Evaluation of Proteasome and Immunoproteasome Levels in Plasma and Peritoneal Fluid in Patients with Endometriosis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Reagents
4.3. Measurement Steps
4.3.1. Structure of the Biosensor Base
4.3.2. Preparation of the Chip for the Determination of the Proteasome 20S in the Sample
4.3.3. Preparation of the Chip for the Determination of the 20Si Immunoproteasome in the Sample
4.3.4. SPRi Measurements
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, M.; Wielgos, M.; Laudanski, P. Corrigendum to “Diagnostic delay of endometriosis in adults and adolescence-current stage of knowledge” [Adv. Med. Sci. (2022 Mar) 67 (1) 148–153]. Adv. Med. Sci. 2023, 68, 60. [Google Scholar] [CrossRef] [PubMed]
- Riazi, H.; Tehranian, N.; Ziaei, S.; Mohammadi, E.; Hajizadeh, E.; Montazeri, A. Clinical diagnosis of pelvic endometriosis: A scoping review. BMC Womens Health 2015, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.A. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Laudanski, P.; Rogalska, G.; Warzecha, D.; Lipa, M.; Manka, G.; Kiecka, M.; Spaczynski, R.; Piekarski, P.; Banaszewska, B.; Jakimiuk, A.; et al. Autoantibody screening of plasma and peritoneal fluid of patients with endometriosis. Hum. Reprod. 2023, 38, 629–643. [Google Scholar] [CrossRef]
- Laudanski, P.; Szamatowicz, J.; Ramel, P. Matrix metalloproteinase-13 and membrane type-1 matrix metalloproteinase in peritoneal fluid of women with endometriosis. Gynecol. Endocrinol. 2005, 21, 106–110. [Google Scholar] [CrossRef]
- Lisowska-Myjak, B.; Skarzynska, E.; Wrobel, M.; Manka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczynski, R.; Piekarski, P.; Banaszewska, B.; et al. Investigation of the Changes in Concentrations of Vitamin D-Binding Protein and Lactoferin in Plasma and Peritoneal Fluid of Patients with Endometriosis. Int. J. Mol. Sci. 2023, 24, 7828. [Google Scholar] [CrossRef]
