The MADF-BESS Protein CP60 Is Recruited to Insulators via CP190 and Has Redundant Functions in Drosophila
Abstract
1. Introduction
2. Results
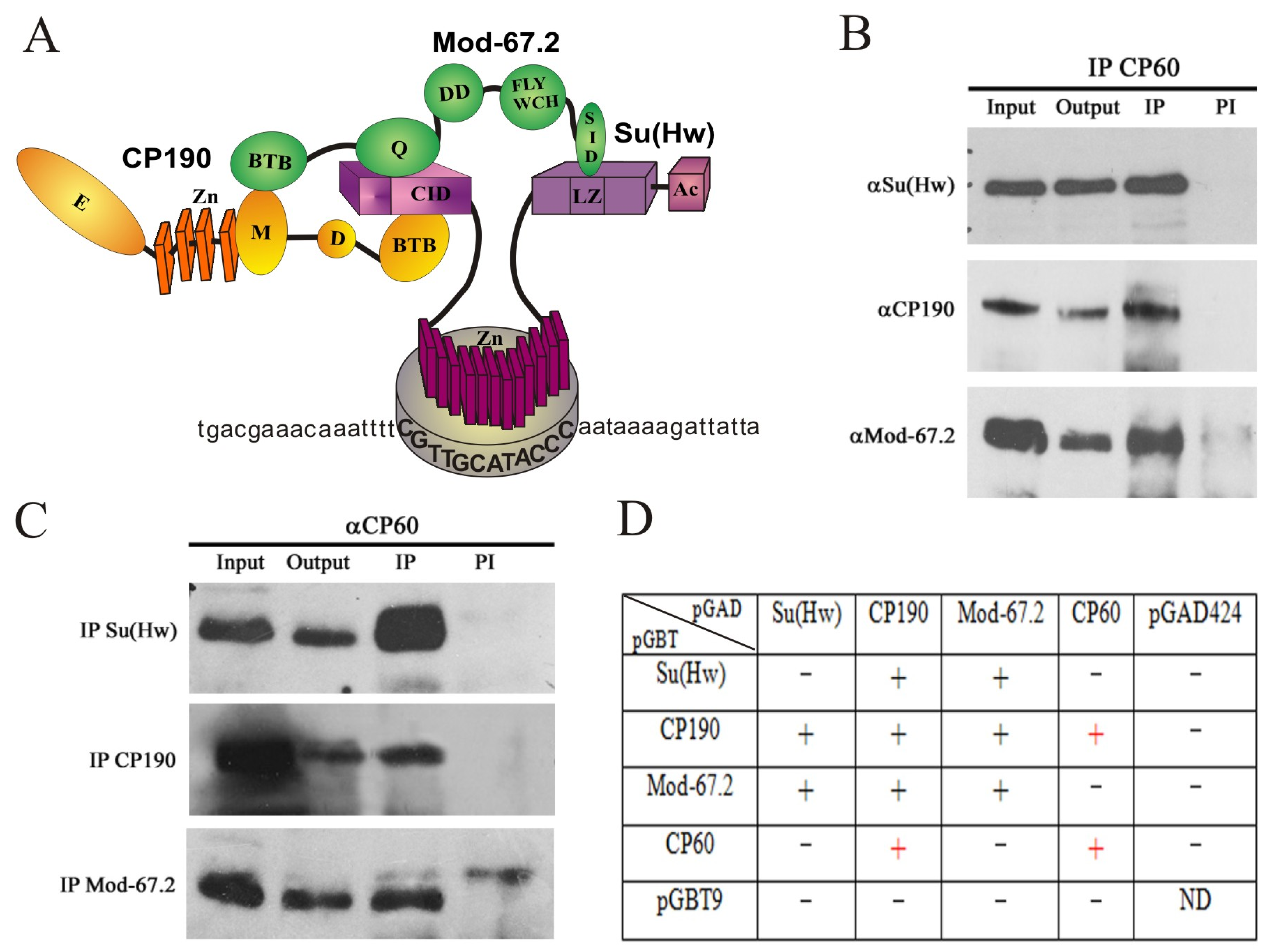
2.1. CP60 Is a Stable Component of the Su(Hw) Insulator Complex
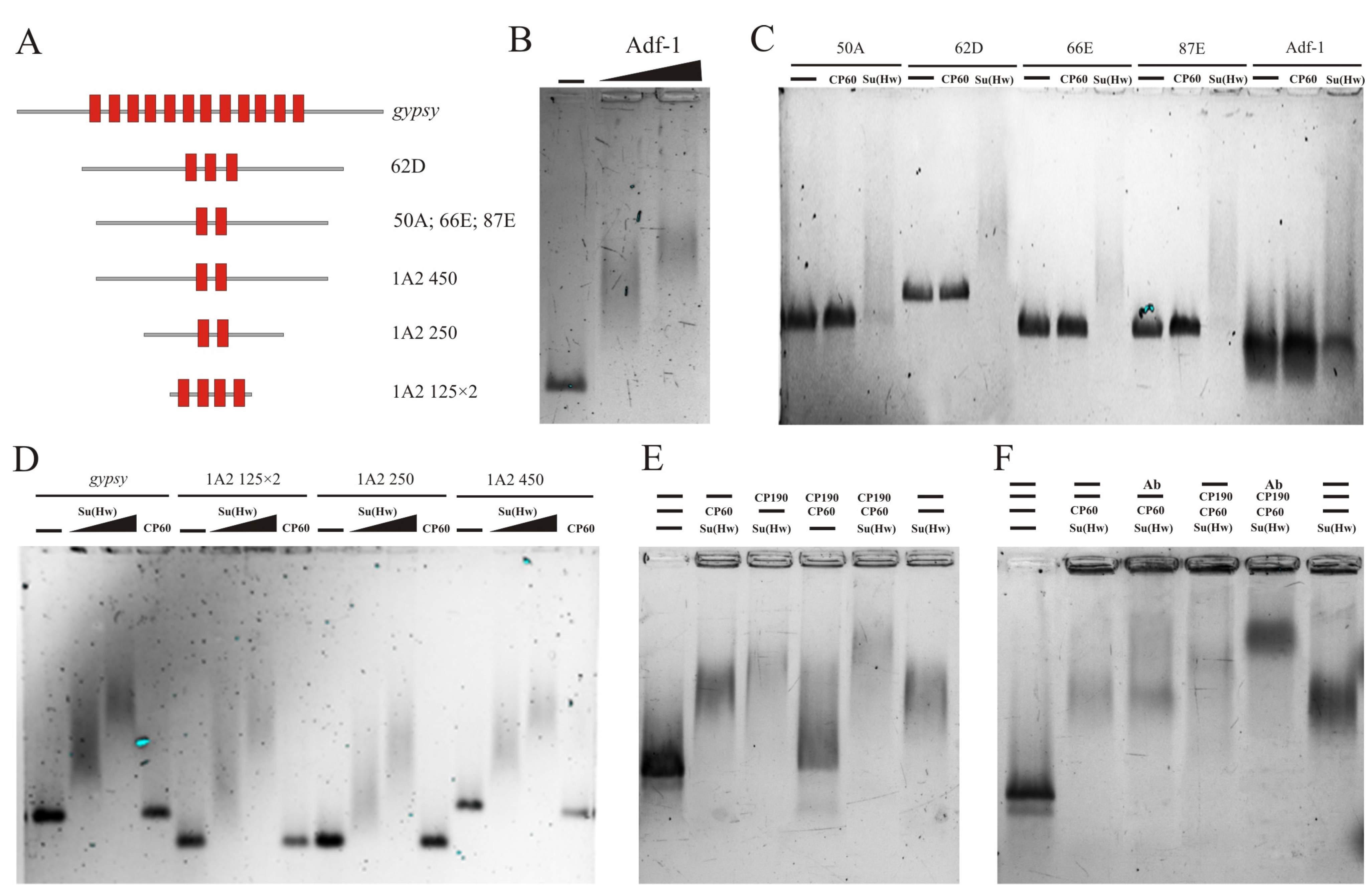
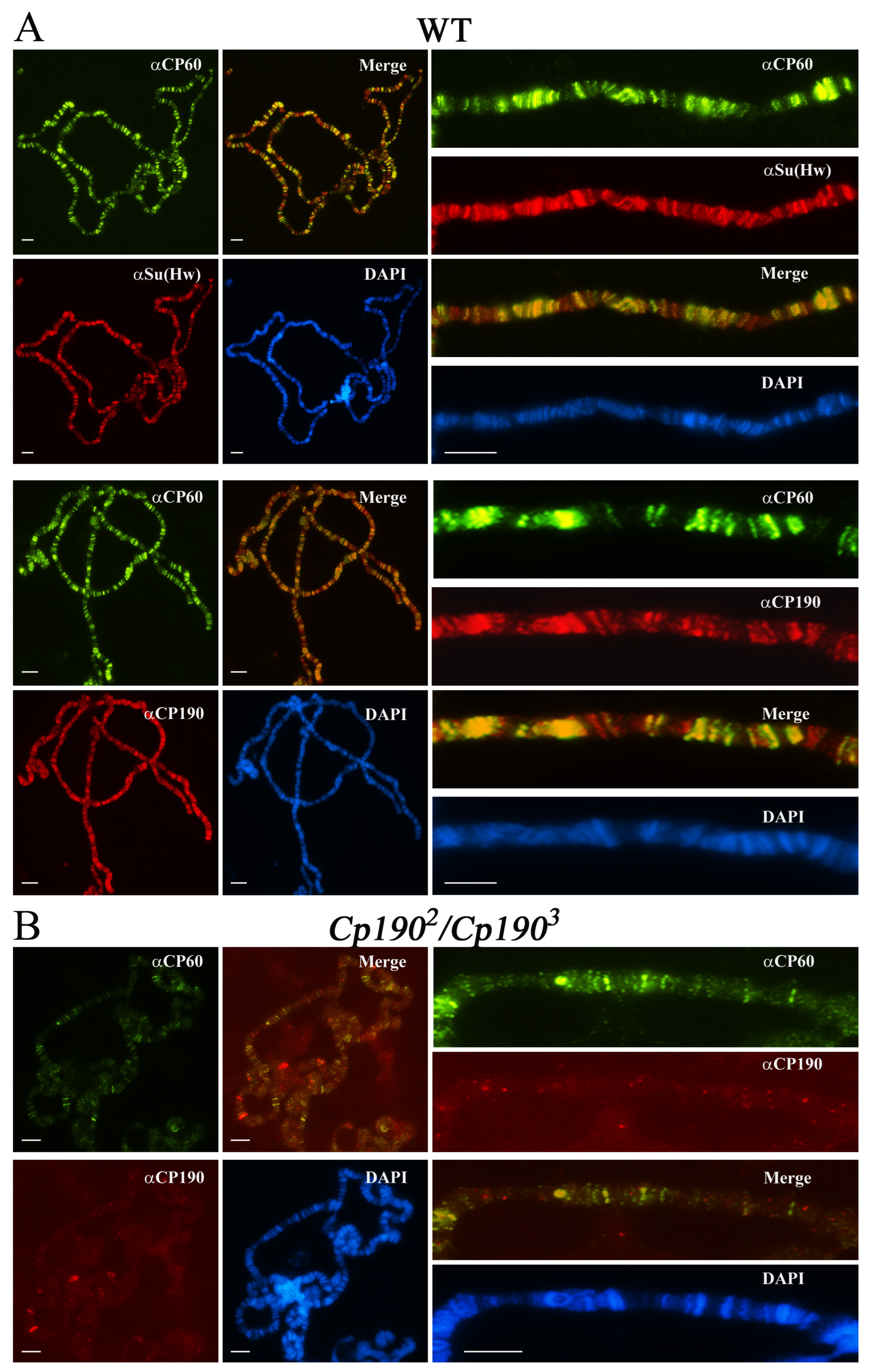
2.2. CP60 Binds to the Su(Hw) Insulator Sites via Interaction with CP190
2.3. The MADF Domain of CP60 Does Not Interact with the Su(Hw) Binding Regions
2.4. Mapping CP60 Domains Involved in the Interaction with CP190 and Homodimerization
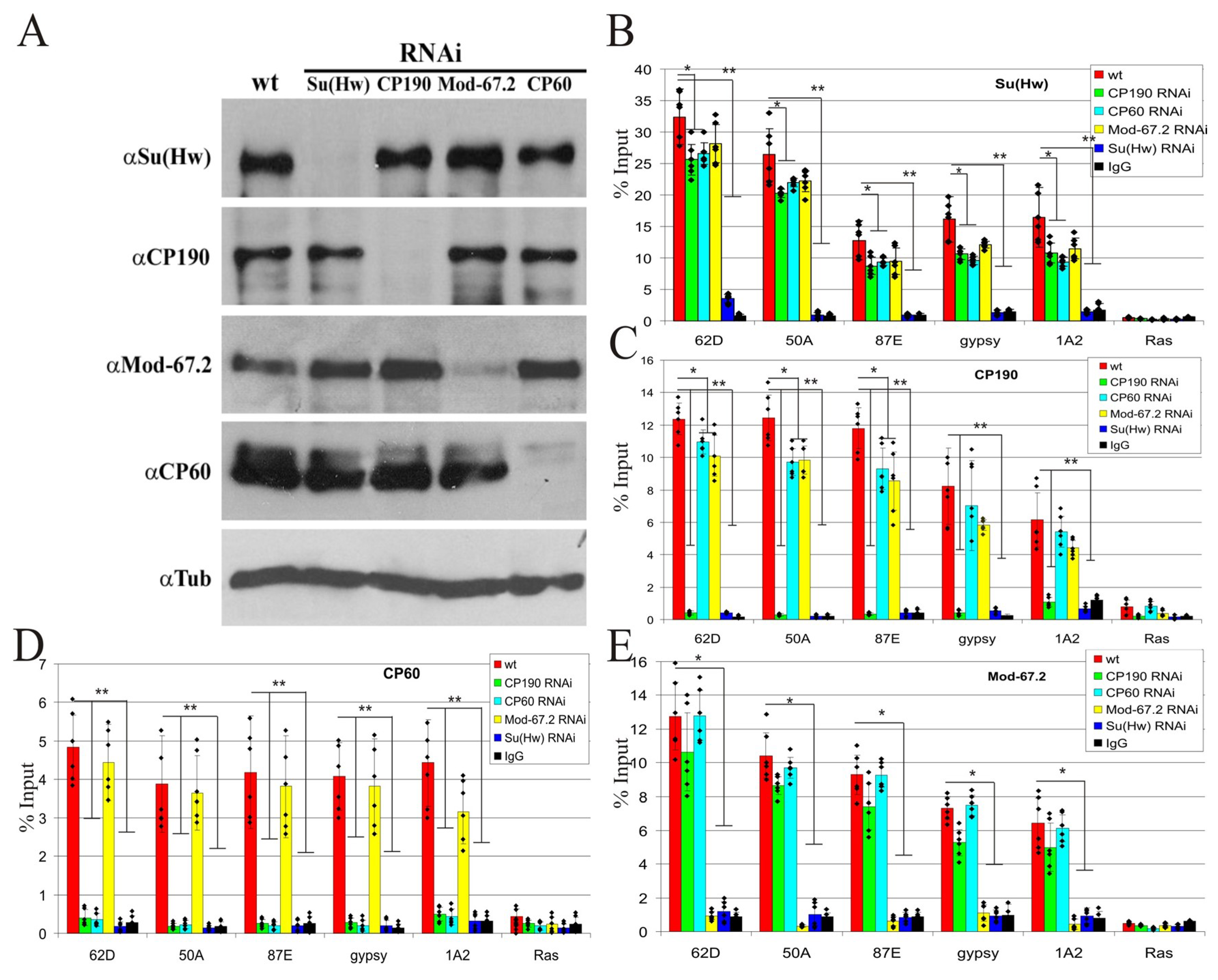
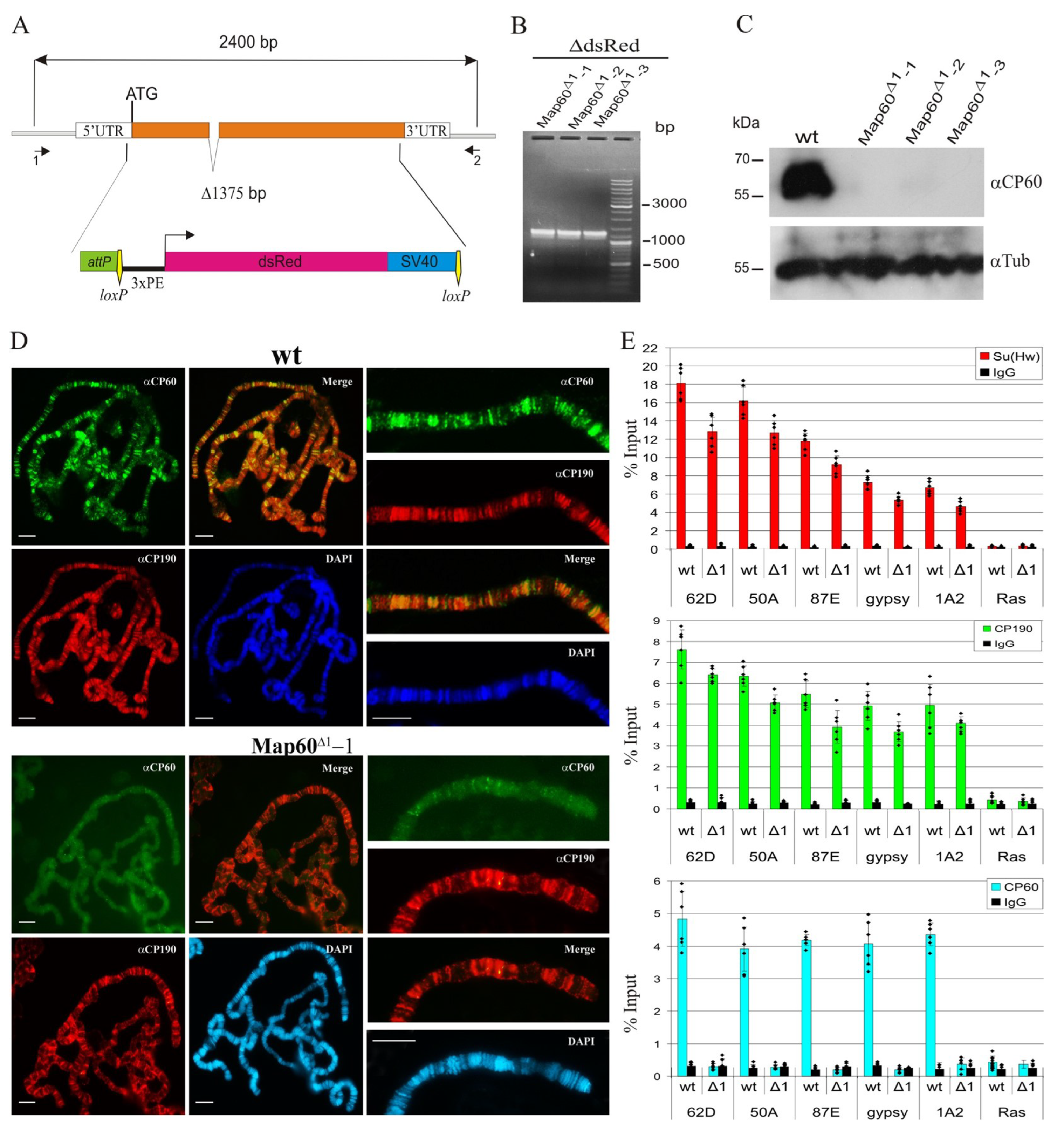
2.5. CP60 Is a Redundant Protein
2.6. CP190 Is the Main Partner of CP60 at Chromatin Sites
3. Discussion
4. Materials and Methods
4.1. Plasmids and Cloning
4.2. RNA Interference in Drosophila S2 Cells
4.3. Immunoprecipitation Assay
4.4. Yeast Two-Hybrid Assay
4.5. Chromatin Preparation and ChIP Analysis
4.6. Protein Expression and Purification
4.7. Electrophoretic Mobility Shift Assay (EMSA)
4.8. Immunostaining of Polytene Chromosomes
4.9. Antibodies
4.10. Germ-Line Transformation, Genetic Crosses, and Phenotypic Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavalheiro, G.R.; Pollex, T.; Furlong, E.E. To Loop or Not to Loop: What Is the Role of TADs in Enhancer Function and Gene Regulation? Curr. Opin. Genet. Dev. 2021, 67, 119–129. [Google Scholar] [CrossRef]
- Furlong, E.E.M.; Levine, M. Developmental Enhancers and Chromosome Topology. Science 2018, 361, 1341–1345. [Google Scholar] [CrossRef]
- Kim, J.; Dean, A. Enhancers Navigate the Three-Dimensional Genome to Direct Cell Fate Decisions. Curr. Opin. Struct. Biol. 2021, 71, 101–109. [Google Scholar] [CrossRef]
- Razin, S.V.; Ulianov, S.V.; Iarovaia, O.V. Enhancer Function in the 3D Genome. Genes 2023, 14, 1277. [Google Scholar] [CrossRef]
- da Costa-Nunes, J.A.; Noordermeer, D. TADs: Dynamic Structures to Create Stable Regulatory Functions. Curr. Opin. Struct. Biol. 2023, 81, 102622. [Google Scholar] [CrossRef]
- Di Stefano, M.; Cavalli, G. Integrative Studies of 3D Genome Organization and Chromatin Structure. Curr. Opin. Struct. Biol. 2022, 77, 102493. [Google Scholar] [CrossRef]
- Razin, S.V.; Kantidze, O.L. The Twisted Path of the 3D Genome: Where Does It Lead? Trends Biochem. Sci. 2022, 47, 736–744. [Google Scholar] [CrossRef]
- Uyehara, C.M.; Apostolou, E. 3D Enhancer-Promoter Interactions and Multi-Connected Hubs: Organizational Principles and Functional Roles. Cell Rep. 2023, 112068. [Google Scholar] [CrossRef]
- Chen, H.; Levo, M.; Barinov, L.; Fujioka, M.; Jaynes, J.B.; Gregor, T. Dynamic Interplay between Enhancer-Promoter Topology and Gene Activity. Nat. Genet. 2018, 50, 1296–1303. [Google Scholar] [CrossRef]
