Homozygous C677T Methylenetetrahydrofolate Reductase (MTHFR) Polymorphism as a Risk Factor for Endometriosis: A Retrospective Case–Control Study
Abstract
1. Introduction
2. Results
3. Discussion
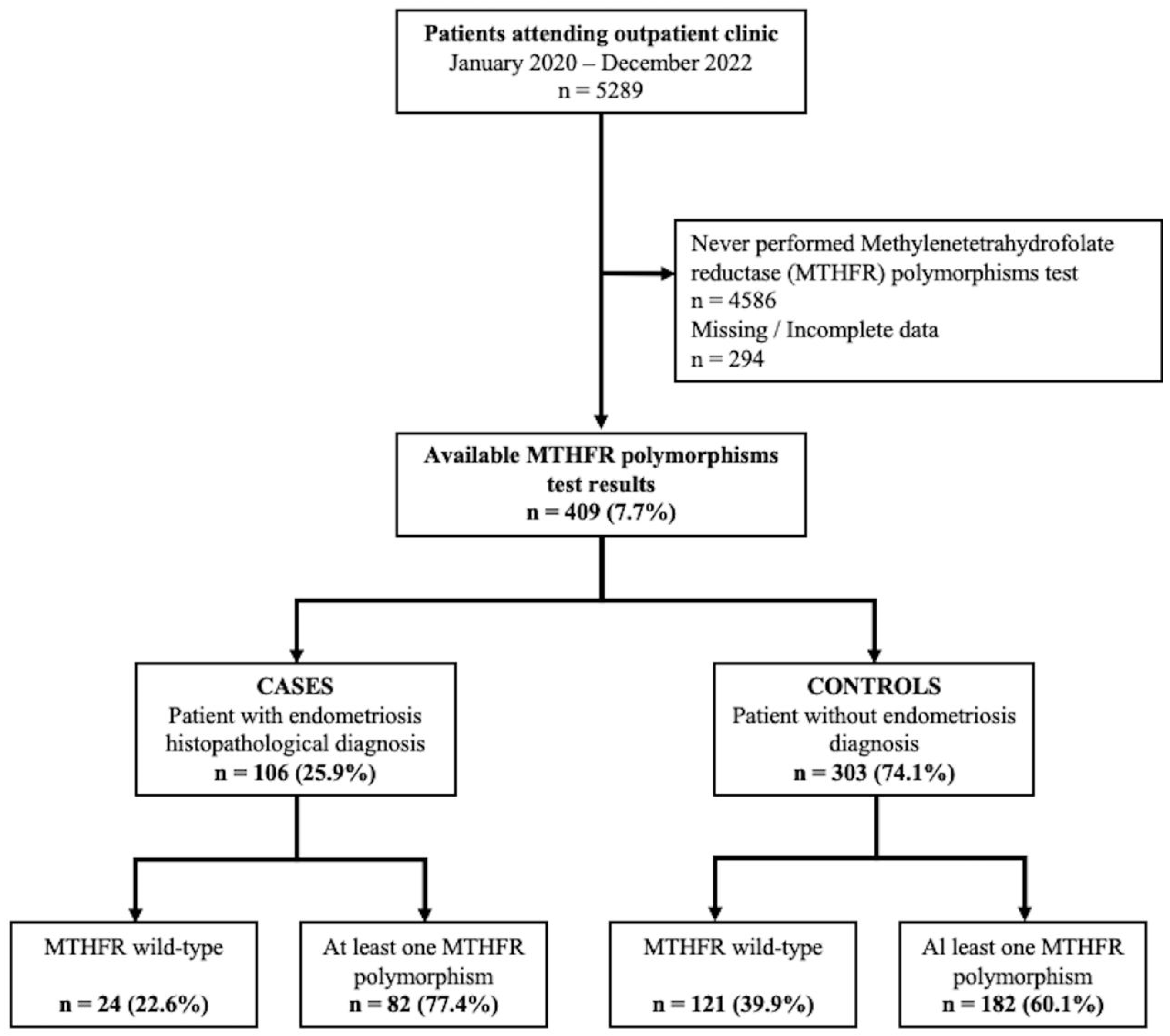
4. Materials and Methods
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viganò, P.; Parazzini, F.; Somigliana, E.; Vercellini, P. Endometriosis: Epidemiology and aetiological factors. Best. Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 177–200. [Google Scholar] [CrossRef] [PubMed]
- Pais, A.S.; Almeida-Santos, T. Recent insights explaining susceptibility to endometriosis-From genetics to environment. WIREs Mech. Dis. 2023, e1624. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, L.; Nisolle, M.; Noël, J.C.; Fastrez, M. Three Types of Endometriosis: Pathogenesis, Diagnosis and Treatment. State of the Art. J. Clin. Med. 2023, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Missmer, S.A. Pathophysiology, diagnosis, and management of endometriosis. BMJ 2022, 379, e070750. [Google Scholar] [CrossRef] [PubMed]
- Ciavattini, A.; Serri, M.; Delli Carpini, G.; Morini, S.; Clemente, N. Ovarian endometriosis and vitamin D serum levels. Gynecol. Endocrinol. 2017, 33, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Ciavattini, A.; Carpini, G.D.; Serri, M.; Tozzi, A.; Leoni, F.; Di Loreto, E.; Saccucci, F. Unfolded protein response, a link between endometrioid ovarian carcinoma and endometriosis: A pilot study. Oncol. Lett. 2018, 16, 5449–5454. [Google Scholar] [CrossRef]
- Guo, S.W. Epigenetics of endometriosis. Mol. Hum. Reprod. 2009, 15, 587–607. [Google Scholar] [CrossRef]
- Wu, Y.; Halverson, G.; Basir, Z.; Strawn, E.; Yan, P.; Guo, S.W. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am. J. Obstet. Gynecol. 2005, 193, 371–380. [Google Scholar] [CrossRef]
- Hsiao, K.Y.; Wu, M.H.; Tsai, S.J. Epigenetic regulation of the pathological process in endometriosis. Reprod. Med. Biol. 2017, 16, 314–319. [Google Scholar] [CrossRef]
- Koukoura, O.; Sifakis, S.; Spandidos, D.A. DNA methylation in endometriosis (Review). Mol. Med. Rep. 2016, 13, 2939–2948. [Google Scholar] [CrossRef]
- Naqvi, H.; Ilagan, Y.; Krikun, G.; Taylor, H.S. Altered genome-wide methylation in endometriosis. Reprod. Sci. 2014, 21, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Mandaviya, P.R.; Stolk, L.; Heil, S.G. Homocysteine and DNA methylation: A review of animal and human literature. Mol. Genet. Metab. 2014, 113, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Palfrey, H.A.; Pathak, R.; Kadowitz, P.J.; Gettys, T.W.; Murthy, S.N. The metabolism and significance of homocysteine in nutrition and health. Nutr. Metab. 