Transcranial Photosensitizer-Free Laser Treatment of Glioblastoma in Rat Brain
Abstract
:1. Introduction
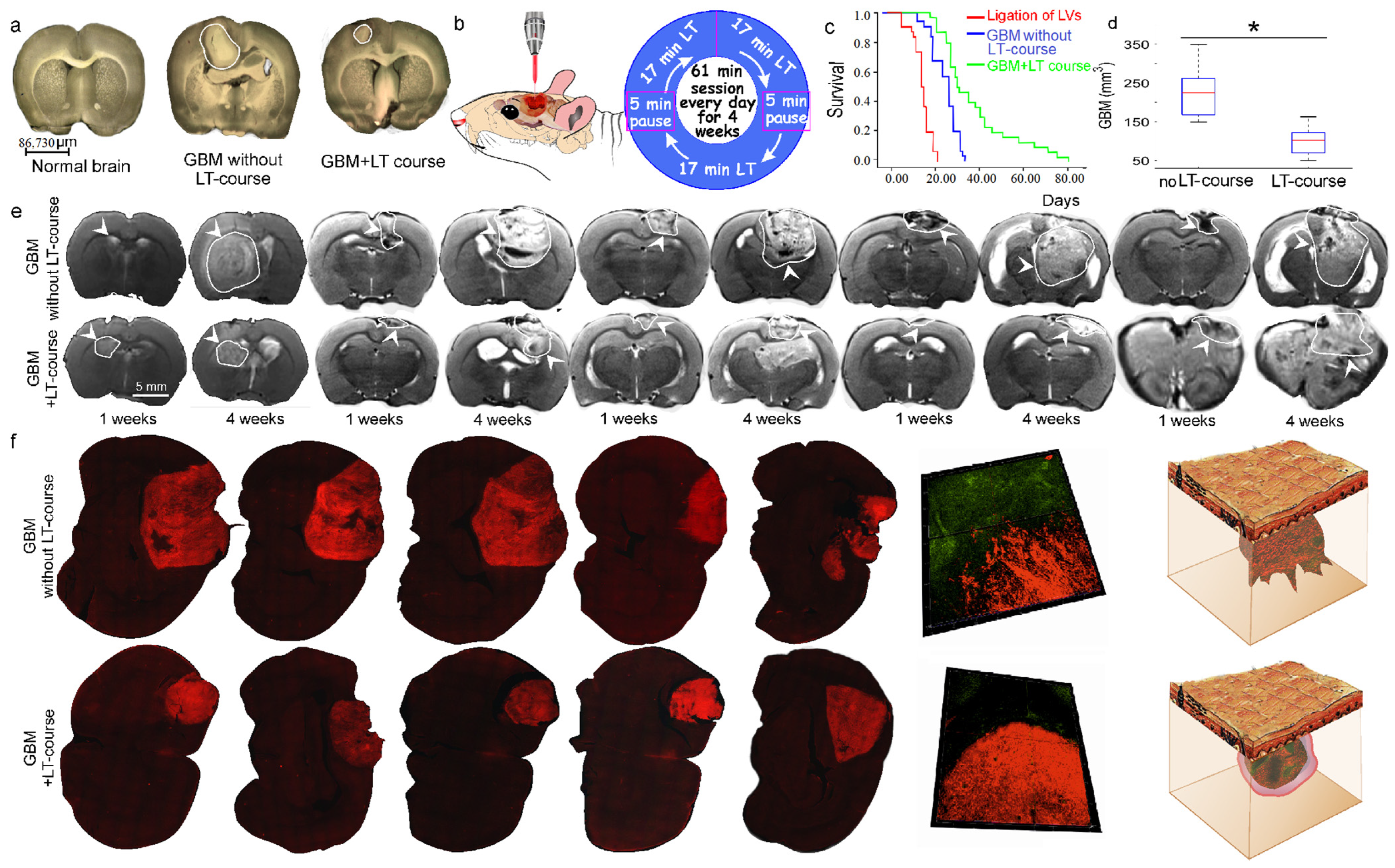
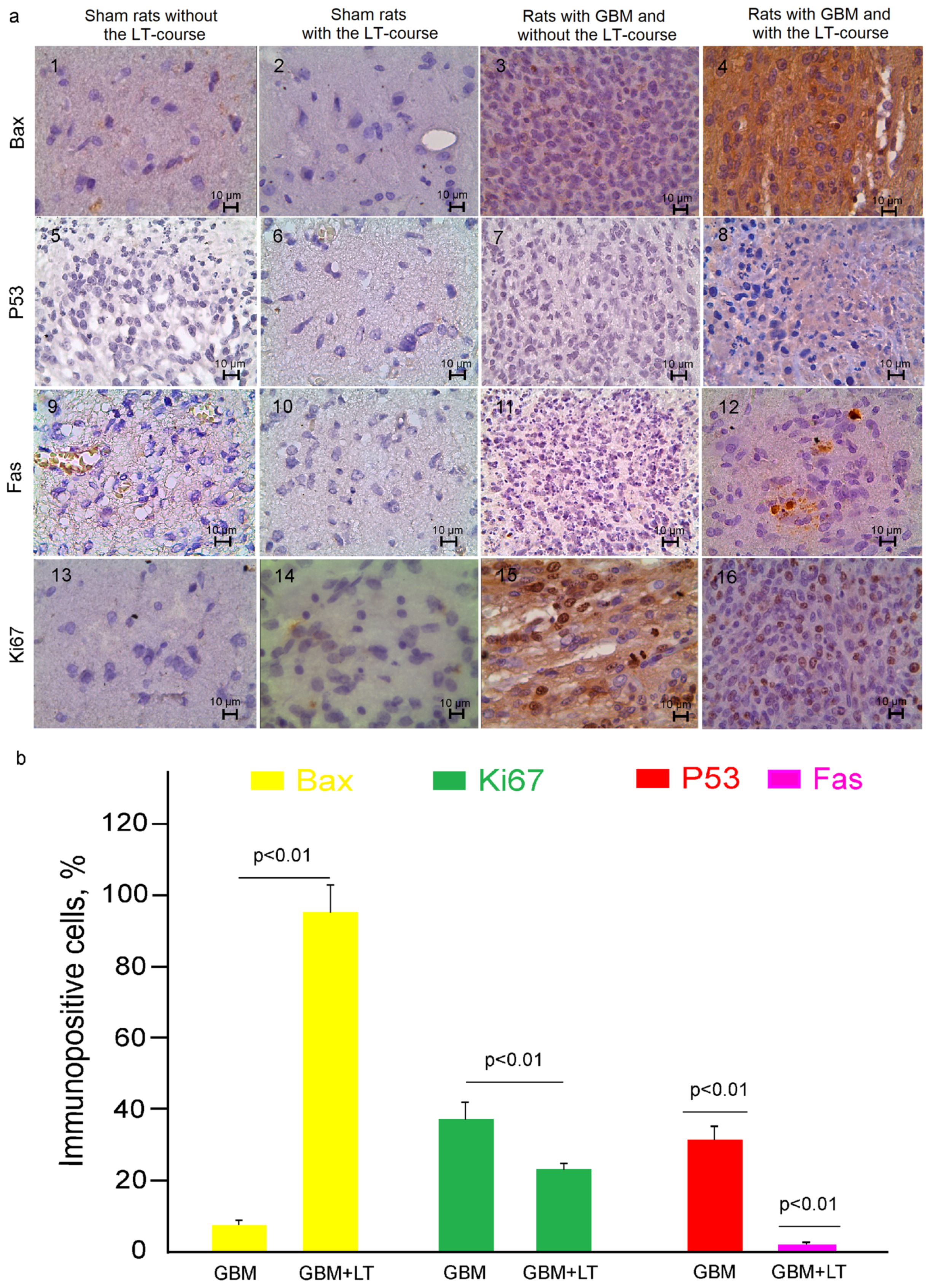
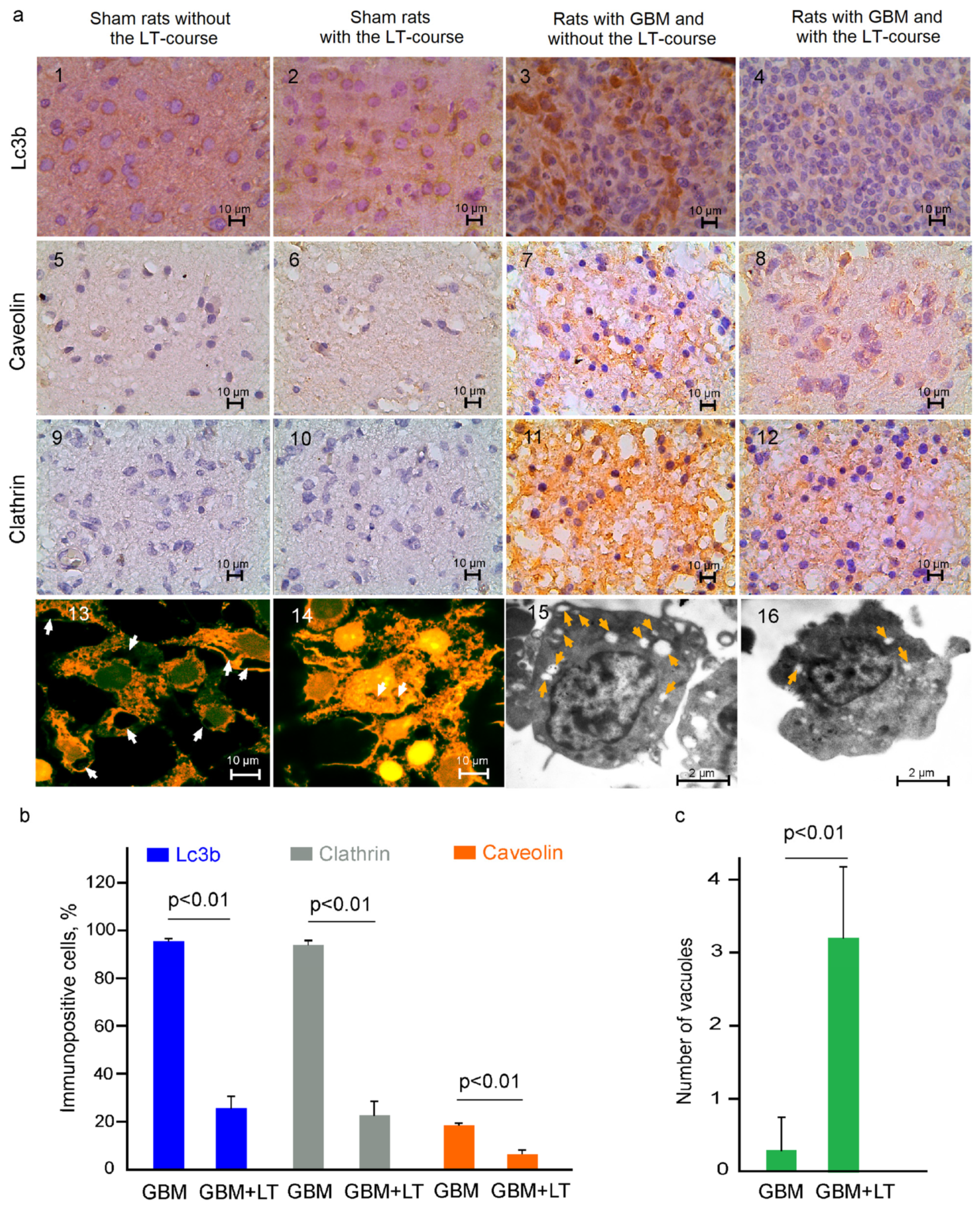
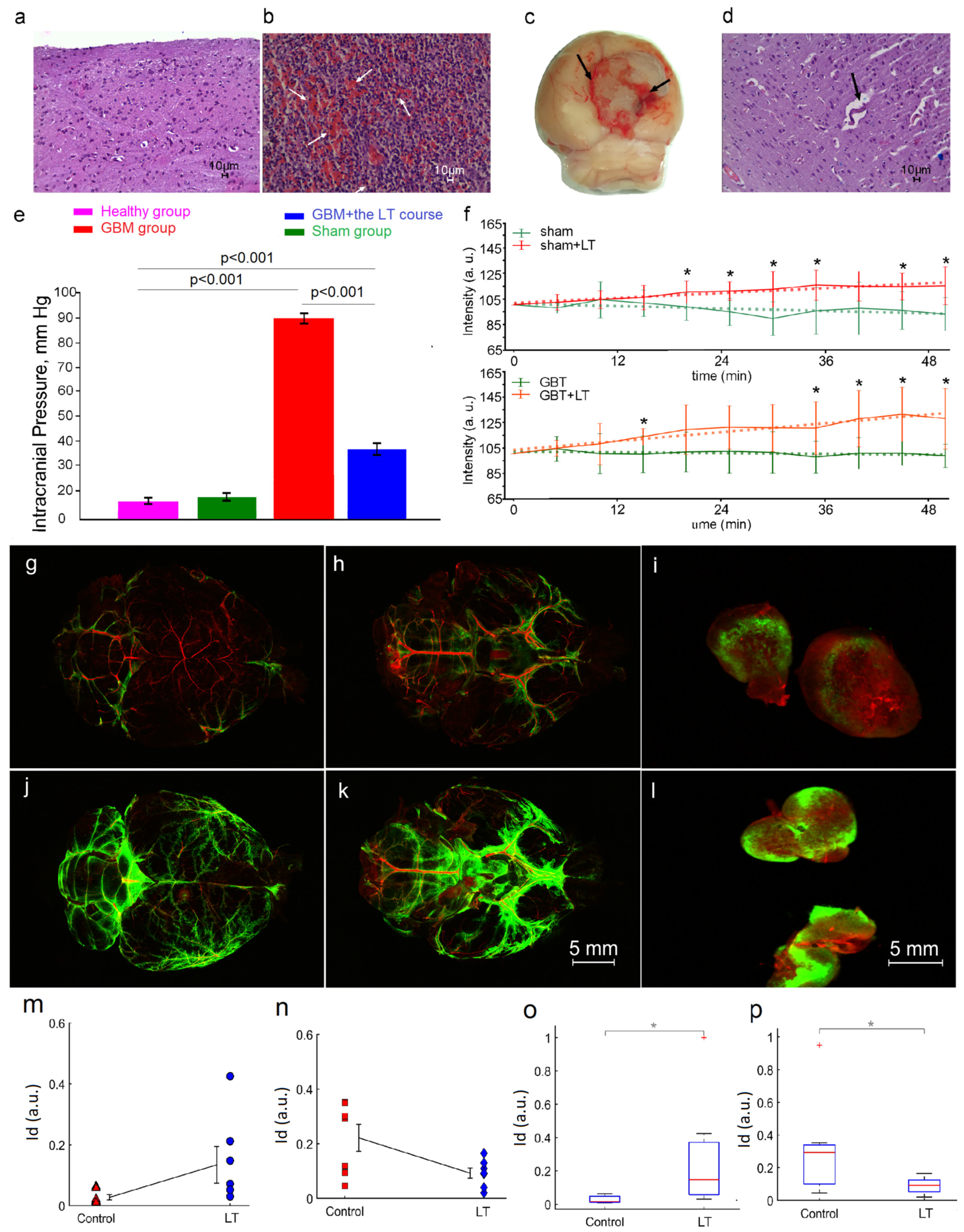
2. Results
2.1. The Effects of an LT Course on GBM Progression and Survival Rate
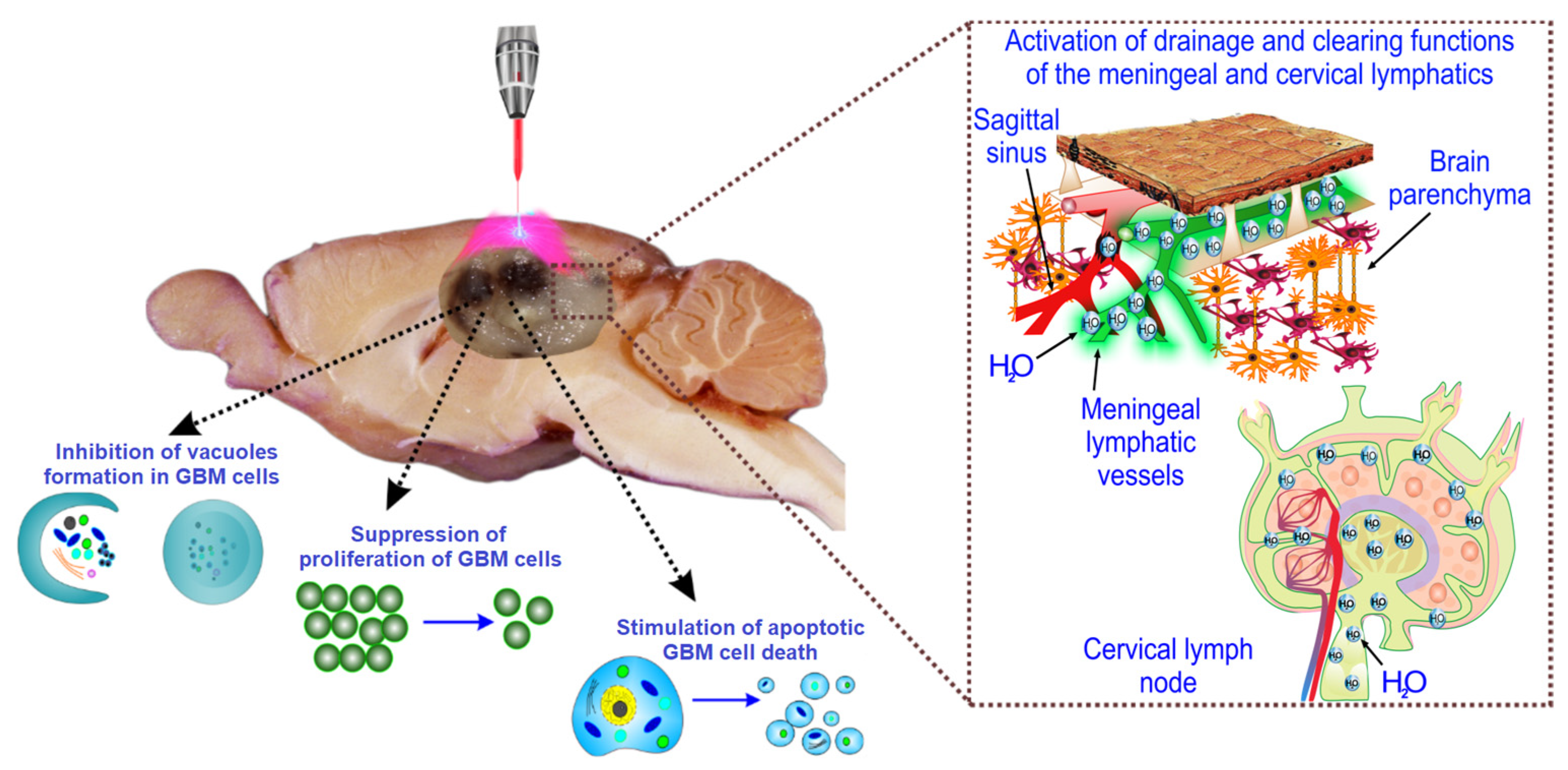
2.2. Cellular Mechanisms of LT-Induced Suppression of GBM Growth
2.3. LT Causes Activation of the Drainage and Clearance of the Brain Tissues
3. Discussion
4. Materials and Methods
4.1. Subjects and Groups
4.2. Implantation of C6 and Fluorescent Glioma Cell Lines [85]
4.3. Laser Radiation Scheme and Dose Calculation
4.3.1. LT Effects: In Vivo Study
4.3.2. The LT Effects: In Vitro Study
4.4. Measure the Thermal Impact of LT
4.5. Measurement of ICP
4.6. Magnetic Resonance Imaging (MRI) of GBMs
4.7. Detection of Vacuoles In Vitro and In Ex Vivo Experiments
4.8. Immunohistochemical (IHC) Assay
4.9. Apoptosis Assessment
4.10. Endothelial Neuronal Cells In Vitro BBB Model
4.11. Analysis of the ROS Production
4.12. Histological Analysis of the Brain Tissues and Vessels
4.13. Measurement of Cerebral Blood Flow (CBF)
4.14. Blocking of the Lymphatic Pathway of CSF Outflow
4.15. In Vivo and Ex Vivo Optical Monitoring of Lymphatic Clearance of FITCD from Rat Brain
4.16. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moreaux, L.; Yatsenko, D.; Sacher, W.; Choi, J.; Lee, C.; Kubat, N.; Cotton, R.; Boyden, E.; Lin, M.; Tian, L.; et al. Integrated Neurophotonics: Toward Dense Volumetric Interrogation of Brain Circuit Activity-at Depth and in Real Time. Neuron 2020, 108, 66–92. