The Effect of Potassium Canrenoate (Mineralocorticoid Receptor Antagonist) on the Markers of Inflammation in the Treatment of COVID-19 Pneumonia and Fibrosis—A Secondary Analysis of Randomized Placebo-Controlled Clinical Trial
Abstract
:1. Introduction
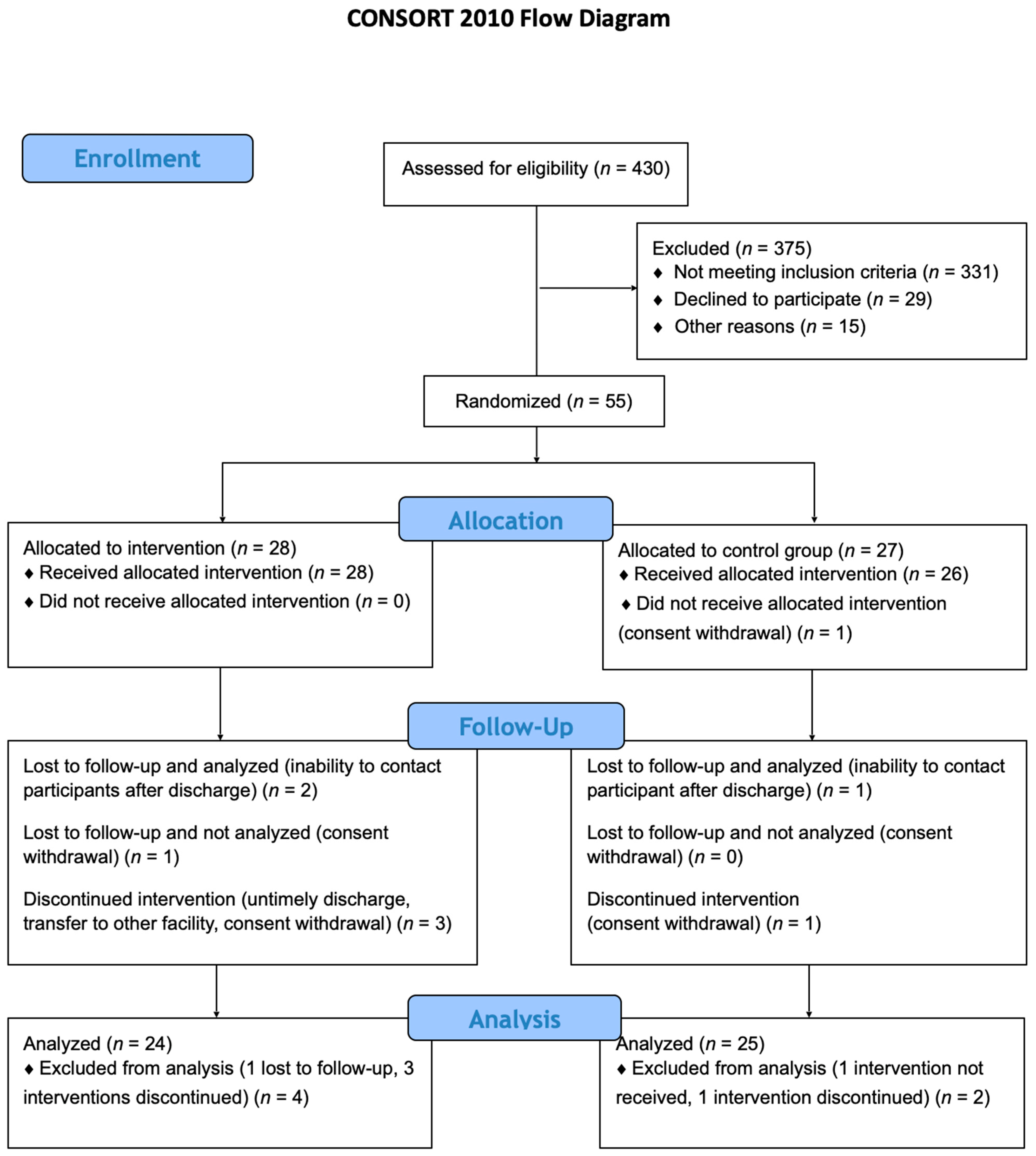
