Soybean (Glycine max) INFOGEST Colonic Digests Attenuated Inflammatory Responses Based on Protein Profiles of Different Varieties
Abstract
1. Introduction
2. Results
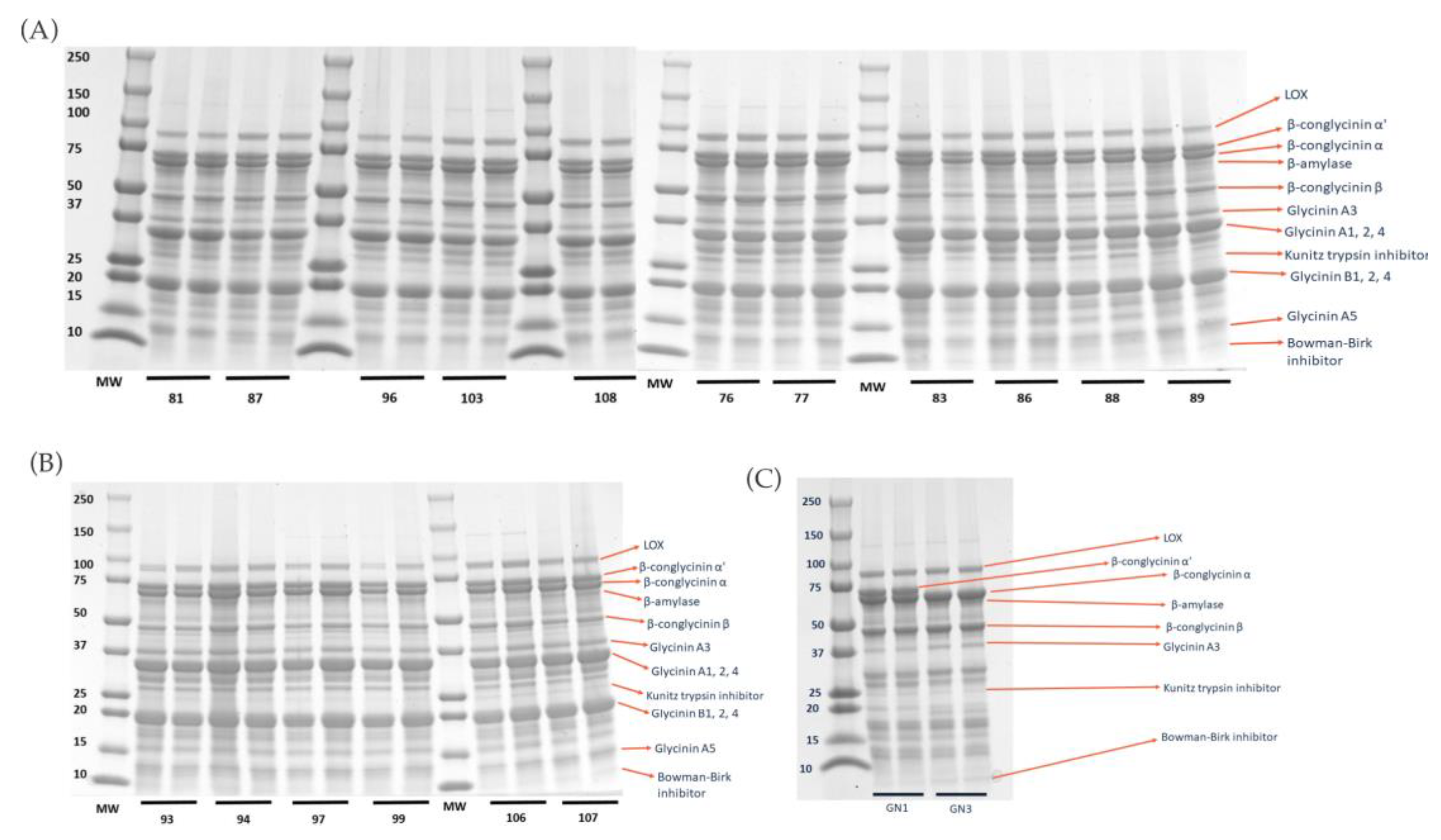
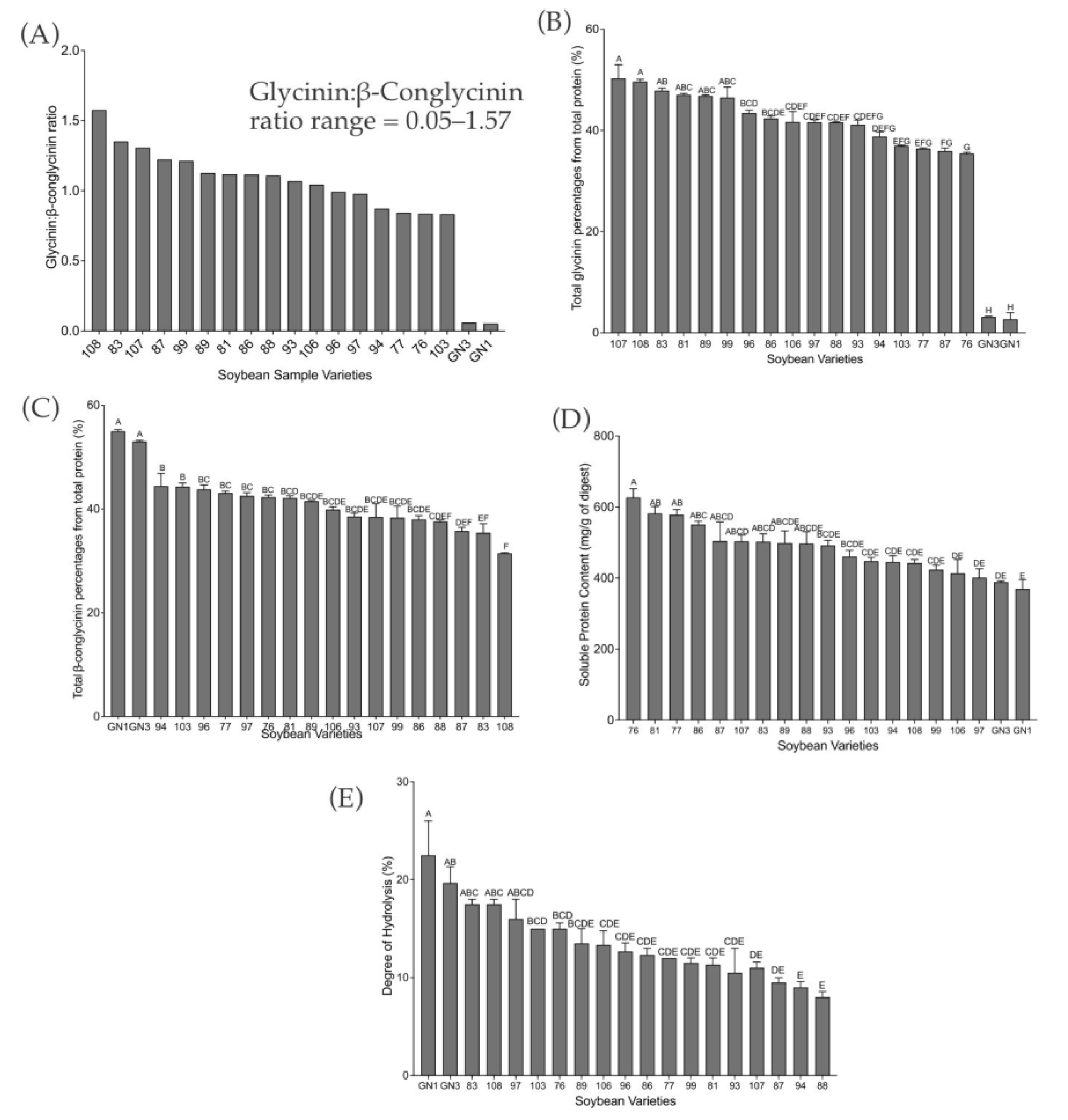
2.1. Degree of Hydrolysis and Protein Profile from Sodium Dodecyl-Sulfate Polyacrylamide Gel Electrophoresis (SDS–PAGE) Analysis
2.2. Peptide Sequencing
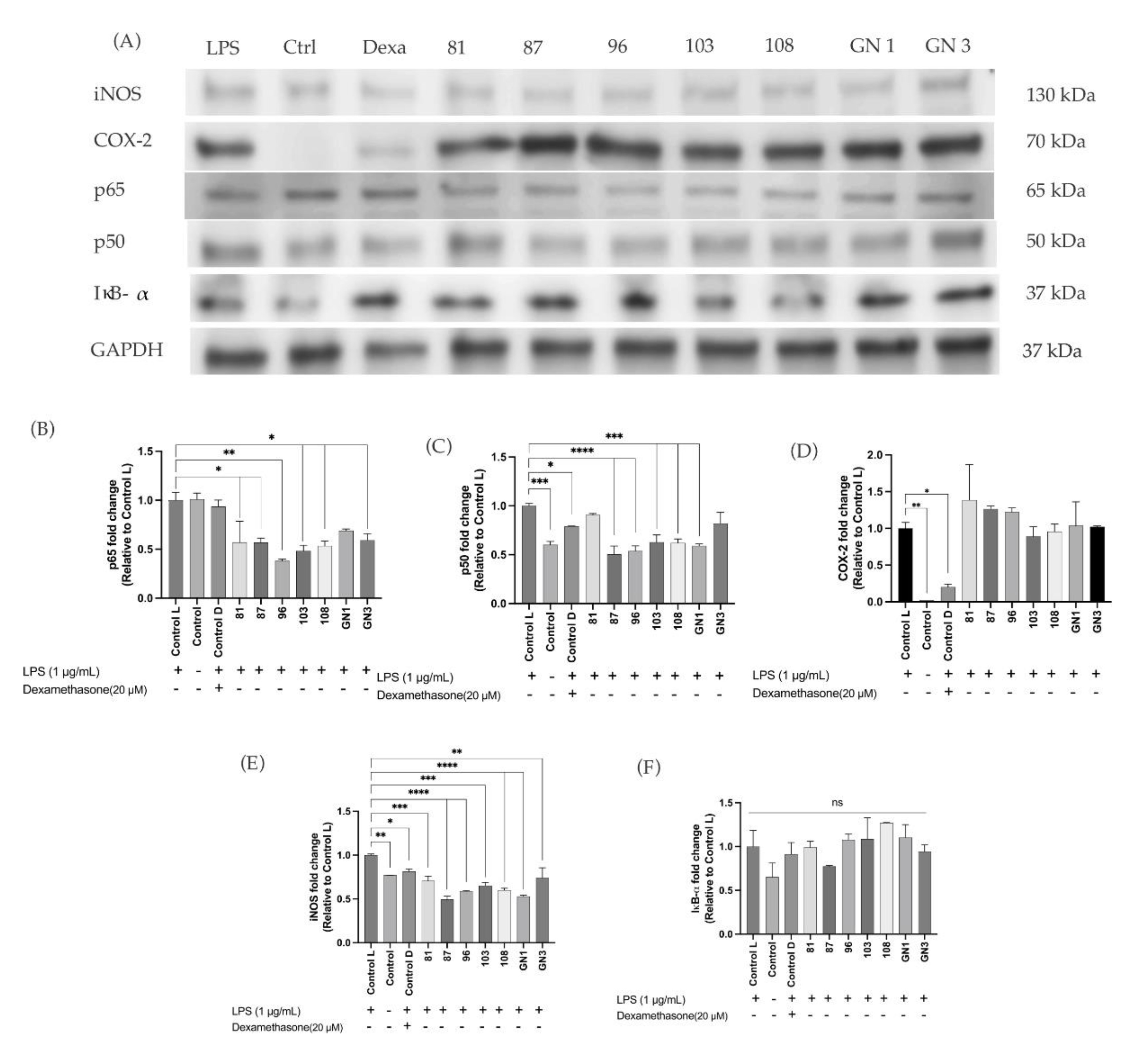
2.3. Biochemical Screening of Pro-Inflammatory Markers
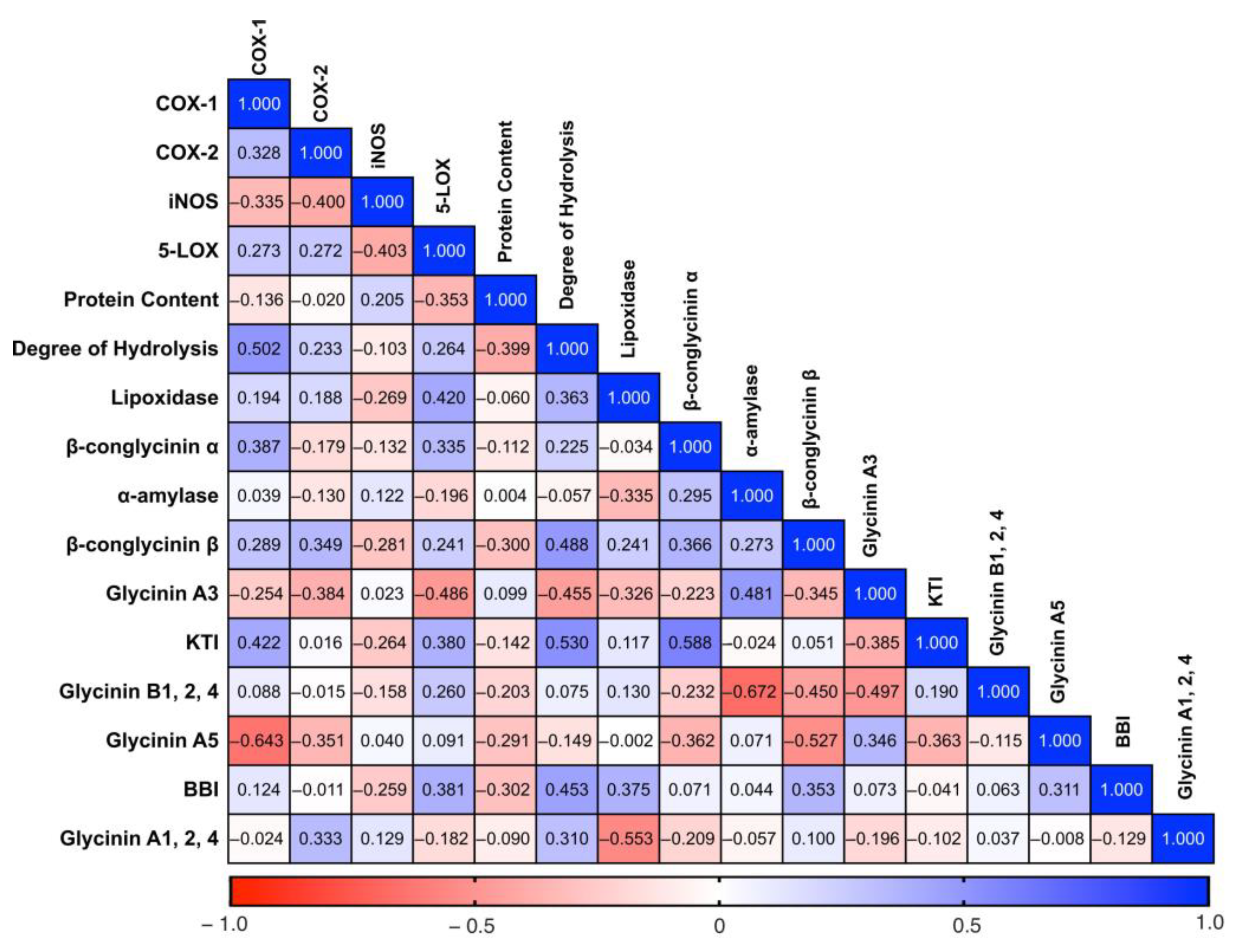
2.4. Correlation Analysis
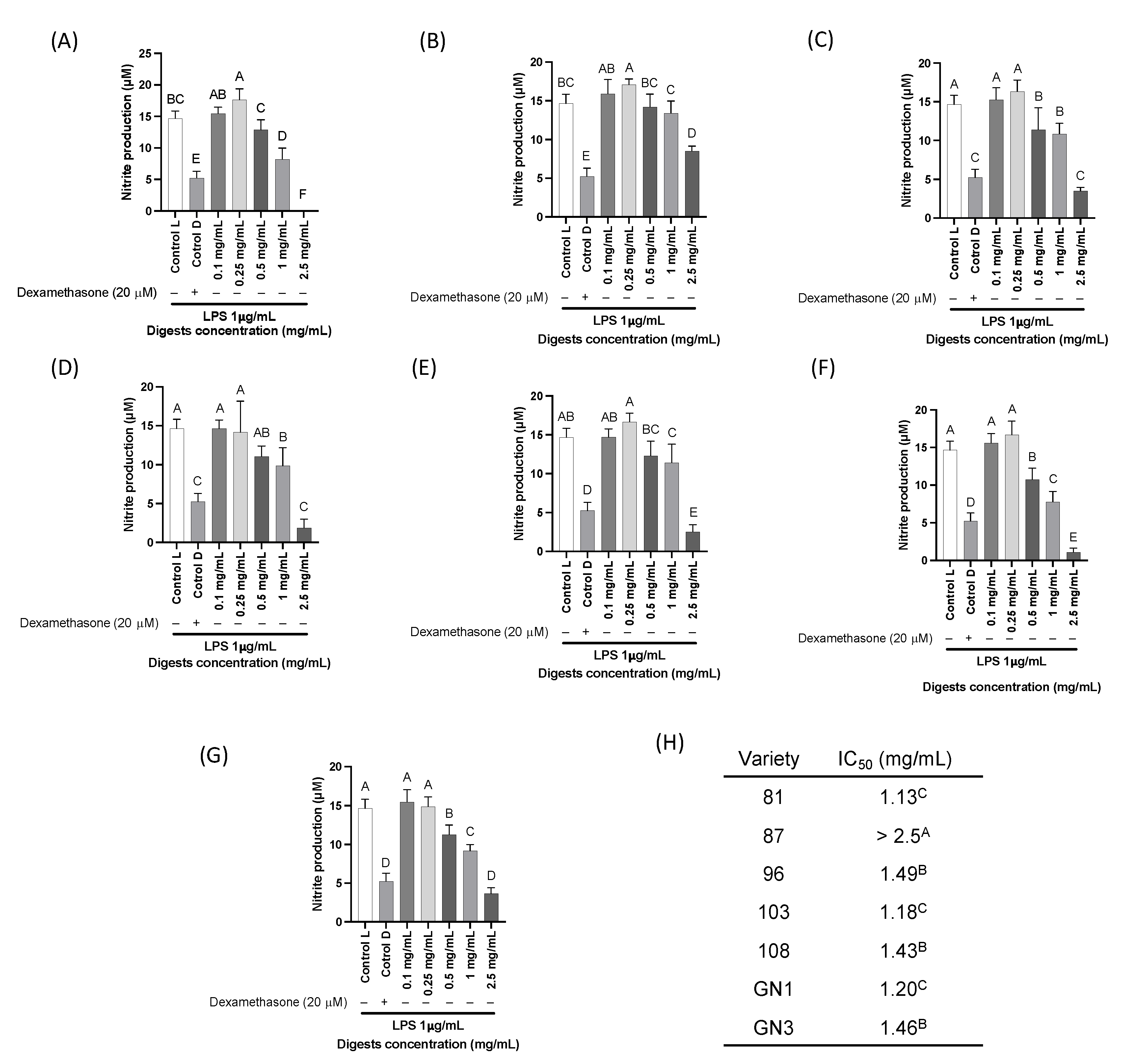
2.5. Cell Viability and In Vitro Pro-Inflammatory Biomarkers
2.6. Enzyme-Linked Immunoassays in In Vitro Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Preparation of Defatted Soybean Flour
4.2.2. Protein Extraction and Quantification
4.2.3. Simulated Gastrointestinal Digestion
4.2.4. Peptide Identification
4.2.5. SDS–PAGE Electrophoresis
4.2.6. Degree of Hydrolysis
4.2.7. Cyclooxygenase (COX)-1 and -2 Inhibitor Screening Fluorometric Biochemical Assay
4.2.8. 5-Lipoxygenase (LOX) Inhibitory Screening Biochemical Assay
4.2.9. Inducible Nitric Oxide Synthase (iNOS) Screening Biochemical Assay
4.2.10. Cell Culture and Cell Viability Assay
4.2.11. Measurement of Nitrite Production in Cell Supernatant
4.2.12. Measurement of IL-1β, IL-6, and TNF-α Levels in Cell Supernatant
4.2.13. Western Blot from Cell Lysates
4.2.14. Inflammation Antibody Array in Cell Supernatant
4.2.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Grassini, P.; La Menza, N.G.; Edreira, J.I.R.; Monzón, J.P.; Tenorio, F.A.; Specht, J.E. Soybean. In Crop Physiology Case Histories for Major Crops; Academic Press: Cambridge, MA, USA, 2021; pp. 282–319. [Google Scholar] [CrossRef]
- He, F.-J.; Chen, J.-Q. Consumption of Soybean, Soy Foods, Soy Isoflavones and Breast Cancer Incidence: Differences between Chinese Women and Women in Western Countries and Possible Mechanisms. Food Sci. Hum. Wellness 2013, 2, 146–161. [Google Scholar] [CrossRef]
- Gilman, J.D.; Bilyeu, K.D. Genes and Alleles for Quality Traits on the Soybean Genetic/Physical Map. In Designing Soybeans for 21st Century Markets; AOCD Press: Kirksville, MO, USA, 2012; pp. 67–96. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Bringe, N.A.; de Mejia, E.G. Selected Soybean Varieties Regulate Hepatic LDL-Cholesterol Homeostasis Depending on Their Glycinin:β-Conglycinin Ratio. Antioxidants 2022, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- van der Wielen, N.; Moughan, P.J.; Mensink, M. Amino Acid Absorption in the Large Intestine of Humans and Porcine Models. J. Nutr. 2017, 147, 1493–1498. [Google Scholar] [CrossRef] [PubMed]