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Penzel, T.; Poluektov, M.; Fedosov, I.; Tzoy, M.; Terskov, A.; Blokhina, I.; Sidorov, V.; Kurths, J. Phototherapy of Alzheimer’s Disease: Photostimulation of Brain Lymphatics during Sleep: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 10946. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G.; Gedik, M.; Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer—A Review of the Current Clinical Status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.; Francis, S.; Barnholtz-Sloan, J. Epidemiology of Brain and Other CNS Tumors. Curr. Neurol. Neurosci. Rep. 2021, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- AlRayahi, J.; Alwalid, O.; Mubarak, W.; Maaz, A.; Mifsud, W. Pediatric Brain Tumors in the Molecular Era: Updates for the Radiologist. Semin. Roentgenol. 2023, 58, 47–66. [Google Scholar] [CrossRef]
- Gonçalves, F.G.; Alves, C.A.P.F.; Vossough, A. Updates in Pediatric Malignant Gliomas. Top. Magn. Reson. Imaging 2020, 2, 83–94. [Google Scholar] [CrossRef]
- Kimberly, M.; Ostrom, Q.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.; Waite, K.; Jemal, A.; Siegel, R.; et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin. 2021, 71, 381–406. [Google Scholar]
- Minniti, G.; Lombardi, G.; Paolini, S. Glioblastoma in Elderly Patients: Current Management and Future Perspectives. Cancers 2019, 11, 336. [Google Scholar] [CrossRef]
- Wang, L.; Banu, M.; Canoll, P.; Bruce, J. Rationale and clinical implications of fluorescein-guided supramarginal resection in newly diagnosed high-grade glioma. Front. Oncol. 2021, 11, 666734. [Google Scholar] [CrossRef]