2. Results
2.1. Baseline Patient Characteristics
2.2. Basic Laboratory Tests
2.3. Immunophenotype Analysis on Day 1 and Day 7
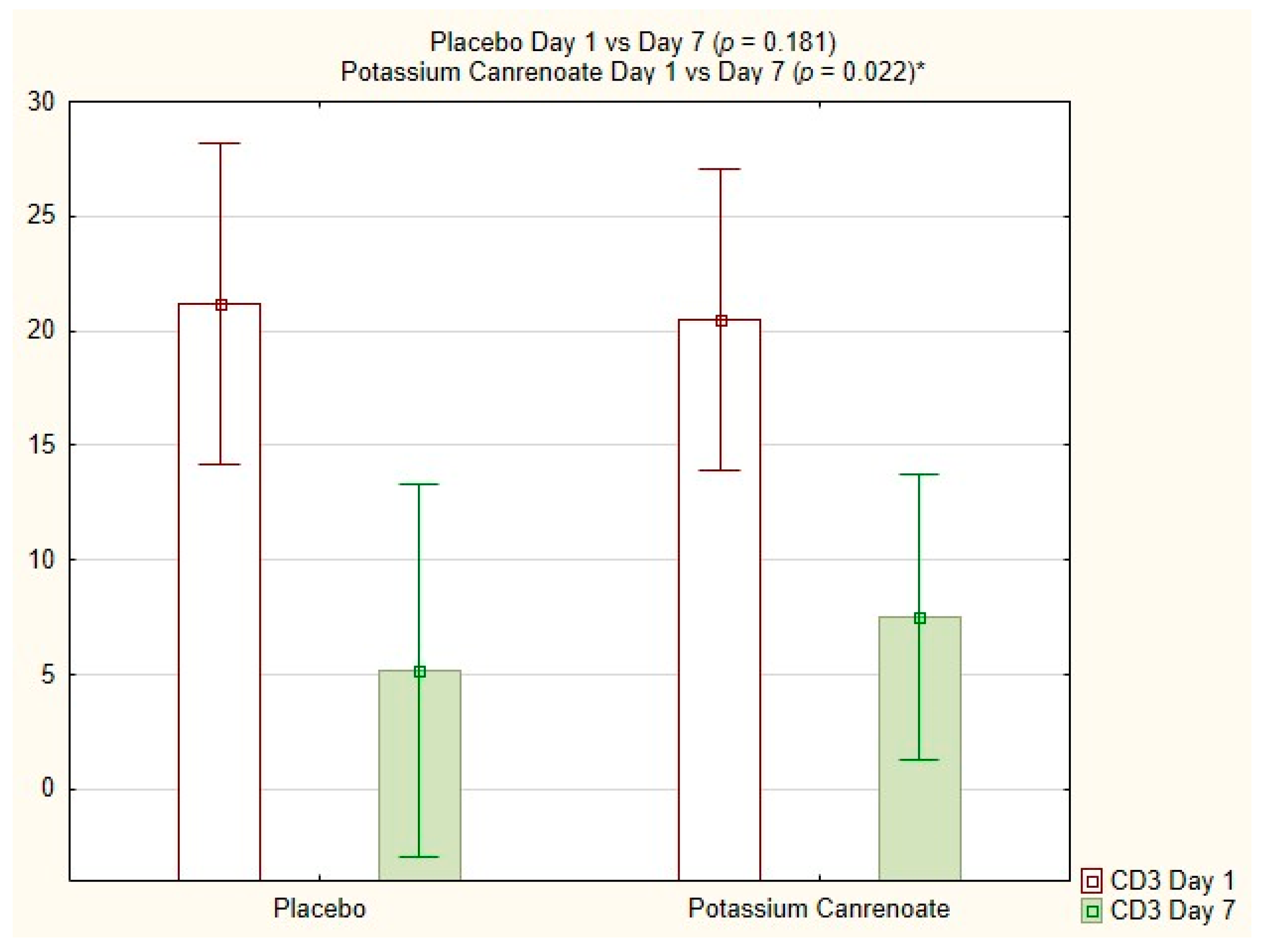
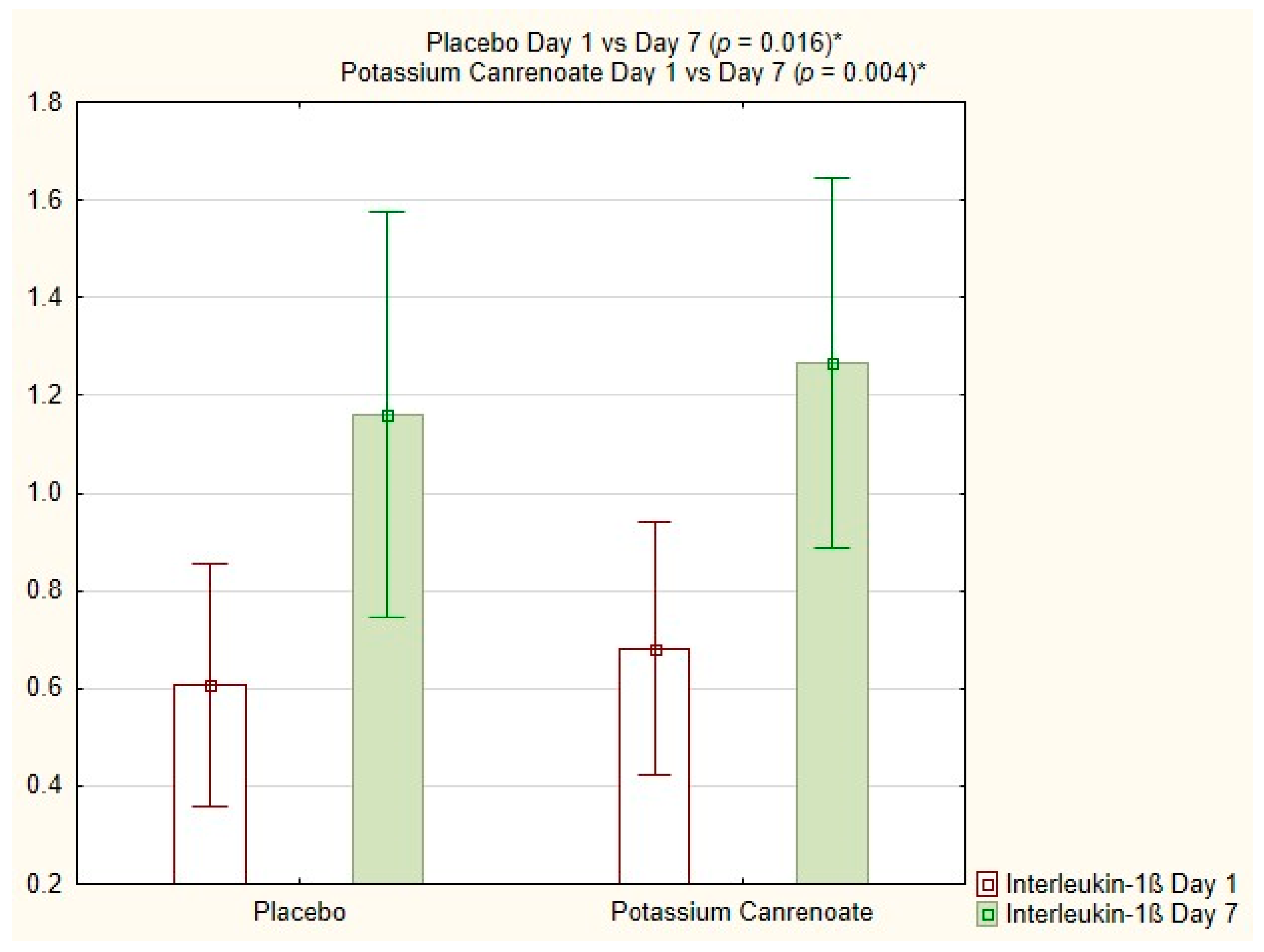
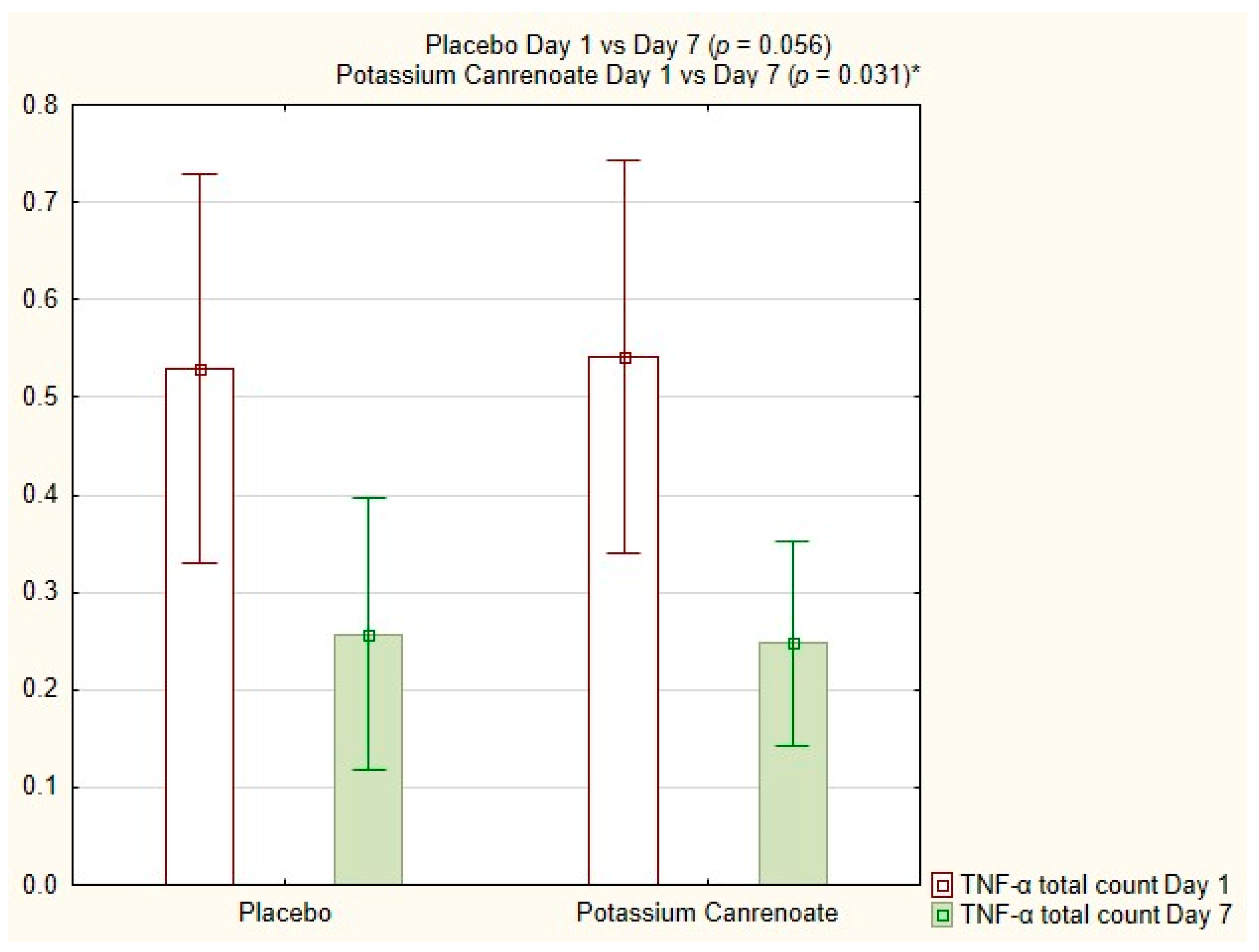