- Skarzynska, E.; Wrobel, M.; Zborowska, H.; Kolek, M.F.; Manka, G.; Kiecka, M.; Lipa, M.; Warzecha, D.; Spaczynski, R.; Piekarski, P.; et al. The Influence of Lactoferrin in Plasma and Peritoneal Fluid on Iron Metabolism in Women with Endometriosis. Int. J. Mol. Sci. 2023, 24, 1619. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Basler, M.; Groettrup, M. On the Role of the Immunoproteasome in Protein Homeostasis. Cells 2021, 10, 3216. [Google Scholar] [CrossRef] [PubMed]
- Budenholzer, L.; Cheng, C.L.; Li, Y.; Hochstrasser, M. Proteasome Structure and Assembly. J. Mol. Biol. 2017, 429, 3500–3524. [Google Scholar] [CrossRef] [PubMed]
- Basler, M.; Mundt, S.; Bitzer, A.; Schmidt, C.; Groettrup, M. The immunoproteasome: A novel drug target for autoimmune diseases. Clin. Exp. Rheumatol. 2015, 33 (Suppl. 92), S74–S79. [Google Scholar] [PubMed]
- Schmidtke, G.; Schregle, R.; Alvarez, G.; Huber, E.M.; Groettrup, M. The 20S immunoproteasome and constitutive proteasome bind with the same affinity to PA28alphabeta and equally degrade FAT10. Mol. Immunol. 2019, 113, 22–30. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Markowska, A.; Lukaszewski, Z.; Puzan, B.; Gorodkiewicz, E. Methods for 20S Immunoproteasome and 20S Constitutive Proteasome Determination Based on SPRI Biosensors. Cell Mol. Bioeng. 2017, 10, 174–185. [Google Scholar] [CrossRef]
- Tanaka, K. The proteasome: Overview of structure and functions. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 12–36. [Google Scholar] [CrossRef]
- Kimura, H.; Caturegli, P.; Takahashi, M.; Suzuki, K. New Insights into the Function of the Immunoproteasome in Immune and Nonimmune Cells. J. Immunol. Res. 2015, 2015, 541984. [Google Scholar] [CrossRef]
- Ettari, R.; Previti, S.; Bitto, A.; Grasso, S.; Zappala, M. Immunoproteasome-Selective Inhibitors: A Promising Strategy to Treat Hematologic Malignancies, Autoimmune and Inflammatory Diseases. Curr. Med. Chem. 2016, 23, 1217–1238. [Google Scholar] [CrossRef]
- Miller, Z.; Ao, L.; Kim, K.B.; Lee, W. Inhibitors of the immunoproteasome: Current status and future directions. Curr. Pharm. Des. 2013, 19, 4140–4151. [Google Scholar] [CrossRef]
- Gandolfi, S.; Laubach, J.P.; Hideshima, T.; Chauhan, D.; Anderson, K.C.; Richardson, P.G. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev. 2017, 36, 561–584. [Google Scholar] [CrossRef]
