Lipid-Based Nanocarriers for Delivery of Neuroprotective Kynurenic Acid: Preparation, Characterization, and BBB Transport
Abstract
1. Introduction
2. Results and Discussion
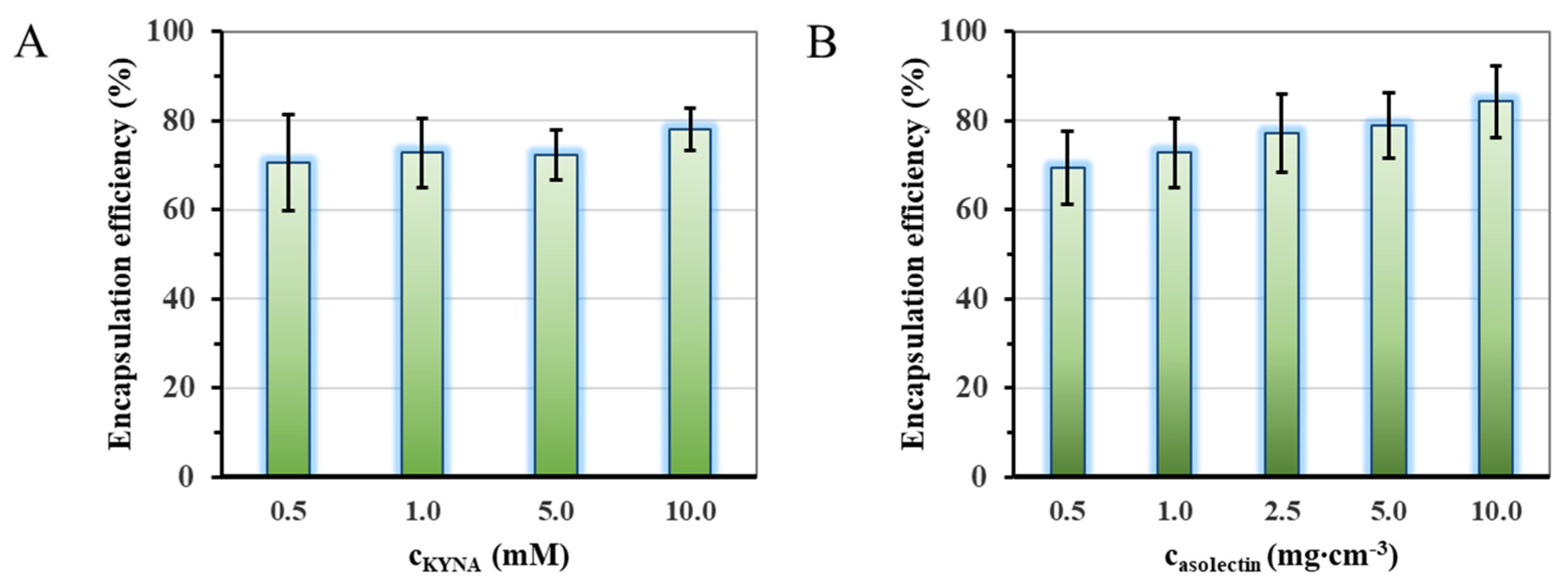
2.1. Optimization of the Preparation of the Liposomal Particles
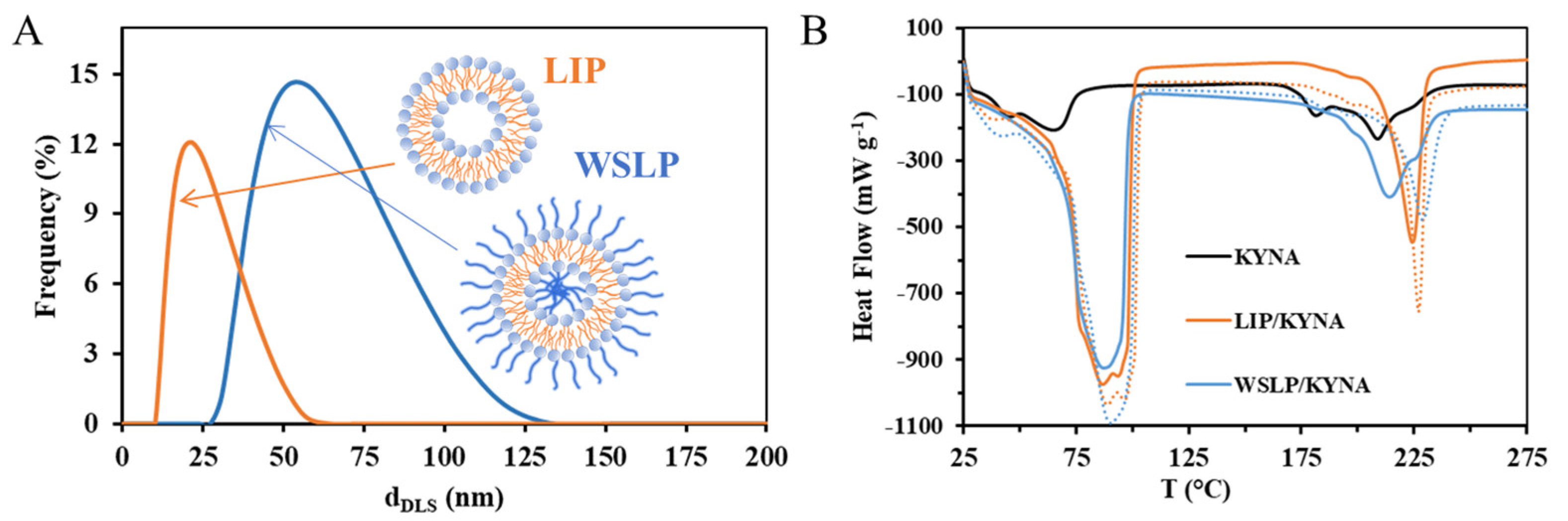
2.2. Physicochemical and Structural Characterization of the Colloidal Carriers
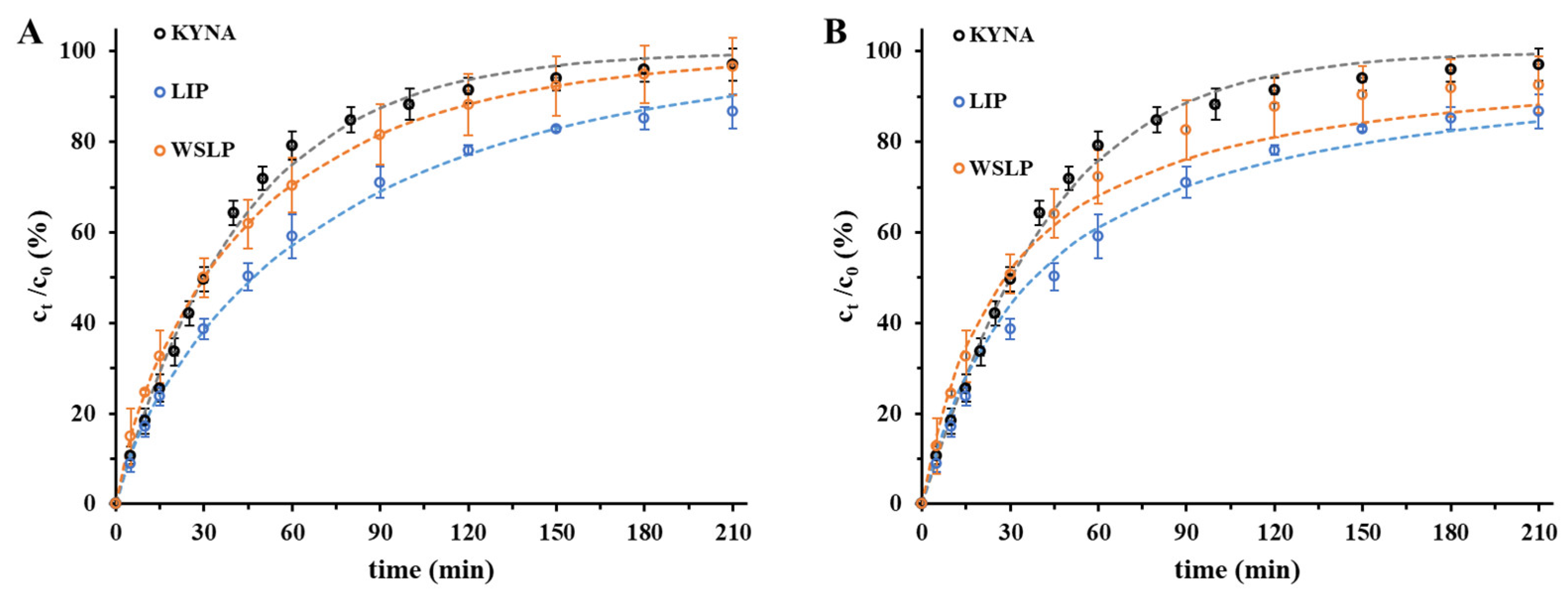
2.3. In Vitro Drug Release Studies of the Drug from Liposomes
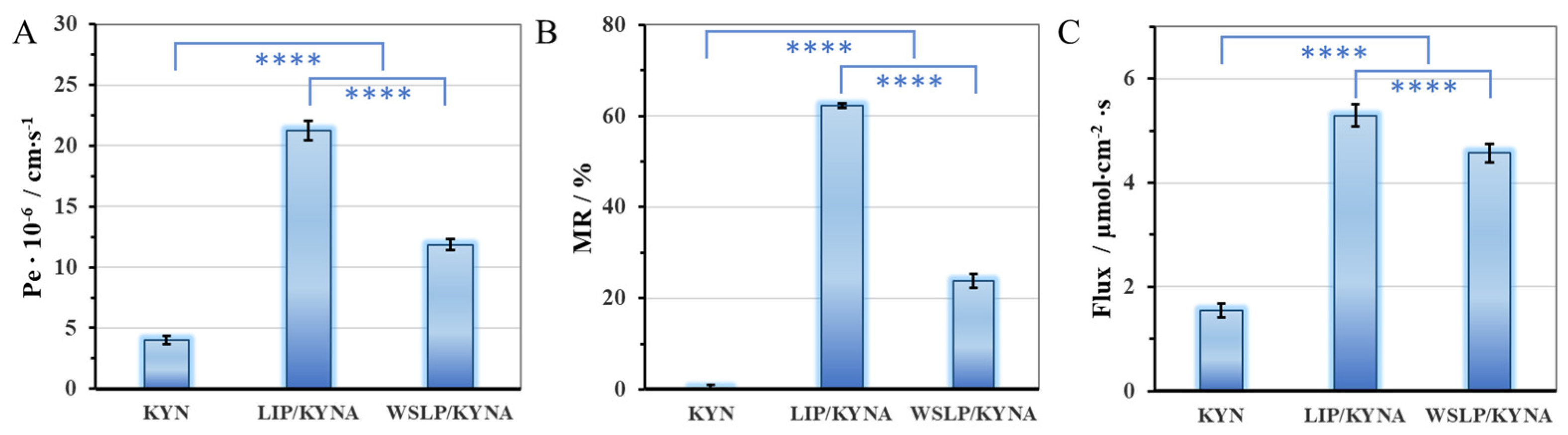
2.4. Blood-Brain Barrier Permeability Study of the Encapsulated Drug
3. Materials and Methods
3.1. Materials
3.2. Preparation of Asolectin-Based Nanocarriers Containing KYNA
3.3. Preparation of Lipopolymer-Based Nanocarriers Containing KYNA
3.4. Methods for Characterization
3.4.1. Structural Characterization of the KYNA-Containing Carriers
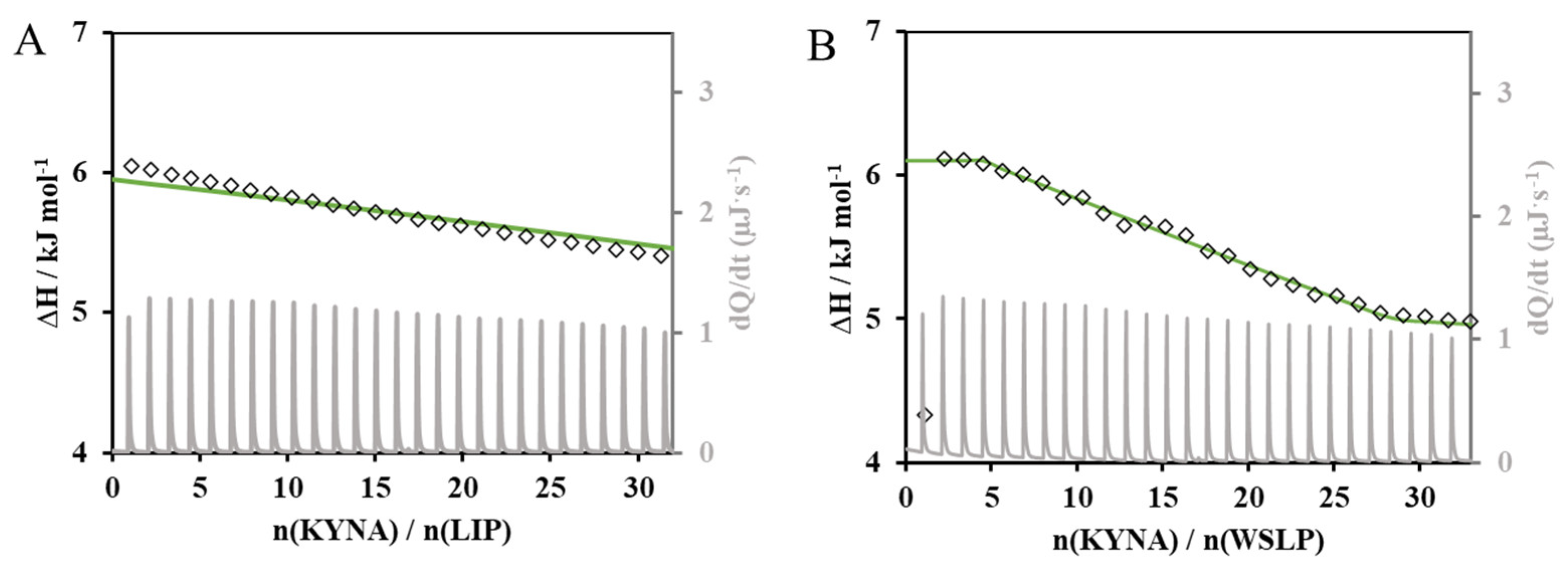
3.4.2. Isothermal Titration Calorimetry (ITC) Based Investigation
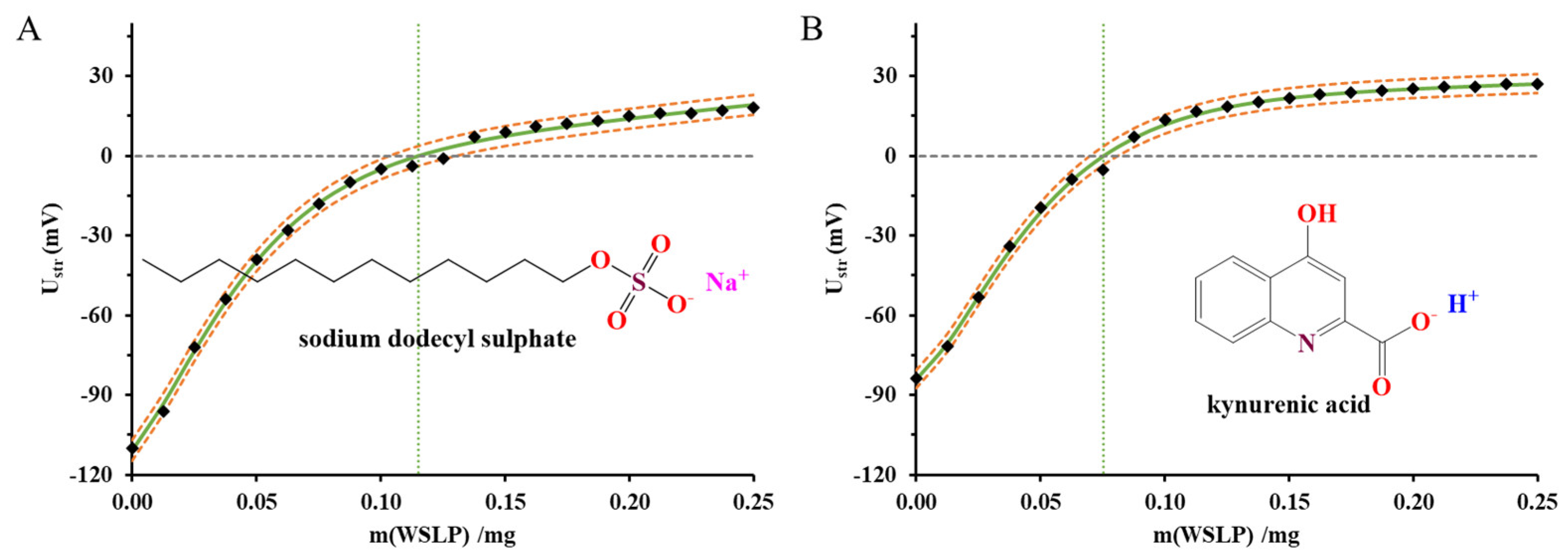
3.4.3. Streaming Potential Measurements by Particle Charge Detector (PCD) Device
3.4.4. In Vitro Dissolution and Release Studies
3.4.5. Permeability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostapiuk, A.; Urbanska, E.M. Kynurenic Acid in Neurodegenerative Disorders—Unique Neuroprotection or Double-Edged Sword? CNS Neurosci. Ther. 2022, 28, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Launer, L.J. Statistics on the Burden of Dementia: Need for Stronger Data. Lancet Neurol. 