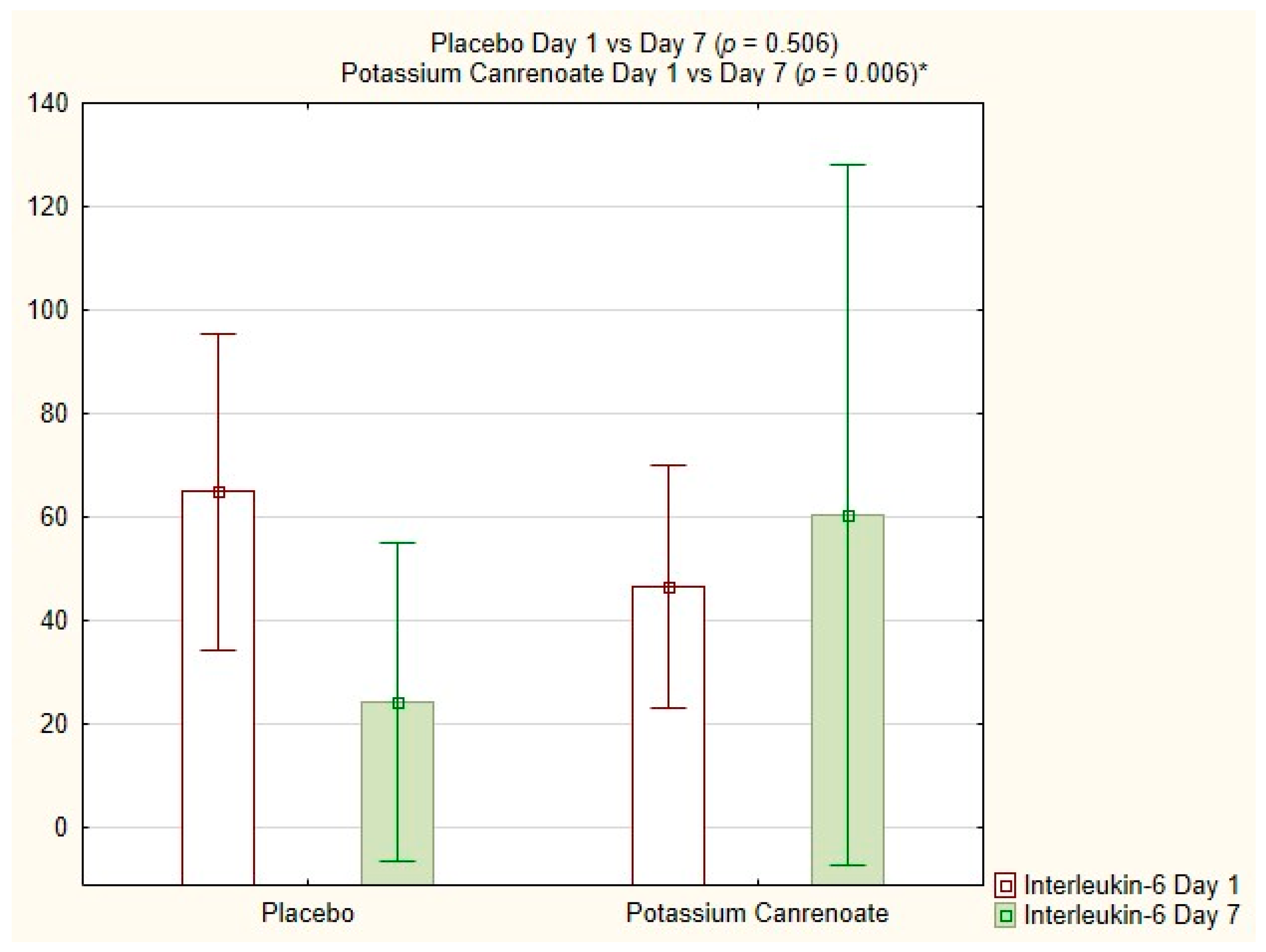
2.4. Intragroup Comparisons between Potassium Canrenoate and Placebo Groups on Day 1 versus Day 7
3. Discussion
4. Materials and Methods
4.1. Ethics
4.2. Study Population
4.3. Inclusion Criteria
- Individuals of all genders, aged between 18 and 90 years.