- Juritsch, A.F.; Moreau, R. Role of Soybean-Derived Bioactive Compounds in Inflammatory Bowel Disease. Nutr. Rev. 2018, 76, 618–638. [Google Scholar] [CrossRef] [PubMed]
- Basson, A.R.; Ahmed, S.; Almutairi, R.; Seo, B.; Cominelli, F. Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds. Foods 2021, 10, 774. [Google Scholar] [CrossRef]
- Das, D.; Sarkar, S.; Borsingh Wann, S.; Kalita, J.; Manna, P. Current Perspectives on the Anti-Inflammatory Potential of Fermented Soy Foods. Food Res. Int. 2022, 152, 110922. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, G.; Li, Z.; Xiao, X.; Tang, J.; Bai, Z. NLRP3 Inflammasome Pharmacological Inhibitors in Glycyrrhiza for NLRP3-Driven Diseases Treatment: Extinguishing the Fire of Inflammation. J. Inflamm. Res. 2022, 15, 409. [Google Scholar] [CrossRef]
- Pahwa, R.; Goyal, A.; Jialal, I. Chronic Inflammation. In Pathobiology of Human Disease: A Dynamic Encyclopedia of Disease Mechanisms; Academic Press: Cambridge, MA, USA, 2021; pp. 300–314. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic Diseases, Inflammation, and Spices: How Are They Linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef]
- Gunaydin, C.; Bilge, S.S. Effects of Nonsteroidal Anti-Inflammatory Drugs at the Molecular Level. Eurasian J. Med. 2018, 50, 116. [Google Scholar] [CrossRef]
- Nørregaard, R.; Kwon, T.H.; Frøkiær, J. Physiology and Pathophysiology of Cyclooxygenase-2 and Prostaglandin E2 in the Kidney. Kidney Res. Clin. Pract. 2015, 34, 194. [Google Scholar] [CrossRef]
- Wisastra, R.; Dekker, F.J. Inflammation, Cancer and Oxidative Lipoxygenase Activity Are Intimately Linked. Cancers 2014, 6, 1500. [Google Scholar] [CrossRef] [PubMed]
- Kusumah, J.; Gonzalez de Mejia, E. Impact of Soybean Bioactive Compounds as Response to Diet-Induced Chronic Inflammation: A Systematic Review. Food Res. Int. 2022, 162, 111928. [Google Scholar] [CrossRef] [PubMed]
- Kusmardi, K.; Nessa, N.; Estuningtyas, A.; Tedjo, A. The Effect of Lunasin from Indonesian Soybean Extract on Histopatologic Examination and Cox-2 Expression in Dextran Sodium Sulfate-Induced Mice Colon. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 154. [Google Scholar] [PubMed]
- Mahesha, H.G.; Singh, S.A.; Rao, A.G.A. Inhibition of Lipoxygenase by Soy Isoflavones: Evidence of Isoflavones as Redox Inhibitors. Arch. Biochem. Biophys. 2007, 461, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Yang, W.S.; Kim, C.H. Beneficial Effects of Soybean-Derived Bioactive Peptides. Int. J. Mol. Sci. 2021, 22, 8570. [Google Scholar] [CrossRef] [PubMed]