2017, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N. Epigenetic modulation of DNA methylation by nutrition and its mechanisms in animals. Anim. Nutr. 2015, 1, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Clément, A.; Cornet, D.N.; Alvarez, S.; Brami, C.; Clément, P.; Menezo, Y. Endometriosis pathogenesis: Role played by the oxidative stress due to MTHFR mutations. ASRM Abstr. 2018, 110, e394–e395. [Google Scholar] [CrossRef]
- Guedes, T.; Santos, A.A.; Vieira-Neto, F.H.; Bianco, B.; Barbosa, C.P.; Christofolini, D.M. Folate metabolism abnormalities in infertile patients with endometriosis. Biomark. Med. 2022, 16, 549–557. [Google Scholar] [CrossRef]
- Clément, P.; Alvarez, S.; Jacquesson-Fournols, L.; Cornet, D.; Clément, A.; Brack, M.; Lalau-Keraly, M.; Delafontaine, D.; Cohen, M.; Menezo, Y. T677T Methylenetetrahydrofolate Reductase Single Nucleotide Polymorphisms Increased Prevalence in a Subgroup of Infertile Patients with Endometriosis. J. Women’s Health 2022, 31, 1501–1506. [Google Scholar] [CrossRef]
- Szczepańska, M.; Mostowska, A.; Wirstlein, P.; Lianeri, M.; Marianowski, P.; Skrzypczak, J.; Jagodziński, P.P. Polymorphic variants of folate and choline metabolism genes and the risk of endometriosis-associated infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 157, 67–72. [Google Scholar] [CrossRef]
- Frosst, P.; Blom, H.J.; Milos, R.; Goyette, P.; Sheppard, C.A.; Matthews, R.G.; Boers, G.J.; den Heijer, M.; Kluijtmans, L.A.; van den Heuvel, L.P.; et al. A candidate genetic risk factor for vascular disease: A common mutation in methylenetetrahydrofolate reductase. Nat. Genet. 1995, 10, 111–113. [Google Scholar] [CrossRef]
- van der Put, N.M.; Gabreëls, F.; Stevens, E.M.; Smeitink, J.A.; Trijbels, F.J.; Eskes, T.K.; van den Heuvel, L.P.; Blom, H.J. A second common mutation in the methylenetetrahydrofolate reductase gene: An additional risk factor for neural-tube defects? Am. J. Hum. Genet. 1998, 62, 1044–1051. [Google Scholar] [CrossRef]
- Bulun, S.E.; Monsivais, D.; Kakinuma, T.; Furukawa, Y.; Bernardi, L.; Pavone, M.E.; Dyson, M. Molecular biology of endometriosis: From aromatase to genomic abnormalities. Semin. Reprod. Med. 2015, 33, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Shao, R.; Cao, S.; Wang, X.; Feng, Y.; Billig, H. The elusive and controversial roles of estrogen and progesterone receptors in human endometriosis. Am. J. Transl. Res. 2014, 6, 104–113. [Google Scholar] [PubMed]