- Dwivedi, V.; Yaniv, K.; Sharon, M. Beyond cells: The extracellular circulating 20S proteasomes. Biochim. Biophys. Acta Mol. Basis. Dis. 2021, 1867, 166041. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nissan, G.; Katzir, N.; Fuzesi-Levi, M.G.; Sharon, M. Biology of the Extracellular Proteasome. Biomolecules 2022, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Shigesi, N.; Kvaskoff, M.; Kirtley, S.; Feng, Q.; Fang, H.; Knight, J.C.; Missmer, S.A.; Rahmioglu, N.; Zondervan, K.T.; Becker, C.M. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Kvaskoff, M.; Mahamat-Saleh, Y.; Farland, L.V.; Shigesi, N.; Terry, K.L.; Harris, H.R.; Roman, H.; Becker, C.M.; As-Sanie, S.; Zondervan, K.T.; et al. Endometriosis and cancer: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 393–420. [Google Scholar] [CrossRef] [PubMed]
- Gorodkiewicz, E.; Sankiewicz, A.; Laudański, P. Surface plasmon resonance imaging biosensors for aromatase based on a potent inhibitor and a specific antibody: Sensor development and application for biological material. Cent. Eur. J. Chem. 2014, 12, 557–567. [Google Scholar] [CrossRef]
- Sankiewicz, A.; Romanowicz, L.; Laudanski, P.; Zelazowska-Rutkowska, B.; Puzan, B.; Cylwik, B.; Gorodkiewicz, E. SPR imaging biosensor for determination of laminin-5 as a potential cancer marker in biological material. Anal. Bioanal. Chem. 2016, 408, 5269–5276. [Google Scholar] [CrossRef]
- Amreen, S.; Kumar, P.; Gupta, P.; Rao, P. Evaluation of Oxidative Stress and Severity of Endometriosis. J. Hum. Reprod. Sci. 2019, 12, 40–46. [Google Scholar] [CrossRef]
- Shang, F.; Taylor, A. Ubiquitin-proteasome pathway and cellular responses to oxidative stress. Free Radic. Biol. Med. 2011, 51, 5–16. [Google Scholar] [CrossRef]
- Fuji, T.; Umeda, Y.; Nyuya, A.; Taniguchi, F.; Kawai, T.; Yasui, K.; Toshima, T.; Yoshida, K.; Fujiwara, T.; Goel, A.; et al. Detection of circulating microRNAs with Ago2 complexes to monitor the tumor dynamics of colorectal cancer patients during chemotherapy. Int. J. Cancer 2019, 144, 2169–2180. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, Y.; Zhai, Y.; Yang, H.; Zhang, C.; Lu, Y.; Wei, W.; Cai, Q.; Ding, X.; Lu, S.; et al. Persistent dysregulation of genes in the development of endometriosis. Ann. Transl. Med. 2022, 10, 1175. [Google Scholar] [CrossRef]