- García-Nebot, M.J.; Recio, I.; Hernández-Ledesma, B. Antioxidant Activity and Protective Effects of Peptide Lunasin against Oxidative Stress in Intestinal Caco-2 Cells. Food Chem. Toxicol. 2014, 65, 155–161. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Dia, V.P. Lunasin and Lunasin-like Peptides Inhibit Inflammation through Suppression of NF-KappaB Pathway in the Macrophage. Peptides 2009, 30, 2388–2398. [Google Scholar] [CrossRef]
- de Mejia, E.G.; Castañeda-Reyes, E.D.; Mojica, L.; Dia, V.; Wang, H.; Wang, T.; Johnson, L.A. Potential Health Benefits Associated with Lunasin Concentration in Dietary Supplements and Lunasin-Enriched Soy Extract. Nutrients 2021, 13, 1618. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Dia, V.P.; Berhow, M.; Bringe, N.A.; de Mejia, E.G. Protein Hydrolysates from Beta-Conglycinin Enriched Soybean Genotypes Inhibit Lipid Accumulation and Inflammation in Vitro. Mol. Nutr. Food Res. 2009, 53, 1007–1018. [Google Scholar] [CrossRef]
- Li, D.; Ikaga, R.; Yamazaki, T. Soya Protein β-Conglycinin Ameliorates Fatty Liver and Obesity in Diet-Induced Obese Mice through the down-Regulation of PPARγ. Br. J. Nutr. 2018, 119, 1220–1232. [Google Scholar] [CrossRef]
- Wanezaki, S.; Saito, S.; Inoue, N.; Tachibana, N.; Yanagita, T.; Nagao, K. Hydrophilic β-Conglycinin Peptide Reduces Hepatic Triglyceride Accumulation in Obese Model OLETF Rats. Food Sci. Technol. Res. 2020, 26, 797–803. [Google Scholar] [CrossRef]
- Young, D.; Ibuki, M.; Nakamori, T.; Fan, M.; Mine, Y. Soy-Derived Di- and Tripeptides Alleviate Colon and Ileum Inflammation in Pigs with Dextran Sodium Sulfate-Induced Colitis3. J. Nutr. 2012, 142, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Kovacs-Nolan, J.; Zhang, H.; Ibuki, M.; Nakamori, T.; Yoshiura, K.; Turner, P.V.; Matsui, T.; Mine, Y. The PepT1-Transportable Soy Tripeptide VPY Reduces Intestinal Inflammation. Biochim. Biophys. Acta 2012, 1820, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, B.; Lv, Y.; Guo, S. Protective and Reparative Effects of Peptides from Soybean β-Conglycinin on Mice Intestinal Mucosa Injury. Int. J. Food Sci. Nutr. 2014, 65, 345–350. [Google Scholar] [CrossRef] [PubMed]