2019, 18, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H. Dementia Epidemiology Fact Sheet 2022. Ann. Rehabil. Med. 2022, 46, 53. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.; Liu, A. What Is the Tryptophan Kynurenine Pathway and Why Is It Important to Neurotherapeutics? Expert Rev. Neurother. 2015, 15, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Perkins, M.N.; Stone, T.W. An Iontophoretic Investigation of the Actions of Convulsant Kynurenines and Their Interaction with the Endogenous Excitant Quinolinic Acid. Brain Res. 1982, 247, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood–Brain Barrier Transport of Kynurenines: Implications for Brain Synthesis and Metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef]
- Jovanovic, F.; Candido, K.D.; Knezevic, N.N. The Role of the Kynurenine Signaling Pathway in Different Chronic Pain Conditions and Potential Use of Therapeutic Agents. Int. J. Mol. Sci. 2020, 21, 6045. [Google Scholar] [CrossRef] [PubMed]
- Tuka, B.; Nyári, A.; Cseh, E.K.; Körtési, T.; Veréb, D.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; Tajti, J.; Vécsei, L. Clinical Relevance of Depressed Kynurenine Pathway in Episodic Migraine Patients: Potential Prognostic Markers in the Peripheral Plasma during the Interictal Period. J. Headache Pain 2021, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Knyihár-Csillik, E.; Toldi, J.; Mihály, A.; Krisztin-Péva, B.; Chadaide, Z.; Németh, H.; Fenyo, R.; Vécsei, L. Kynurenine in Combination with Probenecid Mitigates the Stimulation-Induced Increase of c-Fos Immunoreactivity of the Rat Caudal Trigeminal Nucleus in an Experimental Migraine Model. J. Neural Transm. 2007, 114, 417–421. [Google Scholar] [CrossRef]
