Modulation of the Proliferative Pathway, Neuroinflammation and Pain in Endometriosis
Abstract
1. Introduction
2. Results
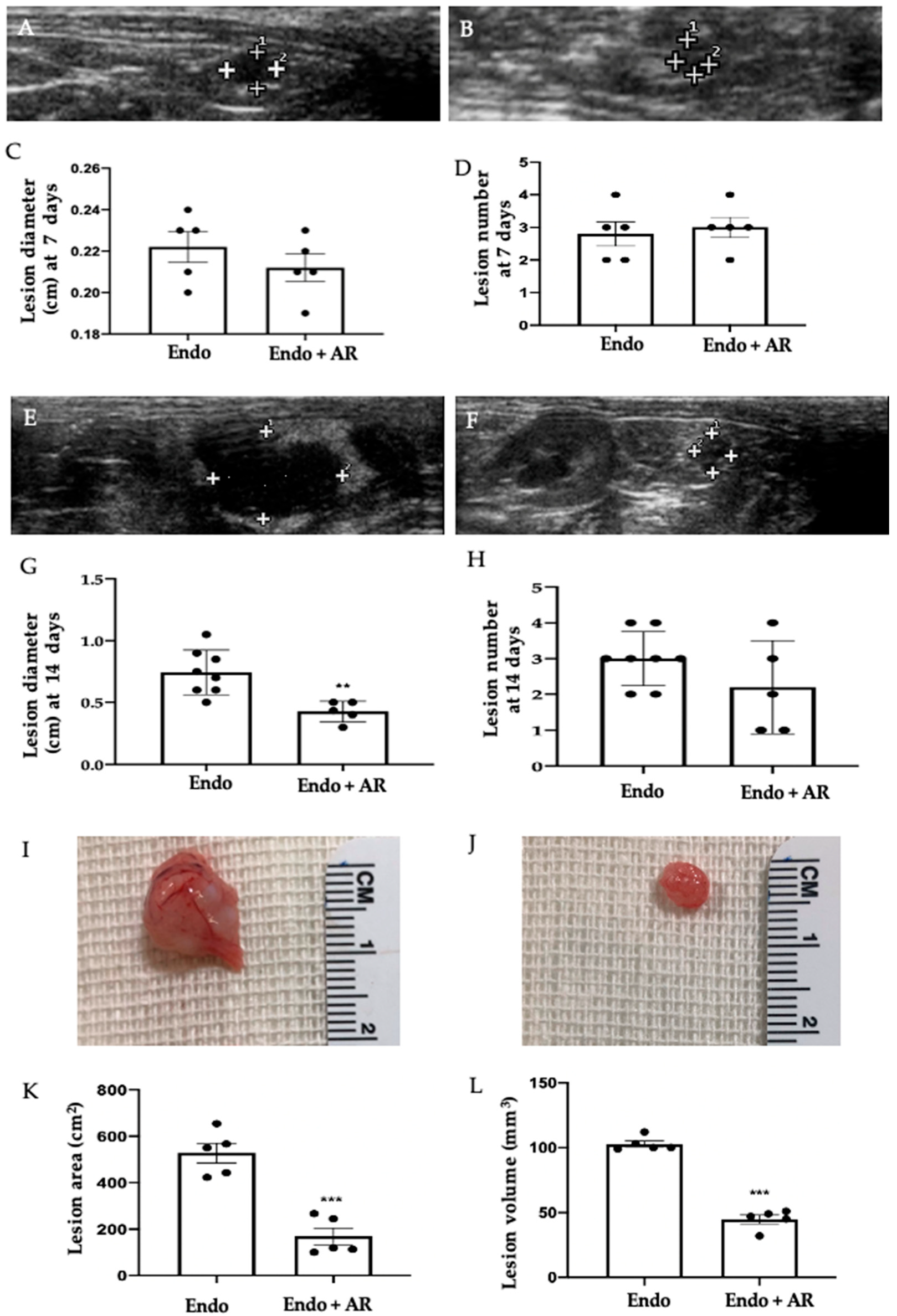
2.1. Effect of AR Administration on Endometriotic Lesions Growth
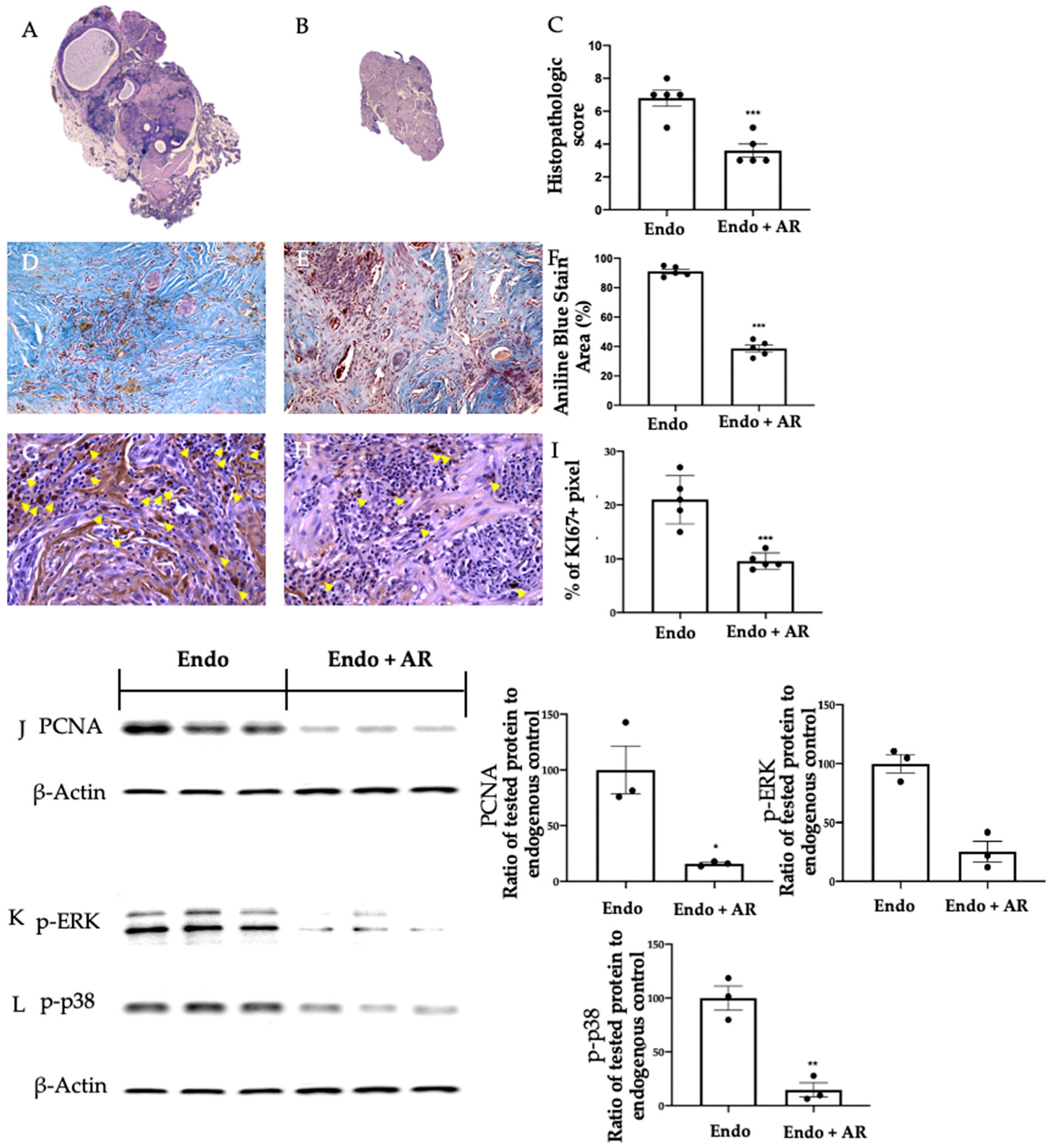
2.2. Effect of AR Administration on Lesion Morphology and Cellular Proliferation
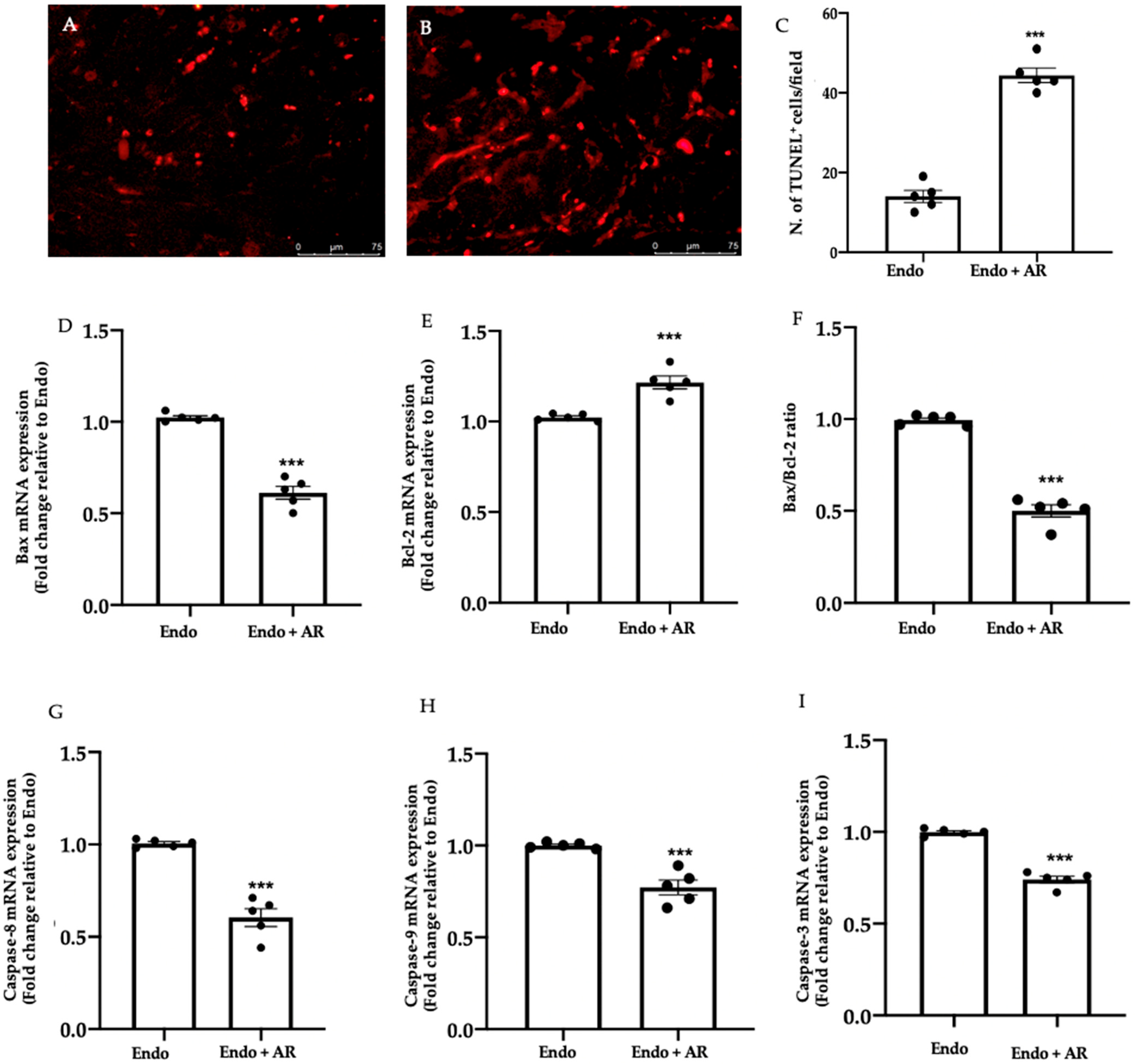