- Ashraf Ahmed Foda, I.A.A.A. Role of some biomarkers in chronic pelvic pain for early detection of endometriosis in infertile women. Middle East Fertil. Soc. J. 2012, 17, 187–194. [Google Scholar] [CrossRef]
- Seifert, U.; Bialy, L.P.; Ebstein, F.; Bech-Otschir, D.; Voigt, A.; Schroter, F.; Prozorovski, T.; Lange, N.; Steffen, J.; Rieger, M.; et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell 2010, 142, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Nathan, J.A.; Spinnenhirn, V.; Schmidtke, G.; Basler, M.; Groettrup, M.; Goldberg, A.L. Immuno- and constitutive proteasomes do not differ in their abilities to degrade ubiquitinated proteins. Cell 2013, 152, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Celik, O.; Ersahin, A.; Acet, M.; Celik, N.; Baykus, Y.; Deniz, R.; Ozerol, E.; Ozerol, I. Disulfiram, as a candidate NF-kappaB and proteasome inhibitor, prevents endometriotic implant growing in a rat model of endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4380–4389. [Google Scholar]
- Wang, Y.; Nicholes, K.; Shih, I.M. The Origin and Pathogenesis of Endometriosis. Annu. Rev. Pathol. 2020, 15, 71–95. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef]
- Gaia-Oltean, A.I.; Braicu, C.; Gulei, D.; Ciortea, R.; Mihu, D.; Roman, H.; Irimie, A.; Berindan-Neagoe, I. Ovarian endometriosis, a precursor of ovarian cancer: Histological aspects, gene expression and microRNA alterations (Review). Exp. Med. 2021, 21, 243. [Google Scholar] [CrossRef]
- Varma, R.; Rollason, T.; Gupta, J.K.; Maher, E.R. Endometriosis and the neoplastic process. Reproduction 2004, 127, 293–304. [Google Scholar] [CrossRef]
- Henry, L.; Lavabre-Bertrand, T.; Vercambre, L.; Ramos, J.; Carillo, S.; Guiraud, I.; Pouderoux, P.; Bismuth, M.; Valats, J.C.; Demattei, C.; et al. Plasma proteasome level is a reliable early marker of malignant transformation of liver cirrhosis. Gut 2009, 58, 833–838. [Google Scholar] [CrossRef]