- Szűcs, E.; Stefanucci, A.; Dimmito, M.P.; Zádor, F.; Pieretti, S.; Zengin, G.; Vécsei, L.; Benyhe, S.; Nalli, M.; Mollica, A. Discovery of Kynurenines Containing Oligopeptides as Potent Opioid Receptor Agonists. Biomolecules 2020, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Demeter, I.; Nagy, K.; Gellért, L.; Vécsei, L.; Fülöp, F.; Toldi, J. A Novel Kynurenic Acid Analog (SZR104) Inhibits Pentylenetetrazole-Induced Epileptiform Seizures. An Electrophysiological Study: Special Issue Related to Kynurenine. J. Neural Transm. 2012, 119, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering Precision Nanoparticles for Drug Delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Fam, S.Y.; Chee, C.F.; Yong, C.Y.; Ho, K.L.; Mariatulqabtiah, A.R.; Tan, W.S. Stealth Coating of Nanoparticles in Drug-Delivery Systems. Nanomaterials 2020, 10, 787. [Google Scholar] [CrossRef]
- López, T.; Ortiz, E.; Gómez, E.; La Cruz, V.P.D.; Carrillo-Mora, P.; Novaro, O. Preparation and Characterization of Kynurenic Acid Occluded in Sol-Gel Silica and Sba-15 Silica as Release Reservoirs. J. Nanomater. 2014, 2014, 507178. [Google Scholar] [CrossRef]
- Deák, Á.; Csapó, E.; Juhász, Á.; Dékány, I.; Janovák, L. Anti-Ulcerant Kynurenic Acid Molecules Intercalated Mg/Al-Layered Double Hydroxide and Its Release Study. Appl. Clay Sci. 2018, 156, 28–35. [Google Scholar] [CrossRef]