2.3. Effect of AR Administration on Apoptosis
2.4. Effect of AR Administration on Oxidative Stress and Inflammation
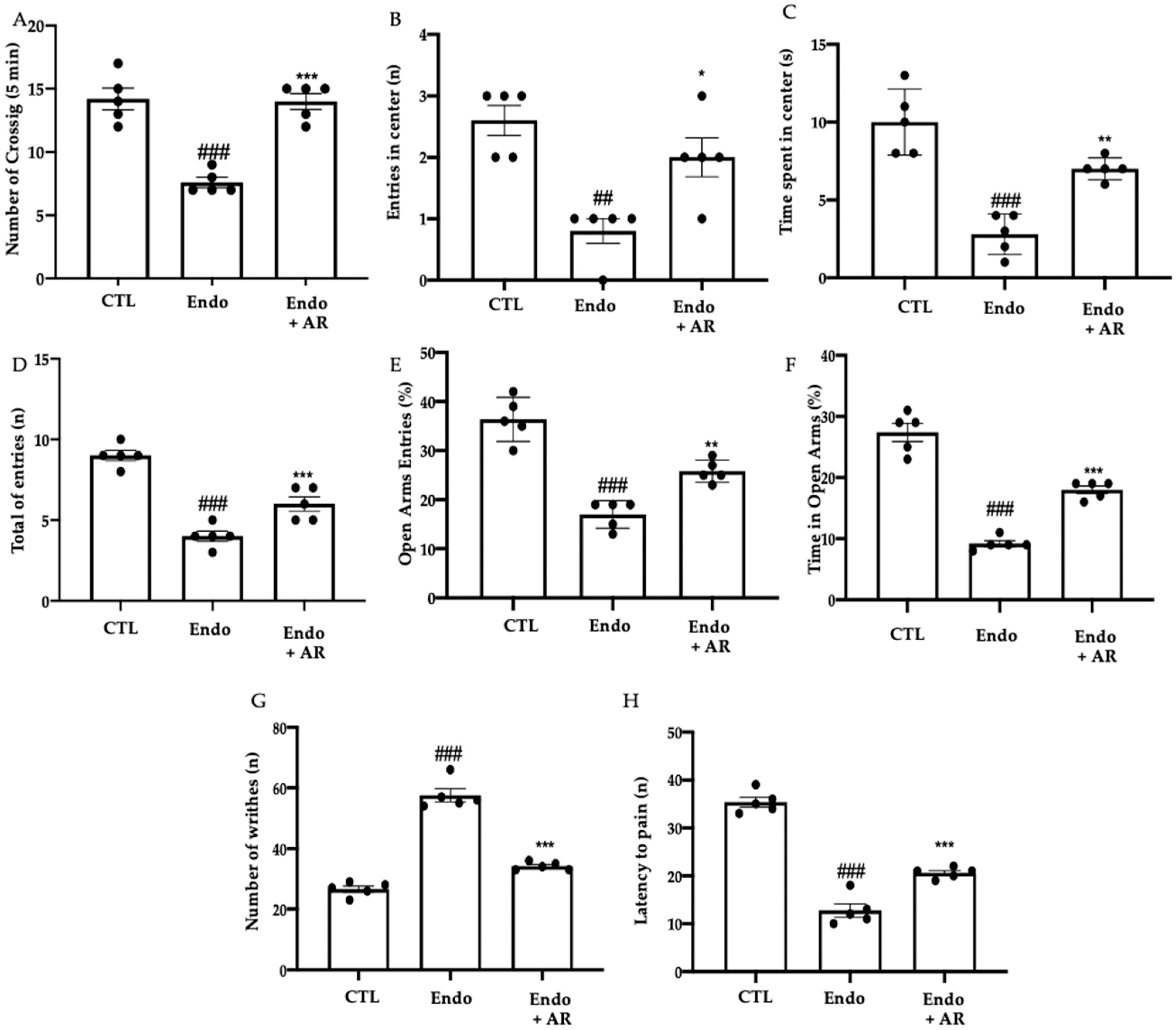
2.5. Effect of AR Administration on the Pain Sensitivity Threshold
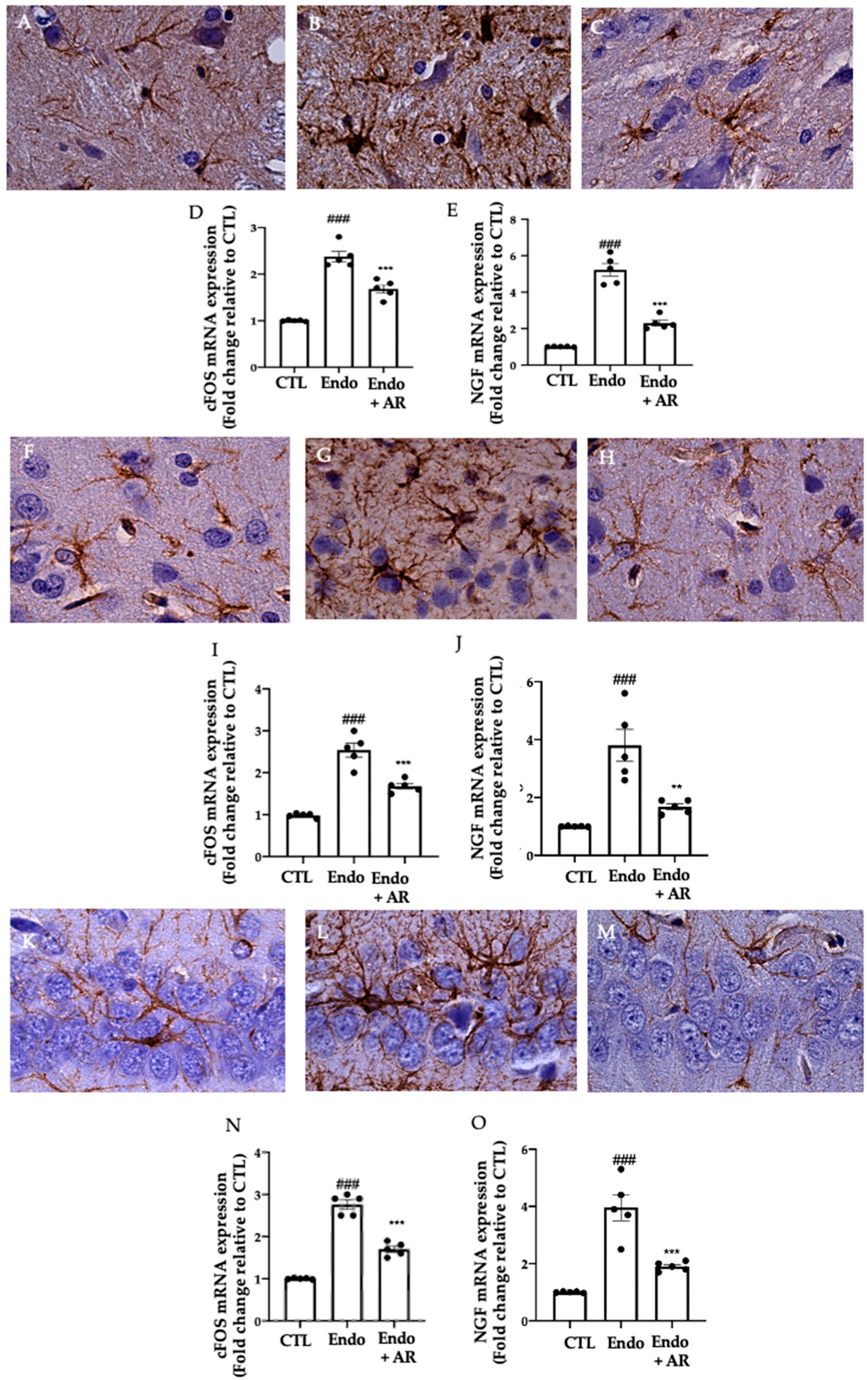
2.6. Effect of AR Administration on Neuroinflammation
3. Discussion
4. Materials and Methods
