 | 15.95 ± 0.37 | 22.51 ± 9.13 | 7.14 ± 0.33 |
| 5q | 14.35 ± 2.13 | 16.25 ± 1.46 | 7.81 ± 0.64 | 6.30 ± 0.03 |
| 5-Fu b | 8.53 ± 2.27 | 8.37 ± 0.79 | 9.17 ± 3.93 | 9.78 ± 1.58 |
| Compounds | IC50 ± SD (μM) | |||
|---|---|---|---|---|
| K562 | A549 | PC-3 | Hep-G2 | |
| 6a | 5.19 ± 0.29 | 8.21 ± 0.56 | 6.12 ± 0.10 | 5.62 ± 1.76 |
| 6b | 18.62 ± 2.14 | 19.90 ± 1.15 | 9.69 ± 0.26 | 1.69 ± 0.68 |
| 6c | >50 | 24.03 ± 2.92 | >50 | 1.23 ± 0.39 |
| 6d | 18.23 ± 2.91 | 17.55 ± 4.11 | 17.58 ± 1.40 | >50 |
| 6e | 8.85 ± 0.26 | 12.78 ± 0.78 | 7.15 ± 0.14 | >50 |
| 6f | 11.91 ± 0.85 | >50 | 41.74 ± 27.89 | 9.56 ± 0.84 |
| 6g | 6.61 ± 0.38 | 15.85 ± 2.98 | 6.81 ± 1.09 | 2.35 ± 0.96 |
| 6h | 13.23 ± 1.75 | 15.13 ± 1.36 | 8.63 ± 0.57 | 2.75 ± 0.78 |
| 6i | 13.23 ± 1.44 | 28.58 ± 2.52 | 12.81 ± 2.95 | 2.58 ± 0.42 |
| 6j | 7.78 ± 0.10 | 20.29 ± 1.24 | 7.58 ± 1.01 | 3.87 ± 0.47 |
| 6k | 11.18 ± 0.56 | 7.83 ± 1.06 | 4.25 ± 0.13 | 1.61 ± 0.28 |
| 6l | 14.01 ± 0.49 | 12.90 ± 0.21 | 2.89 ± 0.16 | 7.65 ± 3.34 |
| 6m | 17.91 ± 3.14 | 22.62 ± 6.53 | 19.94 ± 2.71 | 1.51 ± 0.25 |
| 6n | 13.33 ± 1.40 | 18.66 ± 3.10 | 34.84 ± 4.37 | 4.76 ± 0.63 |
| 6o | 5.15 ± 0.55 | 6.73 ± 1.03 | 8.34 ± 1.55 | 7.37 ± 0.24 |
| 6p | >50 | 13.55 ± 0.53 | 32.10 ± 2.26 | 2.38 ± 0.37 |
| 6q | 5.61 ± 1.14 | 12.85 ± 2.66 | 10.74 ± 1.66 | 2.70 ± 0.47 |
| 6r | 10.03 ± 0.94 | 15.85 ± 1.38 | 27.89 ± 26.14 | >50 |
| 6s | 10.78 ± 0.68 | 20.40 ± 8.08 | 20.26 ± 4.71 | 2.92 ± 1.41 |
| 6t | 11.08 ± 0.69 | 40.88 ± 6.54 | 18.97 ± 0.58 | 1.98 ± 0.35 |
| 6u | >50 | 17.55 ± 4.11 | 27.66 ± 16.92 | 20.04 ± 3.04 |
| 5-Fu | 8.53 ± 2.27 | 8.37 ± 0.79 | 3.47 ± 0.21 | 2.25 ± 0.46 |
| Compounds | IC50 ± SD (μM) | SI a | ||
|---|---|---|---|---|
| Hep-G2 | K562 | HEK-293 | ||
| 5k | 3.32 ± 0.43 | 12.17 ± 2.85 | 3.67 | |
| 6o | 5.15 ± 0.55 | 33.20 ± 3.83 | 6.45 | |
| 5-Fu | 9.78 ± 1.58 | 8.53 ± 2.27 | 1.16 ± 0.11 | 0.12 b; 0.14 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Zhu, M.; Long, X.; Wang, Q.; Wang, Z.; Ouyang, G. Design, Synthesis and Antitumor Activity of 1H-indazole-3-amine Derivatives. Int. J. Mol. Sci. 2023, 24, 8686. https://doi.org/10.3390/ijms24108686
Wang C, Zhu M, Long X, Wang Q, Wang Z, Ouyang G. Design, Synthesis and Antitumor Activity of 1H-indazole-3-amine Derivatives. International Journal of Molecular Sciences. 2023; 24(10):8686. https://doi.org/10.3390/ijms24108686
Chicago/Turabian StyleWang, Congyu, Mei Zhu, Xuesha Long, Qin Wang, Zhenchao Wang, and Guiping Ouyang. 2023. "Design, Synthesis and Antitumor Activity of 1H-indazole-3-amine Derivatives" International Journal of Molecular Sciences 24, no. 10: 8686. https://doi.org/10.3390/ijms24108686
APA StyleWang, C., Zhu, M., Long, X., Wang, Q., Wang, Z., & Ouyang, G. (2023). Design, Synthesis and Antitumor Activity of 1H-indazole-3-amine Derivatives. International Journal of Molecular Sciences, 24(10), 8686. https://doi.org/10.3390/ijms24108686