- González-Montoya, M.; Hernández-Ledesma, B.; Silván, J.M.; Mora-Escobedo, R.; Martínez-Villaluenga, C. Peptides Derived from in Vitro Gastrointestinal Digestion of Germinated Soybean Proteins Inhibit Human Colon Cancer Cells Proliferation and Inflammation. Food Chem. 2018, 242, 75–82. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Papillo, V.A.; Vitaglione, P.; Graziani, G.; Gokmen, V.; Fogliano, V. Release of Antioxidant Capacity from Five Plant Foods during a Multistep Enzymatic Digestion Protocol. J. Agric. Food Chem. 2014, 62, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.K.; Muir, J.G.; Gibson, P.R. Review Article: Insights into Colonic Protein Fermentation, Its Modulation and Potential Health Implications. Aliment. Pharmacol. Ther. 2016, 43, 181–196. [Google Scholar] [CrossRef]
- Windey, K.; de Preter, V.; Verbeke, K. Relevance of Protein Fermentation to Gut Health. Mol. Nutr. Food Res. 2012, 56, 184–196. [Google Scholar] [CrossRef]
- de Jesús Rodríguez-Romero, J.; Durán-Castañeda, A.C.; Cárdenas-Castro, A.P.; Sánchez-Burgos, J.A.; Zamora-Gasga, V.M.; Sáyago-Ayerdi, G.S. What We Know about Protein Gut Metabolites: Implications and Insights for Human Health and Diseases. Food Chem. X 2022, 13, 100195. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Kusumah, J.; Bringe, N.A.; Shen, Y.; de Mejia, E.G. Peptide Release, Radical Scavenging Capacity, and Antioxidant Responses in Intestinal Cells Are Determined by Soybean Variety and Gastrointestinal Digestion under Simulated Conditions. Food Chem. 2023, 405, 134929. [Google Scholar] [CrossRef]
- Nieto-Veloza, A.; Wang, Z.; Zhong, Q.; D’Souza, D.; Krishnan, H.B.; Dia, V.P. Lunasin Protease Inhibitor Concentrate Decreases Pro-Inflammatory Cytokines and Improves Histopathological Markers in Dextran Sodium Sulfate-Induced Ulcerative Colitis. Food Sci. Hum. Wellness 2022, 11, 1508–1514. [Google Scholar] [CrossRef]
- Trommelen, J.; Tomé, D.; van Loon, L.J.C. Gut Amino Acid Absorption in Humans: Concepts and Relevance for Postprandial Metabolism. Clin. Nutr. Open Sci. 2021, 36, 43–55. [Google Scholar] [CrossRef]
- El-Malah, A.A.; Gineinah, M.M.; Deb, P.K.; Khayyat, A.N.; Bansal, M.; Venugopala, K.N.; Aljahdali, A.S. Selective COX-2 Inhibitors: Road from Success to Controversy and the Quest for Repurposing. Pharmaceuticals 2022, 15, 827. [Google Scholar] [CrossRef] [PubMed]
- Radi, Z.A.; Khan, N.K. Effects of Cyclooxygenase Inhibition on the Gastrointestinal Tract. Exp. Toxicol. Pathol. 2006, 58, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Gu, H.; Lan, Z.; Lei, Q.; Wang, W.; Ruan, J.; Yu, M.; Lin, J.; Cui, Q. Downregulation of Cyclooxygenase-1 Stimulates Mitochondrial Apoptosis through the NF-ΚB Signaling Pathway in Colorectal Cancer Cells. Oncol. Rep. 2019, 41, 559–569. [Google Scholar] [CrossRef]
