Microwave-Assisted Synthesis of Zeolite A from Metakaolinite for CO2 Adsorption
Abstract
:1. Introduction
2. Results and Discussion
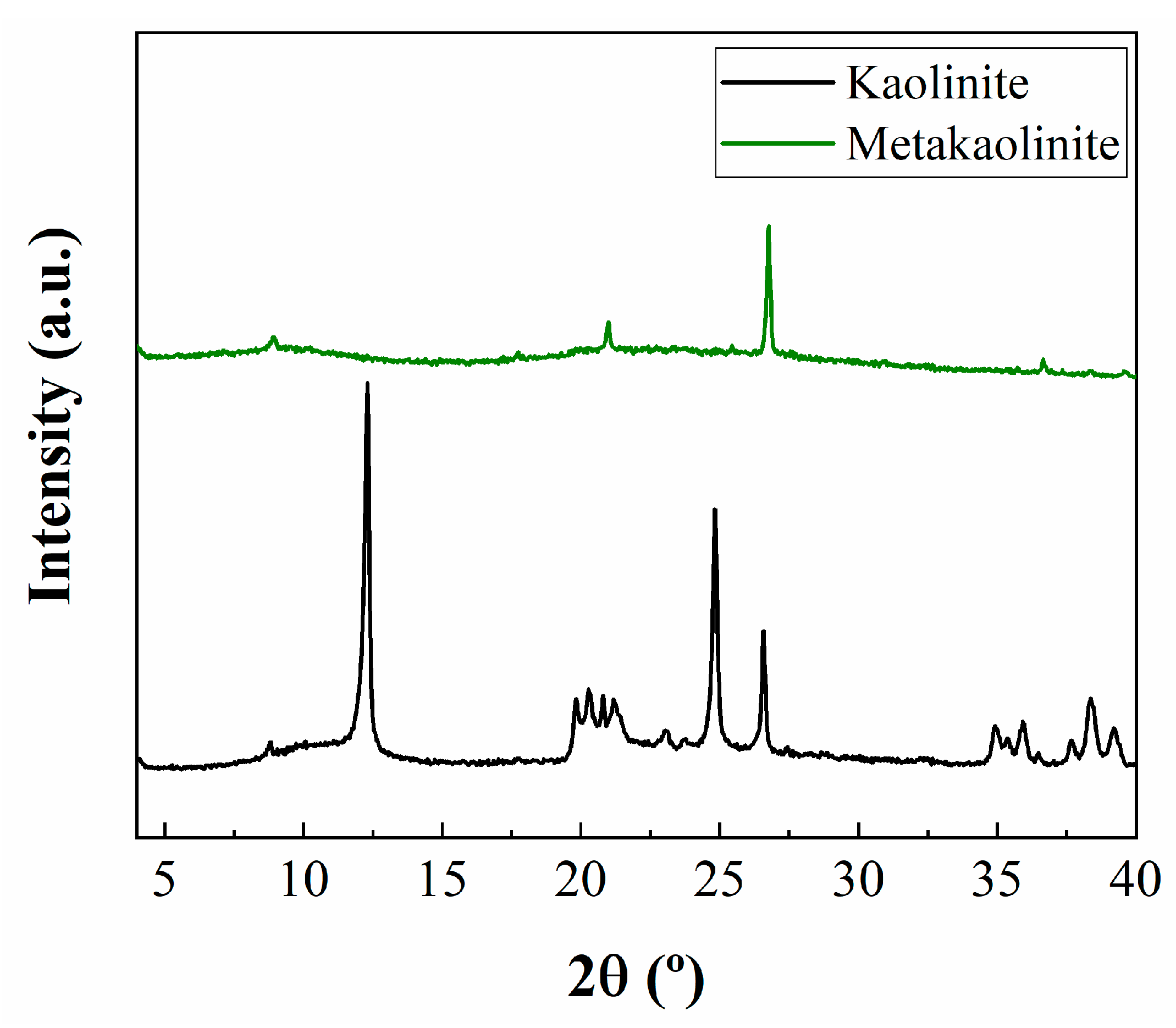
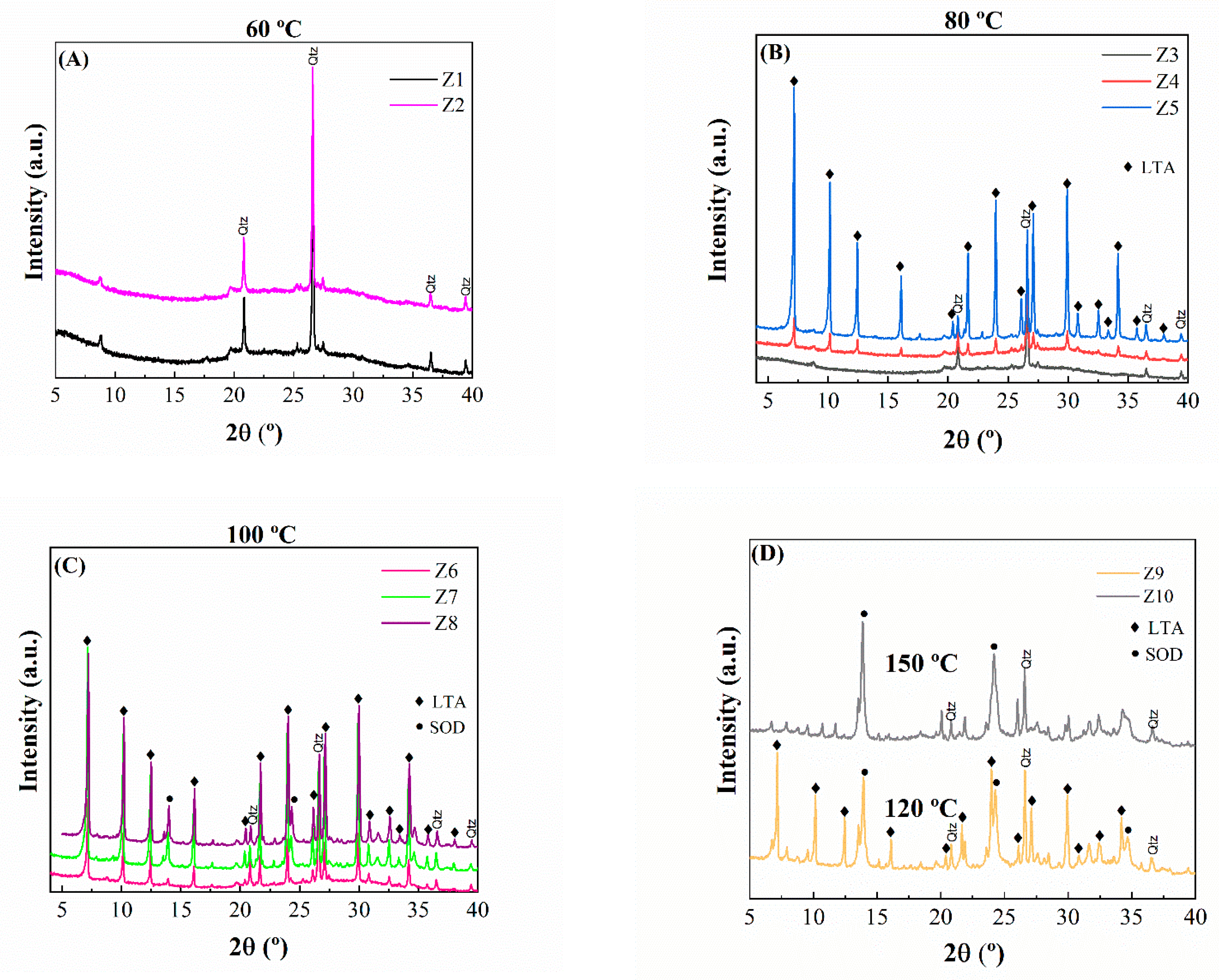
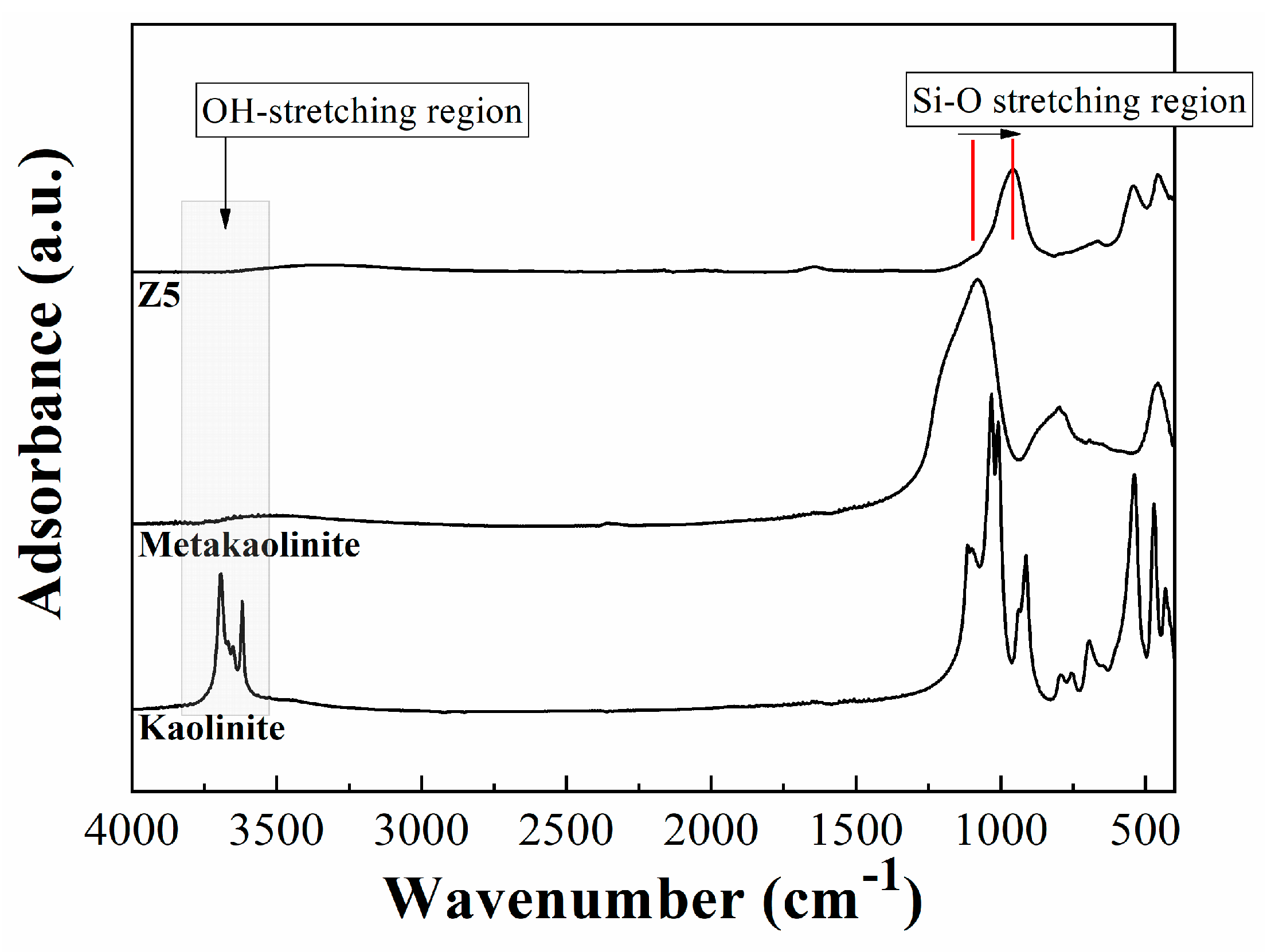
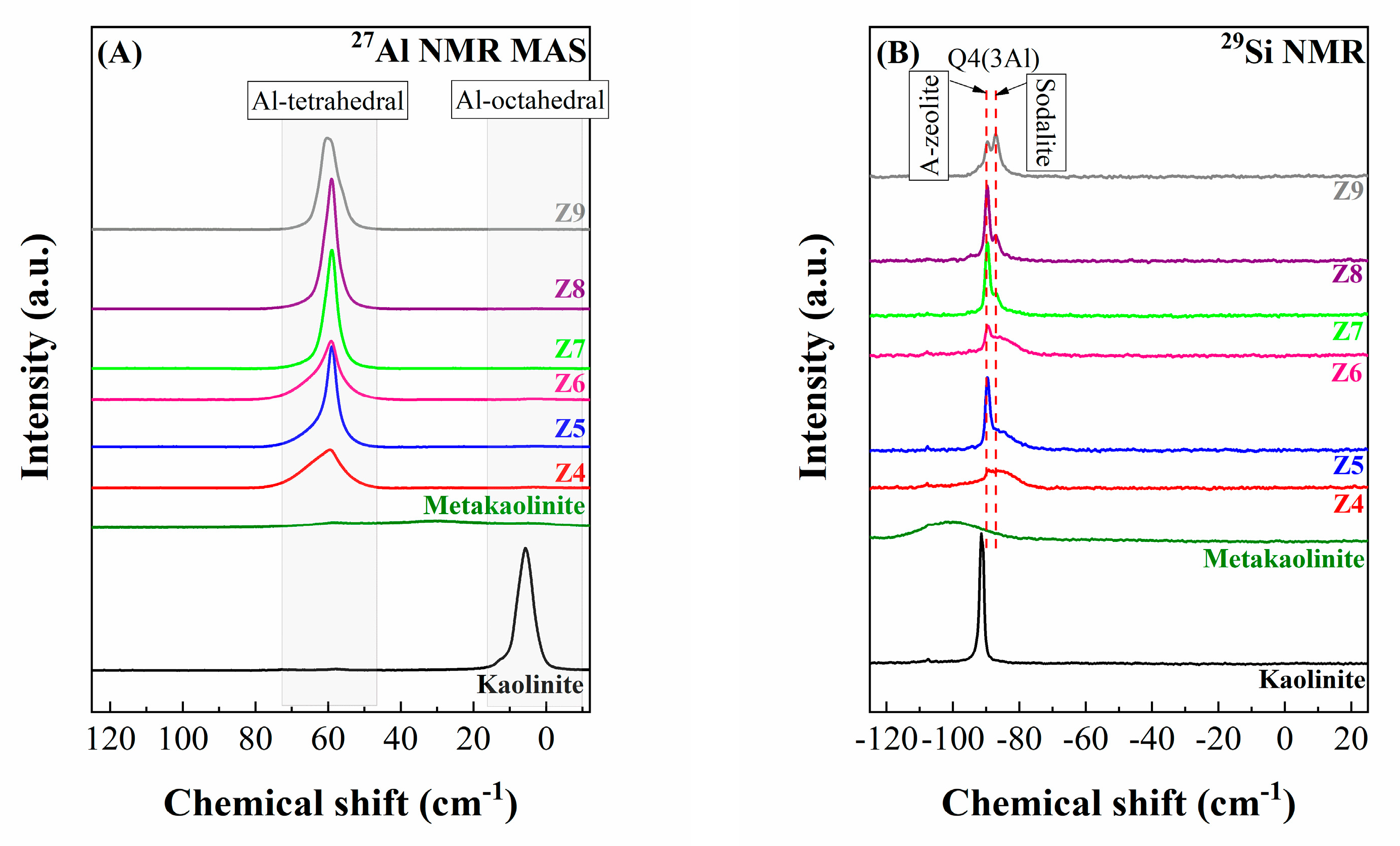
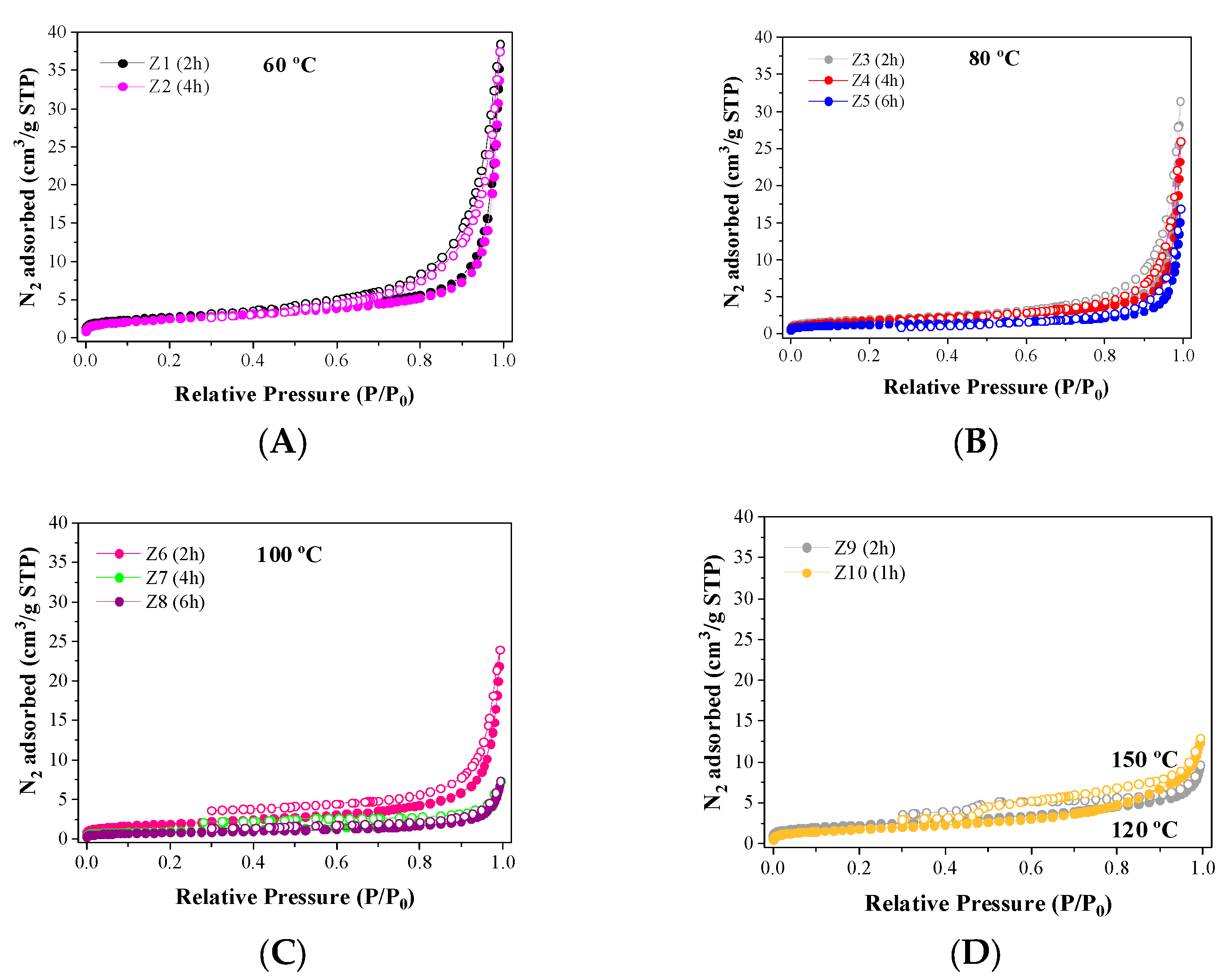
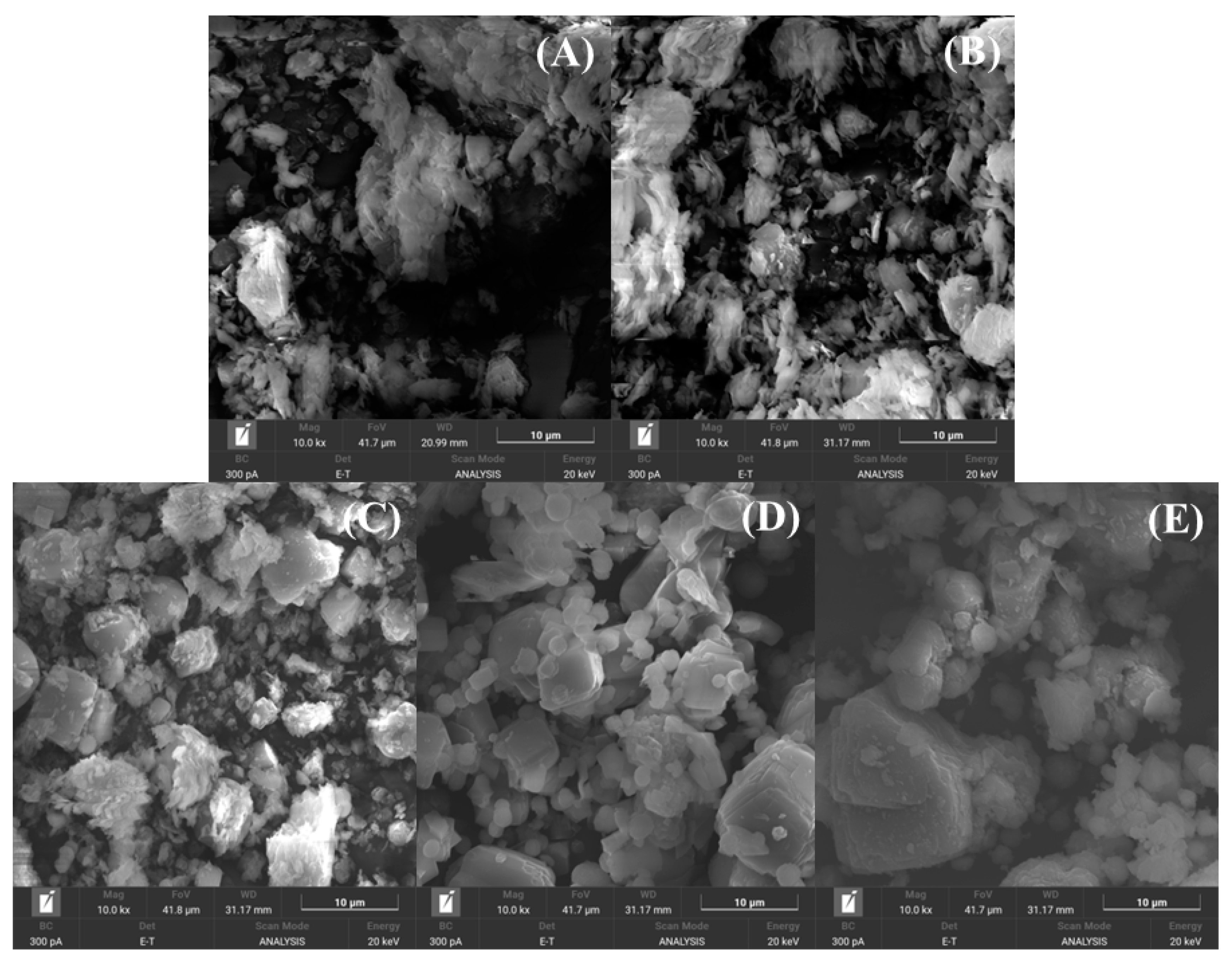
2.1. Characterization
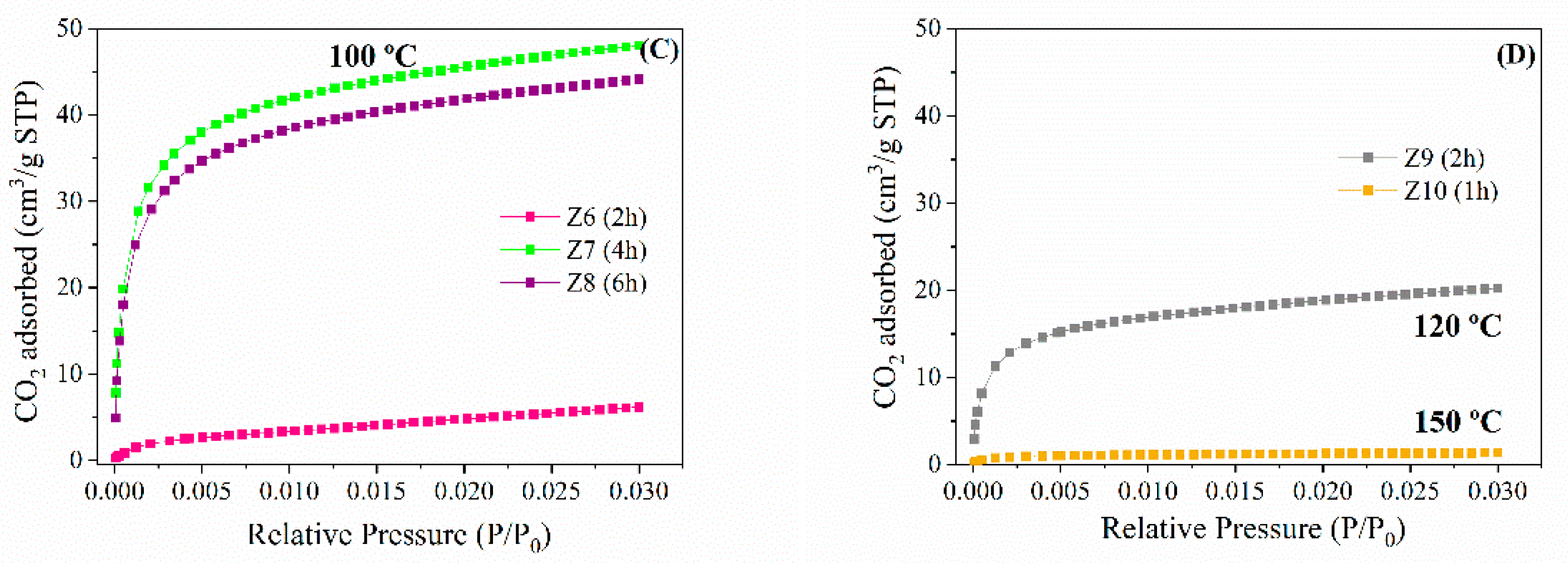
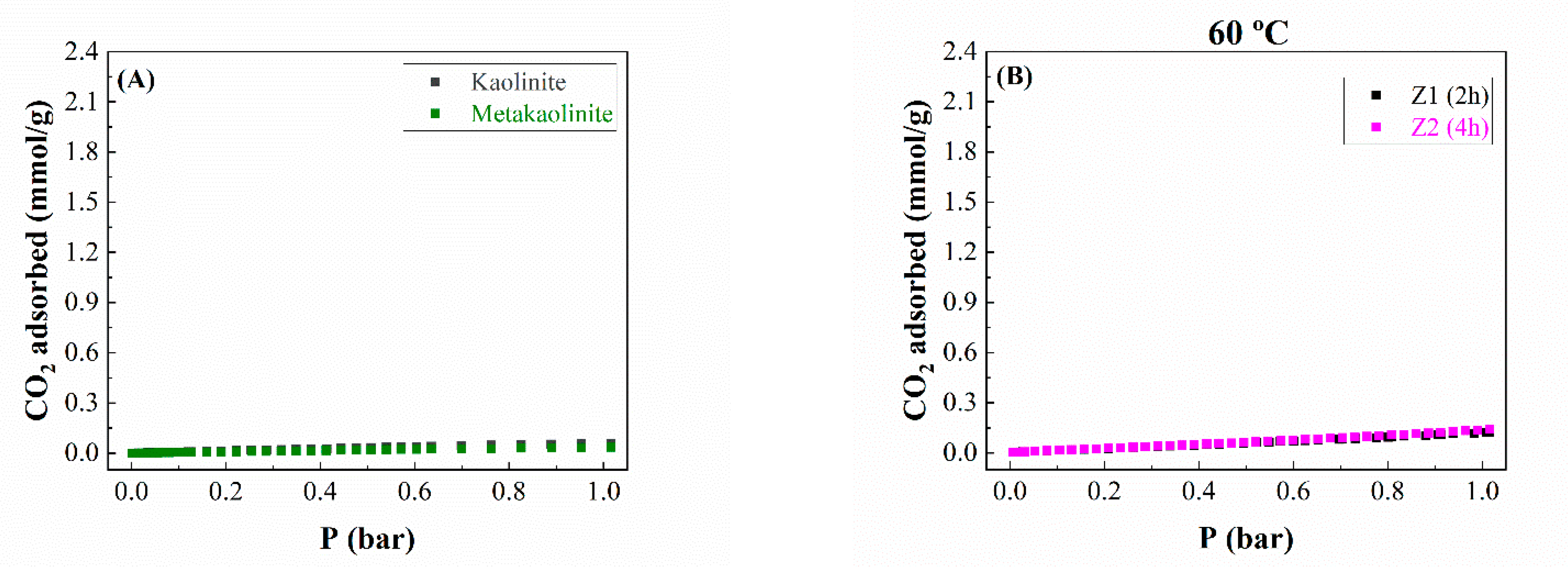
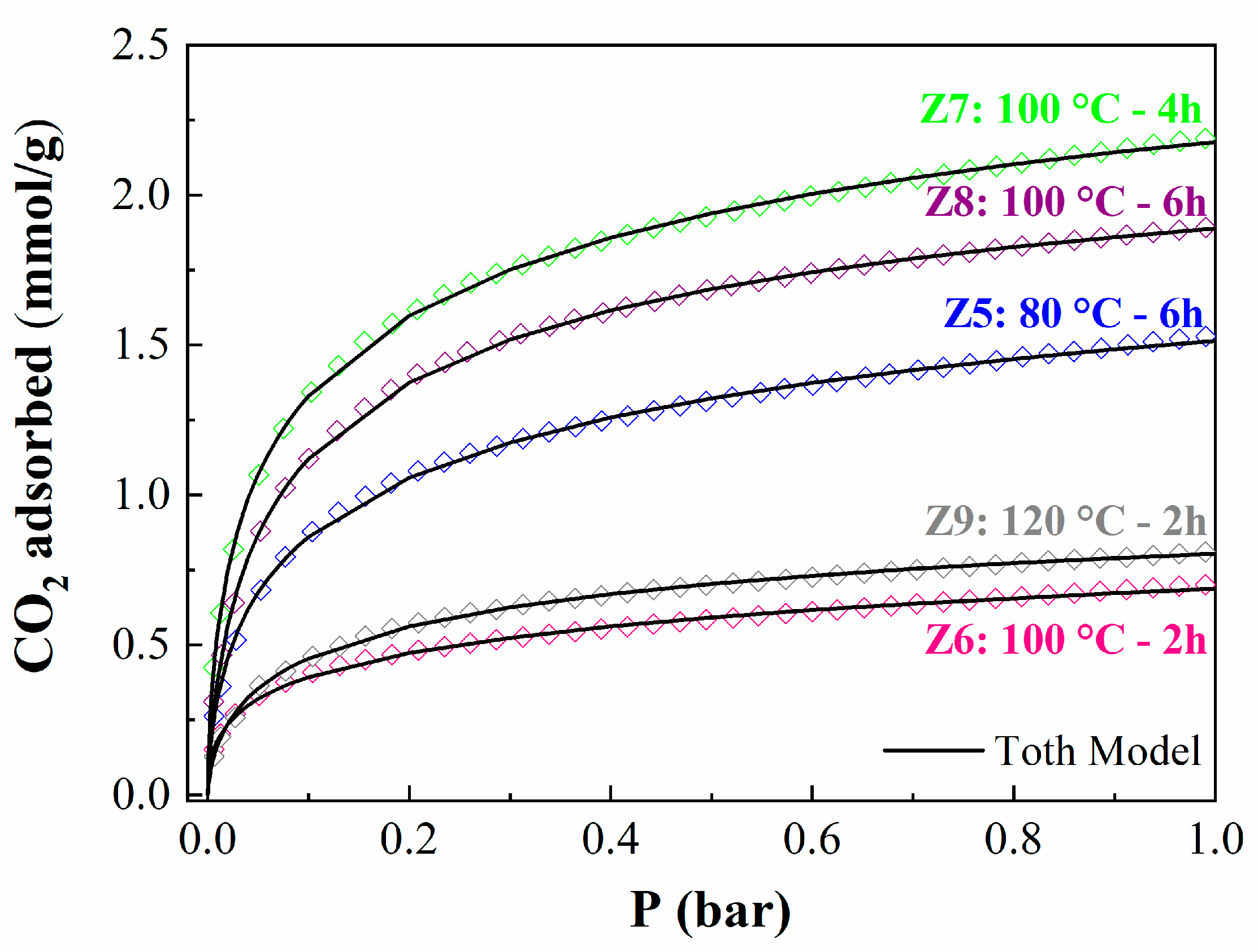
2.2. CO2 Adsorption Studies
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Zeolites
3.3. Characterization
3.4. CO2 Adsorption
3.5. Adsorption Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nie, S.; Zhou, J.; Yang, F.; Lan, M.; Li, J.; Zhang, Z.; Chen, Z.; Xu, M.; Li, H.; Sanjayan, J.G. Analysis of theoretical carbon dioxide emissions from cement production: Methodology and application. J. Clean. Prod. 2022, 334, 130270. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous inorganic membranes for CO2 capture: Present and prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef]
- Yoro, K.O.; Daramola, M.O. CO2 emission sources, greenhouse gases, and the global warming effect. In Advances in Carbon Capture; Woodhead Publishing: Sawston, UK, 2020; pp. 3–28. [Google Scholar] [CrossRef]
- Santos, G. Road transport and CO2 emissions: What are the challenges? Transp. Policy 2017, 59, 71–74. [Google Scholar] [CrossRef]