- Patients requiring oxygen therapy with a blood oxygen saturation level below 94%.
- Confirmed diagnosis of COVID-19 infection through rt-PCR testing.
- Presence of at least one documented risk factor for heightened COVID-19 mortality, as outlined in current scientific literature, such as smoking, hypertension, diabetes, or cardiovascular disease.
- Well-documented informed consent in accordance with ICH-GCP guidelines and national regulations.
4.4. Exclusion Criteria
- Patients with a history of chronic bronchitis, emphysema, interstitial lung disease, or any other preexisting lung conditions.
- Individuals with contraindications for the use of spironolactone.
- Hypersensitivity to spironolactone or any of its components.
- Pregnant individuals (with mandatory pregnancy testing for those of reproductive age) or breastfeeding mothers.
- Patients with mental illness or dementia who are unable to provide informed consent for participation in the study.
- ARDS resulting from another viral infection (testing negative for SARS-CoV-2).
- ARDS caused by other factors or trauma.
- Presence of ionic imbalances, such as hyperkalemia or hyponatremia.
- Adrenal crisis.
- Acute or chronic renal failure, with a creatinine clearance rate below 30 mL/min.
- Anuria.
- Porphyria.
- Chronic use of mineralocorticoid receptor antagonist (MRA) medications from the spironolactone group.
4.5. Clinical Experiment Measures
4.6. Outcome Measures
4.6.1. Primary Outcome Measures for Primary Analysis
- Duration of invasive mechanical ventilation via endotracheal intubation or tracheotomy (observation time 30 days).
- Duration of passive oxygen therapy (observation time 30 days).
4.6.2. Secondary Outcome Measures for Primary Analysis
- Intensive care unit length of stay (LOS) (time frame 30 days).
- Total hospital length of stay (LOS) (time frame 90 days).
- Assessment of the dynamics of recovery of changes in lung ultrasound at 7 days.
- Assessment of the dynamics of recovery of changes in lung ultrasound at 30 days.
- Assessment of the dynamics of recovery of changes in chest computed tomography (CT) at 3 months (90 days).
- Assessment of mortality at 30 days.
- Assessment of mortality at 90 days.
- Six-minute walking test (6MWT) at 30 days.
- Six-minute walking test (6MWT) at 90 days.
4.6.3. Outcome Measures for Secondary Analysis
- Assessment of the dynamics of change in T CD4+ lymphocyte count at 7 days.
- Assessment of the dynamics of change in T CD3+ lymphocytes count at 7 days.
- Assessment of the dynamics of change in IL-1ß on lymphocytes and total count of IL-1ß at 7 days.
- Assessment of the dynamics of change in IL-2 on lymphocytes and total count of IL-2 at 7 days.
- Assessment of the dynamics of change in TNF-α on lymphocytes and total count of TNF-α at 7 days.
- Assessment of the dynamics of change in IL-6 serum level at 7 days.
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Kucewicz-Czech, E.; Damps, M. Triage during the COVID-19 Pandemic. Anaesthesiol. Intensive Ther. 2021, 52, 312–315. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 6 January 2022).
- Lian, J.; Jin, X.; Hao, S.; Jia, H.; Cai, H.; Zhang, X.; Hu, J.; Zheng, L.; Wang, X.; Zhang, S.; et al. Epidemiological, Clinical, and Virological Characteristics of 465 Hospitalized Cases of Coronavirus Disease 2019 (COVID-19) from Zhejiang Province in China. Influenza Other Respi. Viruses 2020, 14, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Roberson, S.W.; Wilson, J.; Pun, B.; Ely, E.W.; Jeżowska, I.; Jezierska, M.; Dabrowski, W. COVID-19: What Do We Need to Know About ICU Delirium during the SARS-CoV-2 Pandemic? Anaesthesiol. Intensive Ther. 2020, 52, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-Month Consequences of COVID-19 in Patients Discharged from Hospital: A Cohort Study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Huang, S.; Yin, L. The Cytokine Storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider Cytokine Storm Syndromes and Immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Jamilloux, Y.; Henry, T.; Belot, A.; Viel, S.; Fauter, M.; El Jammal, T.; Walzer, T.; François, B.; Sève, P. Should We Stimulate or Suppress Immune Responses in COVID-19? Cytokine and Anti-Cytokine Interventions. Autoimmun. Rev. 2020, 19, 102567. [Google Scholar] [CrossRef]
- Takahashi, T.; Wong, P.; Ellingson, M.; Lucas, C.; Klein, J.; Israelow, B.; Silva, J.; Oh, J.; Mao, T.; Tokuyama, M.; et al. Sex Differences in Immune Responses to SARS-CoV-2 That Underlie Disease Outcomes. medRxiv 2020. Update in: Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Sun, X.; Wang, T.; Cai, D.; Hu, Z.; Chen, J.; Liao, H.; Zhi, L.; Wei, H.; Zhang, Z.; Qiu, Y.; et al. Cytokine Storm Intervention in the Early Stages of COVID-19 Pneumonia. Cytokine Growth Factor Rev. 2020, 53, 38–42. [Google Scholar] [CrossRef]
