HSA-ZW800-PEG for Enhanced Optophysical Stability and Tumor Targeting
Abstract
1. Introduction
2. Results
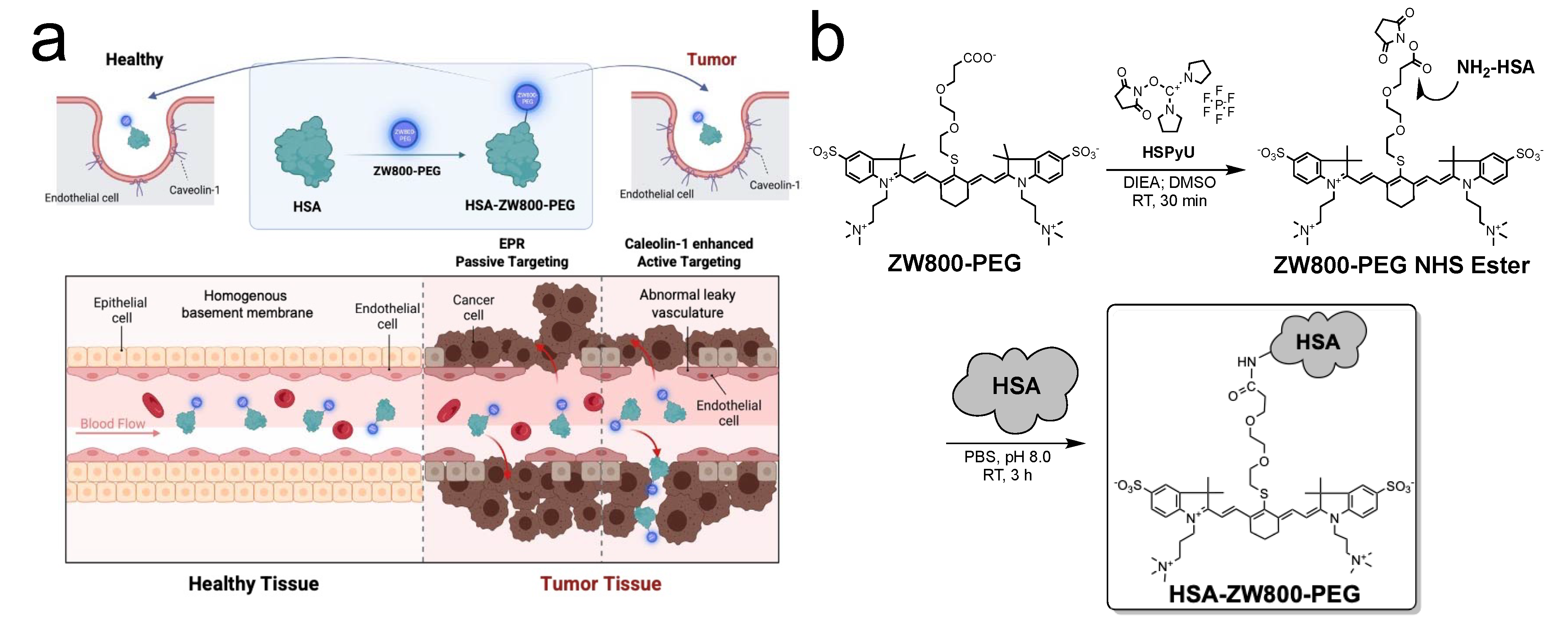
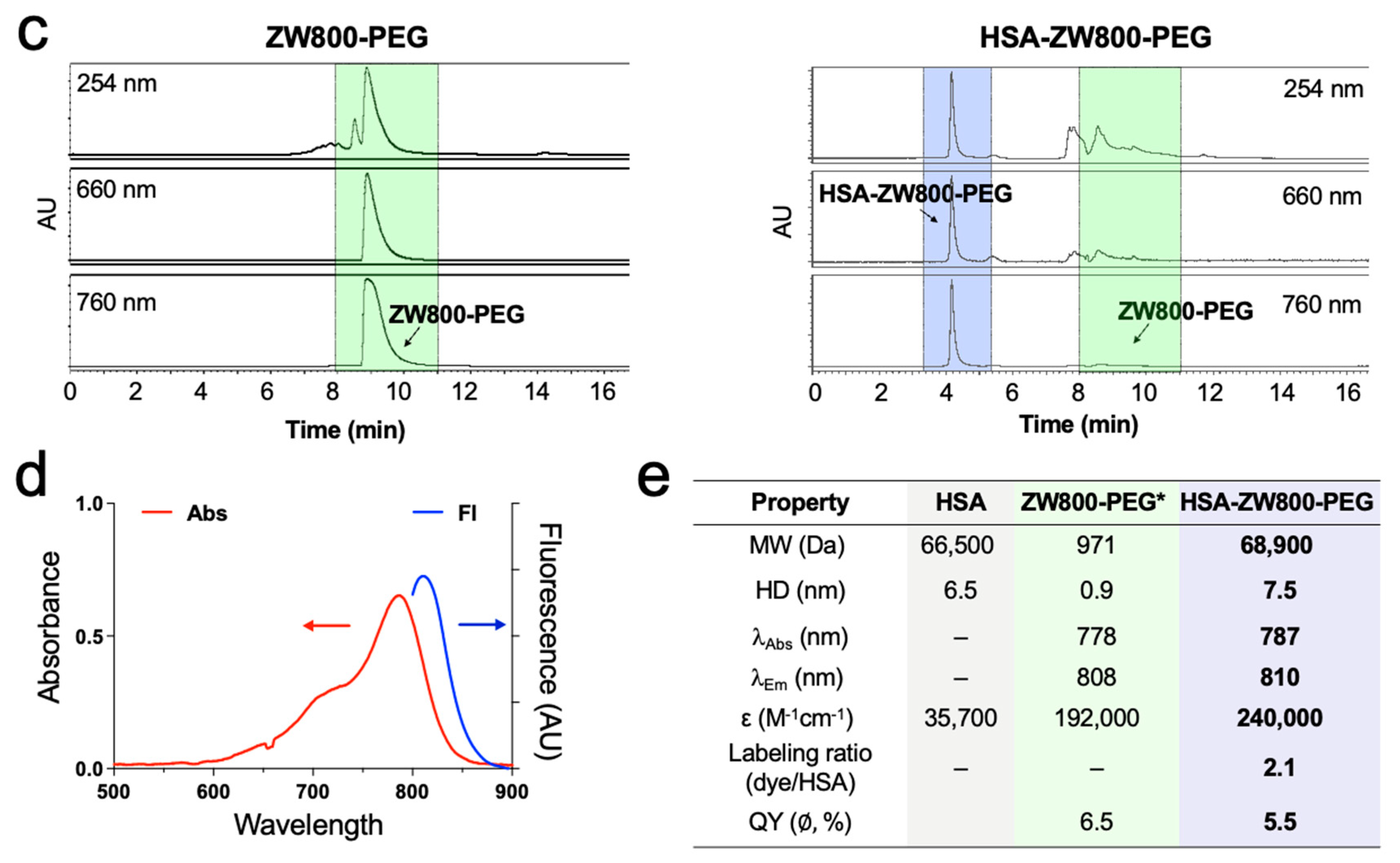
2.1. Synthesis and Optical Characterization of HSA-ZW800-PEG
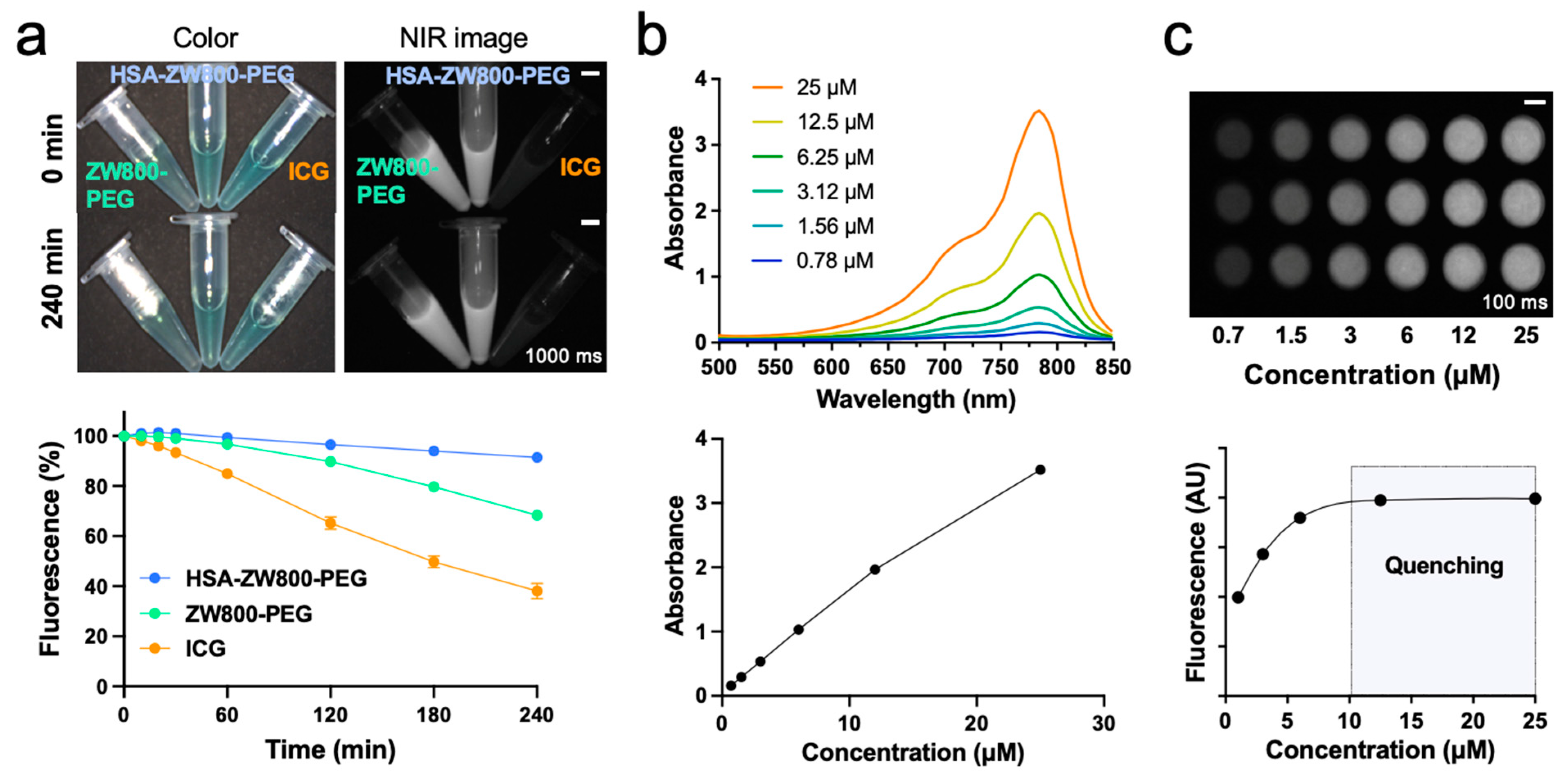
2.2. In Vitro Optophysical Stability
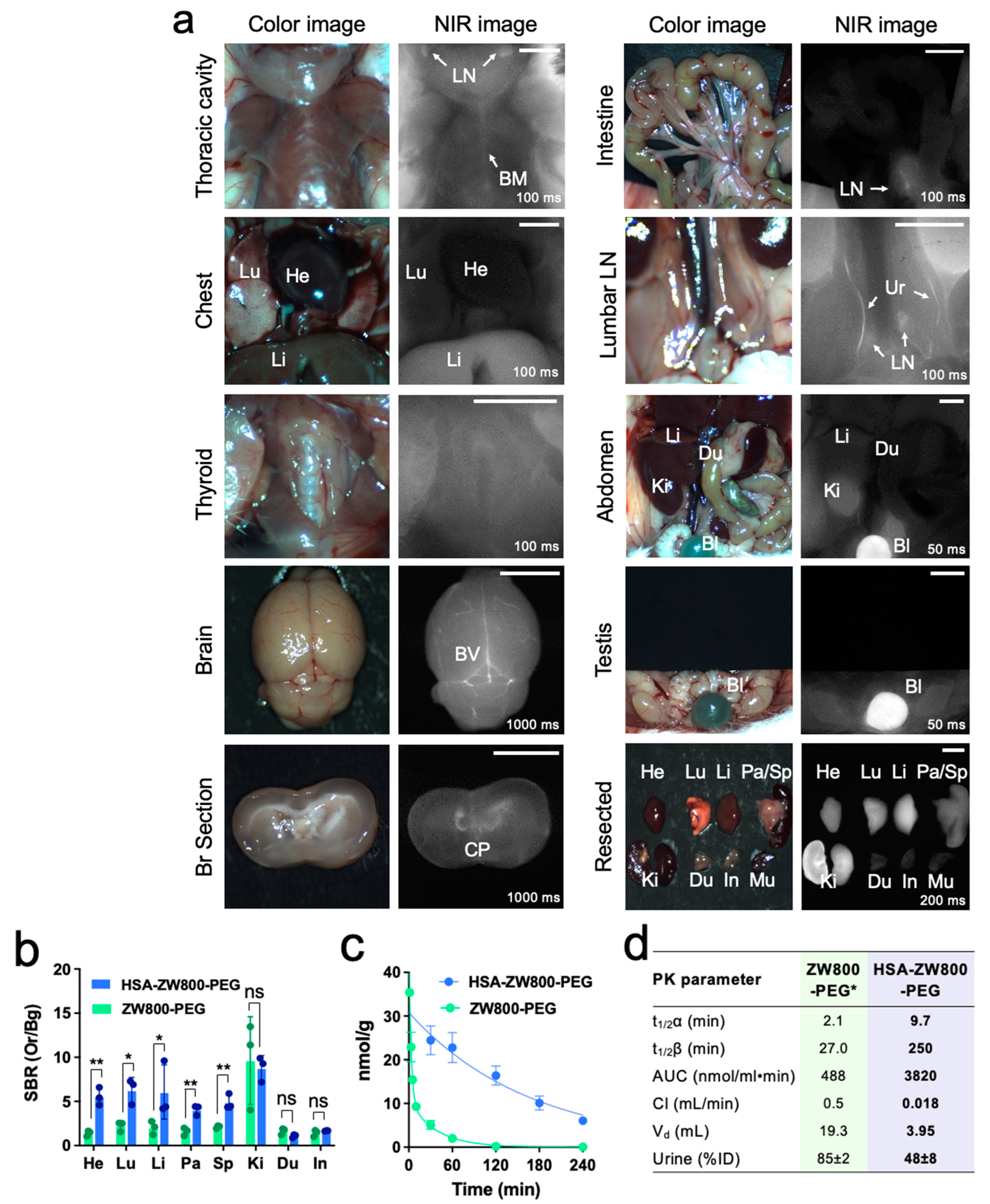
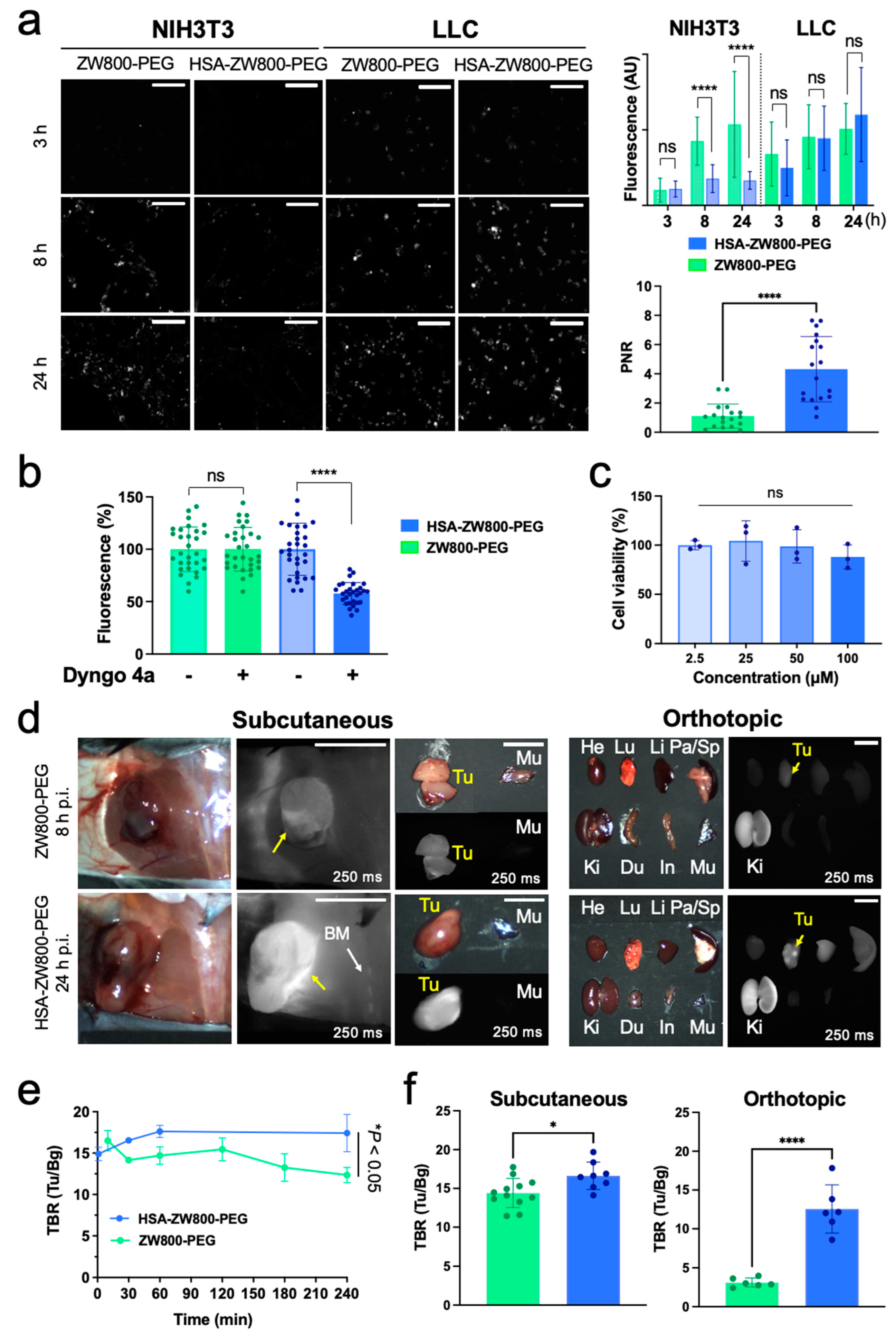
2.3. In Vivo Biodistribution and Pharmacokinetics
2.4. Tumor Targetability and Cytotoxicity Assay
3. Discussion
4. Materials and Methods
4.1. Synthesis and Purification of HSA-ZW800-PEG
4.2. Determination of Molar Absorptivity and Labeling Ratio
4.3. Photostability Assay Using NIR Light
4.4. In Vivo Biodistribution and Pharmacokinetics
4.5. Cellular Uptake and Viability Tests for ZW800-PEG and HSA-ZW800-PEG
4.6. In Vitro Inhibition Study
4.7. Tumor-Bearing Mouse Models
4.8. Quantitative and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, B.; Chu, F.; Bi, A.; Huang, X.; Fang, Y.; Liu, M.; Chen, F.; Li, Y.; Zeng, W. Fidelity-oriented fluorescence imaging probes for beta-galactosidase: From accurate diagnosis to precise treatment. Biotechnol. Adv. 2023, 68, 108244. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Monaghan, M.G.; Niesner, R.; Dmitriev, R.I. Probing organoid metabolism using Fluorescence Lifetime Imaging Microscopy (FLIM): The next frontier of drug discovery and disease understanding. Adv. Drug Deliv. Rev. 2023, 201, 115081. [Google Scholar] [CrossRef] [PubMed]
- Thenuwara, G.; Curtin, J.; Tian, F. Advances in Diagnostic Tools and Therapeutic Approaches for Gliomas: A Comprehensive Review. Sensors 2023, 23, 9842. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Ren, T. Near infrared fluorescent probes for detecting and imaging active small molecules. Coord. Chem. Rev. 2023, 482, 215080. [Google Scholar] [CrossRef]
- Yin, H.; Jiang, W.; Liu, Y.; Zhang, D.; Wu, F.; Zhang, Y.; Li, C.; Chen, G.; Wang, Q. Advanced near-infrared light approaches for neuroimaging and neuromodulation. BMEMat 2023, 1, e12023. [Google Scholar] [CrossRef]