- Sovacool, B.K.; Griffiths, S. Culture and low-carbon energy transitions. Nat. Sustain. 2020, 3, 685–693. [Google Scholar] [CrossRef]
- Acar, S.; Lindmark, M. Periods of converging carbon dioxide emissions from oil combustion in a pre-Kyoto context. Environ. Dev. 2016, 19, 1–9. [Google Scholar] [CrossRef]
- Lakhi, K.S.; Cha, W.S.; Choy, J.H.; Al-Ejji, M.; Abdullah, A.M.; Al-Enizi, A.M.; Vinu, A. Synthesis of mesoporous carbons with controlled morphology and pore diameters from SBA-15 prepared through the microwave-assisted process and their CO2 adsorption capacity. Micropor. Mesopor. Mater. 2016, 233, 44–52. [Google Scholar] [CrossRef]
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 2020, 71, 451–456. [Google Scholar] [CrossRef]
- Pardakhti, M.; Jafari, T.; Tobin, Z.; Dutta, B.; Moharreri, E.; Shemshaki, N.S.; Suib, S.; Srivastava, R. Trends in solid adsorbent materials development for CO2 capture. ACS Appl. Mater. Interfaces 2019, 11, 34533–34559. [Google Scholar] [CrossRef]
- Fu, D.; Davis, M.E. Carbon dioxide capture with zeotype materials. Chem. Soc. Rev. 2022, 51, 9340–9370. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Johnson, E.B.G.; Arshad, S.E. Hydrothermally synthesized zeolites based on kaolinite: A review. Appl. Clay Sci. 2014, 97, 215–221. [Google Scholar] [CrossRef]
- Youssef, H.; Ibrahim, D.; Komarneni, S. Microwave-assisted versus conventional synthesis of zeolite A from metakaolinite. Micropor. Mesopor. Mater. 2008, 115, 527–534. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Deon, M.; Benvenutti, E.V.; Barros, V.A.; de Melo, D.C.; Franceschi, E.; Egues, S.M.; de Conto, J.F. Effect of microwave irradiation on the structural, chemical, and hydrophilicity characteristics of ordered mesoporous silica SBA-15. J. Sol-Gel Sci. Technol. 2020, 94, 708–718. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Cecilia, J.A.; de Conto, J.F.; Egues, S.M.; Rodríguez-Castellón, E. Rapid synthesis of MCM-41 and SBA-15 by microwave irradiation: Promising adsorbents for CO2 adsorption. J. Sol-Gel Sci. Technol. 2023, 105, 370–387. [Google Scholar] [CrossRef]
- Mortada, W.I.; Nabieh, K.A.; Helmy, T.E.; el-Reash, Y.G.A. Microwave-assisted synthesis of MCM-41 composite with rice husk and its functionalization by dithizone for preconcentration of some metal ions from water and food samples. J. Food Compos. Anal. 2022, 106, 104352. [Google Scholar] [CrossRef]
- Zeng, X.; Hu, X.; Song, H.; Xia, G.; Shen, Z.Y.; Yu, R.; Moskovits, M. Microwave synthesis of zeolites and their related applications. Micropor. Mesopor. Mater. 2021, 323, 111262. [Google Scholar] [CrossRef]
- You, H.S.; Jin, H.; Mo, Y.H.; Park, S.E. CO2 adsorption behavior of microwave synthesized zeolite beta. Mater. Lett. 2013, 108, 106–109. [Google Scholar] [CrossRef]
- Makgabutlane, B.; Nthunya, L.N.; Nxumalo, E.N.; Musyoka, N.M.; Mhlanga, S.D. Microwave irradiation-assisted synthesis of zeolites from coal fly ash: An optimization study for a sustainable and efficient production process. ACS Omega 2020, 5, 25000–25008. [Google Scholar] [CrossRef]
