The Pathogenesis of Systemic Sclerosis: The Origin of Fibrosis and Interlink with Vasculopathy and Autoimmunity
Abstract
1. Introduction
2. Vasculopathy as an Initial Step in SSc Pathogenesis
3. Autoimmunity Link with Vasculopathy
3.1. Clinical Evidence
3.2. Immunopathogenesis and Interlink with Fibrosis and Vasculopathy
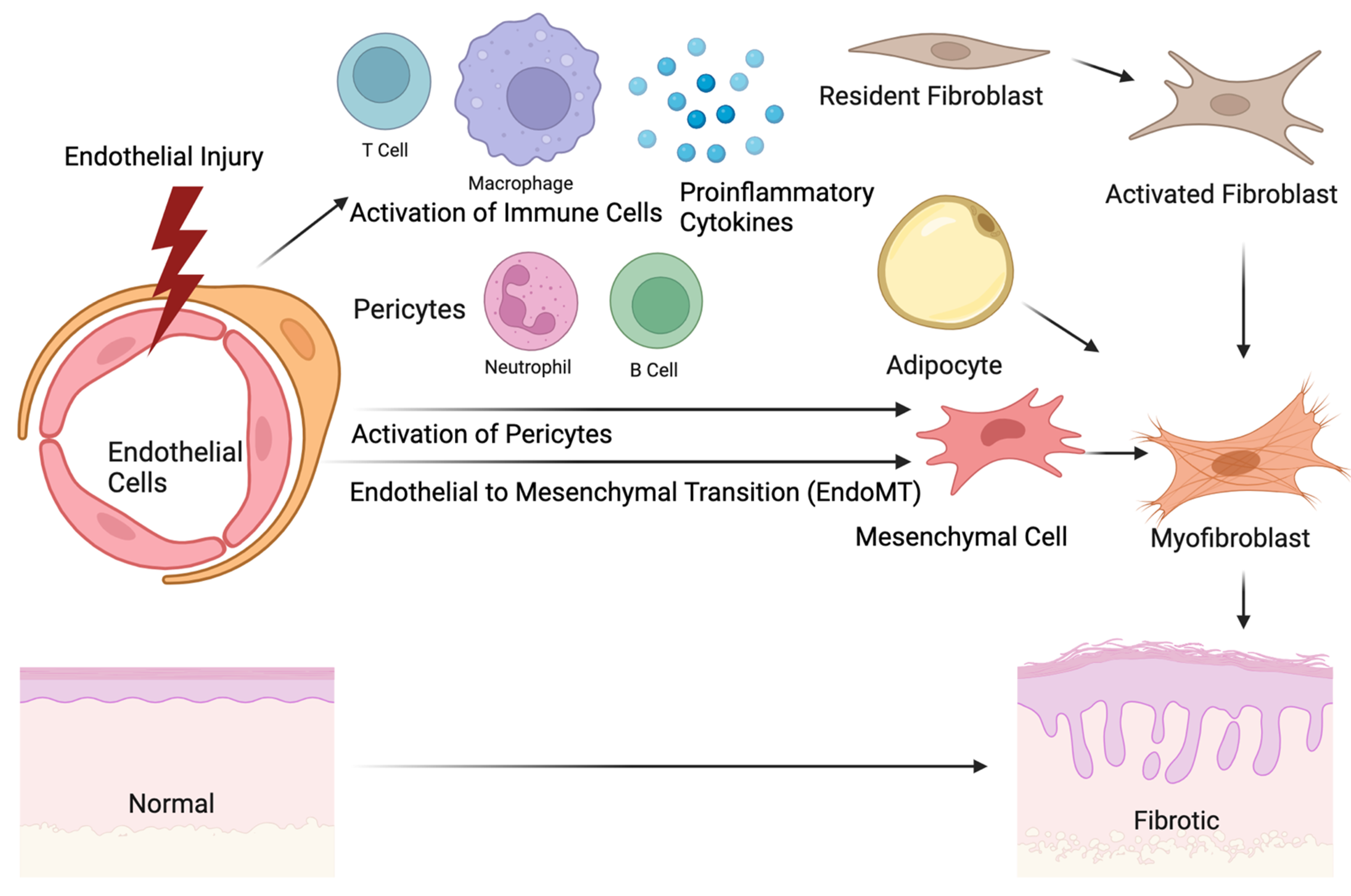
4. The Origin of Fibrosis and Interlink with Vasculopathy
4.1. Contribution of Pericytes to Fibrosis and Vasculopathy
4.2. Signaling Pathways Involved in Vasculopathy and Fibrosis
4.3. Role of Adipocytes in Fibrosis and Vasculopathy
4.4. Fibrocytes
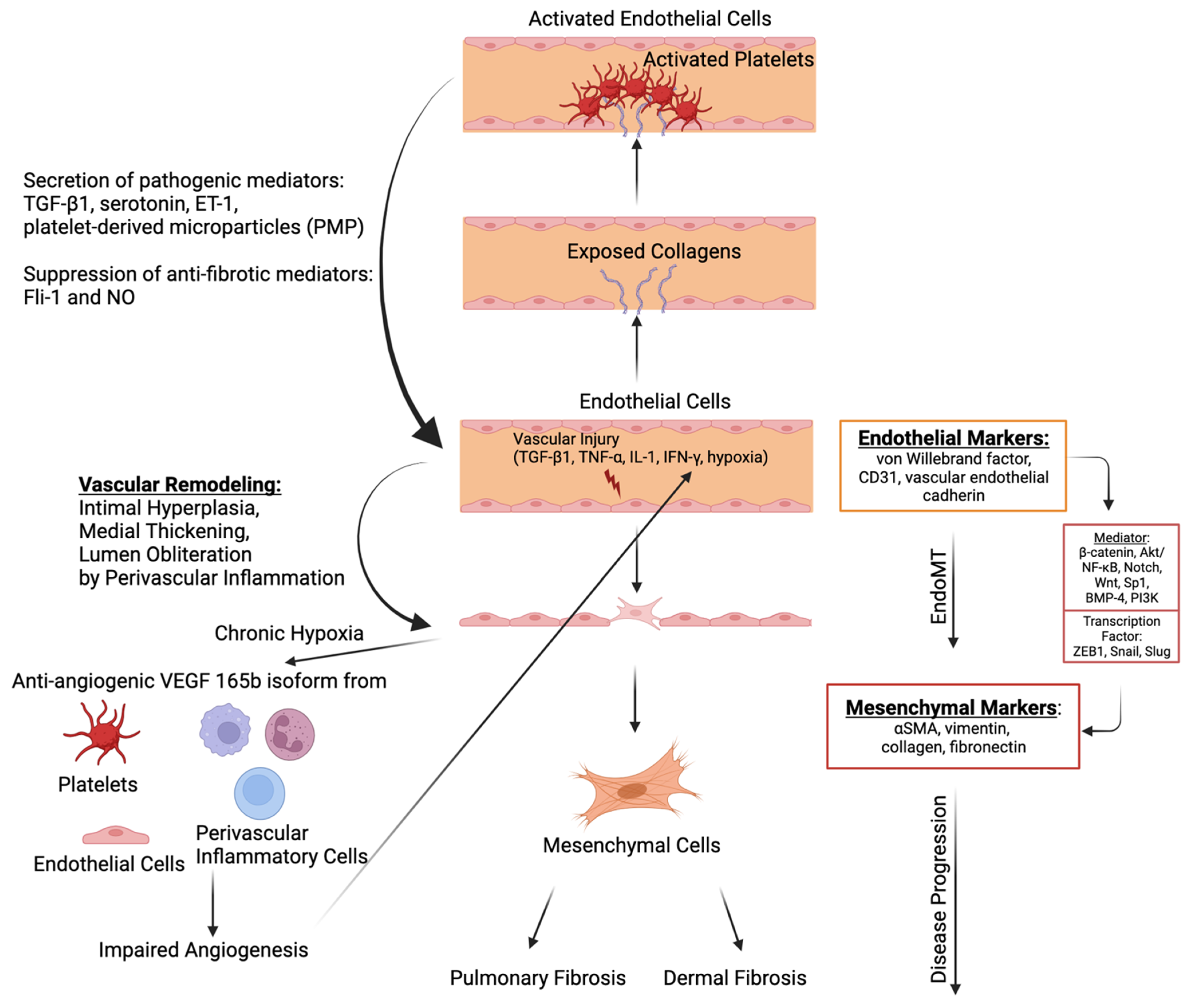
4.5. Endothelial-to-Mesenchymal Transition (EndoMT)
4.6. Platelet Activation in the Interlink between Vasculopathy, Autoimmunity, and Fibrosis
5. Unmet Needs and Future Studies
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Allanore, Y.; Simms, R.; Distler, O.; Trojanowska, M.; Pope, J.; Denton, C.P.; Varga, J. Systemic Sclerosis. Nat. Rev. Dis. Prim. 2015, 1, 15002. [Google Scholar] [CrossRef]
- Ali, H.; Ng, K.R.; Low, A.H.L. A Qualitative Systematic Review of the Prevalence of Coronary Artery Disease in Systemic Sclerosis. Int. J. Rheum. Dis. 2015, 18, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Colaci, M.; Zanoli, L.; Lo Gullo, A.; Sambataro, D.; Sambataro, G.; Aprile, M.L.; Castellino, P.; Malatino, L. The Impaired Elasticity of Large Arteries in Systemic Sclerosis Patients. J. Clin. Med. 2022, 11, 3256. [Google Scholar] [CrossRef]
- Edigin, E.; Ojemolon, P.E.; Eseaton, P.O.; Jamal, S.; Shaka, H.; Akuna, E.; Asemota, I.R.; Manadan, A. Systemic Sclerosis Is Associated with Increased Inpatient Mortality in Patients Admitted for Acute Coronary Syndrome: Analysis of the National Inpatient Sample. J. Clin. Rheumatol. 2022, 28, E110–E117. [Google Scholar] [CrossRef]
- Colaci, M.; Schinocca, C.; Dal Bosco, Y.; Ronsivalle, G.; Guggino, G.; de Andres, I.; Russo, A.A.; Sambataro, D.; Sambataro, G.; Malatino, L. Heart Valve Abnormalities in Systemic Sclerosis Patients: A Multicenter Cohort Study and Review of the Literature. J. Clin. Rheumatol. 2022, 28, E95–E101. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lefebvre, F.; Hoa, S.; Hudson, M. Mortality and Morbidity in Scleroderma Renal Crisis: A Systematic Literature Review. J. Scleroderma Relat. Disord. 2021, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, E.R.; Fischer, A. Update on Morbidity and Mortality in Systemic Sclerosis–Related Interstitial Lung Disease. J. Scleroderma Relat. Disord. 2021, 6, 11. [Google Scholar] [CrossRef]
- De Almeida Chaves, S.; Porel, T.; Mounié, M.; Alric, L.; Astudillo, L.; Huart, A.; Lairez, O.; Michaud, M.; Prévot, G.; Ribes, D.; et al. Sine Scleroderma, Limited Cutaneous, and Diffused Cutaneous Systemic Sclerosis Survival and Predictors of Mortality. Arthritis Res. Ther. 2021, 23, 295. [Google Scholar] [CrossRef]
- Pokeerbux, M.R.; Giovannelli, J.; Dauchet, L.; Mouthon, L.; Agard, C.; Lega, J.C.; Allanore, Y.; Jego, P.; Bienvenu, B.; Berthier, S.; et al. Survival and Prognosis Factors in Systemic Sclerosis: Data of a French Multicenter Cohort, Systematic Review, and Meta-Analysis of the Literature. Arthritis Res. Ther. 2019, 21, 86. [Google Scholar] [CrossRef]
- Tyndall, A.J.; Bannert, B.; Vonk, M.; Airò, P.; Cozzi, F.; Carreira, P.E.; Bancel, D.F.; Allanore, Y.; Müller-Ladner, U.; Distler, O.; et al. Causes and Risk Factors for Death in Systemic Sclerosis: A Study from the EULAR Scleroderma Trials and Research (EUSTAR) Database. Ann. Rheum. Dis. 2010, 69, 1809–1815. [Google Scholar] [CrossRef]
- Steen, V.D.; Medsger, T.A. Changes in Causes of Death in Systemic Sclerosis, 1972–2002. Ann. Rheum. Dis. 2007, 66, 940. [Google Scholar] [CrossRef]
- Kowalska-Kępczyńska, A. Systemic Scleroderma—Definition, Clinical Picture and Laboratory Diagnostics. J. Clin. Med. 2022, 11, 2299. [Google Scholar] [CrossRef]
- Noviani, M.; Chellamuthu, V.R.; Albani, S.; Low, A.H.L. Toward Molecular Stratification and Precision Medicine in Systemic Sclerosis. Front. Med. 2022, 9, 911977. [Google Scholar] [CrossRef] [PubMed]
- Poormoghim, H.; Lucas, M.; Fertig, N.; Medsger, T.A., Jr. Systemic Sclerosis Sine Scleroderma: Demographic, Clinical, and Serologic Features and Survival in Forty-Eight Patients. Arthritis Rheumatol. 2000, 43, 444–451. [Google Scholar] [CrossRef]
- Benjamin, C.; Carlo Alberto, S.; Rosaria, T.; Tobias, A.; Zahir, A.; Tadej, A.; Lorenzo, B.; Andrea, D.; Aurelien, G.; Vera, G.; et al. Mixed Connective Tissue Disease: State of the Art on Clinical Practice Guidelines. RMD Open 2018, 4, e000783. [Google Scholar] [CrossRef]
- Roumm, A.D.; Whiteside, T.L.; Medsger, T.A.; Rodnan, G.P. Lymphocytes in the Skin of Patients with Progressive Systemic Sclerosis. Quantification, Subtyping, and Clinical Correlations. Arthritis Rheum. 1984, 27, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Prescott, R.J.; Freemont, A.J.; Jones, C.J.P.; Hoyland, J.; Fielding, P. Sequential Dermal Microvascular and Perivascular Changes in the Development of Scleroderma. J. Pathol. 1992, 166, 255–263. [Google Scholar] [CrossRef]