- Kyrchanova, O.; Sokolov, V.; Georgiev, P. Mechanisms of Interaction between Enhancers and Promoters in Three Drosophila Model Systems. Int. J. Mol. Sci. 2023, 24, 2855. [Google Scholar] [CrossRef]
- Levo, M.; Raimundo, J.; Bing, X.Y.; Sisco, Z.; Batut, P.J.; Ryabichko, S.; Gregor, T.; Levine, M.S. Transcriptional Coupling of Distant Regulatory Genes in Living Embryos. Nature 2022, 605, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tang, X.; Bing, X.; Catalano, C.; Li, T.; Dolsten, G.; Wu, C.; Levine, M. GAGA-Associated Factor Fosters Loop Formation in the Drosophila Genome. Mol. Cell 2023, 83, 1519–1526.e4. [Google Scholar] [CrossRef] [PubMed]
- Melnikova, L.S.; Georgiev, P.G.; Golovnin, A.K. The Functions and Mechanisms of Action of Insulators in the Genomes of Higher Eukaryotes. Acta Naturae 2020, 12, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lei, E.P. Function and Regulation of Chromatin Insulators in Dynamic Genome Organization. Curr. Opin. Cell Biol. 2019, 58, 61–68. [Google Scholar] [CrossRef]
- Matthews, N.E.; White, R. Chromatin Architecture in the Fly: Living without CTCF/Cohesin Loop Extrusion? Alternating Chromatin States Provide a Basis for Domain Architecture in Drosophila. Bioessays 2019, 41, e1900048. [Google Scholar] [CrossRef]
- Kyrchanova, O.V.; Bylino, O.V.; Georgiev, P.G. Mechanisms of Enhancer-Promoter Communication and Chromosomal Architecture in Mammals and Drosophila. Front. Genet. 2022, 13, 1081088. [Google Scholar] [CrossRef]
- Peterson, S.C.; Samuelson, K.B.; Hanlon, S.L. Multi-Scale Organization of the Drosophila Melanogaster Genome. Genes 2021, 12, 817. [Google Scholar] [CrossRef]
- Maksimenko, O.G.; Fursenko, D.V.; Belova, E.V.; Georgiev, P.G. CTCF As an Example of DNA-Binding Transcription Factors Containing Clusters of C2H2-Type Zinc Fingers. Acta Naturae 2021, 13, 31–46. [Google Scholar] [CrossRef]
- Baxley, R.M.; Bullard, J.D.; Klein, M.W.; Fell, A.G.; Morales-Rosado, J.A.; Duan, T.; Geyer, P.K. Deciphering the DNA Code for the Function of the Drosophila Polydactyl Zinc Finger Protein Suppressor of Hairy-Wing. Nucleic Acids Res. 2017, 45, 4463–4478. [Google Scholar] [CrossRef]
- Cavalheiro, G.R.; Girardot, C.; Viales, R.R.; Pollex, T.; Cao, T.B.N.; Lacour, P.; Feng, S.; Rabinowitz, A.; Furlong, E.E.M. CTCF, BEAF-32, and CP190 Are Not Required for the Establishment of TADs in Early Drosophila Embryos but Have Locus-Specific Roles. Sci. Adv. 2023, 9, eade1085. [Google Scholar] [CrossRef]
- Kahn, T.G.; Savitsky, M.; Kuong, C.; Jacquier, C.; Cavalli, G.; Chang, J.-M.; Schwartz, Y.B. Topological Screen Identifies Hundreds of Cp190- and CTCF-Dependent Drosophila Chromatin Insulator Elements. Sci. Adv. 2023, 9, eade0090. [Google Scholar] [CrossRef]