- Xia, S.; Chen, Y.; Xu, H.; Lv, D.; Yu, J.; Wang, P. Synthesis EMT-type zeolite by microwave and hydrothermal heating. Micropor. Mesopor. Mater. 2019, 278, 54–63. [Google Scholar] [CrossRef]
- Vichaphund, S.; Sricharoenchaikul, V.; Atong, D. Selective aromatic formation from catalytic fast pyrolysis of Jatropha residues using ZSM-5 prepared by microwave-assisted synthesis. J. Anal. Appl. Pyrolysis 2019, 141, 104628. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Dong, M.; Fan, S.; Zhao, T.; Wanga, J.; Fan, W. Strategies to control zeolite particle morphology. Chem. Soc. Rev. 2019, 48, 885–907. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, J. Emerging applications of zeolites in catalysis, separation and host-guest assembly. Nat. Rev. Mater. 2021, 6, 1156–1174. [Google Scholar] [CrossRef]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Vilarrasa-García, E.; Morales-Ospino, R.; Finocchio, E.; Busca, G.; Sapag, K.; Villarroel-Rocha, J.; Bastos-Neto, M.; Azevedo, D.C.S.; Rodríguez-Castellón, E. Kaolinite-based zeolites synthesis and their application in CO2 capture processes. Fuel 2022, 320, 123953. [Google Scholar] [CrossRef]
- Franco, F.; Cecilia, J.A.; Pérez-Maqueda, L.A.; Pérez-Rodríguez, J.L.; Gomes, C.S.F. Particle-size reduction of dickite by ultrasound treatments: Effect on the structure, shape and particle-size distribution. Appl. Clay Sci. 2007, 35, 119–127. [Google Scholar] [CrossRef]
- Cui, Y.; Zheng, Y.; Wang, W. Synthesis of 4A zeolite from kaolinite-type pyrite flotation tailings (KPFT). Minerals 2018, 8, 338. [Google Scholar] [CrossRef]