4.1. Animals
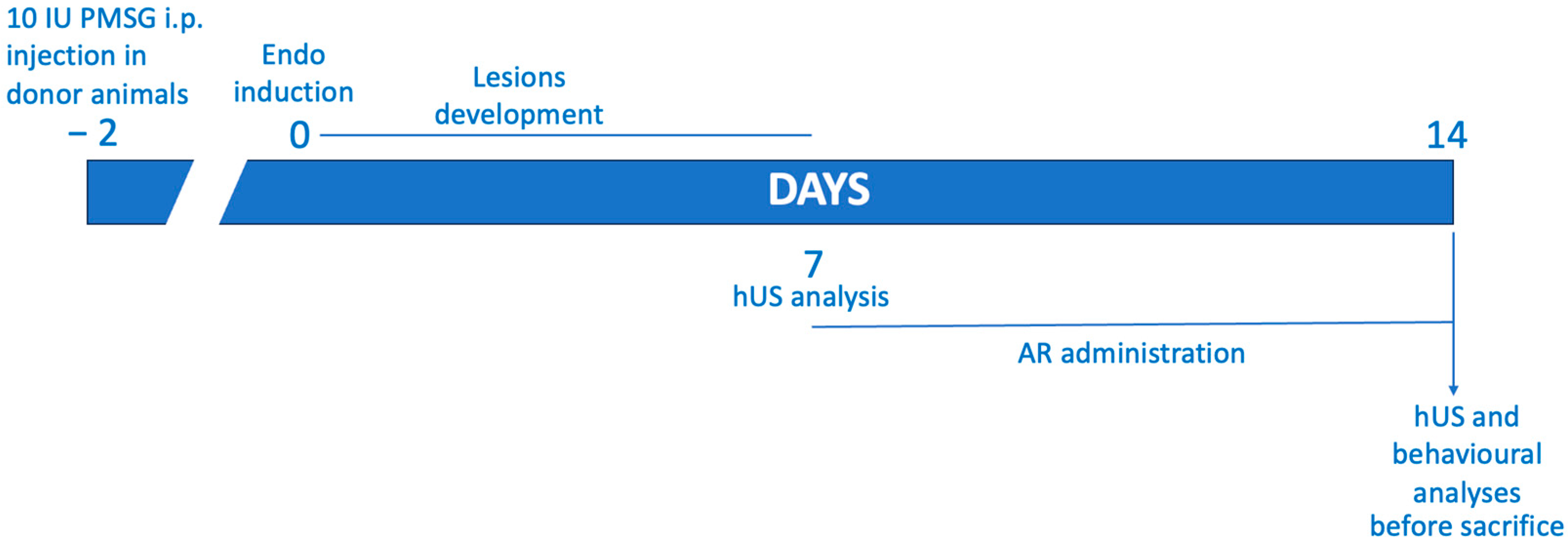
4.2. Experimental Protocol
4.3. Experimental Groups
- (1)
- Endo group: experimental endometriosis was induced in the rats, and they were orally administered with vehicle (saline) using a gavage on the seventh day and subsequently for the following seven days;
- (2)
- Endo + AR group: experimental endometriosis was induced in the rats, and they were orally administered with AR (100 mg/Kg) using a gavage on the seventh day and subsequently for the following seven days;
- (3)
- Control group: the rats were given an intraperitoneal injection of 500 μL of PBS instead of endometrial tissue, and they received a vehicle (saline) via oral gavage on the seventh day and for the subsequent seven days.