- Didziokaite, G.; Biliute, G.; Gudaite, J.; Kvedariene, V. Oxidative Stress as a Potential Underlying Cause of Minimal and Mild Endometriosis-Related Infertility. Int. J. Mol. Sci. 2023, 24, 3809. [Google Scholar] [CrossRef]
- Shamsi, M.; Ghazavi, A.; Saeedifar, A.M.; Mosayebi, G.; Pour, S.K.; Ganji, A. The immune system’s role in PCOS. Mol. Biol. Rep. 2022, 49, 10689–10702. [Google Scholar] [CrossRef] [PubMed]
- Dabravolski, S.A.; Nikiforov, N.G.; Eid, A.H.; Nedosugova, L.V.; Starodubova, A.V.; Popkova, T.V.; Bezsonov, E.E.; Orekhov, A.N. Mitochondrial Dysfunction and Chronic Inflammation in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2021, 22, 3923. [Google Scholar] [CrossRef] [PubMed]
- Cornelli, U.; Belcaro, G.; Cesarone, M.R.; Finco, A. Analysis of oxidative stress during the menstrual cycle. Reprod. Biol. Endocrinol. 2013, 11, 74. [Google Scholar] [CrossRef] [PubMed]
- Karowicz-Bilinska, A.; Plodzidym, M.; Krol, J.; Lewinska, A.; Bartosz, G. Changes of markers of oxidative stress during menstrual cycle. Redox. Rep. 2008, 13, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.S.; Steiner, A.Z.; Faurot, K.R.; Long, A.; Jukic, A.M. Systemic inflammation and menstrual cycle length in a prospective cohort study. Am. J. Obstet. Gynecol. 2023, 228, 215 e211–215 e217. [Google Scholar] [CrossRef]
- Bonavina, G.; Taylor, H.S. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front. Endocrinol. 2022, 13, 1020827. [Google Scholar] [CrossRef]
- Miller, J.E.; Ahn, S.H.; Monsanto, S.P.; Khalaj, K.; Koti, M.; Tayade, C. Implications of immune dysfunction on endometriosis associated infertility. Oncotarget 2017, 8, 7138–7147. [Google Scholar] [CrossRef]
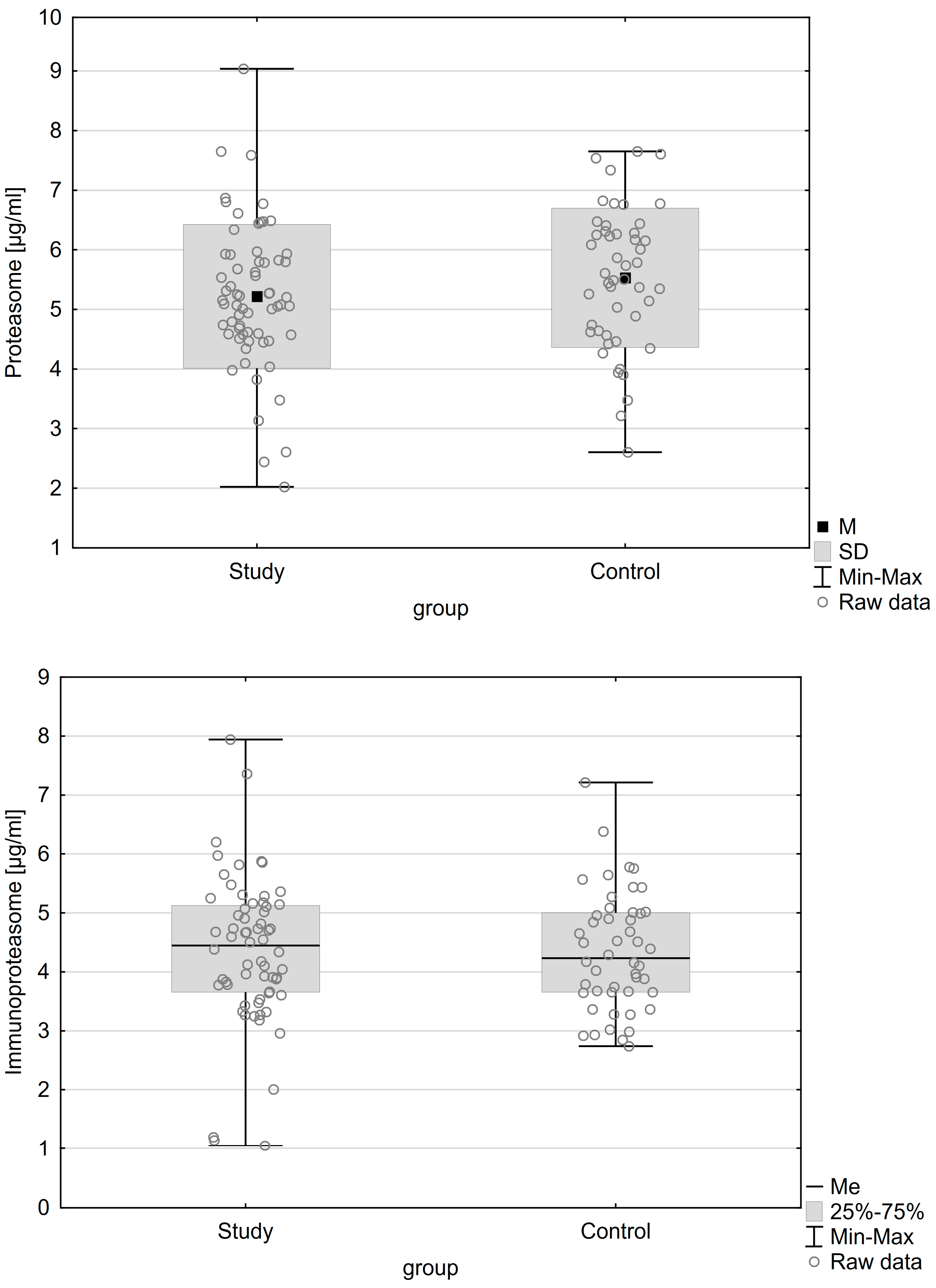
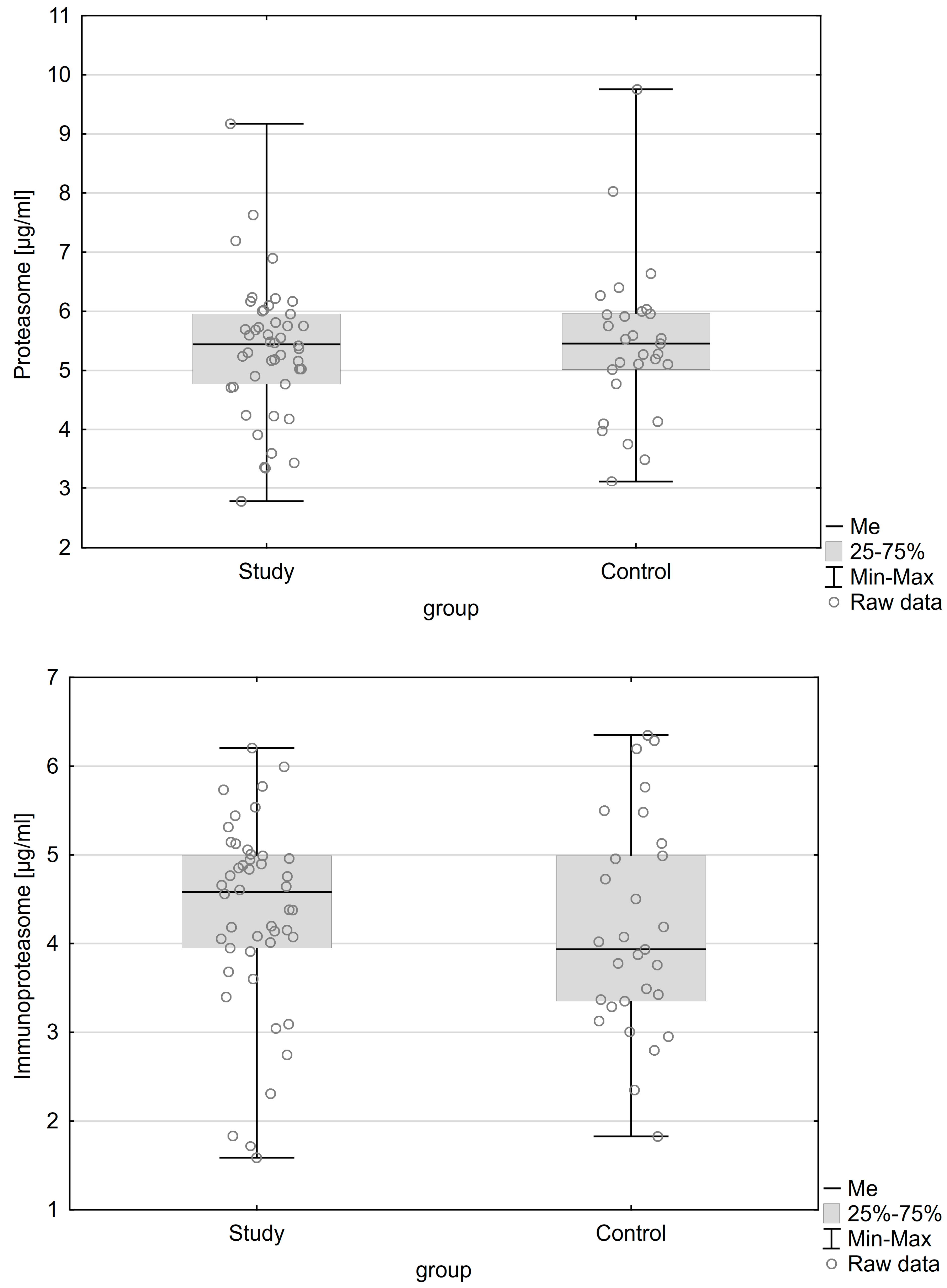

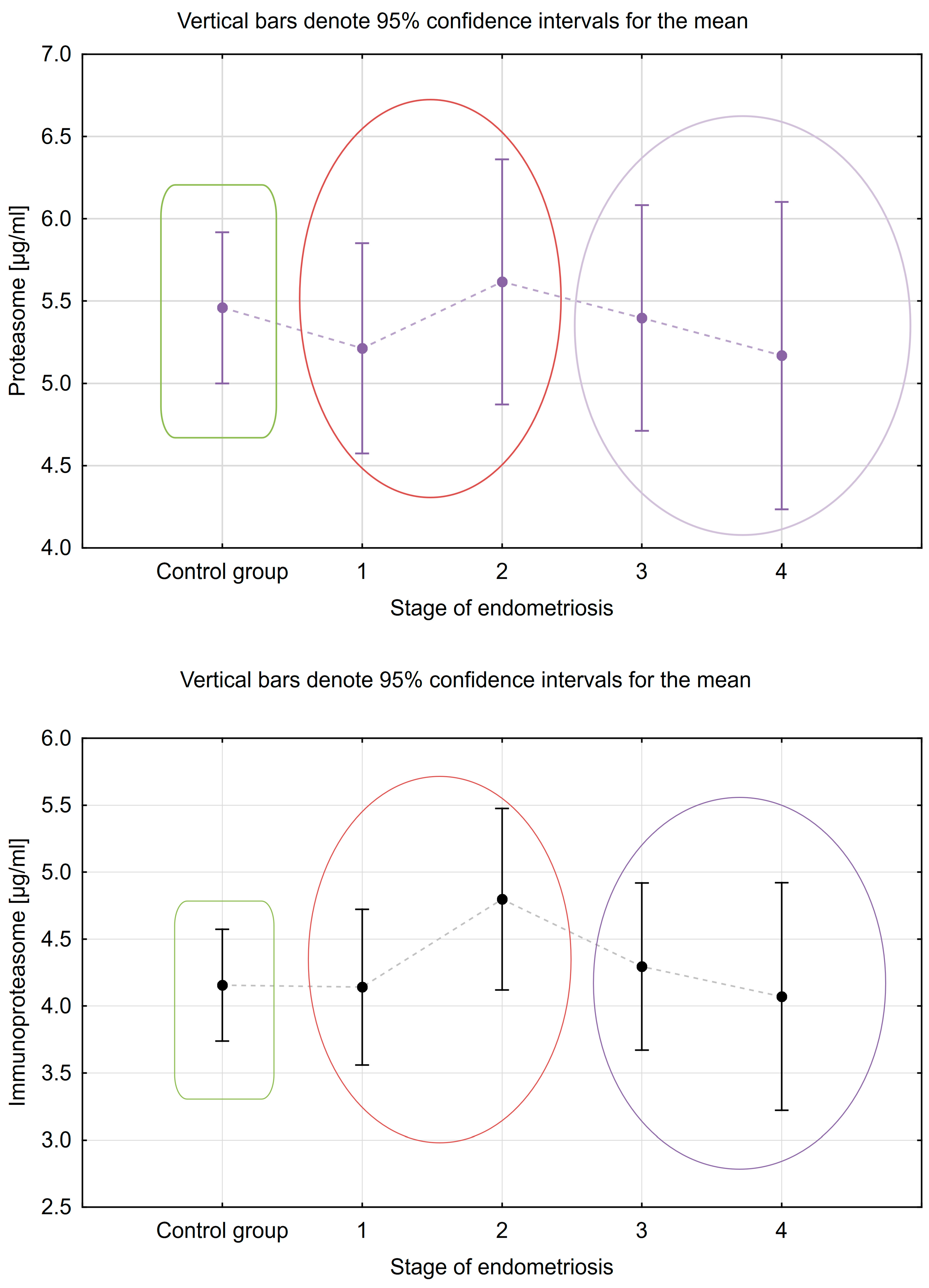
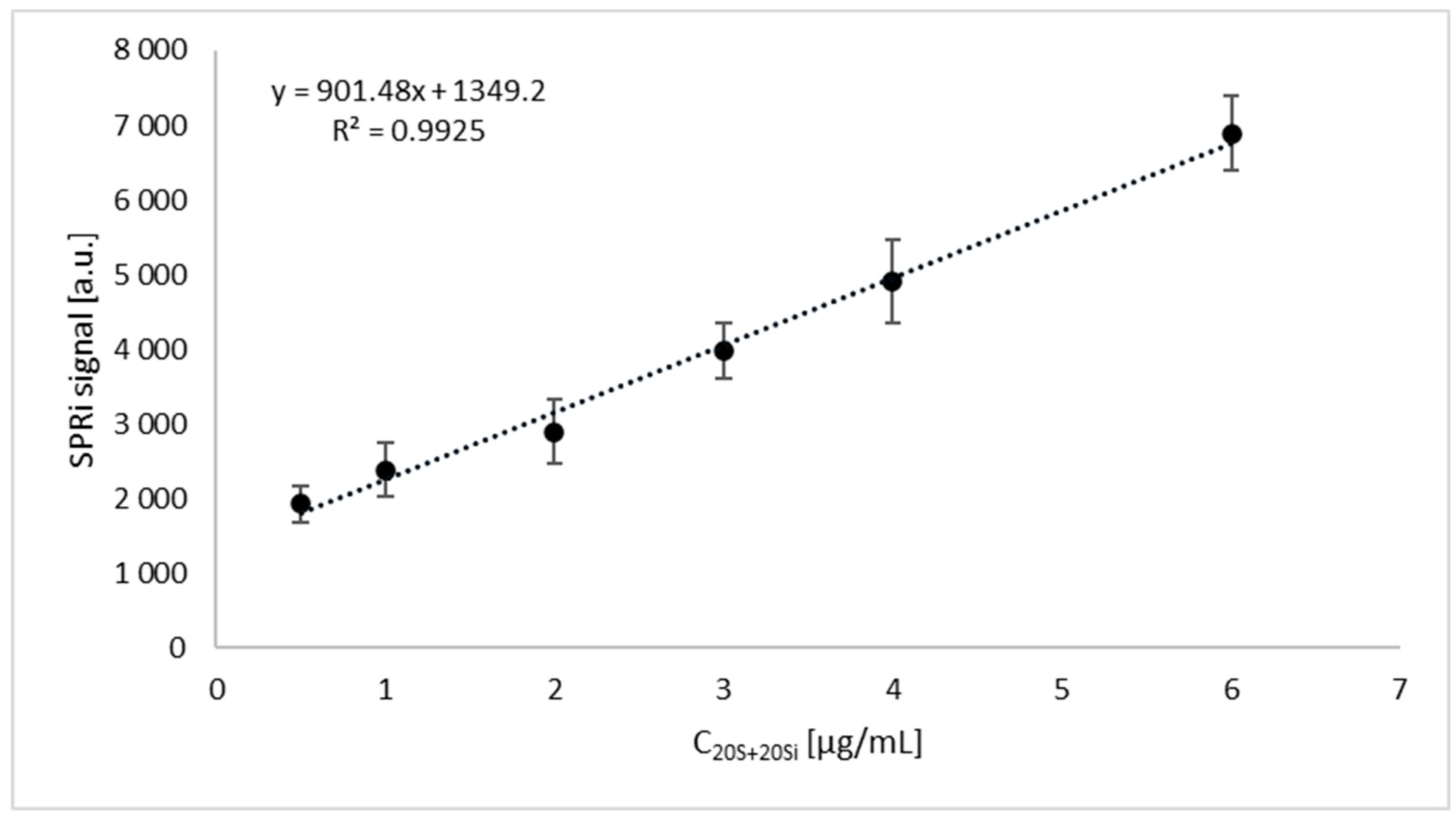
| Variable | Patients with Endometriosis (n = 64) | Patients without Endometriosis (n = 48) | p |
|---|---|---|---|
| Age [years] Me (IQR) | 32.5 (8.0) | 32.0 (9.5) | 0.301 |
| Stage I endometriosis | 24 (37.50%) | Not applicable | - |
| Stage II endometriosis | 14 (21.53%) | Not applicable | - |