- Varga, N.; Csapó, E.; Majláth, Z.; Ilisz, I.; Krizbai, I.A.; Wilhelm, I.; Knapp, L.; Toldi, J.; Vécsei, L.; Dékány, I. Targeting of the Kynurenic Acid across the Blood-Brain Barrier by Core-Shell Nanoparticles. Eur. J. Pharm. Sci. 2016, 86, 67–74. [Google Scholar] [CrossRef]
- Kovács, A.N.; Varga, N.; Juhász, Á.; Csapó, E. Serum Protein-Hyaluronic Acid Complex Nanocarriers: Structural Characterisation and Encapsulation Possibilities. Carbohydr. Polym. 2021, 251, 117047. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Rakesh, K.P.; Manukumar, H.M.; Mohammed, Y.H.E.; Karthik, C.S.; Sumathi, S.; Mallu, P.; Qin, H.L. Innovative Nano-Carriers in Anticancer Drug Delivery-a Comprehensive Review. Bioorganic Chem. 2019, 85, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.F.; Chu, S.M.; Liao, C.C.; Wang, C.J.; Wang, Y.S.; Lai, M.Y.; Wang, H.C.; Huang, H.R.; Tsai, M.H. Nanotechnology and Nanocarrier-Based Drug Delivery as the Potential Therapeutic Strategy for Glioblastoma Multiforme: An Update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef]
- Alshawwa, S.Z.; Kassem, A.A.; Farid, R.M.; Mostafa, S.K.; Labib, G.S. Nanocarrier Drug Delivery Systems: Characterization, Limitations, Future Perspectives and Implementation of Artificial Intelligence. Pharmaceutics 2022, 14, 883. [Google Scholar] [CrossRef] [PubMed]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Zhang, Y.; Obuchi, H.; Toyota, T. A Practical Guide to Preparation and Applications of Giant Unilamellar Vesicles Formed via Centrifugation of Water-in-Oil Emulsion Droplets. Membranes 2023, 13, 440. [Google Scholar] [CrossRef]