- Inagaki, S.; Thomas, K.; Ruaux, V.; Clet, G.; Wakihara, T.; Shinoda, S.; Okamura, S.; Kubota, Y.; Valtchev, V. Crystal growth kinetics as a tool for controlling the catalytic performance of a FAU-type basic catalyst. ACS Catal. 2014, 4, 2333–2341. [Google Scholar] [CrossRef]
- Yao, G.; Lei, J.; Zhang, X.; Sun, Z.; Zheng, S.; Komarneni, S. Mechanism of zeolite X crystallization from diatomite. Mater. Res. Bull. 2018, 107, 132–138. [Google Scholar] [CrossRef]
- Hu, J.Z.; Wan, C.; Vjunov, A.; Wang, M.; Zhao, Z.; Hu, M.Y.; Camaioni, D.M.; Lercher, J.A. 27Al MAS NMR studies of HBEA zeolite at low to high magnetic fields. J. Phys. Chem. C 2017, 121, 12849–12854. [Google Scholar] [CrossRef]
- Hayashi, S.; Ueda, T.; Hayamizu, K.; Akiba, E. NMR study of kaolinite. 1. Silicon-29, aluminum-27, and proton spectra. J. Phys. Chem. 1992, 96, 10922–10928. [Google Scholar] [CrossRef]
- El Idrissi, A.C.; Paris, M.; Rozière, E.; Deneele, D.; Darson, S.; Loukili, A. Alkali- activated grouts with incorporated fly ash: From NMR analysis to mechanical properties. Mater. Today Commun. 2018, 14, 225–232. [Google Scholar] [CrossRef]
- Graham, C.; Imrie, D.A.; Raab, E. Measurement of the electric quadrupole moments of CO2, CO, N2, Cl2 and BF3. Mol. Phys. 1998, 93, 49–56. [Google Scholar] [CrossRef]
- García-Martínez, J.; Cazorla-Amorós, D.; Solano-Linares, A. Further evidences of the usefulness of CO2 microporous solids. Stud. Surf. Sci. Catal. 2000, 128, 485–494. [Google Scholar] [CrossRef]