4.4. Abdominal High-Frequency Ultrasound
4.5. Behavioral Analysis
4.5.1. Open Field Test
4.5.2. Hot Plate
4.5.3. Elevated Plus Maze Test
4.5.4. Acetic-Acid-Induced Abdominal Contractions
4.6. Abdominal High-Frequency Ultrasound
4.7. Histological Examination
4.8. Terminal Deoxynucleotidyl Nick-End Labeling (TUNEL) Assay
4.9. Western Blot Analysis
4.10. Biochemical Analysis
4.11. RNA Extraction and cDNA Synthesis
4.12. Real-Time PCR
4.13. Immunohistochemical Analysis
4.14. ELISA
4.15. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Warner, M.L. Epidemiology of endometriosis. Obstet. Gynecol. Clin. N. Am. 1997, 24, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- Sampson, J.A. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am. J. Obstet. Gynecol. 1927, 14, 422–469. [Google Scholar] [CrossRef]
- Barcz, E.; Kamiński, P.; Marianowski, L. Role of cytokines in pathogenesis of endometriosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2000, 6, 1042–1046. [Google Scholar]
- Bedaiwy, M.A.; Falcone, T.; Sharma, R.K.; Goldberg, J.M.; Attaran, M.; Nelson, D.R.; Agarwal, A. Prediction of endometriosis with serum and peritoneal fluid markers: A prospective controlled trial. Hum. Reprod. 2002, 17, 426–431. [Google Scholar] [CrossRef]
- Scholl, B.; Bersinger, N.A.; Kuhn, A.; Mueller, M.D. Correlation between symptoms of pain and peritoneal fluid inflammatory cytokine concentrations in endometriosis. Gynecol. Endocrinol. 2009, 25, 701–706. [Google Scholar] [CrossRef]
- Santulli, P.; Chouzenoux, S.; Fiorese, M.; Marcellin, L.; Lemarechal, H.; Millischer, A.E.; Batteux, F.; Borderie, D.; Chapron, C. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum. Reprod. 2015, 30, 49–60. [Google Scholar] [CrossRef]
- Wu, J.; Xie, H.; Yao, S.; Liang, Y. Macrophage and nerve interaction in endometriosis. J. Neuroinflamm. 2017, 14, 53. [Google Scholar] [CrossRef]
- Asante, A.; Taylor, R.N. Endometriosis: The role of neuroangiogenesis. Annu. Rev. Physiol. 2011, 73, 163–182. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, H.; Wu, J.; Liu, D.; Yao, S. Villainous role of estrogen in macrophage-nerve interaction in endometriosis. Reprod. Biol. Endocrinol. 2018, 16, 122. [Google Scholar] [CrossRef]
- Bai, W.; Henneicke-von Zepelin, H.-H.; Wang, S.; Zheng, S.; Liu, J.; Zhang, Z.; Geng, L.; Hu, L.; Chunfeng, J.; Liske, E. Efficacy and tolerability of a medicinal product containing an isopropanolic black cohosh extract in Chinese women with menopausal symptoms: A randomized, double blind, parallel-controlled study versus tibolone. Maturitas 2007, 58, 31–41. [Google Scholar] [CrossRef]
- Borrelli, F.; Ernst, E. Black cohosh (Cimicifuga racemosa) for menopausal symptoms: A systematic review of its efficacy. Pharmacol. Res. 2008, 58, 8–14. [Google Scholar] [CrossRef]
- Burdette, J.E.; Liu, J.; Chen, S.-n.; Fabricant, D.S.; Piersen, C.E.; Barker, E.L.; Pezzuto, J.M.; Mesecar, A.; Van Breemen, R.B.; Farnsworth, N.R. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J. Agric. Food Chem. 2003, 51, 5661–5670. [Google Scholar] [CrossRef]
- Mohammad-Alizadeh-Charandabi, S.; Shahnazi, M.; Nahaee, J.; Bayatipayan, S. Efficacy of black cohosh (Cimicifuga racemosa L.) in treating early symptoms of menopause: A randomized clinical trial. Chin. Med. 2013, 8, 20. [Google Scholar] [CrossRef]
- Borrelli, F.; Ernst, E. Cimicifuga racemosa: A systematic review of its clinical efficacy. Eur. J. Clin. Pharmacol. 2002, 58, 235–241. [Google Scholar] [CrossRef]
- Borrelli, F.; Izzo, A.; Ernst, E. Pharmacological effects of Cimicifuga racemosa. Life Sci. 2003, 73, 1215–1229. [Google Scholar] [CrossRef]
- He, K.; Pauli, G.F.; Zheng, B.; Wang, H.; Bai, N.; Peng, T.; Roller, M.; Zheng, Q. Cimicifuga species identification by high performance liquid chromatography-photodiode array/mass spectrometric/evaporative light scattering detection for quality control of black cohosh products. J. Chromatogr. A 2006, 1112, 241–254. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Nikolic, D.; Lankin, D.C.; Chen, S.-N.; Jaki, B.U.; Krunic, A.; van Breemen, R.B.; Fong, H.H.; Farnsworth, N.R.; Pauli, G.F. Cimipronidine, a Cyclic Guanidine Alkaloid from Cimicifuga racemosa. J. Nat. Prod. 2005, 68, 1266–1270. [Google Scholar] [CrossRef]
- Li, W.; Chen, S.; Fabricant, D.; Angerhofer, C.K.; Fong, H.H.; Farnsworth, N.R.; Fitzloff, J.F. High-performance liquid chromatographic analysis of Black Cohosh (Cimicifuga racemosa) constituents with in-line evaporative light scattering and photodiode array detection. Anal. Chim. Acta 2002, 471, 61–75. [Google Scholar] [CrossRef]