- Mardi, A.; Meidaninikjeh, S.; Nikfarjam, S.; Majidi Zolbanin, N.; Jafari, R. Interleukin-1 in COVID-19 Infection: Immunopathogenesis and Possible Therapeutic Perspective. Viral Immunol. 2021, 34, 679–688. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, H.; Dauphars, D.J.; He, Y.W. A Potential Role of Interleukin 10 in COVID-19 Pathogenesis. Trends Immunol. 2021, 42, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Kappelmann, N.; Dantzer, R.; Khandaker, G.M. Interleukin-6 as Potential Mediator of Long-Term Neuropsychiatric Symptoms of COVID-19. Psychoneuroendocrinology 2021, 131, 105295. [Google Scholar] [CrossRef]
- Cadegiani, F.A.; Goren, A.; Wambier, C.G. Spironolactone May Provide Protection from SARS-CoV-2: Targeting Androgens, Angiotensin Converting Enzyme 2 (ACE2), and Renin-Angiotensin-Aldosterone System (RAAS). Med. Hypotheses 2020, 143, 110112. [Google Scholar] [CrossRef]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of Angiotensin-Converting Enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ding, Y.; Zhang, Q.; Che, X.; He, Y.; Shen, H.; Wang, H.; Li, Z.; Zhao, L.; Geng, J.; et al. Expression of Elevated Levels of Pro-Inflammatory Cytokines in SARS-CoV-Infected ACE2+ Cells in SARS Patients: Relation to the Acute Lung Injury and Pathogenesis of SARS. J. Pathol. 2006, 210, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-Y.; Chang, S.C.; Wu, H.-Y.; Yu, T.-C.; Wei, W.-C.; Lin, S.; Chien, C.-L.; Chang, M.-F. Upregulation of the Chemokine (C-C Motif) Ligand 2 via a Severe Acute Respiratory Syndrome Coronavirus Spike-ACE2 Signaling Pathway. J. Virol. 2010, 84, 7703–7712. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Liu, C.Y.-Y.; Chiang, B.-L.; Chao, Y.-C.; Chen, C.-C. Induction of IL-8 Release in Lung Cells via Activator Protein-1 by Recombinant Baculovirus Displaying Severe Acute Respiratory Syndrome-Coronavirus Spike Proteins: Identification of Two Functional Regions. J. Immunol. 2004, 173, 7602–7614. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Xu, M.; Yao, M.; Sun, B. Angiotensin AT1 Receptor Antagonists Exert Anti-Inflammatory Effects in Spontaneously Hypertensive Rats. Br. J. Pharmacol. 2007, 152, 1042–1048. [Google Scholar] [CrossRef]
- Ciaglia, E.; Vecchione, C.; Puca, A.A. COVID-19 Infection and Circulating ACE2 Levels: Protective Role in Women and Children. Front. Pediatr. 2020, 8, 206. [Google Scholar] [CrossRef]
- Xie, X.; Chen, J.; Wang, X.; Zhang, F.; Liu, Y. Age- and Gender-Related Difference of ACE2 Expression in Rat Lung. Life Sci. 2006, 78, 2166–2171. [Google Scholar] [CrossRef]
- Beneteau-Burnat, B.; Baudin, B.; Morgant, G.; Baumann, F.C.; Giboudeau, J. Serum Angiotensin-Converting Enzyme in Healthy and Sarcoidotic Children: Comparison with the Reference Interval for Adults. Clin. Chem. 1990, 36, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, L.; Huang, L.; Zhang, C.; Luo, R.; Zeng, L.; Liang, H.; Li, Q.; Lu, X.; Wang, X.; et al. Distinct Disease Severity Between Children and Older Adults With Coronavirus Disease 2019 (COVID-19): Impacts of ACE2 Expression, Distribution, and Lung Progenitor Cells. Clin. Infect. Dis. 2021, 73, e4154–e4165. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, S.; Hedrich, C.M. SARS-CoV-2 Infections in Children and Young People. Clin. Immunol. 2020, 220, 108588. [Google Scholar] [CrossRef] [PubMed]
- Yavas, G.; Yavas, C.; Celik, E.; Sen, E.; Ata, O.; Afsar, R.E. The Impact of Spironolactone on the Lung Injury Induced By Concomitant Trastuzumab and Thoracic Radiotherapy. Int. J. Radiat. Res. 2019, 17, 87–95. [Google Scholar] [CrossRef]
- Rao, K.-R.; Bao, R.-Y.; Ming, H.; Liu, J.-W.; Dong, Y.-F. The Correlation Between Aldosterone and Leukocyte-Related Inflammation: A Comparison Between Patients with Primary Aldosteronism and Essential Hypertension. J. Inflamm. Res. 2023, 16, 2401–2413. [Google Scholar] [CrossRef]
- Kotfis, K.; Lechowicz, K.; Drożdżal, S.; Niedźwiedzka-Rystwej, P.; Wojdacz, T.K.; Grywalska, E.; Biernawska, J.; Wiśniewska, M.; Parczewski, M. COVID-19—The Potential Beneficial Therapeutic Effects of Spironolactone during SARS-CoV-2 Infection. Pharmaceuticals 2021, 14, 71. [Google Scholar] [CrossRef]
- Kumar, N.; Zuo, Y.; Yalavarthi, S.; Hunker, K.L.; Knight, J.S.; Kanthi, Y.; Obi, A.T.; Ganesh, S.K. SARS-CoV-2 Spike Protein S1-Mediated Endothelial Injury and Pro-Inflammatory State Is Amplified by Dihydrotestosterone and Prevented by Mineralocorticoid Antagonism. Viruses 2021, 13, 2209. [Google Scholar] [CrossRef]
- Wilcox, C.S.; Pitt, B. Is Spironolactone the Preferred Renin–Angiotensin–Aldosterone Inhibitor for Protection Against COVID-19? J. Cardiovasc. Pharmacol. 2021, 77, 323–331. [Google Scholar] [CrossRef]