| Stage III endometriosis | 16 (25.00%) | Not applicable | - |
| Stage IV endometriosis | 10 (15.63%) | Not applicable | - |
| Infertility | 36 (56.25%) | 24 (50%) | 0.672 |
| First phase of cycle | 46 (71.88%) | 37 (77.08%) | 0.534 |
| Second phase of cycle | 18 (28.13%) | 11 (22.92%) | |
| Ovarian cyst | 31 (48.44%) | 0 (0%) | <0.001 |
| Proteasome concentration M (±SD) | 5.220 (±1.206) µg/mL | 5.531 (±1.170) µg/mL | 0.174 |
| Immunoproteasome concentration Me (IQR) | 4.45 (1.48) µg/mL | 4.23 (1.34) µg/mL | 0.696 |
| Variable | Patients with Endometriosis (n = 46) | Patients without Endometriosis (n = 29) | p |
|---|---|---|---|
| Age [years] Me (IQR) | 30.5 (7) | 31.0 (8) | 0.987 |
| Stage I endometriosis | 15 (32.61%) | Not applicable | - |
| Stage II endometriosis | 11 (23.91%) | Not applicable | - |
| Stage III endometriosis | 13 (28.26%) | Not applicable | - |
| Stage IV endometriosis | 7 (15.22%) | Not applicable | - |
| Infertility | 26 (56.52%) | 13 (44.83%) | 0.527 |
| First phase of cycle | 27 (58.70%) | 22 (75.87%) | 0.128 |
| Second phase of cycle | 19 (41.30%) | 7 (24.14%) | |
| Ovarian cyst | 27 (58.70%) | 0 (0%) | <0.001 |
| Proteasome concentration Me (IQR) | 5.445 (1.188) µg/mL | 5.458 (0.945) µg/mL | 0.909 |
| Immunoproteasome concentration Me (IQR) | 4.583 (1.04) µg/mL | 3.936 (1.638) µg/mL | 0.284 |
| Groups | Control Group | Endometriosis Stage | |||
|---|---|---|---|---|---|
| I | II | III | IV | ||
| Proteasome M (±SD) µg/mL | 5.531 ± 1.170 | 5.143 ± 1.059 | 4.860 ± 1.632 | 5.34 ± 1.154 | 5.711 ± 0.862 |
| Immunoproteasome M (±SD) µg/mL | 4.345 ± 0.997 | 3.921 ± 0.960 | 4.386 ± 1.731 | 4.831 ± 1.321 | 4.596 ± 0.618 |
| Proteasome | ||
| t | p | |
| E1 + E2 vs. E3 + E4 (CONTRAST 1) | −1.68 | 0.095 |
| C vs. E1 + E2 (CONTRAST 2) | 2.01 | 0.047 |
| C vs. E3 + E4 (CONTRAST 3) | −0.01 | 0.990 |
| Immunoproteasome | ||
| t | p | |
| E1 + E2 vs. E3 + E4 (CONTRAST 1) | −1.89 | 0.062 |
| C vs. E1 + E2 (CONTRAST 2) | 0.77 | 0.445 |
| C vs. E3 + E4 (CONTRAST 3) | 1.31 | 0.191 |
| Groups | Control Group | I | II | III | IV |
|---|---|---|---|---|---|