- Shan, D.; Yu, H.; Lyu, B.; Fu, H. Soybean β-Conglycinin: Structure Characteristic, Allergenicity, Plasma Lipid-Controlling, Prevention of Obesity and Non-Alcoholic Fatty Liver Disease. Curr. Protein Pept. Sci. 2021, 22, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.L.; Ferreira, E.S.; Silva, M.A.; Neves, V.A.; Demonte, A. The Vicilin Protein (Vigna radiata L.) of Mung Bean as a Functional Food: Evidence of “in Vitro” Hypocholesterolemic Activity. Nutr. Food Sci. 2017, 47, 907–916. [Google Scholar] [CrossRef]
- Philadelpho, B.; Souza, V.; Souza, F.; Santos, J.; Batista, F.; Silva, M.; Capraro, J.; De Benedetti, S.; Heinzl, G.C.; Cilli, E.; et al. Chromatography-Independent Fractionation and Newly Identified Molecular Features of the Adzuki Bean (Vigna Angularis Willd.) β-Vignin Protein. Int. J. Mol. Sci. 2021, 22, 3018. [Google Scholar] [CrossRef]
- De Souza Ferreira, E.; Capraro, J.; Sessa, F.; Magni, C.; Demonte, A.; Consonni, A.; Neves, V.A.; Cilli, E.M.; Duranti, M.; Scarafoni, A. New Molecular Features of Cowpea Bean (Vigna unguiculata, L. Walp) β-Vignin. Biosci. Biotechnol. Biochem. 2018, 82, 285–291. [Google Scholar] [CrossRef]
- Lee, M.; Kim, D.; Kim, H.; Jo, S.; Kim, O.K.; Lee, J. Gastro-Protective Effect of Fermented Soybean (Glycine max (L.) Merr.) in a Rat Model of Ethanol/HCl-Induced Gastric Injury. Nutrients 2022, 14, 2079. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Jiang, Y.; Zhou, X.; Bi, H.; Yang, B. Structure Identification of Soybean Peptides and Their Immunomodulatory Activity. Food Chem. 2021, 359, 129970. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Bi, H.; Zhou, X.; Jiang, Y.; Zhu, H.; Fu, X.; Yang, B. Structure Characterization of Soybean Peptides and Their Protective Activity against Intestinal Inflammation. Food Chem. 2022, 387, 132868. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhang, X.; Cheang, W.S. Isoflavones Daidzin and Daidzein Inhibit Lipopolysaccharide-Induced Inflammation in RAW264.7 Macrophages. Chin. Med. 2022, 17, 95. [Google Scholar] [CrossRef]
- Han, D.G.; Bae, M.J.; An, B.J. Anti-Inflammatory Activity of Velvet Bean (Mucuna pruriens) Substances in LPS-Stimulated RAW 264.7 Macrophages. Molecules 2022, 27, 8797. [Google Scholar] [CrossRef]
- Hwang, K.A.; Hwang, Y.J.; Hwang, H.J.; Lee, S.H.; Kim, Y.J. Sword Bean (Canavalia gladiata) Pod Exerts Anti-Allergic and Anti-Inflammatory Effects through Modulation of Th1/Th2 Cell Differentiation. Nutrients 2022, 14, 2853. [Google Scholar] [CrossRef]