- Sabirov, M.; Kyrchanova, O.; Pokholkova, G.V.; Bonchuk, A.; Klimenko, N.; Belova, E.; Zhimulev, I.F.; Maksimenko, O.; Georgiev, P. Mechanism and Functional Role of the Interaction between CP190 and the Architectural Protein Pita in Drosophila Melanogaster. Epigenetics Chromatin 2021, 14, 16. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, S.H.; Shouche, Y.S.; Mishra, R.K. Functional Sub-Division of the Drosophila Genome via Chromatin Looping: The Emerging Importance of CP190. Nucleus 2013, 4, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.; Sheehan, B.; South, H.; Akbari, O.; Pai, C.Y. The Chromosomal Association/Dissociation of the Chromatin Insulator Protein Cp190 of Drosophila Melanogaster Is Mediated by the BTB/POZ Domain and Two Acidic Regions. BMC Cell Biol. 2010, 11, 101. [Google Scholar] [CrossRef]
- Plevock, K.M.; Galletta, B.J.; Slep, K.C.; Rusan, N.M. Newly Characterized Region of CP190 Associates with Microtubules and Mediates Proper Spindle Morphology in Drosophila Stem Cells. PLoS ONE 2015, 10, e0144174. [Google Scholar] [CrossRef]
- Cuartero, S.; Fresan, U.; Reina, O.; Planet, E.; Espinas, M.L. Ibf1 and Ibf2 Are Novel CP190-Interacting Proteins Required for Insulator Function. EMBO J. 2014, 33, 637–647. [Google Scholar] [CrossRef]
- Pai, C.Y.; Lei, E.P.; Ghosh, D.; Corces, V.G. The Centrosomal Protein CP190 Is a Component of the Gypsy Chromatin Insulator. Mol. Cell 2004, 16, 737–748. [Google Scholar] [CrossRef]
- Bag, I.; Chen, S.; Rosin, L.F.; Chen, Y.; Liu, C.-Y.; Yu, G.-Y.; Lei, E.P. M1BP Cooperates with CP190 to Activate Transcription at TAD Borders and Promote Chromatin Insulator Activity. Nat. Commun. 2021, 12, 4170. [Google Scholar] [CrossRef]
- Santana, J.F.; Parida, M.; Long, A.; Wankum, J.; Lilienthal, A.J.; Nukala, K.M.; Manak, J.R. The Dm-Myb Oncoprotein Contributes to Insulator Function and Stabilizes Repressive H3K27me3 PcG Domains. Cell Rep. 2020, 30, 3218–3228.e5. [Google Scholar] [CrossRef]
- Sabirov, M.; Popovich, A.; Boyko, K.; Nikolaeva, A.; Kyrchanova, O.; Maksimenko, O.; Popov, V.; Georgiev, P.; Bonchuk, A. Mechanisms of CP190 Interaction with Architectural Proteins in Drosophila Melanogaster. Int. J. Mol. Sci. 2021, 22, 12400. [Google Scholar] [CrossRef]
- Kellogg, D.R.; Alberts, B.M. Purification of a Multiprotein Complex Containing Centrosomal Proteins from the Drosophila Embryo by Chromatography with Low-Affinity Polyclonal Antibodies. Mol. Biol. Cell 1992, 3, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oegema, K.; Marshall, W.F.; Sedat, J.W.; Alberts, B.M. Two Proteins That Cycle Asynchronously between Centrosomes and Nuclear Structures: Drosophila CP60 and CP190. J. Cell Sci. 1997, 110, 1573–1583. [Google Scholar] [PubMed]