- Tu, Z.; Xiong, H.; Qiu, Y.; Li, G.; Wang, L.; Peng, S. Limited recurrence distance of glioblastoma under modern radiotherapy era. BMC Cancer 2021, 21, 720. [Google Scholar] [CrossRef]
- Jiang, H.; Yu, K.; Li, M.; Cui, Y.; Ren, X.; Yang, C.; Zhao, X.; Lin, S. Classification of progression patterns in glioblastoma: Analysis of predictive factors and clinical implications. Front. Oncol. 2020, 10, 590648. [Google Scholar] [CrossRef]
- Broniscer, A.; Gajjar, A. Supratentorial high-grade astrocytoma and diffuse brainstem glioma: Two challenges for the pediatric oncologist. Oncologist 2004, 9, 197–206. [Google Scholar] [CrossRef]
- Fangusaro, J. Pediatric highgrade gliomas and diffuse intrinsic pontine gliomas. J. Child. Neurol. 2009, 24, 1409–1417. [Google Scholar] [CrossRef]
- Williams, N.; Rotondo, R.; Bradley, J.; Pincus, D.; Fort, J.; Wynn, T.; Morris, C.; Mendenhall, N.; Indelicato, D. Late Effects After Radiotherapy for Childhood Low-grade Glioma. Am. J. Clin. Oncol. 2018, 41, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Geyer, J.; Finlay, J.; Boyett, J.; Wisoff, J.; Yates, A.; Mao, L.; Packer, R. Survival of infants with malignant astrocytomas. A report from the Children’s Cancer Group. Cancer 1995, 75, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Duffner, P.; Krischer, J.; Burger, P.; Cohen, M.; Backstrom, J.; Horowitz, M.; Sanford, R.; Friedman, H.; Kun, L. Treatment of infants with malignant gliomas: The Pediatric Oncology Group experience. J. Neurooncol. 1996, 28, 245–256. [Google Scholar] [CrossRef]