- Frallicciardi, J.; Melcr, J.; Siginou, P.; Marrink, S.J.; Poolman, B. Membrane Thickness, Lipid Phase and Sterol Type Are Determining Factors in the Permeability of Membranes to Small Solutes. Nat. Commun. 2022, 13, 1605. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Vécsei, L.; Lukács, M.; Tajti, J.; Fülöp, F.; Toldi, J.; Edvinsson, L. The Therapeutic Impact of New Migraine Discoveries. Curr. Med. Chem. 2019, 26, 6261–6281. [Google Scholar] [CrossRef] [PubMed]
- Abd Elhameed, H.A.H.; Ungor, D.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M.; Csapó, E.; Gyurcsik, B. High Molecular Weight Poly(Ethylenimine)-Based Water-Soluble Lipopolymer for Transfection of Cancer Cells. Macromol. Biosci. 2020, 20, 2000040. [Google Scholar] [CrossRef]
- Simonis, B.; Vignone, D.; Gonzalez Paz, O.; Donati, E.; Falchetti, M.L.; Bombelli, C.; Cellucci, A.; Auciello, G.; Fini, I.; Galantini, L.; et al. Transport of Cationic Liposomes in a Human Blood Brain Barrier Model: Role of the Stereochemistry of the Gemini Amphiphile on Liposome Biological Features. J. Colloid Interface Sci. 2022, 627, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, Y.S.; Tsentalovich, Y.P. Acid-Alkaline Properties of Triplet State and Radical of Kynurenic Acid. J. Photochem. Photobiol. A Chem. 2018, 365, 7–12. [Google Scholar] [CrossRef]
- Pereira-Leite, C.; Figueiredo, M.; Burdach, K.; Nunes, C.; Reis, S. Unraveling the Role of Drug-Lipid Interactions in NSAIDs-Induced Cardiotoxicity. Membranes 2020, 11, 24. [Google Scholar] [CrossRef]