- Kellogg, D.R.; Oegema, K.; Raff, J.; Schneider, K.; Alberts, B.M. CP60: A Microtubule-Associated Protein That Is Localized to the Centrosome in a Cell Cycle-Specific Manner. Mol. Biol. Cell 1995, 6, 1673–1684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bartkuhn, M.; Straub, T.; Herold, M.; Herrmann, M.; Rathke, C.; Saumweber, H.; Gilfillan, G.D.; Becker, P.B.; Renkawitz, R. Active Promoters and Insulators Are Marked by the Centrosomal Protein 190. EMBO J. 2009, 28, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Butcher, R.D.; Chodagam, S.; Basto, R.; Wakefield, J.G.; Henderson, D.S.; Raff, J.W.; Whitfield, W.G. The Drosophila Centrosome-Associated Protein CP190 Is Essential for Viability but Not for Cell Division. J. Cell Sci. 2004, 117, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, S.H.; Günther, K.; Weth, O.; Bartkuhn, M.; Bhonde, R.R.; Shouche, Y.S.; Renkawitz, R. Ectopically Tethered CP190 Induces Large-Scale Chromatin Decondensation. Sci. Rep. 2014, 4, 3917. [Google Scholar] [CrossRef]
- Bohla, D.; Herold, M.; Panzer, I.; Buxa, M.K.; Ali, T.; Demmers, J.; Krüger, M.; Scharfe, M.; Jarek, M.; Bartkuhn, M.; et al. A Functional Insulator Screen Identifies NURF and dREAM Components to Be Required for Enhancer-Blocking. PLoS ONE 2014, 9, e107765. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Grisan, V.; Jang, B.; Herbert, J.; Badenhorst, P. Genome-Wide Mapping Targets of the Metazoan Chromatin Remodeling Factor NURF Reveals Nucleosome Remodeling at Enhancers, Core Promoters and Gene Insulators. PLoS Genet. 2016, 12, e1005969. [Google Scholar] [CrossRef]
- Ali, T.; Krüger, M.; Bhuju, S.; Jarek, M.; Bartkuhn, M.; Renkawitz, R. Chromatin Binding of Gcn5 in Drosophila Is Largely Mediated by CP190. Nucleic Acids Res. 2017, 45, 2384–2395. [Google Scholar] [CrossRef]
- Mazina, M.Y.; Ziganshin, R.H.; Magnitov, M.D.; Golovnin, A.K.; Vorobyeva, N.E. Proximity-Dependent Biotin Labelling Reveals CP190 as an EcR/Usp Molecular Partner. Sci. Rep. 2020, 10, 4793. [Google Scholar] [CrossRef]
- Savitsky, M.; Kim, M.; Kravchuk, O.; Schwartz, Y.B. Distinct Roles of Chromatin Insulator Proteins in Control of the Drosophila Bithorax Complex. Genetics 2016, 202, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.; Perry, K.M.; Tjian, R. Adf-1 Is a Nonmodular Transcription Factor That Contains a TAF-Binding Myb-like Motif. Mol. Cell Biol. 1998, 18, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, S.; Wang, J.; Barbash, D.A. Recurrent Positive Selection of the Drosophila Hybrid Incompatibility Gene Hmr. Mol. Biol. Evol. 2008, 25, 2421–2430. [Google Scholar] [CrossRef]