- Freemont, A.J.; Hoyland, J.; Fielding, P.; Hodson, N.; Jayson, M.I.V. Studies of the Microvascular Endothelium in Uninvolved Skin of Patients with Systemic Sclerosis: Direct Evidence for a Generalized Microangiopathy. Br. J. Dermatol. 1992, 126, 561–568. [Google Scholar] [CrossRef]
- Bakst, R.; Merola, J.F.; Franks, A.G.; Sanchez, M. Raynaud’s Phenomenon: Pathogenesis and Management. J. Am. Acad. Dermatol. 2008, 59, 633–653. [Google Scholar] [CrossRef]
- Lambova, S.N.; Müller-Ladner, U. Nailfold Capillaroscopy in Systemic Sclerosis–State of the Art: The Evolving Knowledge about Capillaroscopic Abnormalities in Systemic Sclerosis. J. Scleroderma Relat. Disord. 2019, 4, 200. [Google Scholar] [CrossRef]
- Wielosz, E. The Usefulness of Nailfold Capillaroscopy in “Scleroderma-Spectrum” Disorders. Reumatologia 2021, 59, 273. [Google Scholar] [CrossRef]
- Koenig, M.; Joyal, F.; Fritzler, M.J.; Roussin, A.; Abrahamowicz, M.; Boire, G.; Goulet, J.R.; Rich, É.; Grodzicky, T.; Raymond, Y.; et al. Auto-antibodies and Microvascular Damage Are Independent Predictive Factors for the Progression of Raynaud’s Phenomenon to Systemic Sclerosis: A Twenty-Year Prospective Study of 586 Patients, with Validation of Proposed Criteria for Early Systemic Sclerosi. Arthritis Rheum. 2008, 58, 3902–3912. [Google Scholar] [CrossRef]
- Ingegnoli, F.; Ardoino, I.; Boracchi, P.; Cutolo, M.; Airò, P.; Ananieva, L.P.; Ancuta, C.; Andrade, L.E.; Becvar, R.; Benenati, A.; et al. Nailfold Capillaroscopy in Systemic Sclerosis: Data from the EULAR Scleroderma Trials and Research (EUSTAR) Database. Microvasc. Res. 2013, 89, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Fichel, F.; Baudot, N.; Gaitz, J.P.; Trad, S.; Barbe, C.; Francès, C.; Senet, P. Systemic Sclerosis with Normal or Nonspecific Nailfold Capillaroscopy. Dermatology 2014, 228, 360–367. [Google Scholar] [CrossRef]
- De Santis, M.; Ceribelli, A.; Cavaciocchi, F.; Crotti, C.; Massarotti, M.; Belloli, L.; Marasini, B.; Isailovic, N.; Generali, E.; Selmi, C. Nailfold Videocapillaroscopy and Serum VEGF Levels in Scleroderma Are Associated with Internal Organ Involvement. Auto-Immun. Highlights 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sulli, A.; Ruaro, B.; Alessandri, E.; Pizzorni, C.; Cimmino, M.A.; Zampogna, G.; Gallo, M.; Cutolo, M. Correlations between Nailfold Microangiopathy Severity, Finger Dermal Thickness and Fingertip Blood Perfusion in Systemic Sclerosis Patients. Ann. Rheum. Dis. 2014, 73, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-Vold, A.M.; Midtvedt, Ø.; Tennøe, A.H.; Garen, T.; Lund, M.B.; Aaløkken, T.M.; Andreassen, A.K.; Elhage, F.; Brunborg, C.; Taraldsrud, E.; et al. Cardiopulmonary Disease Development in Anti-RNA Polymerase III-Positive Systemic Sclerosis: Comparative Analyses from an Unselected, Prospective Patient Cohort. J. Rheumatol. 2017, 44, 459–465. [Google Scholar] [CrossRef]
- Callejas-Moraga, E.L.; Guillén-Del-Castillo, A.; Marín-Sánchez, A.M.; Roca-Herrera, M.; Balada, E.; Tolosa-Vilella, C.; Fonollosa-Pla, V.; Simeón-Aznar, C.P. Clinical Features of Systemic Sclerosis Patients with Anti-RNA Polymerase III Antibody in a Single Centre in Spain. Clin. Exp. Rheumatol. 2019, 37, 41–48. [Google Scholar]
- Bhavsar, S.V.; Carmona, R. Anti-RNA Polymerase III Antibodies in the Diagnosis of Scleroderma Renal Crisis in the Absence of Skin Disease. J. Clin. Rheumatol. 2014, 20, 379–382. [Google Scholar] [CrossRef]
- Hamaguchi, Y.; Kodera, M.; Matsushita, T.; Hasegawa, M.; Inaba, Y.; Usuda, T.; Kuwana, M.; Takehara, K.; Fujimoto, M. Clinical and Immunologic Predictors of Scleroderma Renal Crisis in Japanese Systemic Sclerosis Patients with Anti-RNA Polymerase III Auto-antibodies. Arthritis Rheumatol. 2015, 67, 1045–1052. [Google Scholar] [CrossRef]
- Saygin, D.; Domsic, R.T. Pulmonary Arterial Hypertension in Systemic Sclerosis: Challenges in Diagnosis, Screening And Treatment. Open Access Rheumatol. Res. Rev. 2019, 11, 323–333. [Google Scholar] [CrossRef]
- Jiang, Y.; Turk, M.A.; Pope, J.E. Factors Associated with Pulmonary Arterial Hypertension (PAH) in Systemic Sclerosis (SSc). Autoimmun. Rev. 2020, 19, 102602. [Google Scholar] [CrossRef] [PubMed]
- Raschi, E.; Privitera, D.; Bodio, C.; Lonati, P.A.; Borghi, M.O.; Ingegnoli, F.; Meroni, P.L.; Chighizola, C.B. Scleroderma-Specific Auto-antibodies Embedded in Immune Complexes Mediate Endothelial Damage: An Early Event in the Pathogenesis of Systemic Sclerosis. Arthritis Res. Ther. 2020, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Negi, V.S.; Tripathy, N.K.; Misra, R.; Nityanand, S. Antiendothelial Cell Antibodies in Scleroderma Correlate with Severe Digital Ischemia and Pulmonary Arterial Hypertension. J. Rheumatol. 1998, 25, 462–466. [Google Scholar]
- Liu, X.D.; Guo, S.Y.; Yang, L.L.; Zhang, X.L.; Fu, W.Y.; Wang, X.F. Anti-Endothelial Cell Antibodies in Connective Tissue Diseases Associated with Pulmonary Arterial Hypertension. J. Thorac. Dis. 2014, 6, 497–502. [Google Scholar] [CrossRef]