- Buchweitz, O.; Staebler, A.; Wülfing, P.; Hauzman, E.; Greb, R.; Kiesel, L. COX-2 overexpression in peritoneal lesions is correlated with nonmenstrual chronic pelvic pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 124, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Yamada, Y.; Shigemitsu, A.; Akinishi, M.; Kaniwa, H.; Miyake, R.; Yamanaka, S.; Kobayashi, H. Role of Oxidative Stress in Epigenetic Modification in Endometriosis. Reprod. Sci. 2017, 24, 1493–1502. [Google Scholar] [CrossRef] [PubMed]
- Arab, A.; Karimi, E.; Vingrys, K.; Kelishadi, M.R.; Mehrabani, S.; Askari, G. Food groups and nutrients consumption and risk of endometriosis: A systematic review and meta-analysis of observational studies. Nutr. J. 2022, 21, 58. [Google Scholar] [CrossRef]
- Yen, C.F.; Kim, M.R.; Lee, C.L. Epidemiologic Factors Associated with Endometriosis in East Asia. Gynecol. Minim. Invasive Ther. 2019, 8, 4–11. [Google Scholar] [CrossRef]
- Coiplet, E.; Courbiere, B.; Agostini, A.; Boubli, L.; Bretelle, F.; Netter, A. Endometriosis and environmental factors: A critical review. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102418. [Google Scholar] [CrossRef]
- Burghaus, S.; Hildebrandt, T.; Fahlbusch, C.; Heusinger, K.; Antoniadis, S.; Lermann, J.; Hackl, J.; Häberle, L.; Renner, S.P.; Fasching, P.A.; et al. Standards Used by a Clinical and Scientific Endometriosis Center for the Diagnosis and Therapy of Patients with Endometriosis. Geburtshilfe Frauenheilkd. 2019, 79, 487–497. [Google Scholar] [CrossRef]
- Parazzini, F.; Roncella, E.; Cipriani, S.; Trojano, G.; Barbera, V.; Herranz, B.; Colli, E. The frequency of endometriosis in the general and selected populations: A systematic review. J. Endometr. Pelvic Pain Disord. 2020, 12, 176–189. [Google Scholar] [CrossRef]
- Cohen, D.A.; Shirts, B.H.; Jackson, B.R.; Parker, L.S. Laboratory informatics based evaluation of methylene tetrahydrofolate reductase C677T genetic test overutilization. J. Pathol. Inform. 2013, 4, 33. [Google Scholar] [CrossRef]
- Dallapiccola, B.; Torrente, I.; Morena, A.; Dagna-Bricarelli, F.; Mingarelli, R. Genetic testing in Italy, year 2004. Eur. J. Hum. Genet. 2006, 14, 911–916. [Google Scholar] [CrossRef][Green Version]
- Savelli, L.; Fabbri, F.; Zannoni, L.; De Meis, L.; Di Donato, N.; Mollo, F.; Seracchioli, R. Preoperative ultrasound diagnosis of deep endometriosis: Importance of the examiner’s expertise and lesion size. Australas. J. Ultrasound Med. 2012, 15, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Condous, G.; van den Bosch, T.; Valentin, L.; Leone, F.P.; Van Schoubroeck, D.; Exacoustos, C.; Installé, A.J.; Martins, W.P.; Abrao, M.S.; et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: A consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet. Gynecol. 