- Li, X.; Schumann, C.; Albarqi, H.A.; Lee, C.J.; Alani, A.W.; Bracha, S.; Milovancev, M.; Taratula, O.; Taratula, O. A tumor-activatable theranostic nanomedicine platform for NIR fluorescence-guided surgery and combinatorial phototherapy. Theranostics 2018, 8, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Vitorino, R.; Barros, A.S.; Guedes, S.; Caixeta, D.C.; Sabino-Silva, R. Diagnostic and monitoring applications using near infrared (NIR) spectroscopy in cancer and other diseases. Photodiagn. Photodyn. Ther. 2023, 42, 103633. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Zhang, F. Molecular Fluorophores for Deep-Tissue Bioimaging. ACS Cent. Sci. 2020, 6, 1302–1316. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, F.; Lei, Z. The pursuit of polymethine fluorophores with NIR-II emission and high brightness for in vivo applications. Chem. Sci. 2022, 13, 11280–11293. [Google Scholar] [CrossRef]
- Jeon, O.H.; Choi, B.H.; Rho, J.; Kim, K.; Lee, J.H.; Lee, J.; Kim, B.M.; Kim, H.K. Optimization of Indocyanine Green for Intraoperative Fluorescent Image-Guided Localization of Lung Cancer; Analysis Based on Solid Component of Lung Nodule. Cancers 2023, 15, 3643. [Google Scholar] [CrossRef]
- On, K.C.; Rho, J.; Yoon, H.Y.; Chang, H.; Yhee, J.Y.; Yoon, J.S.; Jeong, S.Y.; Kim, H.K.; Kim, K. Tumor-Targeting Glycol Chitosan Nanoparticles for Image-Guided Surgery of Rabbit Orthotopic VX2 Lung Cancer. Pharmaceutics 2020, 12, 621. [Google Scholar] [CrossRef] [PubMed]
- Hutteman, M.; Mieog, J.S.; van der Vorst, J.R.; Liefers, G.J.; Putter, H.; Lowik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Randomized, double-blind comparison of indocyanine green with or without albumin premixing for near-infrared fluorescence imaging of sentinel lymph nodes in breast cancer patients. Breast Cancer Res. Treat. 2011, 127, 163–170. [Google Scholar] [CrossRef]