- Lieber, G.B.; Fernandez, X.; Mingo, G.G.; Jia, Y.; Caniga, M.; Gil, M.A.; Keshwani, S.; Woodhouse, J.D.; Cicmil, M.; Moy, L.Y.; et al. Mineralocorticoid Receptor Antagonists Attenuate Pulmonary Inflammation and Bleomycin-Evoked Fibrosis in Rodent Models. Eur. J. Pharmacol. 2013, 718, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.-J.; Ma, Y.-Q.; Zhou, X.; Zhang, Y.-D.; Lu, R.-Y.; Guo, Z.-Z.; Sun, H.-Y.; Hu, D.-C.; Yang, G.-H.; Li, Y.-M.; et al. Spironolactone Attenuates Bleomycin-Induced Pulmonary Injury Partially via Modulating Mononuclear Phagocyte Phenotype Switching in Circulating and Alveolar Compartments. PLoS ONE 2013, 8, e81090. [Google Scholar] [CrossRef]
- Atalay, C.; Dogan, N.; Aykan, S.; Gundogdu, C.; Keles, M.S. The Efficacy of Spironolactone in the Treatment of Acute Respiratory Distress Syndrome-Induced Rats. Singap. Med. J. 2010, 51, 501–505. [Google Scholar]
- Barut, F.; Ozacmak, V.H.; Turan, I.; Sayan-Ozacmak, H.; Aktunc, E. Reduction of Acute Lung Injury by Administration of Spironolactone After Intestinal Ischemia and Reperfusion in Rats. Clin. Investig. Med. 2016, 39, 15. [Google Scholar] [CrossRef]
- Maleszka, P.; Kruszewski, J. Comparative Evaluation of Inhaling a Single Dose of Furosemide or Spironolactone on Bronchial Hyperreactivity of Patients with Atopic Bronchial Asthma. Pol. Tyg. Lek. 1994, 49, 415–418. [Google Scholar]
- Kolkhof, P.; Bärfacker, L. 30 YEARS OF THE MINERALOCORTICOID RECEPTOR: Mineralocorticoid Receptor Antagonists: 60 Years of Research and Development. J. Endocrinol. 2017, 234, T125–T140. [Google Scholar] [CrossRef] [PubMed]
- Koning, A.-S.C.A.M.; Buurstede, J.C.; van Weert, L.T.C.M.; Meijer, O.C. Glucocorticoid and Mineralocorticoid Receptors in the Brain: A Transcriptional Perspective. J. Endocr. Soc. 2019, 3, 1917–1930. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, F.L.; Karst, H.; de Kloet, E.R.; Joëls, M. Mineralocorticoid and Glucocorticoid Receptors at the Neuronal Membrane, Regulators of Nongenomic Corticosteroid Signalling. Mol. Cell. Endocrinol. 2012, 350, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.-N.; Sun, L.; Wang, B.-R.; Zou, Y.; Xu, S.; Ding, Y.-J.; Shen, L.-J.; Huang, W.-C.; Jiang, X.-J.; Chen, S.-M. The characteristics and evolution of pulmonary fibrosis in COVID-19 patients as assessed by AI-assisted chest HRCT. PLoS ONE 2021, 16, e0248957. [Google Scholar] [CrossRef]
- Grifoni, E.; Valoriani, A.; Cei, F.; Lamanna, R.; Gelli, A.M.G.; Ciambotti, B.; Vannucchi, V.; Moroni, F.; Pelagatti, L.; Tarquini, R.; et al. Interleukin-6 as prognosticator in patients with COVID-19. J. Infect. 2020, 81, 452–482. [Google Scholar] [CrossRef]
- Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M.; Annane, D.; Beane, A.; Van Bentum-Puijk, W.; Berry, L.R.; et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Lechowicz, K.; Drożdżal, S.; Machaj, F.; Rosik, J.; Szostak, B.; Zegan-Barańska, M.; Biernawska, J.; Dabrowski, W.; Rotter, I.; Kotfis, K. COVID-19: The Potential Treatment of Pulmonary Fibrosis Associated with SARS-CoV-2 Infection. J. Clin. Med. 2020, 9, 1917. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Xu, D.; Zhang, R.; Lan, L.; Xu, H. Prediction of the Development of Pulmonary Fibrosis Using Serial Thin-Section Ct and Clinical Features in Patients Discharged after Treatment for COVID-19 Pneumonia. Korean J. Radiol. 2020, 21, 746–755. [Google Scholar] [CrossRef]
- Wynn, T.A. Integrating Mechanisms of Pulmonary Fibrosis. J. Exp. Med. 2011, 208, 1339–1350. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Dai, H. IL-25/IL-33/TSLP Contributes to Idiopathic Pulmonary Fibrosis: Do Alveolar Epithelial Cells and (Myo)Fibroblasts Matter? Exp. Biol. Med. 2020, 245, 897–901. [Google Scholar] [CrossRef]
- Crosby, L.M.; Waters, C.M. Epithelial Repair Mechanisms in the Lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 298, L715–L731. [Google Scholar] [CrossRef] [PubMed]
- Wolszczak-Biedrzycka, B.; Dorf, J.; Milewska, A.; Łukaszyk, M.; Naumnik, W.; Kosidło, J.W.; Dymicka-Piekarska, V. The Diagnostic Value of Inflammatory Markers (CRP, IL6, CRP/IL6, CRP/L, LCR) for Assessing the Severity of COVID-19 Symptoms Based on the MEWS and Predicting the Risk of Mortality. J. Inflamm. Res. 2023, 16, 2173–2188. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Balan, I.; Yadav, S.; Matos, W.F.; Kharawala, A.; Gaddam, M.; Sarabia, N.; Koneru, S.C.; Suddapalli, S.K.; Marzban, S. Post-COVID-19 Pulmonary Fibrosis. Cureus 2022, 14, e22770. [Google Scholar] [CrossRef]
- Jover, E.; Matilla, L.; Garaikoetxea, M.; Fernández-Celis, A.; Muntendam, P.; Jaisser, F.; Rossignol, P.; López-Andrés, N. Beneficial Effects of Mineralocorticoid Receptor Pathway Blockade against Endothelial Inflammation Induced by Sars-COV-2 Spike Protein. Biomedicines 2021, 9, 639. [Google Scholar] [CrossRef]