- Liao, X.; Zhang, Q.Y.; Xu, L.; Zhang, H.Y. Potential Targets of Actein Identified by Systems Chemical Biology Methods. ChemMedChem 2020, 15, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Cicek, S.S.; Girreser, U.; Zidorn, C. Quantification of the total amount of black cohosh cycloartanoids by integration of one specific 1H NMR signal. J. Pharm. Biomed. Anal. 2018, 155, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Wang, Y.H.; Smillie, T.J.; Khan, I.A. Quantitative determination of triterpenoids and formononetin in rhizomes of black cohosh (Actaea racemosa) and dietary supplements by using UPLC-UV/ELS detection and identification by UPLC-MS. Planta Med. 2009, 75, 381–386. [Google Scholar] [CrossRef]
- Tian, Z.; Pan, R.; Chang, Q.; Si, J.; Xiao, P.; Wu, E. Cimicifuga foetida extract inhibits proliferation of hepatocellular cells via induction of cell cycle arrest and apoptosis. J. Ethnopharmacol. 2007, 114, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Gao, Y.; Han, W.; Chen, D. Antioxidant activity and mechanism of Rhizoma Cimicifugae. Chem. Cent. J. 2012, 6, 140. [Google Scholar] [CrossRef]
- Cordaro, M.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Di Paola, D.; Interdonato, L.; Impellizzeri, D.; et al. Actaea racemosa L. Rhizome Protect against MPTP-Induced Neurotoxicity in Mice by Modulating Oxidative Stress and Neuroinflammation. Antioxidants 2022, 12, 40. [Google Scholar] [CrossRef]
- Shams, T.; Setia, M.S.; Hemmings, R.; McCusker, J.; Sewitch, M.; Ciampi, A. Efficacy of black cohosh-containing preparations on menopausal symptoms: A meta-analysis. Altern. Ther. Health Med. 2010, 16, 36. [Google Scholar]
- Arentz, S.; Abbott, J.A.; Smith, C.A.; Bensoussan, A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement. Altern. Med. 2014, 14, 511. [Google Scholar] [CrossRef]
- Seli, E.; Berkkanoglu, M.; Arici, A. Pathogenesis of endometriosis. Obstet. Gynecol. Clin. 2003, 30, 41–61. [Google Scholar] [CrossRef]
- Genovese, T.; Siracusa, R.; D’Amico, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Trovato Salinaro, A.; Raffone, E.; Impellizzeri, D.; et al. Regulation of Inflammatory and Proliferative Pathways by Fotemustine and Dexamethasone in Endometriosis. Int. J. Mol. Sci. 2021, 22, 5998. [Google Scholar] [CrossRef]
- Wingfield, M.; Macpherson, A.; Healy, D.L.; Rogers, P.A. Cell proliferation is increased in the endometrium of women with endometriosis. Fertil. Steril. 1995, 64, 340–346. [Google Scholar] [CrossRef]
- Duchrow, M.; Schlüter, C.; Wohlenberg, C.; Flad, H.D.; Gerdes, J. Molecular characterization of the gene locus of the human cell proliferation-associated nuclear protein defined by monoclonal antibody Ki-67. Cell Prolif. 1996, 29, 1–12. [Google Scholar] [CrossRef]
- Foyouzi, N.; Berkkanoglu, M.; Arici, A.; Kwintkiewicz, J.; Izquierdo, D.; Duleba, A.J. Effects of oxidants and antioxidants on proliferation of endometrial stromal cells. Fertil. Steril. 2004, 82 (Suppl. S3), 1019–1022. [Google Scholar] [CrossRef]
- Yi, L.; Lilan, L.; Haibo, Z. Levels of lipid perioxides and superoxide dismutase in peritoneal fluid of patients with endometriosis. J. Tongji Med. Univ. 2001, 21, 166–167. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar] [CrossRef]
- Arnhold, J.; Flemmig, J. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 2010, 500, 92–106. [Google Scholar] [CrossRef]
- Li, Y.; Adur, M.K.; Kannan, A.; Davila, J.; Zhao, Y.; Nowak, R.A.; Bagchi, M.K.; Bagchi, I.C.; Li, Q. Progesterone alleviates endometriosis via inhibition of uterine cell proliferation, inflammation and angiogenesis in an immunocompetent mouse model. PLoS ONE 2016, 11, e0165347. [Google Scholar] [CrossRef]
- Hernandez, S.; Cruz, M.L.; Seguinot, I.I.; Torres-Reveron, A.; Appleyard, C.B. Impact of psychological stress on pain perception in an animal model of endometriosis. Reprod. Sci. 2017, 24, 1371–1381. [Google Scholar] [CrossRef]