- Chouikhi, N.; Cecilia, J.A.; Vilarrasa-García, E.; Besghaier, S.; Chlendi, M.; Franco-Duro, F.I.; Rodríguez-Castellón, E.; Bagane, M. CO2 adsorption of materials synthesized from clay minerals: A review. Minerals 2019, 9, 514. [Google Scholar] [CrossRef]
- Tun, H.; Chen, C.C. Isosteric heat of adsorption from thermodynamic Langmuir isotherm. Adsorption 2021, 27, 979–989. [Google Scholar] [CrossRef]
- Candamano, S.; Policicchio, A.; Conte, G.; Abarca, R.; Algieri, C.; Chakraborty, S.; Curcio, S.; Calabrò, V.; Crea, F.; Agostino, R.G. Preparation of foamed and unfoamed geopolymer/NaX zeolite/activated carbon composites for CO2 adsorption. J. Clean. Prod. 2022, 330, 129843. [Google Scholar] [CrossRef]
- Gasparini, E.; Tarantino, S.C.; Ghigna, P.; Riccardi, M.P.; Cedillo-González, E.I.; Siligardi, C.; Zema, M. Thermal dehydroxylation of kaolinite under isothermal conditions. Appl. Clay Sci. 2013, 80, 417–425. [Google Scholar] [CrossRef]
- Redfern, S.A.T. The kinetics of dehydroxylation of kaolinite. Clay Miner. 1987, 22, 447–456. [Google Scholar] [CrossRef]
- Sperinck, S.; Raiteri, P.; Marks, N.; Wright, K. Dehydroxylation of kaolinite to metakaolin—A molecular dynamics study. J. Mater. Chem. 2011, 21, 2118–2125. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Dubinin, M.M. Fundamentals of the theory of adsorption in micropores of carbon adsorbents: Characteristics of their adsorption properties and microporous structures. Carbon 1989, 27, 457–467. [Google Scholar] [CrossRef]