- Dufour, C.; Grill, J.; Lellouch-Tubiana, A.; Puget, S.; Chastagner, P.; Frappaz, D.; Doz, F.; Pichon, F.; Plantaz, D.; Gentet, J.; et al. High-grade glioma in children under 5 years of age: A chemotherapy only approach with the BBSFOP protocol. Eur. J. Cancer 2006, 42, 2939–2945. [Google Scholar] [CrossRef]
- Sanders, R.; Kocak, M.; Burger, P.; Merchant, T.; Gajjar, A.; Broniscer, A. High-grade astrocytoma in very young children. Pediatr. Blood Cancer 2007, 49, 888–893. [Google Scholar] [CrossRef]
- Fallai, C.; Olmi, P. Hyperfractionated and accelerated radiation therapy in central nervous system tumors (malignant gliomas, pediatric tumors, and brain metastases). Radiother. Oncol. 1997, 43, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Desouky, O.; Ding, N.; Zhou, G. Targeted and non-targeted effects of ionizing radiation. J. Radiat. Res. Appl. Sci. 2015, 8, 247–254. [Google Scholar] [CrossRef]
- Thorbinson, C.; Kilday, J.-P. Childhood Malignant Brain Tumors: Balancing the Bench and Bedside. Cancers 2021, 13, 6099. [Google Scholar] [CrossRef]
- Guidi, M.; Giunti, L.; Buccoliero, A.; Santi, M.; Spacca, B.; De Masi, S.; Genitori, L.; Sardi, I. Use of High-Dose Chemotherapy in Front-Line Therapy of Infants Aged Less Than 12 Months Treated for Aggressive Brain Tumors. Front. Pediatr. 2020, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Saito, T.; Nitta, M.; Muragaki, Y.; Kawamata, T. Photodynamic therapy for malignant brain tumors in children and young adolescents. Front. Oncol. 2022, 12, 957267. [Google Scholar]