- As-Sanie, S.; Harris, R.E.; Harte, S.E.; Tu, F.F.; Neshewat, G.; Clauw, D.J. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet. Gynecol. 2013, 122, 1047. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Beissner, F.; Preibisch, C.; Schweizer-Arau, A.; Popovici, R.M.; Meissner, K. Psychotherapy with somatosensory stimulation for endometriosis-associated pain: The role of the anterior hippocampus. Biol. Psychiatry 2018, 84, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Mutso, A.A.; Petre, B.; Huang, L.; Baliki, M.N.; Torbey, S.; Herrmann, K.M.; Schnitzer, T.J.; Apkarian, A.V. Reorganization of hippocampal functional connectivity with transition to chronic back pain. J. Neurophysiol. 2014, 111, 1065–1076. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Mendoza, F.; Jauregui-Huerta, F.; Aguilar-Delgadillo, A.; Garcia-Estrada, J.; Luquin, S. Immediate Early Gene c-fos in the Brain: Focus on Glial Cells. Brain Sci. 2022, 12, 687. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Trovato Salinaro, A.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Interdonato, L.; Crea, R.; Fusco, R.; et al. Hidrox® and Endometriosis: Biochemical Evaluation of Oxidative Stress and Pain. Antioxidants 2021, 10, 720. [Google Scholar] [CrossRef]
- Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Salinaro, A.T.; Raffone, E.; Genovese, T.; et al. Autophagy and Mitophagy Promotion in a Rat Model of Endometriosis. Int. J. Mol. Sci. 2021, 22, 5074. [Google Scholar] [CrossRef]
- D’Amico, R.; Impellizzeri, D.; Cordaro, M.; Siracusa, R.; Interdonato, L.; Marino, Y.; Crupi, R.; Gugliandolo, E.; Macri, F.; Di Paola, D.; et al. Complex Interplay between Autophagy and Oxidative Stress in the Development of Endometriosis. Antioxidants 2022, 11, 2484. [Google Scholar] [CrossRef]
- Fusco, R.; D’amico, R.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Absence of formyl peptide receptor 1 causes endometriotic lesion regression in a mouse model of surgically-induced endometriosis. Oncotarget 2018, 9, 31355. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Peritore, A.F.; Cordaro, M.; Gugliandolo, E.; Siracusa, R.; Crupi, R.; D’Amico, R.; Fusco, R.; Evangelista, M.; Cuzzocrea, S. The neuroprotective effects of micronized PEA (PEA-m) formulation on diabetic peripheral neuropathy in mice. FASEB J. 2019, 33, 11364–11380. [Google Scholar] [CrossRef]
- D’Amico, R.; Siracusa, R.; Fusco, R.; Cordaro, M.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Protective effects of Colomast®, A new formulation of adelmidrol and sodium hyaluronate, in a mouse model of acute restraint stress. Int. J. Mol. Sci. 2020, 21, 8136. [Google Scholar] [CrossRef]
- de Carvalho, A.M.R.; Vasconcelos, L.F.; Rocha, N.F.M.; Rios, E.R.V.; Dias, M.L.; de França Fonteles, M.M.; Gaspar, D.M.; Barbosa Filho, J.M.; Gutierrez, S.J.C.; de Sousa, F.C.F. Antinociceptive activity of Riparin II from Aniba riparia: Further elucidation of the possible mechanisms. Chem. Biol. Interact. 2018, 287, 49–56. [Google Scholar] [CrossRef]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Combined toxicity of xenobiotics Bisphenol A and heavy metals on zebrafish embryos (Danio rerio). Toxics 2021, 9, 344. [Google Scholar] [CrossRef]
- Di Paola, D.; Capparucci, F.; Abbate, J.M.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Genovese, T.; Impellizzeri, D.; et al. Environmental Risk Assessment of Oxaliplatin Exposure on Early Life Stages of Zebrafish (Danio rerio). Toxics 2022, 10, 81. [Google Scholar] [CrossRef]
- Genovese, T.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Caudullo, S.; Raffone, E.; Macri, F.; Interdonato, L.; Gugliandolo, E.; Interlandi, C.; et al. Molecular and Biochemical Mechanism of Cannabidiol in the Management of the Inflammatory and Oxidative Processes Associated with Endometriosis. Int. J. Mol. Sci. 2022, 23, 5427. [Google Scholar] [CrossRef]
- Fusco, R.; Salinaro, A.T.; Siracusa, R.; D’Amico, R.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Crea, R.; Cordaro, M.; Cuzzocrea, S.; et al. Hidrox® Counteracts Cyclophosphamide-Induced Male Infertility through NRF2 Pathways in a Mouse Model. Antioxidants 2021, 10, 778. [Google Scholar] [CrossRef]
- Di Paola, R.; Fusco, R.; Gugliandolo, E.; D’Amico, R.; Campolo, M.; Latteri, S.; Carughi, A.; Mandalari, G.; Cuzzocrea, S. The Antioxidant Activity of Pistachios Reduces Cardiac Tissue Injury of Acute Ischemia/Reperfusion (I/R) in Diabetic Streptozotocin (STZ)-Induced Hyperglycaemic Rats. Front. Pharmacol. 2018, 9, 51. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; D’Amico, R.; Crupi, R.; Smeriglio, A.; Mandalari, G.; et al. Consumption of Anacardium occidentale L. (Cashew Nuts) Inhibits Oxidative Stress through Modulation of the Nrf2/HO-1 and NF-kB Pathways. Molecules 2020, 25, 4426. [Google Scholar] [CrossRef]
- Peritore, A.F.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021, 22, 5533. [Google Scholar] [CrossRef]