- Shukla, V.; Habib, F.; Kulkarni, A.; Ratnaparkhi, G.S. Gene Duplication, Lineage-Specific Expansion, and Subfunctionalization in the MADF-BESS Family Patterns the Drosophila Wing Hinge. Genetics 2014, 196, 481–496. [Google Scholar] [CrossRef] [PubMed]
- England, B.P.; Heberlein, U.; Tjian, R. Purified Drosophila Transcription Factor, Adh Distal Factor-1 (Adf-1), Binds to Sites in Several Drosophila Promoters and Activates Transcription. J. Biol. Chem. 1990, 265, 5086–5094. [Google Scholar]
- Zimmermann, G.; Furlong, E.E.; Suyama, K.; Scott, M.P. Mes2, a MADF-Containing Transcription Factor Essential for Drosophila Development. Dev. Dyn. 2006, 235, 3387–3395. [Google Scholar] [CrossRef]
- Bhaskar, V.; Courey, A.J. The MADF-BESS Domain Factor Dip3 Potentiates Synergistic Activation by Dorsal and Twist. Gene 2002, 299, 173–184. [Google Scholar] [CrossRef]
- Ratnaparkhi, G.S.; Duong, H.A.; Courey, A.J. Dorsal Interacting Protein 3 Potentiates Activation by Drosophila Rel Homology Domain Proteins. Dev. Comp. Immunol. 2008, 32, 1290–1300. [Google Scholar] [CrossRef]
- Song, H.; Goetze, S.; Bischof, J.; Spichiger-Haeusermann, C.; Kuster, M.; Brunner, E.; Basler, K. Coop Functions as a Corepressor of Pangolin and Antagonizes Wingless Signaling. Genes Dev. 2010, 24, 881–886. [Google Scholar] [CrossRef]
- Clark, K.A.; McKearin, D.M. The Drosophila Stonewall Gene Encodes a Putative Transcription Factor Essential for Germ Cell Development. Development 1996, 122, 937–950. [Google Scholar] [CrossRef]
- Maines, J.Z.; Park, J.K.; Williams, M.; McKearin, D.M. Stonewalling Drosophila Stem Cell Differentiation by Epigenetic Controls. Development 2007, 134, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Pfreundt, U.; James, D.P.; Tweedie, S.; Wilson, D.; Teichmann, S.A.; Adryan, B. FlyTF: Improved Annotation and Enhanced Functionality of the Drosophila Transcription Factor Database. Nucleic Acids Res. 2010, 38, D443–D447. [Google Scholar] [CrossRef] [PubMed]
- Geyer, P.K.; Corces, V.G. DNA Position-Specific Repression of Transcription by a Drosophila Zinc Finger Protein. Genes Dev. 1992, 6, 1865–1873. [Google Scholar] [PubMed]
- Holdridge, C.; Dorsett, D. Repression of Hsp70 Heat Shock Gene Transcription by the Suppressor of Hairy-Wing Protein of Drosophila Melanogaster. Mol. Cell Biol. 1991, 11, 1894–1900. [Google Scholar]
- Roseman, R.R.; Swan, J.M.; Geyer, P.K. A Drosophila Insulator Protein Facilitates Dosage Compensation of the X Chromosome Min-White Gene Located at Autosomal Insertion Sites. Development 1995, 121, 3573–3582. [Google Scholar] [CrossRef]