- Nunes, C.; Brezesinski, G.; Lopes, D.; Lima, J.L.F.C.; Reis, S.; Lúcio, M. Lipid-Drug Interaction: Biophysical Effects of Tolmetin on Membrane Mimetic Systems of Different Dimensionality. J. Phys. Chem. B 2011, 115, 12615–12623. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Ramírez, M.; Silva-Jiménez, H.; Banat, I.M.; Díaz De Rienzo, M.A. Surfactants: Physicochemical Interactions with Biological Macromolecules. Biotechnol. Lett. 2021, 43, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberg, D.; Ahyayauch, H.; Alonso, A.; Goñi, F.M. Detergent Solubilization of Lipid Bilayers: A Balance of Driving Forces. Trends Biochem. Sci. 2013, 38, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Corsaro, C.; Neri, G.; Mezzasalma, A.M.; Fazio, E. Weibull Modeling of Controlled Drug Release from Ag-PMA Nanosystems. Polymers 2021, 13, 2897. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting Non-Linear Drug Diffusion Data: Utilizing Korsmeyer-Peppas Model to Study Drug Release from Liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Higuchi Equation: Derivation, Applications, Use and Misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Zhu, W.; Long, J.; Shi, M. Release Kinetics Model Fitting of Drugs with Different Structures from Viscose Fabric. Materials 2023, 16, 3282. [Google Scholar] [CrossRef]
- del Valle, J.C.; Catalán, J.; Amat-Guerri, F. Comparative Photophysical Study of Rose Bengal, Eosin Y and Their Monomethyl and Dimethyl Derivatives. J. Photochem. Photobiol. A Chem. 1993, 72, 49–53. [Google Scholar] [CrossRef]