- Crupi, R.; Palma, E.; Siracusa, R.; Fusco, R.; Gugliandolo, E.; Cordaro, M.; Impellizzeri, D.; De Caro, C.; Calzetta, L.; Cuzzocrea, S.; et al. Protective Effect of Hydroxytyrosol against Oxidative Stress Induced by the Ochratoxin in Kidney Cells: In Vitro and In Vivo Study. Front. Vet. Sci. 2020, 7, 136. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Cordaro, M.; Fusco, R.; Peritore, A.F.; Siracusa, R.; Genovese, T.; D’Amico, R.; Impellizzeri, D.; Di Paola, R.; Cuzzocrea, S.; et al. Protective effect of snail secretion filtrate against ethanol-induced gastric ulcer in mice. Sci. Rep. 2021, 11, 3638. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. Biochemical evaluation of the antioxidant effects of hydroxytyrosol on pancreatitis-associated gut injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Siracusa, R.; Fusco, R.; D’Amico, R.; Peritore, A.F.; Gugliandolo, E.; Genovese, T.; Scuto, M.; Crupi, R.; Mandalari, G. Cashew (Anacardium occidentale L.) nuts counteract oxidative stress and inflammation in an acute experimental model of Carrageenan-induced Paw edema. Antioxidants 2020, 9, 660. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Trovato Salinaro, A.; Fusco, R.; Cordaro, M.; Impellizzeri, D.; Scuto, M.; Ontario, M.L.; Lo Dico, G.; Cuzzocrea, S.; Di Paola, R. Hericium erinaceus and coriolus versicolor modulate molecular and biochemical changes after traumatic brain injury. Antioxidants 2021, 10, 898. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; D’Amico, R.; Licata, P.; Crupi, R.; Gugliandolo, E. Effects of hydroxytyrosol against lipopolysaccharide-induced inflammation and oxidative stress in bovine mammary epithelial cells: A natural therapeutic tool for bovine mastitis. Antioxidants 2020, 9, 693. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, D.; Capparucci, F.; Lanteri, G.; Crupi, R.; Marino, Y.; Franco, G.A.; Cuzzocrea, S.; Spano, N.; Gugliandolo, E.; Peritore, A.F. Environmental Toxicity Assessment of Sodium Fluoride and Platinum-Derived Drugs Co-Exposure on Aquatic Organisms. Toxics 2022, 10, 272. [Google Scholar] [CrossRef]
- Di Paola, D.; D’Amico, R.; Genovese, T.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; Interdonato, L.; Impellizzeri, D.; et al. Chronic Exposure to Vinclozolin Induced Fibrosis, Mitochondrial Dysfunction, Oxidative Stress, and Apoptosis in Mice Kidney. Int. J. Mol. Sci. 2022, 23, 1296. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Fusco, R.; Crupi, R.; Rizzarelli, E.; Cuzzocrea, S.; et al. Protective effect of a new hyaluronic acid -carnosine conjugate on the modulation of the inflammatory response in mice subjected to collagen-induced arthritis. Biomed. Pharmacother. 2020, 125, 110023. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Campolo, M.; Evangelista, M.; Di Paola, R.; Cuzzocrea, S. Effect of a new formulation of micronized and ultramicronized N-palmitoylethanolamine in a tibia fracture mouse model of complex regional pain syndrome. PLoS ONE 2017, 12, e0178553. [Google Scholar] [CrossRef]
- Peritore, A.F.; Crupi, R.; Scuto, M.; Gugliandolo, E.; Siracusa, R.; Impellizzeri, D.; Cordaro, M.; D’Amico, R.; Fusco, R.; Di Paola, R.; et al. The Role of Annexin A1 and Formyl Peptide Receptor 2/3 Signaling in Chronic Corticosterone-Induced Depression-Like Behaviors and Impairment in Hippocampal-Dependent Memory. CNS Neurol. Disord. Drug Targets 2020, 19, 27–43. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Interdonato, L.; Marino, Y.; D’Amico, R.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Macrì, F.; Fusco, R.; Cuzzocrea, S.; Di Paola, R. Modulation of the Proliferative Pathway, Neuroinflammation and Pain in Endometriosis. Int. J. Mol. Sci. 2023, 24, 11741. https://doi.org/10.3390/ijms241411741
Interdonato L, Marino Y, D’Amico R, Cordaro M, Siracusa R, Impellizzeri D, Macrì F, Fusco R, Cuzzocrea S, Di Paola R. Modulation of the Proliferative Pathway, Neuroinflammation and Pain in Endometriosis. International Journal of Molecular Sciences. 2023; 24(14):11741. https://doi.org/10.3390/ijms241411741
Chicago/Turabian StyleInterdonato, Livia, Ylenia Marino, Ramona D’Amico, Marika Cordaro, Rosalba Siracusa, Daniela Impellizzeri, Francesco Macrì, Roberta Fusco, Salvatore Cuzzocrea, and Rosanna Di Paola. 2023. "Modulation of the Proliferative Pathway, Neuroinflammation and Pain in Endometriosis" International Journal of Molecular Sciences 24, no. 14: 11741. https://doi.org/10.3390/ijms241411741
APA StyleInterdonato, L., Marino, Y., D’Amico, R., Cordaro, M., Siracusa, R., Impellizzeri, D., Macrì, F., Fusco, R., Cuzzocrea, S., & Di Paola, R. (2023). Modulation of the Proliferative Pathway, Neuroinflammation and Pain in Endometriosis. International Journal of Molecular Sciences, 24(14), 11741. https://doi.org/10.3390/ijms241411741