| Proteasome M (±SD) µg/mL | 5.460 ± 1.318 | 5.213 ± 1.615 | 5.617 ± 0.433 | 5.397 ± 1.206 | 5.169 ± 0.719 |
| Immunoproteasome M (±SD) µg/mL | 4.156 ± 1.199 | 4.142 ± 1.190 | 4.798 ± 0.609 | 4.295 ± 1.134 | 4.072 ± 1.286 |
| Proteasome | ||
| t | p | |
| E1 + E2 vs. E3 + E4 (CONTRAST 1) | 0.35 | 0.729 |
| K vs. E1 + E2 (CONTRAST 2) | 0.13 | 0.895 |
| K vs. E3 + E4 (CONTRAST 3) | −0.48 | 0.635 |
| Immunoproteasome | ||
| t | p | |
| E1 + E2 vs. E3 + E4 (CONTRAST 1) | 0.825 | 0.412 |
| K vs. E1 + E2 (CONTRAST 2) | −1.024 | 0.309 |
| K vs. E3 + E4 (CONTRAST 3) | 0.083 | 0.934 |
| PROTEASOME | |||
| Feature | Present | Absent | p |
| Ovarian cyst M (±SD) µg/mL | 5.458 (±1.027) | 5.313 (±1.258) | 0.567 |
| Infertility M (±SD) µg/mL | |||
| Patients with endometriosis | 5.005 (±1.256) | 5.496 (±1.100) | 0.107 |
| Patient without endometriosis | 5.460 (±1.250) | 5.602 (±1.106) | 0.265 |
| First | Second | p | |
| Menstrual cycle phase M (±SD) µg/mL | |||
| Patients with endometriosis | 5.249 (±1.209) | 5.145 (±1.233) | 0.758 |
| Patient without endometriosis | 5.399 (±1.177) | 5.976 (±1.078) | 0.153 |
| IMMUNOPROTEASOME | |||
| Feature | Present | Absent | p |
| Ovarian cyst Me (IQR) µg/mL | 4.817 (1.291) | 4.103 (1.261) | 0.017 |
| Infertility M (±SD) µg/mL | |||
| Patients with endometriosis | 4.341 (±1.359) | 4.373 (±1.120) | 0.912 |
| Patient without endometriosis | 4.183 (±0.794) | 4.507 (±1.159) | 0.265 |
| First | Second | p | |
| Menstrual cycle phase Me (IQR) µg/mL | |||
| Patients with endometriosis | 4.364 (1.295) | 4.653 (1.826) | 0.478 |
| Patient without endometriosis | 4.174 (1.311) | 4.291 (1.336) | 0.556 |
| PROTEASOME | |||
| Feature | Present | Absent | p |
| Ovarian cyst Me (IQR) | 5.47 (1.054) | 5.415 (1.207) | 0.987 |
| Infertility (M ± SD) | |||
| Patients with endometriosis | 5.454 ± 1.294 | 5.227 ± 0.968 | 0.516 |
| Patient without endometriosis | 5.693 ± 1.057 | 5.270 ± 1.504 | 0.399 |
| Menstrual cycle phase Me (IQR) | First | Second | p |
| Patients with endometriosis | 5.265 (1.714) | 5.599 (0.845) | 0.284 |
| Patient without endometriosis | 5.537 (0.984) | 5.197 (1.862) | 0.665 |
| IMMUNOPROTEASOME | |||