- Pouw, J.N.; Leijten, E.F.A.; van Laar, J.M.; Boes, M. Revisiting B Cell Tolerance and Auto-antibodies in Seropositive and Seronegative Autoimmune Rheumatic Disease (AIRD). Clin. Exp. Immunol. 2021, 203, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Young, C.; Lau, A.W.Y.; Burnett, D.L. B Cells in the Balance: Offsetting Self-Reactivity Avoidance with Protection against Foreign. Front. Immunol. 2022, 13, 951385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Huang, C.; Luo, H.; Li, J.; Huang, H.; Liu, X.; Zhan, S. Identification of Key Genes and Immune Profile in Limited Cutaneous Systemic Sclerosis-Associated Pulmonary Arterial Hypertension by Bioinformatics Analysis. Life Sci. 2021, 271, 119151. [Google Scholar] [CrossRef]
- Mekinian, A.; Mahevas, T.; Mohty, M.; Jachiet, V.; Rivière, S.; Fain, O. Mucosal-Associated Invariant Cells Are Deficient in Systemic Sclerosis. Scand. J. Immunol. 2017, 86, 216–220. [Google Scholar] [CrossRef]
- Paleja, B.; Low, A.H.L.; Kumar, P.; Saidin, S.; Lajam, A.; Nur Hazirah, S.; Chua, C.; Li Yun, L.; Albani, S. Systemic Sclerosis Perturbs the Architecture of the Immunome. Front. Immunol. 2020, 11, 1602. [Google Scholar] [CrossRef]
- Fan, Q.; Nan, H.; Li, Z.; Li, B.; Zhang, F.; Bi, L. New Insights into MAIT Cells in Autoimmune Diseases. Biomed. Pharmacother. 2023, 159, 114250. [Google Scholar] [CrossRef]
- Giordano, N.; Puccetti, L.; Papakostas, P.; Di Pietra, N.; Bruni, F.; Pasqui, A.L.; Acampa, M.; Bocchi, V.; Donati, V.; Voglino, M.; et al. Bosentan Treatment for Raynauds Phenomenon and Skin Fibrosis in Patients with Systemic Sclerosis and Pulmonary Arterial Hypertension: An Open-Label, Observational, Retrospective Study. Int. J. Immunopathol. Pharmacol. 2010, 23, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Haust, M.; Ruland, V.; Weber, R.; Verde, P.; Felder, G.; Ohmann, C.; Gensch, K.; Ruzicka, T. Effect of Bosentan on Skin Fibrosis in Patients with Systemic Sclerosis: A Prospective, Open-Label, Non-Comparative Trial. Rheumatology 2010, 49, 1336–1345. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sandner, P.; Stasch, J.P. Anti-Fibrotic Effects of Soluble Guanylate Cyclase Stimulators and Activators: A Review of the Preclinical Evidence. Respir. Med. 2017, 122, S1–S9. [Google Scholar] [CrossRef]
- Khanna, D.; Allanore, Y.; Denton, C.P.; Kuwana, M.; Matucci-Cerinic, M.; Pope, J.E.; Atsumi, T.; Bečvár, R.; Czirják, L.; Hachulla, E.; et al. Riociguat in Patients with Early Diffuse Cutaneous Systemic Sclerosis (RISE-SSc): Randomised, Double-Blind, Placebo-Controlled Multicentre Trial. Ann. Rheum. Dis. 2020, 79, 618–625. [Google Scholar] [CrossRef]
- Dunkern, T.R.; Feurstein, D.; Rossi, G.A.; Sabatini, F.; Hatzelmann, A. Inhibition of TGF-Beta Induced Lung Fibroblast to Myofibroblast Conversion by Phosphodiesterase Inhibiting Drugs and Activators of Soluble Guanylyl Cyclase. Eur. J. Pharmacol. 2007, 572, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Kawaguchi, Y.; Takagi, K.; Tochimoto, A.; Ota, Y.; Katsumata, Y.; Ichida, H.; Hanaoka, M.; Kawasumi, H.; Tochihara, M.; et al. Sildenafil Attenuates the Fibrotic Phenotype of Skin Fibroblasts in Patients with Systemic Sclerosis. Clin. Immunol. 2015, 161, 333–338. [Google Scholar] [CrossRef]
- Khanna, D.; Denton, C.P.; Jahreis, A.; van Laar, J.M.; Frech, T.M.; Anderson, M.E.; Baron, M.; Chung, L.; Fierlbeck, G.; Lakshminarayanan, S.; et al. Safety and Efficacy of Subcutaneous Tocilizumab in Adults with Systemic Sclerosis (FaSScinate): A Phase 2, Randomised, Controlled Trial. Lancet 2016, 387, 2630–2640. [Google Scholar] [CrossRef]
- Manetti, M.; Pratesi, S.; Romano, E.; Bellando-Randone, S.; Rosa, I.; Guiducci, S.; Fioretto, B.S.; Ibba-Manneschi, L.; Maggi, E.; Matucci-Cerinic, M. Angiogenic T Cell Expansion Correlates with Severity of Peripheral Vascular Damage in Systemic Sclerosis. PLoS ONE 2017, 12, e0183102. [Google Scholar] [CrossRef] [PubMed]
- Krasimirova, E.; Velikova, T.; Ivanova-Todorova, E.; Tumangelova-Yuzeir, K.; Kalinova, D.; Boyadzhieva, V.; Stoilov, N.; Yoneva, T.; Rashkov, R.; Kyurkchiev, D. Treg/Th17 Cell Balance and Phytohaemagglutinin Activation of T Lymphocytes in Peripheral Blood of Systemic Sclerosis Patients. World J. Exp. Med. 2017, 7, 84. [Google Scholar] [CrossRef]
- Klein, S.; Kretz, C.C.; Ruland, V.; Stumpf, C.; Haust, M.; Hartschuh, W.; Hartmann, M.; Enk, A.; Suri-Payer, E.; Oberle, N.; et al. Reduction of Regulatory T Cells in Skin Lesions but Not in Peripheral Blood of Patients with Systemic Scleroderma. Ann. Rheum. Dis. 2011, 70, 1475–1481. [Google Scholar] [CrossRef]
- Asano, Y.; Sato, S. Vasculopathy in Scleroderma. Semin. Immunopathol. 2015, 37, 489–500. [Google Scholar] [CrossRef]
- Tan, F.K.; Zhou, X.; Mayes, M.D.; Gourh, P.; Guo, X.; Marcum, C.; Jin, L.; Arnett, F.C. Signatures of Differentially Regulated Interferon Gene Expression and Vasculotrophism in the Peripheral Blood Cells of Systemic Sclerosis Patients. Rheumatology 2006, 45, 694–702. [Google Scholar] [CrossRef]
- Yoshizaki, A.; Yanaba, K.; Iwata, Y.; Komura, K.; Ogawa, A.; Akiyama, Y.; Muroi, E.; Hara, T.; Ogawa, F.; Takenaka, M.; et al. Cell Adhesion Molecules Regulate Fibrotic Process via Th1/Th2/Th17 Cell Balance in a Bleomycin-Induced Scleroderma Model. J. Immunol. 2010, 185, 2502. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Pezet, S.; Avouac, J.; Allanore, Y. Soluble CD163 as a Potential Biomarker in Systemic Sclerosis. Dis. Markers 2018, 2018, 8509583. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ogawa, F.; Yoshizaki, A.; Akiyama, Y.; Kuwatsuka, Y.; Okazaki, S.; Tomita, H.; Takenaka, M.; Sato, S. Increased Serum Levels of Soluble CD163 in Patients with Scleroderma. Clin. Rheumatol. 2012, 31, 1059–1064. [Google Scholar] [CrossRef]