- Toth, J. State equation of the solid gas interface layer. Acta Chim. Acad. Sci. Hung. 1971, 69, 311–317. [Google Scholar]


| Samples | Time (h) | Temperature (°C) |
|---|---|---|
| Zeolite A 60 °C–2 h: Z1 | 2 | 60 |
| Zeolite A 60 °C–4 h: Z2 | 4 | |
| Zeolite A 80 °C–2 h: Z3 | 2 | 80 |
| Zeolite A 80 °C–4 h: Z4 | 4 | |
| Zeolite A 80 °C–6 h: Z5 | 6 | |
| Zeolite A 100 °C–2 h: Z6 | 2 | 100 |
| Zeolite A 100 °C–4 h: Z7 | 4 | |
| Zeolite A 100 °C–6 h: Z8 | 6 | |
| Zeolite A 120 °C–2 h: Z9 | 2 | 120 |
| Zeolite A 150 °C–1 h: Z10 | 1 | 150 |
| Samples | SBET (m2/g) | Smp (m2/g) | VP (cm3/g) | VMP (cm3/g) |
|---|---|---|---|---|
| Kaolinite | 10.8 | 1.40 | 0.022 | 0.0011 |
| Metakaolinite | 12.2 | 1.70 | 0.024 | 0.0012 |
| Z1 | 9.7 | 2.13 | 0.019 | 0.0010 |
| Z2 | 8.9 | 1.35 | 0.017 | 0.0005 |
| Z3 | 6.9 | 1.58 | 0.013 | 0.0007 |
| Z4 | 6.4 | 0.88 | 0.011 | 0.0003 |
| Z5 | 4.5 | 1.78 | 0.007 | 0.0007 |
| Z6 | 6.8 | 0.7 | 0.013 | 0.0003 |
| Z7 | 3.4 | 0.78 | 0.005 | 0.0003 |
| Z8 | 2.8 | 0.89 | 0.005 | 0.0004 |
| Z9 | 8.1 | 2.14 | 0.009 | 0.0010 |
| Z10 | 6.4 | -- | 0.012 | -- |
| Samples | VMP (cm3/g) | Equivalent Surface Area (m2/g) |
|---|---|---|
| Kaolinite | 0.0008 | 2 |
| Metakaolinite | 0.0003 | 1 |
| Z1 | 0.0029 | 7 |
| Z2 | 0.0029 | 7 |
| Z3 | 0.0027 | 7 |
| Z4 | 0.0104 | 26 |
| Z5 | 0.0867 | 216 |
| Z6 | 0.0140 | 35 |
| Z7 | 0.1177 | 294 |
| Z8 | 0.0967 | 241 |
| Z9 | 0.0490 | 122 |
| Z10 | 0.0028 | 7 |
| Samples | qm (mmol/g) | B (bar−1) | n | (ΔH) (kJ/mol) |
|---|---|---|---|---|
| Z5 | 2.65 | 5.62 × 10−5 | 0.35 | −35.2 |