- Abdurashitov, A.; Tuchin, V.; Semyachkina-Glushkovskaya, O. Photodynamic therapy of brain tumors and novel optical coherence tomography strategies for in vivo monitoring of cerebral fluid dynamics. J. Innov. Opt. Health Sci. 2020, 13, 2030004. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Żołyniak, A.; Barnaś, E.; Machorowska-Pieniążek, A.; Oleś, P.; Kawczyk-Krupka, A.; Aebisher, D. The Use of Photodynamic Therapy in the Treatment of Brain Tumors—A Review of the Literature. Molecules 2022, 27, 6847. [Google Scholar] [CrossRef]
- Schipmann, S.; Müther, M.; Stögbauer, L.; Zimmer, S.; Brokinkel, B.; Holling, M.; Grauer, O.; Suero Molina, E.; Warneke, N.; Stummer, W. Combination of ALA-induced fluorescence-guided resection and intraoperative open photodynamic therapy for recurrent glioblastoma: Case series on a promising dual strategy for local tumor control. J. Neurosurg. 2020, 134, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Newman, T.; Wickremasinghe, A.; Walsh, E.; Grimes, B.; McCulloch, C.; Kuzniewicz, M. Retrospective Cohort Study of Phototherapy and Childhood Cancer in Northern California. Pediatrics 2016, 137, e20151354. [Google Scholar] [CrossRef] [PubMed]
- Whelan, H.T. Photodynamic Therapy (PDT) for Recurrent Pediatric Brain Tumors. Clinical Trial Report U.S. National Laboratory of Medicine. 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT01682746 (accessed on 29 July 2023).
- Wickremasinghe, A.; Kuzniewicz, M.; Grimes, B.; McCulloch, C.; Newman, T. Neonatal Phototherapy and Infantile Cancer. Pediatrics 2016, 137, e20151353. [Google Scholar] [CrossRef]