- Lee, M.; Rentz, J.; Han, S.O.; Bull, D.A.; Kim, S.W. Water-Soluble Lipopolymer as an Efficient Carrier for Gene Delivery to Myocardium. Gene Ther. 2003, 10, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Juhász, Á.; Ungor, D.; Berta, K.; Seres, L.; Csapó, E. Spreadsheet-Based Nonlinear Analysis of in Vitro Release Properties of a Model Drug from Colloidal Carriers. J. Mol. Liq. 2021, 328, 115405. [Google Scholar] [CrossRef]
- Kovács, A.N.; Katona, G.; Juhász, Á.; Balogh, G.T.; Csapó, E. Albumin-Hyaluronic Acid Colloidal Nanocarriers: Effect of Human and Bovine Serum Albumin for Intestinal Ibuprofen Release Enhancement. J. Mol. Liq. 2022, 351, 118614. [Google Scholar] [CrossRef]
- Dragan, E.S.; Bucatariu, F. Cross-Linked Multilayers of Poly(Vinyl Amine) as a Single Component and Their Interaction with Proteins. Macromol. Rapid Commun. 2010, 31, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Böckenhoff, K.; Fischer, W.R. Determination of Electrokinetic Charge with a Particle-Charge Detector, and Its Relationship to the Total Charge. Fresenius’ J. Anal. Chem. 2001, 371, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Piekarski, H.; Łudzik, K. A Microcalorimetric Titration Study on the Micelle Formation of Alkanediyl-α,ω-Bis(Dimethylalkylammonium Bromide) Surfactants at a 283.15–343.15 K Temperature Range. J. Therm. Anal. Calorim. 2012, 110, 263–271. [Google Scholar] [CrossRef][Green Version]
- Pethő, B.; Zwillinger, M.; Csenki, J.T.; Káncz, A.E.; Krámos, B.; Müller, J.; Balogh, G.T.; Novák, Z. Palladium-Catalyzed 2,2,2-Trifluoroethoxylation of Aromatic and Heteroaromatic Chlorides Utilizing Borate Salt and the Synthesis of a Trifluoro Analogue of Sildenafil. Chem.-A Eur. J. 2017, 23, 15628–15632. [Google Scholar] [CrossRef] [PubMed]