- van der Vorst, J.R.; Schaafsma, B.E.; Verbeek, F.P.; Hutteman, M.; Mieog, J.S.; Lowik, C.W.; Liefers, G.J.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Randomized comparison of near-infrared fluorescence imaging using indocyanine green and 99m technetium with or without patent blue for the sentinel lymph node procedure in breast cancer patients. Ann. Surg. Oncol. 2012, 19, 4104–4111. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, F.P.; Troyan, S.L.; Mieog, J.S.; Liefers, G.J.; Moffitt, L.A.; Rosenberg, M.; Hirshfield-Bartek, J.; Gioux, S.; van de Velde, C.J.; Vahrmeijer, A.L.; et al. Near-infrared fluorescence sentinel lymph node mapping in breast cancer: A multicenter experience. Breast Cancer Res. Treat. 2014, 143, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Sevieri, M.; Silva, F.; Bonizzi, A.; Sitia, L.; Truffi, M.; Mazzucchelli, S.; Corsi, F. Indocyanine Green Nanoparticles: Are They Compelling for Cancer Treatment? Front. Chem. 2020, 8, 535. [Google Scholar] [CrossRef] [PubMed]
- Porcu, E.; Salis, A.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol. Adv. 2016, 34, 768–789. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Nasr, K.; Alyabyev, S.; Feith, D.; Lee, J.H.; Kim, S.H.; Ashitate, Y.; Hyun, H.; Patonay, G.; Strekowski, L.; et al. Synthesis and in vivo fate of zwitterionic near-infrared fluorophores. Angew. Chem. Int. Ed. Engl. 2011, 50, 6258–6263. [Google Scholar] [CrossRef]
- Hyun, H.; Owens, E.A.; Narayana, L.; Wada, H.; Gravier, J.; Bao, K.; Frangioni, J.V.; Choi, H.S.; Henary, M. Central C-C bonding increases optical and chemical stability of NIR fluorophores. RSC Adv. 2014, 4, 58762–58768. [Google Scholar] [CrossRef]
- Yang, C.; Wang, H.; Yokomizo, S.; Hickey, M.; Chang, H.; Kang, H.; Fukuda, T.; Song, M.Y.; Lee, S.Y.; Park, J.W.; et al. ZW800-PEG: A Renal Clearable Zwitterionic Near-Infrared Fluorophore for Potential Clinical Translation. Angew. Chem. Int. Ed. Engl. 2021, 60, 13847–13852. [Google Scholar] [CrossRef]