| Z6 | 2.42 | 5.48 × 10−5 | 0.38 | −39.8 |
| Z7 | 3.39 | 7.49 × 10−5 | 0.36 | −36.1 |
| Z8 | 2.60 | 5.59 × 10−5 | 0.48 | −33.6 |
| Z9 | 1.35 | 1.61 × 10−4 | 0.37 | −32.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, M.R.; Cecilia, J.A.; Ballesteros-Plata, D.; Barroso-Martín, I.; Núñez, P.; Infantes-Molina, A.; Rodríguez-Castellón, E. Microwave-Assisted Synthesis of Zeolite A from Metakaolinite for CO2 Adsorption. Int. J. Mol. Sci. 2023, 24, 14040. https://doi.org/10.3390/ijms241814040
Oliveira MR, Cecilia JA, Ballesteros-Plata D, Barroso-Martín I, Núñez P, Infantes-Molina A, Rodríguez-Castellón E. Microwave-Assisted Synthesis of Zeolite A from Metakaolinite for CO2 Adsorption. International Journal of Molecular Sciences. 2023; 24(18):14040. https://doi.org/10.3390/ijms241814040
Chicago/Turabian StyleOliveira, Marilia R., Juan A. Cecilia, Daniel Ballesteros-Plata, Isabel Barroso-Martín, Pedro Núñez, Antonia Infantes-Molina, and Enrique Rodríguez-Castellón. 2023. "Microwave-Assisted Synthesis of Zeolite A from Metakaolinite for CO2 Adsorption" International Journal of Molecular Sciences 24, no. 18: 14040. https://doi.org/10.3390/ijms241814040
APA StyleOliveira, M. R., Cecilia, J. A., Ballesteros-Plata, D., Barroso-Martín, I., Núñez, P., Infantes-Molina, A., & Rodríguez-Castellón, E. (2023). Microwave-Assisted Synthesis of Zeolite A from Metakaolinite for CO2 Adsorption. International Journal of Molecular Sciences, 24(18), 14040. https://doi.org/10.3390/ijms241814040