- Guo, F.; Tsao, R.; Li, C.; Wang, X.; Zhang, H.; Jiang, L.; Sun, Y.; Xiong, H. Polyphenol Content of Green Pea (Pisum sativum L.) Hull under in Vitro Digestion and Effects of Digestive Products on Anti-Inflammatory Activity and Intestinal Barrier in the Caco-2/Raw264.7 Coculture Model. J. Agric. Food Chem. 2022, 70, 3477–3488. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, L.; Li, H.; Song, W.; Li, J.; Yuan, L. Selenium-Enriched Black Soybean Protein Prevents Benzo(a)Pyrene-Induced Pyroptotic Colon Damage and Gut Dysbacteriosis. J. Agric. Food Chem. 2022, 70, 12629–12640. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-ΚB, IκB, and IKK: Integral Components of Immune System Signaling. Adv. Exp. Med. Biol. 2019, 1172, 207–226. [Google Scholar] [CrossRef]
- Genovese, T.; Cordaro, M.; Siracusa, R.; Impellizzeri, D.; Caudullo, S.; Raffone, E.; Macrí, F.; Interdonato, L.; Gugliandolo, E.; Interlandi, C.; et al. Molecular and Biochemical Mechanism of Cannabidiol in the Management of the Inflammatory and Oxidative Processes Associated with Endometriosis. Int. J. Mol. Sci. 2022, 23, 5427. [Google Scholar] [CrossRef]
- Ma, X.; Li, X.; Ma, L.L.; Chen, Y.; He, S. Soy Isoflavones Alleviate Polycystic Ovary Syndrome in Rats by Regulating NF-ΚB Signaling Pathway. Bioengineered 2021, 12, 7215–7223. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Indiano-Romacho, P.; Mora-Gutiérrez, I.; Pérez-Rodríguez, L.; Ortega Moreno, L.; Marin, A.C.; Baldán-Martín, M.; Moreno-Monteagudo, J.A.; Santander, C.; Chaparro, M.; et al. Lunasin Peptide Is a Modulator of the Immune Response in the Human Gastrointestinal Tract. Mol. Nutr. Food Res. 2021, 65, 2001034. [Google Scholar] [CrossRef]
- Hirano, T. IL-6 in Inflammation, Autoimmunity and Cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Signalling in Health and Disease. F1000Research 2020, 9. [Google Scholar] [CrossRef]
- Chen, J.; Wei, Y.; Yang, W.; Huang, Q.; Chen, Y.; Zeng, K.; Chen, J. IL-6: The Link Between Inflammation, Immunity and Breast Cancer. Front. Oncol. 2022, 12, 3472. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, 16295–16296. [Google Scholar] [CrossRef]
- Achuthan, A.A.; Lee, K.M.C.; Hamilton, J.A. Targeting GM-CSF in Inflammatory and Autoimmune Disorders. In Seminars in Immunology; Academic Press: Cambridge, MA, USA, 2021; Volume 54. [Google Scholar] [CrossRef]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef]