- Bao, K.; Tully, M.; Cardenas, K.; Wang, H.; Srinivas, S.; Rho, J.; Jeon, O.H.; Dinh, J.; Yokomizo, S.; McDonnell, R. Ultralow Background Near-Infrared Fluorophores with Dual-Channel Intraoperative Imaging Capability. Adv. Healthc. Mater. 2023, 12, 2203134. [Google Scholar] [CrossRef]
- Deng, H.; Li, X.; Ju, J.; Mo, X.; Ge, G.; Zhu, X. Multifunctional nanoprobes for macrophage imaging. Biomaterials 2022, 290, 121824. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Deliv. Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zhang, J.; Yang, F.; Song, W.; Han, D.; Wen, W.; Qin, W. Quicker, deeper and stronger imaging: A review of tumor-targeted, near-infrared fluorescent dyes for fluorescence guided surgery in the preclinical and clinical stages. Eur. J. Pharm. Biopharm. 2020, 152, 123–143. [Google Scholar] [CrossRef]
- Deng, H.; Konopka, C.J.; Prabhu, S.; Sarkar, S.; Medina, N.G.; Fayyaz, M.; Arogundade, O.H.; Vidana Gamage, H.E.; Shahoei, S.H.; Nall, D.; et al. Dextran-Mimetic Quantum Dots for Multimodal Macrophage Imaging In Vivo, Ex Vivo, and In Situ. ACS Nano 2022, 16, 1999–2012. [Google Scholar] [CrossRef]
- Sabu, A.; Lin, J.-Y.; Doong, R.-A.; Huang, Y.-F.; Chiu, H.-C. Prospects of an engineered tumor-targeted nanotheranostic platform based on NIR-responsive upconversion nanoparticles. Mater. Adv. 2021, 2, 7101–7117. [Google Scholar] [CrossRef]
- Parungo, C.P.; Ohnishi, S.; De Grand, A.M.; Laurence, R.G.; Soltesz, E.G.; Colson, Y.L.; Kang, P.M.; Mihaljevic, T.; Cohn, L.H.; Frangioni, J.V. In vivo optical imaging of pleural space drainage to lymph nodes of prognostic significance. Ann. Surg. Oncol. 2004, 11, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Troyan, S.L.; Kianzad, V.; Gibbs-Strauss, S.L.; Gioux, S.; Matsui, A.; Oketokoun, R.; Ngo, L.; Khamene, A.; Azar, F.; Frangioni, J.V. The FLARE intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann. Surg. Oncol. 2009, 16, 2943–2952. [Google Scholar] [CrossRef]