- Bielecki, M.; Kowal, K.; Lapinska, A.; Chyczewski, L.; Kowal-Bielecka, O. Increased Release of Soluble CD163 by the Peripheral Blood Mononuclear Cells Is Associated with Worse Prognosis in Patients with Systemic Sclerosis. Adv. Med. Sci. 2013, 58, 126–133. [Google Scholar] [CrossRef][Green Version]
- Brown, M.; O’Reilly, S. The Immunopathogenesis of Fibrosis in Systemic Sclerosis. Clin. Exp. Immunol. 2019, 195, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Al-Adwi, Y.; Westra, J.; van Goor, H.; Burgess, J.K.; Denton, C.P.; Mulder, D.J. Macrophages as Determinants and Regulators of Fibrosis in Systemic Sclerosis. Rheumatology 2023, 62, 535–545. [Google Scholar] [CrossRef]
- Sakkas, L.I.; Bogdanos, D.P. The Role of B Cells in the Pathogenesis of Systemic Sclerosis. Isr. Med. Assoc. J. 2016, 18, 516–518. [Google Scholar] [CrossRef]
- Talotta, R.; Atzeni, F.; Ditto, M.C.; Gerardi, M.C.; Batticciotto, A.; Bongiovanni, S.; Puttini, P.S. Certainties and Uncertainties Concerning the Contribution of Pericytes to the Pathogenesis of Systemic Sclerosis. J. Scleroderma Relat. Disord. 2018, 3, 14–20. [Google Scholar] [CrossRef]
- Bergers, G.; Song, S. The Role of Pericytes in Blood-Vessel Formation and Maintenance. Neuro. Oncol. 2005, 7, 452. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Nalbandian, A.; Uchida, Y.; Li, W.; Arnold, T.D.; Kubota, Y.; Yamamoto, S.; Ema, M.; Mukouyama, Y. suke Tissue Myeloid Progenitors Differentiate into Pericytes through TGFβ Signaling in Developing Skin Vasculature. Cell Rep. 2017, 18, 2991–3004. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Friman, T.; Kowanetz, M.; Van Wieringen, T.; Gustafsson, R.; Sundberg, C. Phenotypical Differences in Connective Tissue Cells Emerging from Microvascular Pericytes in Response to Overexpression of PDGF-B and TGFΒ1 in Normal Skin in Vivo. Am. J. Pathol. 2013, 182, 2132–2146. [Google Scholar] [CrossRef]
- Rajkumar, V.S.; Howell, K.; Csiszar, K.; Denton, C.P.; Black, C.M.; Abraham, D.J. Shared Expression of Phenotypic Markers in Systemic Sclerosis Indicates a Convergence of Pericytes and Fibroblasts to a Myofibroblast Lineage in Fibrosis. Arthritis Res. Ther. 2005, 7, R1113–R1123. [Google Scholar] [CrossRef]
- Rajkumar, V.S.; Sundberg, C.; Abraham, D.J.; Rubin, K.; Black, C.M. Activation of Microvascular Pericytes in Autoimmune Raynaud’s Phenomenon and Systemic Sclerosis. Arthritis Rheum. 1999, 42, 930–941. [Google Scholar] [CrossRef]
- Makino, K.; Makino, T.; Stawski, L.; Mantero, J.C.; Lafyatis, R.; Simms, R.; Trojanowska, M. Blockade of PDGF Receptors by Crenolanib Has Therapeutic Effect in Patient Fibroblasts and in Preclinical Models of Systemic Sclerosis. J. Investig. Dermatol. 2017, 137, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Trojanowska, M. Cellular and Molecular Aspects of Vascular Dysfunction in Systemic Sclerosis. Nat. Rev. Rheumatol. 2010, 6, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Lafyatis, R. Transforming Growth Factor β–at the Centre of Systemic Sclerosis. Nat. Rev. Rheumatol. 2014, 10, 706–719. [Google Scholar] [CrossRef]
- Liakouli, V.; Cipriani, P.; Marrelli, A.; Alvaro, S.; Ruscitti, P.; Giacomelli, R. Angiogenic Cytokines and Growth Factors in Systemic Sclerosis. Autoimmun. Rev. 2011, 10, 590–594. [Google Scholar] [CrossRef]
- Pattanaik, D.; Brown, M.; Postlethwaite, B.C.; Postlethwaite, A.E. Pathogenesis of Systemic Sclerosis. Front. Immunol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Shiwen, X.; Leask, A.; Abraham, D.J.; Fonseca, C. Endothelin Receptor Selectivity: Evidence from in Vitro and Pre-Clinical Models of Scleroderma. Eur. J. Clin. Investig. 2009, 39 (Suppl. 2), 19–26. [Google Scholar] [CrossRef] [PubMed]
- Avouac, J.; Vallucci, M.; Smith, V.; Senet, P.; Ruiz, B.; Sulli, A.; Pizzorni, C.; Frances, C.; Chiocchia, G.; Cutolo, M.; et al. Correlations between Angiogenic Factors and Capillaroscopic Patterns in Systemic Sclerosis. Arthritis Res. Ther. 2013, 15, R55. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGFβ: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Shi-Wen, X.; Rodríguez-Pascual, F.; Lamas, S.; Holmes, A.; Howat, S.; Pearson, J.D.; Dashwood, M.R.; du Bois, R.M.; Denton, C.P.; Black, C.M.; et al. Constitutive ALK5-Independent c-Jun N-Terminal Kinase Activation Contributes to Endothelin-1 Overexpression in Pulmonary Fibrosis: Evidence of an Autocrine Endothelin Loop Operating through the Endothelin A and B Receptors. Mol. Cell. Biol. 2006, 26, 5518–5527. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y.; Stawski, L.; Hant, F.; Highland, K.; Silver, R.; Szalai, G.; Watson, D.K.; Trojanowska, M. Endothelial Fli1 Deficiency Impairs Vascular Homeostasis: A Role in Scleroderma Vasculopathy. Am. J. Pathol. 2010, 176, 1983. [Google Scholar] [CrossRef]