2016, 48, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Carfagna, P.; De Cicco Nardone, C.; De Cicco Nardone, A.; Testa, A.C.; Scambia, G.; Marana, R.; De Cicco Nardone, F. Role of transvaginal ultrasound in evaluation of ureteral involvement in deep infiltrating endometriosis. Ultrasound Obstet. Gynecol. 2018, 51, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T.; Dueholm, M.; Leone, F.P.; Valentin, L.; Rasmussen, C.K.; Votino, A.; Van Schoubroeck, D.; Landolfo, C.; Installé, A.J.; Guerriero, S.; et al. Terms, definitions and measurements to describe sonographic features of myometrium and uterine masses: A consensus opinion from the Morphological Uterus Sonographic Assessment (MUSA) group. Ultrasound Obstet. Gynecol. 2015, 46, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Savelli, L. Transvaginal sonography for the assessment of ovarian and pelvic endometriosis: How deep is our understanding? Ultrasound Obstet. Gynecol. 2009, 33, 497–501. [Google Scholar] [CrossRef]
- Munro, M.G.; Critchley, H.O.D.; Fraser, I.S.; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. Int. J. Gynaecol. Obstet. 2018, 143, 393–408. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, N.Y. Environmental Risk Factors for Endometriosis: An Umbrella Review of a Meta-Analysis of 354 Observational Studies with over 5 Million Populations. Front. Med. 2021, 8, 680833. [Google Scholar] [CrossRef]
- Parasar, P.; Ozcan, P.; Terry, K.L. Endometriosis: Epidemiology, Diagnosis and Clinical Management. Curr. Obstet. Gynecol. Rep. 2017, 6, 34–41. [Google Scholar] [CrossRef]
- Peterson, C.M.; Johnstone, E.B.; Hammoud, A.O.; Stanford, J.B.; Varner, M.W.; Kennedy, A.; Chen, Z.; Sun, L.; Fujimoto, V.Y.; Hediger, M.L.; et al. Risk factors associated with endometriosis: Importance of study population for characterizing disease in the ENDO Study. Am. J. Obstet. Gynecol. 2013, 208, 451.e1–451.e11. [Google Scholar] [CrossRef]
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Delli Carpini, G.; Giannella, L.; Di Giuseppe, J.; Montanari, M.; Fichera, M.; Pizzagalli, D.; Meccariello, M.L.; Palazzo, P.; Valenza, C.; Francucci, A.; et al. Effect of the mode of delivery on the risk of endometriosis recurrence: A retrospective cohort study. Fertil. Steril. 2022, 118, 1080–1087. [Google Scholar] [CrossRef]
- Hsieh, F.Y.; Bloch, D.A.; Larsen, M.D. A simple method of sample size calculation for linear and logistic regression. Stat. Med. 1998, 17, 1623–1634. [Google Scholar] [CrossRef]