- Inoue, K.; Gibbs, S.L.; Liu, F.; Lee, J.H.; Xie, Y.; Ashitate, Y.; Fujii, H.; Frangioni, J.V.; Choi, H.S. Microscopic validation of macroscopic in vivo images enabled by same-slide optical and nuclear fusion. J. Nucl. Med. 2014, 55, 1899–1904. [Google Scholar] [CrossRef]
- Sleep, D.; Cameron, J.; Evans, L.R. Albumin as a versatile platform for drug half-life extension. Biochim. Biophys. Acta (BBA) Gen. Subj. 2013, 1830, 5526–5534. [Google Scholar] [CrossRef]
- Usama, S.M.; Park, G.K.; Nomura, S.; Baek, Y.; Choi, H.S.; Burgess, K. Role of albumin in accumulation and persistence of tumor-seeking cyanine dyes. Bioconjug. Chem. 2020, 31, 248–259. [Google Scholar] [CrossRef]
- Deng, H.; Konopka, C.J.; Cross, T.-W.L.; Swanson, K.S.; Dobrucki, L.W.; Smith, A.M. Multimodal Nanocarrier Probes Reveal Superior Biodistribution Quantification by Isotopic Analysis over Fluorescence. ACS Nano 2020, 14, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Merlot, A.M.; Kalinowski, D.S.; Richardson, D.R. Unraveling the mysteries of serum albumin-more than just a serum protein. Front. Physiol. 2014, 5, 299. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Shin, G.; Lim, S.I. Human serum albumin binders: A piggyback ride for long-acting therapeutics. Drug Discov. Today 2023, 28, 103738. [Google Scholar] [CrossRef] [PubMed]
- Colyer, C. Noncovalent labeling of proteins in capillary electrophoresis with laser-induced fluorescence detection. Cell Biochem. Biophys. 2000, 33, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Aslan, K.; Leonenko, Z.; Lakowicz, J.R.; Geddes, C.D. Fast and slow deposition of silver nanorods on planar surfaces: Application to metal-enhanced fluorescence. J. Phys. Chem. B 2005, 109, 3157–3162. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, S.; Lomnes, S.J.; Laurence, R.G.; Gogbashian, A.; Mariani, G.; Frangioni, J.V. Organic alternatives to quantum dots for intraoperative near-infrared fluorescent sentinel lymph node mapping. Mol. Imaging 2005, 4, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Kim, H.; Sohn, D.K.; Eom, J.B.; Seo, Y.S.; Yoon, H.M.; Choi, Y. Indocyanine green-loaded injectable alginate hydrogel as a marker for precision cancer surgery. Quant. Imaging Med. Surg. 2020, 10, 779–788. [Google Scholar] [CrossRef]
- Li, X.; Fu, Y.; Ma, L.; Wang, Z.; Zhang, H. Spectrometric study on the interaction of indocyanine green with human serum albumin. Chem. Res. Chin. Univ. 2016, 32, 343–347. [Google Scholar] [CrossRef]
- Kummari, R.; Bose, K. Gel Filtration Chromatography; Springer: Berlin/Heidelberg, Germany, 2022; pp. 199–219. [Google Scholar]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Al-Ghobashy, M.A.; Mostafa, M.M.; Abed, H.S.; Fathalla, F.A.; Salem, M.Y. Correlation between Dynamic Light Scattering and Size Exclusion High Performance Liquid Chromatography for Monitoring the Effect of pH on Stability of Biopharmaceuticals. J. Chromatogr. B 2017, 1060, 1–9. [Google Scholar] [CrossRef]
- James, S.; Neuhaus, K.; Murphy, M.; Leahy, M. Contrast agents for photoacoustic imaging: A review of stem cell tracking. Stem Cell Res. Ther. 2021, 12, 511. [Google Scholar] [CrossRef]
- Aird, W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 2007, 100, 158–173. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Frangioni, J.V. Nanoparticles for biomedical imaging: Fundamentals of clinical translation. Mol. Imaging 2010, 9, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Komiya, K.; Nakamura, T.; Nakashima, C.; Takahashi, K.; Umeguchi, H.; Watanabe, N.; Sato, A.; Takeda, Y.; Kimura, S.; Sueoka-Aragane, N. SPARC is a possible predictive marker for albumin-bound paclitaxel in non-small-cell lung cancer. OncoTargets Ther. 2016, 9, 6663–6668. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Park, G.; Hong, G.H.; Choi, J.; Choi, H.S. Design considerations for targeted optical contrast agents. Quant. Imaging Med. Surg. 2012, 2, 266–273. [Google Scholar] [PubMed]