| Sample | Sonication Time (min) | dDLS (nm) | ζ-Potential (mV) | EE% |
|---|---|---|---|---|
| 1. | 0 | 152 ± 34 | −67 ± 4 | 58.0 |
| 2. | 10 | 34 ± 18 | −62 ± 5 | 55.4 |
| 3. | 20 | 42 ± 21 | −62 ± 3 | 70.0 |
| 4. | 30 | 23 ± 10 | −58 ± 3 | 69.4 |
| 5. | 40 | 36 ± 10 | −53 ± 4 | 48.8 |
| 6. | 50 | 44 ± 13 | −61 ± 4 | 54.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juhász, Á.; Ungor, D.; Varga, N.; Katona, G.; Balogh, G.T.; Csapó, E. Lipid-Based Nanocarriers for Delivery of Neuroprotective Kynurenic Acid: Preparation, Characterization, and BBB Transport. Int. J. Mol. Sci. 2023, 24, 14251. https://doi.org/10.3390/ijms241814251
Juhász Á, Ungor D, Varga N, Katona G, Balogh GT, Csapó E. Lipid-Based Nanocarriers for Delivery of Neuroprotective Kynurenic Acid: Preparation, Characterization, and BBB Transport. International Journal of Molecular Sciences. 2023; 24(18):14251. https://doi.org/10.3390/ijms241814251
Chicago/Turabian StyleJuhász, Ádám, Ditta Ungor, Norbert Varga, Gábor Katona, György T. Balogh, and Edit Csapó. 2023. "Lipid-Based Nanocarriers for Delivery of Neuroprotective Kynurenic Acid: Preparation, Characterization, and BBB Transport" International Journal of Molecular Sciences 24, no. 18: 14251. https://doi.org/10.3390/ijms241814251
APA StyleJuhász, Á., Ungor, D., Varga, N., Katona, G., Balogh, G. T., & Csapó, E. (2023). Lipid-Based Nanocarriers for Delivery of Neuroprotective Kynurenic Acid: Preparation, Characterization, and BBB Transport. International Journal of Molecular Sciences, 24(18), 14251. https://doi.org/10.3390/ijms241814251