- Bujor, A.M.; El Adili, F.; Parvez, A.; Marden, G.; Trojanowska, M. Fli1 Downregulation in Scleroderma Myeloid Cells Has Profibrotic and Proinflammatory Effects. Front. Immunol. 2020, 11, 800. [Google Scholar] [CrossRef]
- Toyama, T.; Asano, Y.; Miyagawa, T.; Nakamura, K.; Hirabayashi, M.; Yamashita, T.; Saigusa, R.; Miura, S.; Ichimura, Y.; Takahashi, T.; et al. The Impact of Transcription Factor Fli1 Deficiency on the Regulation of Angiogenesis. Exp. Dermatol. 2017, 26, 912–918. [Google Scholar] [CrossRef]
- Kubo, M.; Czuwara-Ladykowska, J.; Moussa, O.; Markiewicz, M.; Smith, E.; Silver, R.M.; Jablonska, S.; Blaszczyk, M.; Watson, D.K.; Trojanowska, M. Persistent Down-Regulation of Fli1, a Suppressor of Collagen Transcription, in Fibrotic Scleroderma Skin. Am. J. Pathol. 2003, 163, 571–581. [Google Scholar] [CrossRef]
- Nakamura, K.; Taniguchi, T.; Hirabayashi, M.; Yamashita, T.; Saigusa, R.; Miura, S.; Takahashi, T.; Toyama, T.; Ichimura, Y.; Yoshizaki, A.; et al. Altered Properties of Endothelial Cells and Mesenchymal Stem Cells Underlying the Development of Scleroderma-like Vasculopathy in KLF5+/−;Fli-1+/− Mice. Arthritis Rheumatol. 2020, 72, 2136–2146. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, P.S.; Kahaleh, B. Association between Enhanced Type I Collagen Expression and Epigenetic Repression of the FLI1 Gene in Scleroderma Fibroblasts. Arthritis Rheum. 2006, 54, 2271–2279. [Google Scholar] [CrossRef]
- Larsson, E.; Fuchs, P.F.; Heldin, J.; Barkefors, I.; Bondjers, C.; Genové, G.; Arrondel, C.; Gerwins, P.; Kurschat, C.; Schermer, B.; et al. Discovery of Microvascular MiRNAs Using Public Gene Expression Data: MiR-145 Is Expressed in Pericytes and Is a Regulator of Fli1. Genome Med. 2009, 1, 108. [Google Scholar] [CrossRef]
- Manetti, M.; Allanore, Y.; Saad, M.; Fatini, C.; Cohignac, V.; Guiducci, S.; Romano, E.; Airó, P.; Caramaschi, P.; Tinazzi, I.; et al. Evidence for Caveolin-1 as a New Susceptibility Gene Regulating Tissue Fibrosis in Systemic Sclerosis. Ann. Rheum. Dis. 2012, 71, 1034–1041. [Google Scholar] [CrossRef]
- Del Galdo, F.; Sotgia, F.; De Almeida, C.J.; Jasmin, J.F.; Musick, M.; Lisanti, M.P.; Jiménez, S.A. Decreased Expression of Caveolin-1 in Systemic Sclerosis: Crucial Role in the Pathogenesis of Tissue Fibrosis. Arthritis Rheum. 2008, 58, 2854–2865. [Google Scholar] [CrossRef]
- Tourkina, E.; Bonner, M.; Oates, J.; Hofbauer, A.; Richard, M.; Znoyko, S.; Visconti, R.P.; Zhang, J.; Hatfield, C.M.; Silver, R.M.; et al. Altered Monocyte and Fibrocyte Phenotype and Function in Scleroderma Interstitial Lung Disease: Reversal by Caveolin-1 Scaffolding Domain Peptide. Fibrogenesis Tissue Repair 2011, 4, 15. [Google Scholar] [CrossRef]
- Li, Z.; Wermuth, P.J.; Benn, B.S.; Lisanti, M.P.; Jimenez, S.A. Caveolin-1 Deficiency Induces Spontaneous Endothelial-to-Mesenchymal Transition in Murine Pulmonary Endothelial Cells in Vitro. Am. J. Pathol. 2013, 182, 325–331. [Google Scholar] [CrossRef]
- Cipriani, P.; Di Benedetto, P.; Capece, D.; Zazzeroni, F.; Liakouli, V.; Ruscitti, P.; Pantano, I.; Berardicurti, O.; Carubbi, F.; Alesse, E.; et al. Impaired Cav-1 Expression in SSc Mesenchymal Cells Upregulates VEGF Signaling: A Link between Vascular Involvement and Fibrosis. Fibrogenesis Tissue Repair 2014, 7, 13. [Google Scholar] [CrossRef]
- Fleischmajer, R.; Damiano, V.; Nedwich, A. Scleroderma and the Subcutaneous Tissue. Science 1971, 171, 1019–1021. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Melichian, D.S.; Chang, E.; Warner-Blankenship, M.; Ghosh, A.K.; Varga, J. Rosiglitazone Abrogates Bleomycin-Induced Scleroderma and Blocks Profibrotic Responses through Peroxisome Proliferator-Activated Receptor-Gamma. Am. J. Pathol. 2009, 174, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Stawski, L.; Han, R.; Bujor, A.M.; Trojanowska, M. Angiotensin II Induces Skin Fibrosis: A Novel Mouse Model of Dermal Fibrosis. Arthritis Res. Ther. 2012, 14, R194. [Google Scholar] [CrossRef] [PubMed]
- Sonnylal, S.; Denton, C.P.; Zheng, B.; Keene, D.R.; He, R.; Adams, H.P.; VanPelt, C.S.; Geng, Y.J.; Deng, J.M.; Behringer, R.R.; et al. Postnatal Induction of Transforming Growth Factor Beta Signaling in Fibroblasts of Mice Recapitulates Clinical, Histologic, and Biochemical Features of Scleroderma. Arthritis Rheum. 2007, 56, 334–344. [Google Scholar] [CrossRef]
- Christner, P.J.; Peters, J.; Hawkins, D.; Siracusa, L.D.; Jiménez, S.A. The Tight Skin 2 Mouse. An Animal Model of Scleroderma Displaying Cutaneous Fibrosis and Mononuclear Cell Infiltration. Arthritis Rheum. 1995, 38, 1791–1798. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Korman, B.; Varga, J. Adipocytic Progenitor Cells Give Rise to Pathogenic Myofibroblasts: Adipocyte-to-Mesenchymal Transition and Its Emerging Role in Fibrosis in Multiple Organs. Curr. Rheumatol. Rep. 2020, 22, 79. [Google Scholar] [CrossRef]
- Marangoni, R.G.; Korman, B.D.; Wei, J.; Wood, T.A.; Graham, L.V.; Whitfield, M.L.; Scherer, P.E.; Tourtellotte, W.G.; Varga, J. Myofibroblasts in Murine Cutaneous Fibrosis Originate from Adiponectin-Positive Intradermal Progenitors. Arthritis Rheumatol. 2015, 67, 1062–1073. [Google Scholar] [CrossRef]