- Roseman, R.R.; Pirrotta, V.; Geyer, P.K. The Su(Hw) Protein Insulates Expression of the Drosophila Melanogaster White Gene from Chromosomal Position-Effects. EMBO J. 1993, 12, 435–442. [Google Scholar] [CrossRef]
- Scott, K.C.; Taubman, A.D.; Geyer, P.K. Enhancer Blocking by the Drosophila Gypsy Insulator Depends upon Insulator Anatomy and Enhancer Strength. Genetics 1999, 153, 787–798. [Google Scholar] [CrossRef]
- Melnikova, L.; Molodina, V.; Erokhin, M.; Georgiev, P.; Golovnin, A. HIPP1 Stabilizes the Interaction between CP190 and Su(Hw) in the Drosophila Insulator Complex. Sci. Rep. 2019, 9, 19102. [Google Scholar] [CrossRef]
- Melnikova, L.; Kostyuchenko, M.; Molodina, V.; Parshikov, A.; Georgiev, P.; Golovnin, A. Interactions between BTB Domain of CP190 and Two Adjacent Regions in Su(Hw) Are Required for the Insulator Complex Formation. Chromosoma 2018, 127, 59–71. [Google Scholar] [CrossRef]
- Melnikova, L.; Kostyuchenko, M.; Molodina, V.; Parshikov, A.; Georgiev, P.; Golovnin, A. Multiple Interactions Are Involved in a Highly Specific Association of the Mod(Mdg4)-67.2 Isoform with the Su(Hw) Sites in Drosophila. Open Biol. 2017, 7, 170150. [Google Scholar] [CrossRef]
- Glenn, S.E.; Geyer, P.K. Investigation of the Developmental Requirements of Drosophila HP1 and Insulator Protein Partner, HIPP1. G3 (Bethesda) 2019, 9, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Stow, E.C.; Simmons, J.R.; An, R.; Schoborg, T.A.; Davenport, N.M.; Labrador, M. A Drosophila Insulator Interacting Protein Suppresses Enhancer-Blocking Function and Modulates Replication Timing. Gene 2022, 819, 146208. [Google Scholar] [CrossRef]
- King, M.R.; Matzat, L.H.; Dale, R.K.; Lim, S.J.; Lei, E.P. The RNA-Binding Protein Rumpelstiltskin Antagonizes Gypsy Chromatin Insulator Function in a Tissue-Specific Manner. J. Cell Sci. 2014, 127, 2956–2966. [Google Scholar] [CrossRef] [PubMed]
- Matzat, L.H.; Dale, R.K.; Moshkovich, N.; Lei, E.P. Tissue-Specific Regulation of Chromatin Insulator Function. PLoS Genet. 2012, 8, e1003069. [Google Scholar] [CrossRef] [PubMed]
- Soshnev, A.A.; Baxley, R.M.; Manak, J.R.; Tan, K.; Geyer, P.K. The Insulator Protein Suppressor of Hairy-Wing Is an Essential Transcriptional Repressor in the Drosophila Ovary. Development 2013, 140, 3613–3623. [Google Scholar] [CrossRef]
- Melnikova, L.; Elizar’ev, P.; Erokhin, M.; Molodina, V.; Chetverina, D.; Kostyuchenko, M.; Georgiev, P.; Golovnin, A. The Same Domain of Su(Hw) Is Required for Enhancer Blocking and Direct Promoter Repression. Sci. Rep. 2019, 9, 5314. [Google Scholar] [CrossRef]
- Cai, H.; Levine, M. Modulation of Enhancer-Promoter Interactions by Insulators in the Drosophila Embryo. Nature 1995, 376, 533–536. [Google Scholar] [CrossRef]