- Perria, C.; Capuzzo, T.; Cavagnaro, G.; Datti, R.; Francaviglia, N.; Rivano, C.; Tercero, V. Fast attempts at the photodynamic treatment of human gliomas. J. Neurosurg. Sci. 1980, 24, 119–129. [Google Scholar]
- Eljamel, S. Photodynamic applications in brain tumors: A comprehensive review of the literature. Photodiagn. Photodyn. Ther. 2010, 7, 76–85. [Google Scholar] [CrossRef]
- Cramer, S.; Chen, C. Photodynamic Therapy for the Treatment of Glioblastoma. Front. Surg. 2020, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Hirschberg, H.; Berg, K.; Peng, Q. Photodynamic therapy mediated immune therapy of brain tumors. Neuroimmunol. Neuroinflamm. 2018, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Hernández, I.; Yu, Y.; Ossendorp, F.; Korbelik, M.; Oliveira, S. Preclinical and Clinical Evidence of Immune Responses Triggered in Oncologic PhotodynamicTherapy: Clinical Recommendations. J. Clin. Med. 2020, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. [Google Scholar] [CrossRef]
- Canti, G.; Lattuada, D.; Nicolin, A.; Taroni, P.; Valentini, G.; Cubeddu, R. Antitumor immunity induced by photodynamic therapy with aluminum disulfonated phthalocyanines and laser light. Anticancer Drugs 1994, 5, 443–447. [Google Scholar] [CrossRef]
- Garg, A.; Agostinis, P. Cell death and immunity in cancer: From danger signals to mimicry of pathogen defense responses. Immunol. Rev. 2017, 280, 126–148. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Garg, A.; Martin, S.; Golab, J.; Agostinis, P. Danger signaling during cancer cell death: Origins, plasticity and regulation. Cell Death Differ. 2014, 21, 26–38. [Google Scholar] [CrossRef]