- Martins, V.; Gonzalez De Los Santos, F.; Wu, Z.; Capelozzi, V.; Phan, S.H.; Liu, T. FIZZ1-Induced Myofibroblast Transdifferentiation from Adipocytes and Its Potential Role in Dermal Fibrosis and Lipoatrophy. Am. J. Pathol. 2015, 185, 2768–2776. [Google Scholar] [CrossRef]
- Korman, B.; Marangoni, R.G.; Lord, G.; Olefsky, J.; Tourtellotte, W.; Varga, J. Adipocyte-Specific Repression of PPAR-Gamma by NCoR Contributes to Scleroderma Skin Fibrosis. Arthritis Res. Ther. 2018, 20, 145. [Google Scholar] [CrossRef]
- Tomčík, M.; Arima, K.; Hulejová, H.; Kuklová, M.; Filková, M.; Braun, M.; Beláček, J.; Novák, M.; Bečvář, R.; Vencovský, J.; et al. Adiponectin Relation to Skin Changes and Dyslipidemia in Systemic Sclerosis. Cytokine 2012, 58, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Miura, S.; Asano, Y.; Saigusa, R.; Yamashita, T.; Taniguchi, T.; Takahashi, T.; Ichimura, Y.; Toyama, T.; Tamaki, Z.; Tada, Y.; et al. Serum Vaspin Levels: A Possible Correlation with Digital Ulcers in Patients with Systemic Sclerosis. J. Dermatol. 2015, 42, 528–531. [Google Scholar] [CrossRef]
- Takahashi, T.; Asano, Y.; Noda, S.; Aozasa, N.; Akamata, K.; Taniguchi, T.; Ichimura, Y.; Toyama, T.; Sumida, H.; Kuwano, Y.; et al. A Possible Contribution of Lipocalin-2 to the Development of Dermal Fibrosis, Pulmonary Vascular Involvement and Renal Dysfunction in Systemic Sclerosis. Br. J. Dermatol. 2015, 173, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Asano, Y.; Takahashi, T.; Aozasa, N.; Akamata, K.; Noda, S.; Taniguchi, T.; Ichimura, Y.; Sumida, H.; Tamaki, Z.; et al. Clinical Significance of Serum Retinol Binding Protein-4 Levels in Patients with Systemic Sclerosis. J. Eur. Acad. Dermatology Venereol. 2013, 27, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Masui, Y.; Asano, Y.; Shibata, S.; Noda, S.; Aozasa, N.; Akamata, K.; Yamada, D.; Tamaki, Z.; Tada, Y.; Sugaya, M.; et al. Serum Adiponectin Levels Inversely Correlate with the Activity of Progressive Skin Sclerosis in Patients with Diffuse Cutaneous Systemic Sclerosis. J. Eur. Acad. Dermatology Venereol. 2012, 26, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Marangoni, R.G.; Masui, Y.; Fang, F.; Korman, B.; Lord, G.; Lee, J.; Lakota, K.; Wei, J.; Scherer, P.E.; Otvos, L.; et al. Adiponectin Is an Endogenous Anti-Fibrotic Mediator and Therapeutic Target. Sci. Rep. 2017, 7, 4397. [Google Scholar] [CrossRef]
- Yamashita, T.; Lakota, K.; Taniguchi, T.; Yoshizaki, A.; Sato, S.; Hong, W.; Zhou, X.; Sodin-Semrl, S.; Fang, F.; Asano, Y.; et al. An Orally-Active Adiponectin Receptor Agonist Mitigates Cutaneous Fibrosis, Inflammation and Microvascular Pathology in a Murine Model of Systemic Sclerosis. Sci. Rep. 2018, 8, 11843. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.C.; Dong, R.; Long, X.; Wang, X. Aesthetic and Therapeutic Outcome of Fat Grafting for Localized Scleroderma Treatment: From Basic Study to Clinical Application. J. Cosmet. Dermatol. 2021, 20, 2723–2728. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Cai, J.; Zhang, Q.; Wen, J.; Liao, Y.; Lu, F. Fat Transplantation Induces Dermal Adipose Regeneration and Reverses Skin Fibrosis through Dedifferentiation and Redifferentiation of Adipocytes. Stem Cell Res. Ther. 2022, 13, 499. [Google Scholar] [CrossRef]
- Bayati, P.; Kalantari, M.; Assarehzadegan, M.A.; Poormoghim, H.; Mojtabavi, N. MiR-27a as a Diagnostic Biomarker and Potential Therapeutic Target in Systemic Sclerosis. Sci. Rep. 2022, 12, 18932. [Google Scholar] [CrossRef]
- Bucala, R.; Spiegel, L.A.; Chesney, J.; Hogan, M.; Cerami, A. Circulating Fibrocytes Define a New Leukocyte Subpopulation That Mediates Tissue Repair. Mol. Med. 1994, 1, 71. [Google Scholar] [CrossRef]
- Borie, R.; Quesnel, C.; Phin, S.; Debray, M.P.; Marchal-Somme, J.; Tiev, K.; Bonay, M.; Fabre, A.; Soler, P.; Dehoux, M.; et al. Detection of Alveolar Fibrocytes in Idiopathic Pulmonary Fibrosis and Systemic Sclerosis. PLoS ONE 2013, 8, e53736. [Google Scholar] [CrossRef]
- Ruaro, B.; Soldano, S.; Smith, V.; Paolino, S.; Contini, P.; Montagna, P.; Pizzorni, C.; Casabella, A.; Tardito, S.; Sulli, A.; et al. Correlation between Circulating Fibrocytes and Dermal Thickness in Limited Cutaneous Systemic Sclerosis Patients: A Pilot Study. Rheumatol. Int. 2019, 39, 1369–1376. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct Fibroblast Lineages Determine Dermal Architecture in Skin Development and Repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef]
- Mostmans, Y.; Cutolo, M.; Giddelo, C.; Decuman, S.; Melsens, K.; Declercq, H.; Vandecasteele, E.; De Keyser, F.; Distler, O.; Gutermuth, J.; et al. The Role of Endothelial Cells in the Vasculopathy of Systemic Sclerosis: A Systematic Review. Autoimmun. Rev. 2017, 16, 774–786. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Ruscitti, P.; Berardicurti, O.; Vomero, M.; Navarini, L.; Dolo, V.; Cipriani, P.; Giacomelli, R. Endothelial-to-Mesenchymal Transition in Systemic Sclerosis. Clin. Exp. Immunol. 2021, 205, 12–27. [Google Scholar] [CrossRef]
- Wang, E.; Wang, H.; Chakrabarti, S. Endothelial-to-Mesenchymal Transition: An Underappreciated Mediator of Diabetic Complications. Front. Endocrinol. 2023, 14, 1050540. [Google Scholar] [CrossRef]