- Parnell, T.J.; Kuhn, E.J.; Gilmore, B.L.; Helou, C.; Wold, M.S.; Geyer, P.K. Identification of Genomic Sites That Bind the Drosophila Suppressor of Hairy-Wing Insulator Protein. Mol. Cell Biol. 2006, 26, 5983–5993. [Google Scholar] [CrossRef]
- England, B.P.; Admon, A.; Tjian, R. Cloning of Drosophila Transcription Factor Adf-1 Reveals Homology to Myb Oncoproteins. Proc. Natl. Acad. Sci. USA 1992, 89, 683–687. [Google Scholar] [CrossRef]
- Orsi, G.A.; Kasinathan, S.; Hughes, K.T.; Saminadin-Peter, S.; Henikoff, S.; Ahmad, K. High-Resolution Mapping Defines the Cooperative Architecture of Polycomb Response Elements. Genome Res. 2014, 24, 809–820. [Google Scholar] [CrossRef]
- Port, F.; Chen, H.-M.; Lee, T.; Bullock, S.L. Optimized CRISPR/Cas Tools for Efficient Germline and Somatic Genome Engineering in Drosophila. Proc. Natl. Acad. Sci. USA 2014, 111, E2967–E2976. [Google Scholar] [CrossRef] [PubMed]
- Gdula, D.A.; Corces, V.G. Characterization of Functional Domains of the Su(Hw) Protein That Mediate the Silencing Effect of Mod(Mdg4) Mutations. Genetics 1997, 145, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Chetverina, D.; Vorobyeva, N.E.; Mazina, M.Y.; Fab, L.V.; Lomaev, D.; Golovnina, A.; Mogila, V.; Georgiev, P.; Ziganshin, R.H.; Erokhin, M. Comparative Interactome Analysis of the PRE DNA-Binding Factors: Purification of the Combgap-, Zeste-, Psq-, and Adf1-Associated Proteins. Cell. Mol. Life Sci. 2022, 79, 353. [Google Scholar] [CrossRef] [PubMed]
- Heberlein, U.; England, B.; Tjian, R. Characterization of Drosophila Transcription Factors That Activate the Tandem Promoters of the Alcohol Dehydrogenase Gene. Cell 1985, 41, 965–977. [Google Scholar] [CrossRef]
- Kaushal, A.; Dorier, J.; Wang, B.; Mohana, G.; Taschner, M.; Cousin, P.; Waridel, P.; Iseli, C.; Semenova, A.; Restrepo, S.; et al. Essential Role of Cp190 in Physical and Regulatory Boundary Formation. Sci. Adv. 2022, 8, eabl8834. [Google Scholar] [CrossRef] [PubMed]
- Maksimenko, O.; Bartkuhn, M.; Stakhov, V.; Herold, M.; Zolotarev, N.; Jox, T.; Buxa, M.K.; Kirsch, R.; Bonchuk, A.; Fedotova, A.; et al. Two New Insulator Proteins, Pita and ZIPIC, Target CP190 to Chromatin. Genome Res. 2015, 25, 89–99. [Google Scholar] [CrossRef]
- Shukla, V.; Dhiman, N.; Nayak, P.; Dahanukar, N.; Deshpande, G.; Ratnaparkhi, G.S. Stonewall and Brickwall: Two Partially Redundant Determinants Required for the Maintenance of Female Germline in Drosophila. G3 (Bethesda) 2018, 8, 2027–2041. [Google Scholar] [CrossRef]
- Zinshteyn, D.; Barbash, D.A. Stonewall Prevents Expression of Ectopic Genes in the Ovary and Accumulates at Insulator Elements in D. Melanogaster. PLoS Genet. 2022, 18, e1010110. [Google Scholar] [CrossRef]