| Feature | Present | Absent | p |
| Ovarian cyst Me (IQR) | 4.605 (1.06) | 4.048 (1.614) | 0.124 |
| Infertility (M ± SD) | |||
| Patients with endometriosis | 4.279 ± 1.072 | 4.399 ± 1.110 | 0.714 |
| Patient without endometriosis | 4.481 ± 1.178 | 3.891 ± 1.185 | 0.192 |
| Menstrual cycle phase M (±SD) | First | Second | p |
| Patients with endometriosis | 4.527 (±1.061) | 4.053 (±1.069) | 0.144 |
| Patient without endometriosis | 4.239 (±1.211) | 3.894 (±1.209) | 0.517 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel, M.; Zuzanna, Z.; Ołdak, Ł.; Kalicka, A.; Mańka, G.; Kiecka, M.; Spaczyński, R.Z.; Piekarski, P.; Banaszewska, B.; Jakimiuk, A.; et al. Evaluation of Proteasome and Immunoproteasome Levels in Plasma and Peritoneal Fluid in Patients with Endometriosis. Int. J. Mol. Sci. 2023, 24, 14363. https://doi.org/10.3390/ijms241814363
Wróbel M, Zuzanna Z, Ołdak Ł, Kalicka A, Mańka G, Kiecka M, Spaczyński RZ, Piekarski P, Banaszewska B, Jakimiuk A, et al. Evaluation of Proteasome and Immunoproteasome Levels in Plasma and Peritoneal Fluid in Patients with Endometriosis. International Journal of Molecular Sciences. 2023; 24(18):14363. https://doi.org/10.3390/ijms241814363
Chicago/Turabian StyleWróbel, Monika, Zielińska Zuzanna, Łukasz Ołdak, Aleksandra Kalicka, Grzegorz Mańka, Mariusz Kiecka, Robert Z. Spaczyński, Piotr Piekarski, Beata Banaszewska, Artur Jakimiuk, and et al. 2023. "Evaluation of Proteasome and Immunoproteasome Levels in Plasma and Peritoneal Fluid in Patients with Endometriosis" International Journal of Molecular Sciences 24, no. 18: 14363. https://doi.org/10.3390/ijms241814363
APA StyleWróbel, M., Zuzanna, Z., Ołdak, Ł., Kalicka, A., Mańka, G., Kiecka, M., Spaczyński, R. Z., Piekarski, P., Banaszewska, B., Jakimiuk, A., Issat, T., Rokita, W., Młodawski, J., Szubert, M., Sieroszewski, P., Raba, G., Szczupak, K., Kluz, T., Kluza, M., ... Laudański, P. (2023). Evaluation of Proteasome and Immunoproteasome Levels in Plasma and Peritoneal Fluid in Patients with Endometriosis. International Journal of Molecular Sciences, 24(18), 14363. https://doi.org/10.3390/ijms241814363