- 1000 Genomes Project Consortium; Auton, A.; Brooks, L.D.; Durbin, R.M.; Garrison, E.P.; Kang, H.M.; Korbel, J.O.; Marchini, J.L.; McCarthy, S.; McVean, G.A.; et al. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J. Principles of confounder selection. Eur. J. Epidemiol. 2019, 34, 211–219. [Google Scholar] [CrossRef]
| Variable | Cases n = 106 | Controls n = 303 | p * |
|---|---|---|---|
| Age (years) | 41.7 ± 9.0 | 43.6 ± 11.3 | 0.1035 |
| Body Mass Index (BMI) | 24.4 ± 4.2 | 24.9 ± 4.1 | 0.2321 |
| History of Mullerian anomalies | 4 (3.8) | 16 (5.3) | 0.5390 |
| Tobacco use | 3 (2.8) | 6 (2.0) | 0.6299 |
| Alcohol use | 1 (0.9) | 0 (0.0) | 0.2591 |
| Frequent menstrual cycle | 7 (6.6) | 26 (8.5) | 0.5355 |
| Heavy menstrual cycle | 16 (15.1) | 53 (17.4) | 0.5861 |
| History of infertility | 16 (15.1) | 34 (11.2) | 0.2918 |
| N° previous pregnancies | 0 (0–1) | 1 (0–2) | 0.0002 |
| No previous pregnancy | 54 (50.9) | 106 (35.0) | 0.0039 |
| At least one spontaneous miscarriage ^ | 10 (19.2) | 59 (29.9) | 0.1257 |
| Recurrent miscarriage ^ | 0 (0.0) | 16 (2.7) | 0.2319 |
| At least one cesarean section ^ | 21 (40.4) | 53 (17.5) | 0.0004 |
| Homocysteinemia (μmol/L) | 11.5 ± 5.6 | 11.9 ± 4.4 | 0.5013 |
| MTHFR Status | Cases n = 106 | Controls n = 303 | p * | |
|---|---|---|---|---|
| C677T | A1298C | |||
| wild-type | wild-type | 24 (22.6) | 121 (39.9) | 0.0014 |
| homozygous | wild-type | 26 (24.5) | 48 (15.8) | 0.0453 |
| heterozygous | wild-type | 25 (23.6) | 64 (21.1) | 0.5917 |
| wild-type | homozygous | 7 (6.6) | 13 (4.3) | 0.3455 |
| wild-type | heterozygous | 11 (10.4) | 18 (5.9) | 0.1201 |
| heterozygous | heterozygous | 12 (11.3) | 37 (12.2) | 0.8061 |
| homozygous | heterozygous | 0 (0.0) | 2 (0.7) | 0.3884 |
| heterozygous | homozygous | 0 (0.0) | 0 (0.0) | - |
| homozygous | homozygous | 1 (0.9) | 0 (0.0) | 0.0987 |
| MTHFR Status | Ovarian Endometriosis n = 81 | Deeply Infiltrative Endometriosis n = 25 | p * | |
|---|---|---|---|---|
| C677T | A1298C | |||
| wild-type | wild-type | 17 (21.1) | 7 (28.0) | 0.6462 |
| homozygous | wild-type | 21 (25.9) | 5 (20.0) | 0.7368 |
| heterozygous | wild-type | 21 (25.9) | 4 (16.0) | 0.4518 |
| wild-type | homozygous | 4 (4.9) | 3 (12.0) | 0.4341 |
| wild-type | heterozygous | 9 (11.1) | 2 (8.0) | 0.9436 |
| heterozygous | heterozygous | 9 (11.1) | 3 (12.0) | 0.8116 |
| homozygous | heterozygous | 0 (0.0) | 0 (0.0) | - |
| heterozygous | homozygous | 0 (0.0) | 0 (0.0) | - |
| homozygous | homozygous | 0 (0.0) | 1 (4.0) | 0.5319 |
| Variable | adOR | 95% CI | B | Std. Error | Wald | p |
|---|---|---|---|---|---|---|