- Cho, H.; Jeon, S.I.; Ahn, C.-H.; Shim, M.K.; Kim, K. Emerging albumin-binding anticancer drugs for tumor-targeted drug delivery: Current understandings and clinical translation. Pharmaceutics 2022, 14, 728. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.P.; Trieu, V.; Hwang, L.Y.; Wu, R.; Soon-Shiong, P.; Gradishar, W.J. Improved effectiveness of nanoparticle albumin-bound (nab) paclitaxel versus polysorbate-based docetaxel in multiple xenografts as a function of HER2 and SPARC status. Anti-Cancer Drugs 2008, 19, 899–909. [Google Scholar] [CrossRef]
- Feng, J.; Tang, L. SPARC in Tumor Pathophysiology and as a Potential Therapeutic Target. Curr. Pharm. Des. 2014, 20, 6182–6190. [Google Scholar] [CrossRef]
- Zsila, F. Subdomain IB is the third major drug binding region of human serum albumin: Toward the three-sites model. Mol. Pharm. 2013, 10, 1668–1682. [Google Scholar] [CrossRef]
- Park, C.R.; Song, M.G.; Park, J.Y.; Youn, H.; Chung, J.K.; Jeong, J.M.; Lee, Y.S.; Cheon, G.J.; Kang, K.W. Conjugation of arginylglycylaspartic acid to human serum albumin decreases the tumor-targeting effect of albumin by hindering its secreted protein acidic and rich in cysteine-mediated accumulation in tumors. Am. J. Transl. Res. 2020, 12, 2488–2498. [Google Scholar]
- Kim, J.; Cho, H.; Lim, D.K.; Joo, M.K.; Kim, K. Perspectives for Improving the Tumor Targeting of Nanomedicine via the EPR Effect in Clinical Tumors. Int. J. Mol. Sci. 2023, 24, 10082. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Shen, N.; Sun, J.; Tang, Z.; Chen, X. Destruction of tumor vasculature by vascular disrupting agents in overcoming the limitation of EPR effect. Adv. Drug Deliv. Rev. 2022, 183, 114138. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Min, H.S.; Ku, S.H.; Son, S.; Kwon, I.C.; Kim, S.H.; Kim, K. Tumor-targeting glycol chitosan nanoparticles as a platform delivery carrier in cancer diagnosis and therapy. Nanomedicine 2014, 9, 1697–1713. [Google Scholar] [CrossRef]