- Fels, B.; Acharya, S.; Vahldieck, C.; Graf, T.; Käding, N.; Rupp, J.; Kusche-Vihrog, K. Mineralocorticoid Receptor-Antagonism Prevents COVID-19-Dependent Glycocalyx Damage. Pflug. Arch. 2022, 474, 1069–1076. [Google Scholar] [CrossRef]
- Mareev, V.Y.; Orlova, Y.A.; Plisyk, A.G.; Pavlikova, E.P.; Matskeplishvili, S.; Akopyan, Z.A.; Seredenina, E.M.; Potapenko, A.V.; Agapov, M.A.; Asratyan, D.A.; et al. Results of Open-Label Non-Randomized Comparative Clinical Trial: “Bromhexine and Spironolactone for Coronavirus Infection Requiring Hospitalization (BISCUIT). Kardiologiya 2020, 60, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Umemura, Y.; Mitsuyama, Y.; Minami, K.; Nishida, T.; Watanabe, A.; Okada, N.; Yamakawa, K.; Nochioka, K.; Fujimi, S. Efficacy and Safety of Nintedanib for Pulmonary Fibrosis in Severe Pneumonia Induced by COVID-19: An Interventional Study. Int. J. Infect. Dis. 2021, 108, 454–460. [Google Scholar] [CrossRef]
- Combet, M.; Pavot, A.; Savale, L.; Humbert, M.; Monnet, X. Rapid Onset Honeycombing Fibrosis in Spontaneously Breathing Patient with COVID-19. Eur. Respir. J. 2020, 56, 2001808. [Google Scholar] [CrossRef] [PubMed]
- Alhiyari, M.A.; Ata, F.; Alghizzawi, M.I.; I Bilal, A.B.; Abdulhadi, A.S.; Yousaf, Z. Post COVID-19 fibrosis, an emerging complicationof SARS-CoV-2 infection. IDCases 2021, 23, e01041. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Bruen, C.; Schnaus, M.; Zhang, J.; Ali, S.; Lind, A.; Stoecker, Z.; Stauderman, K.; Hebbar, S. Auxora versus Standard of Care for the Treatment of Severe or Critical COVID-19 Pneumonia: Results from a Randomized Controlled Trial. Crit. Care 2020, 24, 502. [Google Scholar] [CrossRef]
- Jeon, D.; Son, M.; Choi, J. Effect of Spironolactone on COVID-19 in Patients With Underlying Liver Cirrhosis: A Nationwide Case-Control Study in South Korea. Front. Med. 2021, 8, 629176. [Google Scholar] [CrossRef]
- Wadhwa, B.; Malhotra, V.; Kerai, S.; Husain, F.; Pandey, N.B.; Saxena, K.N.; Singh, V.; Quinn, T.M.; Li, F.; Gaughan, E.; et al. Phase 2 randomised placebo-controlled trial of spironolactone and dexamethasone versus dexamethasone in COVID-19 hospitalised patients in Delhi. BMC Infect. Dis. 2023, 23, 326. [Google Scholar] [CrossRef]
| Variables | Potassium Canrenoate (n = 24) | Placebo (n = 25) | p-Value Significance ≤ 0.05 | |
|---|---|---|---|---|
| Age [years], mean ± SD; Me | 61.54 ± 9.06; 64.00 | 63.84 ± 14.75; 66.00 | 0.513 | |
| Gender [male], n (%) | 10 (41.67) | 16 (64.00) | 0.200 | |
| BMI [kg/m2], mean ± SD; Me | 30.92 ± 4.10; 30.78 | 30.57 ± 4.63; 29.05 | 0.780 | |
| Smoking, n (%) | No | 14 (58.33) | 12 (48.00) | 0.204 |
| Yes | 0 (0.00) | 3 (12.00) | ||
| Quit >1 month | 10 (41.67) | 10 (40.00) | ||
| Alcohol use, n (%) | No | 9 (37.50) | 7 (29.17) | 0.734 |
| Yes | 1 (4.17) | 2 (8.33) | ||
| Occasionally | 14 (58.33) | 15 (62.50) | ||
| CFS [1,2,3,4,5,6,7], (mean± SD; Me) | 3.17 ± 0.70; 3.00 | 3.76 ± 1.01; 4.00 | 0.034 | |
| Comorbidities | ||||
| Arterial hypertension, n (%) | 15 (62.50) | 16 (64.00) | 0.851 | |
| Ischemic heart disease, n (%) | 0 (0.00) | 7 (28.00) | 0.017 | |
| Myocardial infarction, n (%) | 0 (0.00) | 4 (16.00) | 0.128 | |
| Chronic heart failure, n (%) | 0 (0.00) | 3 (12.00) | 0.248 | |
| Atrial fibrillation, n (%) | 0 (0.00) | 4 (16.00) | 0.128 | |
| Hypercholesterolemia, n (%) | 3 (12.50) | 8 (32.00) | 0.196 | |
| Transient ischemic attack, n (%) | 0 (0.00) | 1 (4.00) | 0.984 | |
| Diabetes, n (%) | 10 (41.67) | 4 (16.00) | 0.095 | |
| Peripheral vascular disease, n (%) | 1 (4.17) | 5 (20.00) | 0.209 | |
| Peptic ulcer disease, n (%) | 0 (0.00) | 1 (4.00) | 0.984 | |
| Thyroid disease, n (%) | 4 (16.67) | 4 (16.00) | 0.746 | |
| Active neoplasm, n (%) | 2 (8.33) | 2 (8.00) | 0.632 | |
| Depression, n (%) | 0 (0.00) | 1 (4.00) | 0.984 | |
| Variables | Potassium Canrenoate Mean ± SD; Me | Placebo Mean ± SD; Me | p-Value Significance ≤ 0.05 |
|---|---|---|---|
| White blood cells [G/L] | 8.19 ± 3.00; 8.41 | 7.52 ± 3.06; 7.59 | 0.440 |
| Neutrophils [G/L] | 6.64 ± 2.98; 6.69 | 6.02 ± 2.84; 5.74 | 0.461 |
| Lymphocytes [G/L] | 1.05 ± 0.30; 0.98 | 0.95 ± 0.32; 0.90 | 0.266 |
| Red blood cells [T/L] | 4.30 ± 0.41; 4.20 | 4.20 ± 0.63; 4.27 | 0.523 |
| Platelets [G/L] | 317.04 ± 132.56; 265.00 | 260.08 ± 93.54; 245.00 | 0.091 |
| Hemoglobin [mmol/L] | 7.97 ± 1.11; 7.90 | 7.90 ± 0.99; 7.90 | 0.836 |
| C-reactive protein [mg/dL] | 95.58 ± 65.37; 80.14 | 71.08 ± 44.78; 76.04 | 0.135 |
| Procalcitonin [ng/mL] | 0.23 ± 0.35; 0.09 | 0.15 ± 0.12; 0.12 | 0.327 |
| D-dimer [ng/mL] | 2329.58 ± 2695.07; 1016.00 | 1799.32 ± 1902.33; 1158.00 | 0.432 |