- Lin, F.; Wang, N.; Zhang, T.C. The Role of Endothelial-Mesenchymal Transition in Development and Pathological Process. IUBMB Life 2012, 64, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, P.A.; Tombetti, E.; Maugeri, N.; Rovere-Querini, P.; Brunelli, S.; Manfredi, A.A. Vascular Remodelling and Mesenchymal Transition in Systemic Sclerosis. Stem Cells Int. 2016, 2016, 4636859. [Google Scholar] [CrossRef] [PubMed]
- Rosa, I.; Romano, E.; Fioretto, B.S.; Manetti, M. The Contribution of Mesenchymal Transitions to the Pathogenesis of Systemic Sclerosis. Eur. J. Rheumatol. 2020, 7, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Manetti, M.; Guiducci, S.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Impaired Angiogenesis in Systemic Sclerosis: The Emerging Role of the Antiangiogenic VEGF(165)b Splice Variant. Trends Cardiovasc. Med. 2011, 21, 204–210. [Google Scholar] [CrossRef]
- Hirigoyen, D.; Burgos, P.I.; Mezzano, V.; Duran, J.; Barrientos, M.; Saez, C.G.; Panes, O.; Mezzano, D.; Iruretagoyena, M. Inhibition of Angiogenesis by Platelets in Systemic Sclerosis Patients. Arthritis Res. Ther. 2015, 17, 332. [Google Scholar] [CrossRef]
- Sobierajska, K.; Wawro, M.E.; Ciszewski, W.M.; Niewiarowska, J. Transforming Growth Factor-β Receptor Internalization via Caveolae Is Regulated by Tubulin-Β2 and Tubulin-Β3 during Endothelial-Mesenchymal Transition. Am. J. Pathol. 2019, 189, 2531–2546. [Google Scholar] [CrossRef]
- Li, Z.; Jimenez, S.A. Protein Kinase Cδ and C-Abl Kinase Are Required for Transforming Growth Factor β Induction of Endothelial-Mesenchymal Transition in Vitro. Arthritis Rheum. 2011, 63, 2473–2483. [Google Scholar] [CrossRef]
- Cipriani, P.; Di Benedetto, P.; Ruscitti, P.; Capece, D.; Zazzeroni, F.; Liakouli, V.; Pantano, I.; Berardicurti, O.; Carubbi, F.; Pecetti, G.; et al. The Endothelial-Mesenchymal Transition in Systemic Sclerosis Is Induced by Endothelin-1 and Transforming Growth Factor-β and May Be Blocked by Macitentan, a Dual Endothelin-1 Receptor Antagonist. J. Rheumatol. 2015, 42, 1808–1816. [Google Scholar] [CrossRef] [PubMed]
- Lambers, C.; Roth, M.; Zhong, J.; Campregher, C.; Binder, P.; Burian, B.; Petkov, V.; Block, L.H. The Interaction of Endothelin-1 and TGFΒ1 Mediates Vascular Cell Remodeling. PLoS ONE 2013, 8, e73399. [Google Scholar] [CrossRef]
- Lee, W.J.; Park, J.H.; Shin, J.U.; Noh, H.; Lew, D.H.; Yang, W.I.; Yun, C.O.; Lee, K.H.; Lee, J.H. Endothelial-to-Mesenchymal Transition Induced by Wnt 3a in Keloid Pathogenesis. Wound Repair Regen. 2015, 23, 435–442. [Google Scholar] [CrossRef]
- Yoshimatsu, Y.; Wakabayashi, I.; Kimuro, S.; Takahashi, N.; Takahashi, K.; Kobayashi, M.; Maishi, N.; Podyma-Inoue, K.A.; Hida, K.; Miyazono, K.; et al. TNF-α Enhances TGFβ-Induced Endothelial-to-Mesenchymal Transition via TGFβ Signal Augmentation. Cancer Sci. 2020, 111, 2385–2399. [Google Scholar] [CrossRef] [PubMed]
- Chrobak, I.; Lenna, S.; Stawski, L.; Trojanowska, M. Interferon-γ Promotes Vascular Remodeling in Human Microvascular Endothelial Cells by Upregulating Endothelin (ET)-1 and Transforming Growth Factor (TGF) Β2. J. Cell. Physiol. 2013, 228, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, P.; Di Benedetto, P.; Ruscitti, P.; Verzella, D.; Fischietti, M.; Zazzeroni, F.; Liakouli, V.; Carubbi, F.; Berardicurti, O.; Alesse, E.; et al. Macitentan Inhibits the Transforming Growth Factor-β Profibrotic Action, Blocking the Signaling Mediated by the ETR/TβRI Complex in Systemic Sclerosis Dermal Fibroblasts. Arthritis Res. Ther. 2015, 17, 247. [Google Scholar] [CrossRef]
- Corallo, C.; Cutolo, M.; Kahaleh, B.; Pecetti, G.; Montella, A.; Chirico, C.; Soldano, S.; Nuti, R.; Giordano, N. Bosentan and Macitentan Prevent the Endothelial-to-Mesenchymal Transition (EndoMT) in Systemic Sclerosis: In Vitro Study. Arthritis Res. Ther. 2016, 18, 228. [Google Scholar] [CrossRef]
- Tsou, P.S.; Palisoc, P.J.; Flavahan, N.A.; Khanna, D. Dissecting the Cellular Mechanism of Prostacyclin Analog Iloprost in Reversing Vascular Dysfunction in Scleroderma. Arthritis Rheumatol. 2021, 73, 520–529. [Google Scholar] [CrossRef]
- Ntelis, K.; Bogdanos, D.; Dimitroulas, T.; Sakkas, L.; Daoussis, D. Platelets in Systemic Sclerosis: The Missing Link Connecting Vasculopathy, Autoimmunity, and Fibrosis? Curr. Rheumatol. Rep. 2019, 21, 15. [Google Scholar] [CrossRef]
- Ramirez, G.A.; Franchini, S.; Rovere-Querini, P.; Sabbadini, M.G.; Manfredi, A.A.; Maugeri, N. The Role of Platelets in the Pathogenesis of Systemic Sclerosis. Front. Immunol. 2012, 3, 29071. [Google Scholar] [CrossRef]
- Matucci-Cerinic, M.; Kahaleh, B.; Wigley, F.M. Review: Evidence That Systemic Sclerosis Is a Vascular Disease. Arthritis Rheum. 2013, 65, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Čolić, J.; Cerinic, M.M.; Guiducci, S.; Damjanov, N. Microparticles in Systemic Sclerosis, Targets or Tools to Control: This Is the Question! J. Scleroderma Relat. Disord. 2020, 5, 6. [Google Scholar] [CrossRef]