- Castro-Dopico, T.; Fleming, A.; Dennison, T.W.; Ferdinand, J.R.; Harcourt, K.; Stewart, B.J.; Cader, Z.; Tuong, Z.K.; Jing, C.; Lok, L.S.C.; et al. GM-CSF Calibrates Macrophage Defense and Wound Healing Programs during Intestinal Infection and Inflammation. Cell Rep. 2020, 32, 107857. [Google Scholar] [CrossRef]
- Pandur, E.; Tamási, K.; Pap, R.; Jánosa, G.; Sipos, K. Modulatory Effects of Fractalkine on Inflammatory Response and Iron Metabolism of Lipopolysaccharide and Lipoteichoic Acid-Activated THP-1 Macrophages. Int. J. Mol. Sci. 2022, 23, 2629. [Google Scholar] [CrossRef]
- Ruytinx, P.; Proost, P.; Van Damme, J.; Struyf, S. Chemokine-Induced Macrophage Polarization in Inflammatory Conditions. Front. Immunol. 2018, 9, 1930. [Google Scholar] [CrossRef] [PubMed]
- Kishi, H.; Sato, M.; Shibata, Y.; Sato, K.; Inoue, S.; Abe, S.; Kimura, T.; Nishiwaki, M.; Yamauchi, K.; Nemoto, T.; et al. Role of Chemokine C-C Motif Ligand-1 in Acute and Chronic Pulmonary Inflammations. Springerplus 2016, 5, 1241. [Google Scholar] [CrossRef] [PubMed]
- Knipfer, L.; Schulz-Kuhnt, A.; Kindermann, M.; Greif, V.; Symowski, C.; Voehringer, D.; Neurath, M.F.; Atreya, I.; Wirtz, S. A CCL1/CCR8-Dependent Feed-Forward Mechanism Drives ILC2 Functions in Type 2-Mediated Inflammation. J. Exp. Med. 2019, 216, 2763–2777. [Google Scholar] [CrossRef] [PubMed]
- Fujikawa, M.; Koma, Y.-I.; Hosono, M.; Urakawa, N.; Tanigawa, K.; Shimizu, M.; Kodama, T.; Sakamoto, H.; Nishio, M.; Shigeoka, M.; et al. Chemokine (C-C Motif) Ligand 1 Derived from Tumor-Associated Macrophages Contributes to Esophageal Squamous Cell Carcinoma Progression via CCR8-Mediated Akt/Proline-Rich Akt Substrate of 40 KDa/Mammalian Target of Rapamycin Pathway. Am. J. Pathol. 2021, 191, 686–703. [Google Scholar] [CrossRef]
- Vila-Caballer, M.; González-Granado, J.M.; Zorita, V.; Abu Nabah, Y.N.; Silvestre-Roig, C.; del Monte-Monge, A.; Molina-Sánchez, P.; Ait-Oufella, H.; Andrés-Manzano, M.J.; Sanz, M.J.; et al. Disruption of the CCL1-CCR8 Axis Inhibits Vascular Treg Recruitment and Function and Promotes Atherosclerosis in Mice. J. Mol. Cell. Cardiol. 2019, 132, 154–163. [Google Scholar] [CrossRef]
- Kang, L.; Schmalzl, A.; Leupold, T.; Gonzalez-Acera, M.; Atreya, R.; Neurath, M.F.; Becker, C.; Wirtz, S. CCR8 Signaling via CCL1 Regulates Responses of Intestinal IFN-γ Producing Innate Lymphoid CelIs and Protects From Experimental Colitis. Front. Immunol. 2021, 11, 609400. [Google Scholar] [CrossRef]