| Variables | Potassium Canrenoate Mean ± SD; Me | Placebo Mean ± SD; Me | p-Value Significance ≤ 0.05 |
|---|---|---|---|
| White blood cells [G/L] | 9.90 ± 4.19; 9.27 | 10.88 ± 5.77; 10.09 | 0.512 |
| Neutrophils [G/L] | 7.49 ± 4.41; 6.93 | 8.36 ± 5.81; 6.12 | 0.574 |
| Lymphocytes [G/L] | 1.60 ± 0.62; 1.78 | 1.57 ± 0.72; 1.44 | 0.875 |
| Red blood cells [T/L] | 4.26 ± 0.43; 4.19 | 4.31 ± 0.55; 4.33 | 0.714 |
| Platelets [G/L] | 385.87 ± 112.78; 380.00 | 372.70 ± 119.06; 365.00 | 0.702 |
| Hemoglobin [mmol/L] | 7.89 ± 0.97; 7.80 | 8.08 ± 0.77; 8.10 | 0.463 |
| C-reactive protein [mg/dL] | 28.35 ± 45.98; 10.50 | 25.71 ± 41.52; 7.60 | 0.841 |
| Procalcitonin [ng/mL] | 0.20 ± 0.49; 0.06 | 5.43 ± 23.14; 0.07 | 0.337 |
| D-dimer [ng/mL] | 1782.45 ± 1607.25; 1312.00 | 1719.48 ± 1826.09; 1105.00 | 0.903 |
| Variables | Potassium Canrenoate Mean ± SD; Me | Placebo Mean ± SD; Me | p-Value Significance ≤ 0.05 |
|---|---|---|---|
| CD4 [%] | 23.48 ± 14.64; 22.05 | 26.20 ± 1.,42; 24.40 | 0.482 |
| CD3 [%] | 12.85 ± 9.46; 11.55 | 15.66 ± 11.39; 12.65 | 0.482 |
| IL-1ß on lymphocytes [%] | 0.24 ± 0.25; 0.15 | 0.28 ± 0.43; 0.10 | 0.605 |
| IL-1ß total [%] | 0.68 ± 0.58; 0.45 | 0.61 ± 0.59; 0.40 | 0.843 |
| IL-2 on lymphocytes [%] | 1.27 ± 1.62; 0.75 | 1.35 ± 1.31; 1.05 | 0.475 |
| IL-2 total [%] | 4.39 ± 3.76; 3.60 | 5.24 ± 7.71; 3.75 | 0.956 |
| TNF-α on lymphocytes [%] | 0.15 ± 0.16; 0.10 | 0.15 ± 0.16; 0.10 | 0.835 |
| TNF-α total count [%] | 0.54 ± 0.45; 0.40 | 0.53 ± 0.47; 0.35 | 0.792 |
| IL-6 [pg/mL] | 64.97 ± 72.52; 41.00 | 46.68 ± 56.79; 24.90 | 0.332 |
| Variables | Potassium Canrenoate Mean ± SD; Me | Placebo Mean ± SD; Me | p-Value Significance ≤ 0.05 |
|---|---|---|---|
| CD4 [%] | 31.66 ± 14.92; 28.10 | 32.92 ± 11.16; 31.10 | 0.421 |
| CD3 [%] | 20.50 ± 14.40; 17.80 | 21.16 ± 15.37; 16.40 | 0.950 |
| IL-1ß on lymphocytes [%] | 0.43 ± 0.57; 0.20 | 0.40 ± 0.39; 0.30 | 0.850 |
| IL-1ß total [%] | 1.27 ± 0.83; 1.20 | 1.16 ± 0.91; 1.00 | 0.563 |
| IL-2 on lymphocytes [%] | 1.42 ± 1.28; 1.20 | 1.67 ± 0.98; 1.70 | 0.247 |
| IL-2 total [%] | 2.59 ± 1.67; 2.70 | 2.45 ± 1.94; 2.30 | 0.554 |
| TNF-α on lymphocytes [%] | 0.04 ± 0.12; 0.00 | 0.02 ± 0.05; 0.00 | 0.870 |
| TNF-α total count [%] | 0.25 ± 0.23; 0.10 | 0.26 ± 0.31; 0.20 | 0.841 |
| Interleukin-6 [pg/mL] | 24.20 ± 69.38; 5.30 | 60.56 ± 152.47; 11.00 | 0.317 |
| Day 7 versus Day 1 | Potassium Canrenoate p-Value Significance ≤ 0.05 | Placebo p-Value Significance ≤ 0.05 |
|---|---|---|
| CD4 [%] | 0.051 | 0.131 |
| CD3 [%] | 0.022 | 0.181 |
| Interleukin-1ß on lymphocytes [%] | 0.177 | 0.185 |
| Interleukin-1ß total [%] | 0.004 | 0.016 |
| Interleukin-2 on lymphocytes [%] | 0.649 | 0.265 |
| Interleukin-2 total count [%] | 0.070 | 0.040 |
| TNF-α on lymphocytes [%] | 0.152 | 0.003 |
| TNF-α total count [%] | 0.031 | 0.056 |
| Interleukin-6 [pg/mL] | 0.006 | 0.506 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karolak, I.; Hrynkiewicz, R.; Niedźwiedzka-Rystwej, P.; Lechowicz, K.; Sieńko, J.; Szylińska, A.; Dabrowski, W.; Kotfis, K. The Effect of Potassium Canrenoate (Mineralocorticoid Receptor Antagonist) on the Markers of Inflammation in the Treatment of COVID-19 Pneumonia and Fibrosis—A Secondary Analysis of Randomized Placebo-Controlled Clinical Trial. Int. J. Mol. Sci. 2023, 24, 14247. https://doi.org/10.3390/ijms241814247
Karolak I, Hrynkiewicz R, Niedźwiedzka-Rystwej P, Lechowicz K, Sieńko J, Szylińska A, Dabrowski W, Kotfis K. The Effect of Potassium Canrenoate (Mineralocorticoid Receptor Antagonist) on the Markers of Inflammation in the Treatment of COVID-19 Pneumonia and Fibrosis—A Secondary Analysis of Randomized Placebo-Controlled Clinical Trial. International Journal of Molecular Sciences. 2023; 24(18):14247. https://doi.org/10.3390/ijms241814247
Chicago/Turabian StyleKarolak, Igor, Rafał Hrynkiewicz, Paulina Niedźwiedzka-Rystwej, Kacper Lechowicz, Jerzy Sieńko, Aleksandra Szylińska, Wojciech Dabrowski, and Katarzyna Kotfis. 2023. "The Effect of Potassium Canrenoate (Mineralocorticoid Receptor Antagonist) on the Markers of Inflammation in the Treatment of COVID-19 Pneumonia and Fibrosis—A Secondary Analysis of Randomized Placebo-Controlled Clinical Trial" International Journal of Molecular Sciences 24, no. 18: 14247. https://doi.org/10.3390/ijms241814247
APA StyleKarolak, I., Hrynkiewicz, R., Niedźwiedzka-Rystwej, P., Lechowicz, K., Sieńko, J., Szylińska, A., Dabrowski, W., & Kotfis, K. (2023). The Effect of Potassium Canrenoate (Mineralocorticoid Receptor Antagonist) on the Markers of Inflammation in the Treatment of COVID-19 Pneumonia and Fibrosis—A Secondary Analysis of Randomized Placebo-Controlled Clinical Trial. International Journal of Molecular Sciences, 24(18), 14247. https://doi.org/10.3390/ijms241814247