- Rohrbaugh, M.; Clore, A.; Davis, J.; Johnson, S.; Jones, B.; Jones, K.; Kim, J.; Kithuka, B.; Lunsford, K.; Mitchell, J.; et al. Identification and Characterization of Proteins Involved in Nuclear Organization Using Drosophila GFP Protein Trap Lines. PLoS ONE 2013, 8, e53091. [Google Scholar] [CrossRef]
- Lomaev, D.; Mikhailova, A.; Erokhin, M.; Shaposhnikov, A.V.; Moresco, J.J.; Blokhina, T.; Wolle, D.; Aoki, T.; Ryabykh, V.; Yates, J.R.; et al. The GAGA Factor Regulatory Network: Identification of GAGA Factor Associated Proteins. PLoS ONE 2017, 12, e0173602. [Google Scholar] [CrossRef]
- Georgieva, S.G.; Nabirochkina, E.N.; Pustovoĭtov, M.V.; Krasnov, A.N.; Soldatov, A.V. [Molecular characteristics of the new evolutionary-conserved nuclear protein e(y)2]. Genetika 2001, 37, 18–23. [Google Scholar]
- Loubiere, V.; Delest, A.; Schuettengruber, B.; Martinez, A.-M.; Cavalli, G. Chromatin Immunoprecipitation Experiments from Whole Drosophila Embryos or Larval Imaginal Discs. Bio Protoc. 2017, 7, e2327. [Google Scholar] [CrossRef]
- Murawska, M.; Brehm, A. Immunostaining of Drosophila Polytene Chromosomes to Investigate Recruitment of Chromatin-Binding Proteins. Methods Mol. Biol. 2012, 809, 267–277. [Google Scholar] [CrossRef]
- Golovnin, A.; Volkov, I.; Georgiev, P. SUMO Conjugation Is Required for the Assembly of Drosophila Su(Hw) and Mod(Mdg4) into Insulator Bodies That Facilitate Insulator Complex Formation. J. Cell Sci. 2012, 125, 2064–2074. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melnikova, L.; Molodina, V.; Babosha, V.; Kostyuchenko, M.; Georgiev, P.; Golovnin, A. The MADF-BESS Protein CP60 Is Recruited to Insulators via CP190 and Has Redundant Functions in Drosophila. Int. J. Mol. Sci. 2023, 24, 15029. https://doi.org/10.3390/ijms241915029
Melnikova L, Molodina V, Babosha V, Kostyuchenko M, Georgiev P, Golovnin A. The MADF-BESS Protein CP60 Is Recruited to Insulators via CP190 and Has Redundant Functions in Drosophila. International Journal of Molecular Sciences. 2023; 24(19):15029. https://doi.org/10.3390/ijms241915029
Chicago/Turabian StyleMelnikova, Larisa, Varvara Molodina, Valentin Babosha, Margarita Kostyuchenko, Pavel Georgiev, and Anton Golovnin. 2023. "The MADF-BESS Protein CP60 Is Recruited to Insulators via CP190 and Has Redundant Functions in Drosophila" International Journal of Molecular Sciences 24, no. 19: 15029. https://doi.org/10.3390/ijms241915029
APA StyleMelnikova, L., Molodina, V., Babosha, V., Kostyuchenko, M., Georgiev, P., & Golovnin, A. (2023). The MADF-BESS Protein CP60 Is Recruited to Insulators via CP190 and Has Redundant Functions in Drosophila. International Journal of Molecular Sciences, 24(19), 15029. https://doi.org/10.3390/ijms241915029