| Age | 0.985 | 0.963–1.008 | −0.015 | 0.012 | 1.655 | 0.1983 |
| BMI | 0.964 | 0.910–1.020 | −0.037 | 0.029 | 1.624 | 0.2026 |
| Mullerian anomalies | 0.541 | 0.169–1.732 | −0.614 | 0.593 | 1.070 | 0.3009 |
| Frequent menstrual cycle | 0.835 | 0.339–2.057 | −0.180 | 0.460 | 0.153 | 0.6956 |
| Heavy menstrual cycle | 0.853 | 0.450–1.619 | −0.160 | 0.326 | 0.239 | 0.6248 |
| History of infertility | 1.398 | 0.724–2.700 | 0.335 | 0.336 | 0.993 | 0.3189 |
| No previous pregnancy | 2.191 | 1.295–3.708 | 0.784 | 0.268 | 8.538 | 0.0035 |
| 1+ cesarean section | 1.750 | 0.916–3.346 | 0.560 | 0.331 | 2.867 | 0.0904 |
| MTHFR C677T homozygous | 1.889 | 1.076–3.318 | 0.636 | 0.287 | 4.899 | 0.0269 |
| Homocysteinemia | 0.985 | 0.939–1.033 | −0.015 | 0.025 | 0.396 | 0.5291 |
| Constant | 0.985 | 0.963–1.008 | −0.015 | 0.012 | 1.655 | 0.1983 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delli Carpini, G.; Giannella, L.; Di Giuseppe, J.; Montik, N.; Montanari, M.; Fichera, M.; Crescenzi, D.; Marzocchini, C.; Meccariello, M.L.; Di Biase, D.; et al. Homozygous C677T Methylenetetrahydrofolate Reductase (MTHFR) Polymorphism as a Risk Factor for Endometriosis: A Retrospective Case–Control Study. Int. J. Mol. Sci. 2023, 24, 15404. https://doi.org/10.3390/ijms242015404
Delli Carpini G, Giannella L, Di Giuseppe J, Montik N, Montanari M, Fichera M, Crescenzi D, Marzocchini C, Meccariello ML, Di Biase D, et al. Homozygous C677T Methylenetetrahydrofolate Reductase (MTHFR) Polymorphism as a Risk Factor for Endometriosis: A Retrospective Case–Control Study. International Journal of Molecular Sciences. 2023; 24(20):15404. https://doi.org/10.3390/ijms242015404
Chicago/Turabian StyleDelli Carpini, Giovanni, Luca Giannella, Jacopo Di Giuseppe, Nina Montik, Michele Montanari, Mariasole Fichera, Daniele Crescenzi, Carolina Marzocchini, Maria Liberata Meccariello, Donato Di Biase, and et al. 2023. "Homozygous C677T Methylenetetrahydrofolate Reductase (MTHFR) Polymorphism as a Risk Factor for Endometriosis: A Retrospective Case–Control Study" International Journal of Molecular Sciences 24, no. 20: 15404. https://doi.org/10.3390/ijms242015404
APA StyleDelli Carpini, G., Giannella, L., Di Giuseppe, J., Montik, N., Montanari, M., Fichera, M., Crescenzi, D., Marzocchini, C., Meccariello, M. L., Di Biase, D., Vignini, A., & Ciavattini, A. (2023). Homozygous C677T Methylenetetrahydrofolate Reductase (MTHFR) Polymorphism as a Risk Factor for Endometriosis: A Retrospective Case–Control Study. International Journal of Molecular Sciences, 24(20), 15404. https://doi.org/10.3390/ijms242015404