- De Mejia, E.G.; Vásconez, M.; De Lumen, B.O.; Nelson, R. Lunasin Concentration in Different Soybean Genotypes, Commercial Soy Protein, and Isoflavone Products. J. Agric. Food Chem. 2004, 52, 5882–5887. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Adler-Nissen, J. ISSPH-the production and properties of an enzymic food protein hydrolysate. In Enzymatic Hydrolysis of Food Proteins; Elsevier Applied Science Publisher: New York, NY, USA, 1986; pp. 263–313. [Google Scholar]
- Gómez-Maqueo, A.; Soccio, M.; Cano, M.P. In Vitro Antioxidant Capacity of Opuntia Spp. Fruits Measured by the LOX-FL Method and Its High Sensitivity Towards Betalains. Plant Foods Hum. Nutr. 2021, 76, 354–362. [Google Scholar] [CrossRef]




| Marker | Abbreviation | Role | Fold Change Relative to Control LPS | |
|---|---|---|---|---|
| LPS | GN1 | |||
| Granulocyte–macrophage colony-stimulating factor | GM-CSF | Stimulating the production of white blood cells including granulocytes and macrophages | 1.00 ± 0.02 a | 0.01 ± 0.05 b |
| Interleukin-6 | IL-6 | Pro-inflammatory cytokine; stimulates synthesis of acute phase proteins (i.e., CRP) and serum amyloid A; inhibits albumin production | 1.00 ± 0.03 a | 0.45 ± 0.03 b |
| Fractalkine | TNFSF8 | Migration, adhesion, and proliferation of multiple types of cells including T-cells | 1.00 ± 0.03 a | 1.42 ± 0.03 b |
| C-C motif ligand 1 | I-309 (TCA-3/CCL1) | Attracts macrophages to the inflammation site | 1.00 ± 0.07 a | 1.80 ± 0.07 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusumah, J.; Castañeda-Reyes, E.D.; Bringe, N.A.; Gonzalez de Mejia, E. Soybean (Glycine max) INFOGEST Colonic Digests Attenuated Inflammatory Responses Based on Protein Profiles of Different Varieties. Int. J. Mol. Sci. 2023, 24, 12396. https://doi.org/10.3390/ijms241512396
Kusumah J, Castañeda-Reyes ED, Bringe NA, Gonzalez de Mejia E. Soybean (Glycine max) INFOGEST Colonic Digests Attenuated Inflammatory Responses Based on Protein Profiles of Different Varieties. International Journal of Molecular Sciences. 2023; 24(15):12396. https://doi.org/10.3390/ijms241512396
Chicago/Turabian StyleKusumah, Jennifer, Erick Damian Castañeda-Reyes, Neal A. Bringe, and Elvira Gonzalez de Mejia. 2023. "Soybean (Glycine max) INFOGEST Colonic Digests Attenuated Inflammatory Responses Based on Protein Profiles of Different Varieties" International Journal of Molecular Sciences 24, no. 15: 12396. https://doi.org/10.3390/ijms241512396
APA StyleKusumah, J., Castañeda-Reyes, E. D., Bringe, N. A., & Gonzalez de Mejia, E. (2023). Soybean (Glycine max) INFOGEST Colonic Digests Attenuated Inflammatory Responses Based on Protein Profiles of Different Varieties. International Journal of Molecular Sciences, 24(15), 12396. https://doi.org/10.3390/ijms241512396