- de Oliveira, S.M.; de Azevedo Teixeira, I.L.; França, C.N.; de Oliveira Izar, M.C.; Kayser, C. Microparticles: Potential New Contributors to the Pathogenesis of Systemic Sclerosis? Adv. Rheumatol. 2023, 63, 19. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, N.; Capobianco, A.; Rovere-Querini, P.; Ramirez, G.A.; Tombetti, E.; Della Valle, P.; Monno, A.; D’Alberti, V.; Gasparri, A.M.; Franchini, S.; et al. Platelet Microparticles Sustain Autophagy-Associated Activation of Neutrophils in Systemic Sclerosis. Sci. Transl. Med. 2018, 10, eaao3089. [Google Scholar] [CrossRef]
- Didier, K.; Giusti, D.; Le Jan, S.; Terryn, C.; Muller, C.; Pham, B.N.; Le Naour, R.; Antonicelli, F.D.; Servettaz, A. Neutrophil Extracellular Traps Generation Relates with Early Stage and Vascular Complications in Systemic Sclerosis. J. Clin. Med. 2020, 9, 2136. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, E.F.; Martyanov, V.; Fiorentino, D.; Wood, T.A.; Haddon, D.J.; Jarrell, J.A.; Utz, P.J.; Genovese, M.C.; Whitfield, M.L.; Chung, L. Gene Expression Changes Reflect Clinical Response in a Placebo-Controlled Randomized Trial of Abatacept in Patients with Diffuse Cutaneous Systemic Sclerosis. Arthritis Res. Ther. 2015, 17, 159. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Fiorentino, D.F.; BenBarak, M.J.; Adler, A.S.; Mariano, M.M.; Paniagua, R.T.; Milano, A.; Connolly, M.K.; Ratiner, B.D.; Wiskocil, R.L.; et al. Molecular Framework for Response to Imatinib Mesylate in Systemic Sclerosis. Arthritis Rheum. 2009, 60, 584–591. [Google Scholar] [CrossRef]
- Gordon, J.K.; Martyanov, V.; Magro, C.; Wildman, H.F.; Wood, T.A.; Huang, W.T.; Crow, M.K.; Whitfield, M.L.; Spiera, R.F. Nilotinib (TasignaTM) in the Treatment of Early Diffuse Systemic Sclerosis: An Open-Label, Pilot Clinical Trial. Arthritis Res. Ther. 2015, 17, 213. [Google Scholar] [CrossRef]
- Martyanov, V.; Kim, G.H.J.; Hayes, W.; Du, S.; Ganguly, B.J.; Sy, O.; Lee, S.K.; Bogatkevich, G.S.; Schieven, G.L.; Schiopu, E.; et al. Novel Lung Imaging Biomarkers and Skin Gene Expression Subsetting in Dasatinib Treatment of Systemic Sclerosis-Associated Interstitial Lung Disease. PLoS ONE 2017, 12, e0187580. [Google Scholar] [CrossRef]
- Toledo, D.M.; Pioli, P.A. Macrophages in Systemic Sclerosis: Novel Insights and Therapeutic Implications. Curr. Rheumatol. Rep. 2019, 21, 31. [Google Scholar] [CrossRef]
- Christmann, R.B.; Hayes, E.; Pendergrass, S.; Padilla, C.; Farina, G.; Affandi, A.J.; Whitfield, M.L.; Farber, H.W.; Lafyatis, R. Interferon and Alternative Activation of Monocyte/Macrophages in Systemic Sclerosis-Associated Pulmonary Arterial Hypertension. Arthritis Rheum. 2011, 63, 1718–1728. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef]
- Rusek, M.; Krasowska, D. Non-Coding RNA in Systemic Sclerosis: A Valuable Tool for Translational and Personalized Medicine. Genes 2021, 12, 1296. [Google Scholar] [CrossRef] [PubMed]
- Ramahi, A.; Altorok, N.; Kahaleh, B. Epigenetics and Systemic Sclerosis: An Answer to Disease Onset and Evolution? Eur. J. Rheumatol. 2020, 7, 147–156. [Google Scholar] [CrossRef]
- Thomson-Luque, R.; Wang, C.; Ntumngia, F.B.; Xu, S.; Szekeres, K.; Conway, A.; Adapa, S.R.; Barnes, S.J.; Adams, J.H.; Jiang, R.H.Y. In-Depth Phenotypic Characterization of Reticulocyte Maturation Using Mass Cytometry. Blood Cells. Mol. Dis. 2018, 72, 22–33. [Google Scholar] [CrossRef]
- Rybakowska, P.; Van Gassen, S.; Martorell Marugán, J.; Quintelier, K.; Saeys, Y.; Alarcón-Riquelme, M.E.; Marañón, C. Protocol for Large Scale Whole Blood Immune Monitoring by Mass Cytometry and Cyto Quality Pipeline. STAR Protoc. 2022, 3, 101697. [Google Scholar] [CrossRef] [PubMed]
- Spurgeon, B.E.J.; Michelson, A.D.; Frelinger, A.L. Platelet Immunophenotyping by High-Dimensional Mass Cytometry. Curr. Protoc. 2021, 1, e112. [Google Scholar] [CrossRef]
- Yeo, J.G.; Wasser, M.; Kumar, P.; Pan, L.; Poh, S.L.; Ally, F.; Arkachaisri, T.; Lim, A.J.M.; Leong, J.Y.; Lai, L.; et al. The Extended Polydimensional Immunome Characterization (EPIC) Web-Based Reference and Discovery Tool for Cytometry Data. Nat. Biotechnol. 2020, 38, 679–684. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, J.; Noviani, M.; Chellamuthu, V.R.; Albani, S.; Low, A.H.L. The Pathogenesis of Systemic Sclerosis: The Origin of Fibrosis and Interlink with Vasculopathy and Autoimmunity. Int. J. Mol. Sci. 2023, 24, 14287. https://doi.org/10.3390/ijms241814287
Ko J, Noviani M, Chellamuthu VR, Albani S, Low AHL. The Pathogenesis of Systemic Sclerosis: The Origin of Fibrosis and Interlink with Vasculopathy and Autoimmunity. International Journal of Molecular Sciences. 2023; 24(18):14287. https://doi.org/10.3390/ijms241814287
Chicago/Turabian StyleKo, Junsuk, Maria Noviani, Vasuki Ranjani Chellamuthu, Salvatore Albani, and Andrea Hsiu Ling Low. 2023. "The Pathogenesis of Systemic Sclerosis: The Origin of Fibrosis and Interlink with Vasculopathy and Autoimmunity" International Journal of Molecular Sciences 24, no. 18: 14287. https://doi.org/10.3390/ijms241814287
APA StyleKo, J., Noviani, M., Chellamuthu, V. R., Albani, S., & Low, A. H. L. (2023). The Pathogenesis of Systemic Sclerosis: The Origin of Fibrosis and Interlink with Vasculopathy and Autoimmunity. International Journal of Molecular Sciences, 24(18), 14287. https://doi.org/10.3390/ijms241814287