- Bertino, E.M.; Williams, T.M.; Nana-Sinkam, S.P.; Shilo, K.; Chatterjee, M.; Mo, X.; Rahmani, M.; Phillips, G.S.; Villalona-Calero, M.A.; Otterson, G.A. Stromal caveolin-1 is associated with response and survival in a phase II trial of nab-paclitaxel with carboplatin for advanced NSCLC patients. Clin. Lung Cancer 2015, 16, 466–474.e4. [Google Scholar] [CrossRef][Green Version]
- Um, W.; Park, J.; Youn, A.; Cho, H.; Lim, S.; Lee, J.W.; Yoon, H.Y.; Lim, D.-K.; Park, J.H.; Kim, K. A comparative study on albumin-binding molecules for targeted tumor delivery through covalent and noncovalent approach. Bioconjug. Chem. 2019, 30, 3107–3118. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, P.; Ser, J.; Cardenas, K.; Kim, H.J.; Hickey, M.; Jang, J.; Gladstone, J.; Bailey, A.; Dinh, J.; Nguyen, V.; et al. HSA-ZW800-PEG for Enhanced Optophysical Stability and Tumor Targeting. Int. J. Mol. Sci. 2024, 25, 559. https://doi.org/10.3390/ijms25010559
Jang P, Ser J, Cardenas K, Kim HJ, Hickey M, Jang J, Gladstone J, Bailey A, Dinh J, Nguyen V, et al. HSA-ZW800-PEG for Enhanced Optophysical Stability and Tumor Targeting. International Journal of Molecular Sciences. 2024; 25(1):559. https://doi.org/10.3390/ijms25010559
Chicago/Turabian StyleJang, Paul, Jinhui Ser, Kevin Cardenas, Hajin Joanne Kim, Morgan Hickey, Jiseon Jang, Jason Gladstone, Aisha Bailey, Jason Dinh, Vy Nguyen, and et al. 2024. "HSA-ZW800-PEG for Enhanced Optophysical Stability and Tumor Targeting" International Journal of Molecular Sciences 25, no. 1: 559. https://doi.org/10.3390/ijms25010559
APA StyleJang, P., Ser, J., Cardenas, K., Kim, H. J., Hickey, M., Jang, J., Gladstone, J., Bailey, A., Dinh, J., Nguyen, V., DeMarco, E., Srinivas, S., Kang, H., Kashiwagi, S., Bao, K., Yamashita, A., & Choi, H. S. (2024). HSA-ZW800-PEG for Enhanced Optophysical Stability and Tumor Targeting. International Journal of Molecular Sciences, 25(1), 559. https://doi.org/10.3390/ijms25010559