- Vandenabeele, P.; Vandecasteele, B.; Krysko, O.; Krysko, D. Immunogenic apoptotic cell death and anticancer immunity. Adv. Exp. Med. Biol. 2016, 930, 133–149. [Google Scholar]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic cell death in cancer therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Anzengruber, F.; Avci, P.; Freitas, L.; Hamblin, M. T-cell mediated anti-tumor immunity after photodynamic therapy: Why does it not always work and how can we improve it? Photochem. Photobiol. Sci. 2015, 14, 1492–1509. [Google Scholar] [CrossRef]
- Song, E.; Mao, T.; Dong, H.; Simoes, L.; Boisserand, B.; Antila, S.; Bosenberg, M.; Alitalo, K.; Thomas, J.; Iwasak, A. VEGF-C-driven Lymphatic Drainage Enables Immunosurveillance of Brain Tumours. Nature 2020, 577, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Deng, Q.; Ma, L.; Li, Q.; Chen, Y.; Liao, Y.; Zhou, F.l.; Zhang, C.l.; Shao, L.; Feng, J.; et al. Meningeal lymphatic vessels regulate brain tumor drainage and immunity. Cell Res. 2020, 30, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Fedosov, I.; Shirokov, A.; Vodovozov, E.; Alekseev, A.; Khorovodov, A.; Blokhina, I.; Terskov, A.; Mamedova, A.; Klimova, M.; et al. Photomodulation of lymphatic delivery of liposomes to the brain bypassing the blood-brain barrier: New perspectives for glioma therapy. Nanophotonics 2021, 10, 3215–3227. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Karavaev, A.; Prokhorov, M.; Runnova, A.; Borovkova, E.; Ishbulatov, Y.; Hramkov, A.; Kulminskiy, D.; Semenova, N.; Sergeev, K.; et al. EEG biomarkers of activation of the lymphatic drainage system of the brain during sleep and opening of the blood-brain barrier. Comput. Struct. Biotechnol. J. 2023, 21, 758–768. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Penzel, T.; Blokhina, I.; Khorovodov, A.; Fedosov, I.; Yu, T.; Karandin, G.; Evsukova, A.; Elovenko, D.; Adushkina, V.; et al. Night Photostimulation of Clearance of Beta-Amyloid from Mouse Brain: New Strategies in Preventing Alzheimer’s Disease. Cells 2021, 10, 3289. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-glushkovskaya, O.; Abdurashitov, A.; Dubrovsky, A.; Klimova, M.; Agranovich, I.; Terskov, A.; Shirokov, A.; Vinnik, V.; Kuznecova, A.; Lezhnev, N.; et al. Photobiomodulation of lymphatic drainage and clearance: Perspective strategy for augmentation of meningeal lymphatic functions. Biomed. Opt. Express 2020, 11, 725–734. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Shirokov, A.; Blokhina, I.; Telnova, V.; Vodovozova, E.; Alekseeva, A.; Boldyrev, I.; Fedosov, I.; Dubrovsky, A.; Khorovodov, A.; et al. Intranasal delivery of liposomes to glioblastoma by photostimulation of the lymphatic system. Pharmaceutics 2023, 15, 36. [Google Scholar] [CrossRef]
- Da Mesquita, S.; Louveau, A.; Vaccari, A.; Smirnov, I.; Cornelison, R.; Kingsmore, K.; Contarino, C.; Onengut-Gumuscu, S.; Farber, E.; Raper, D.; et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature 2018, 560, 185–191. [Google Scholar] [CrossRef]
- Louveau, A.; Smirnov, I.; Keyes, T.; Eccles, J.; Rouhani, S.; Peske, J.; Derecki, N.; Castle, D.; Mandell, J.; Lee, K.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Zinchenko, E.; Navolokin, N.; Shirokov, A.; Khlebtsov, B.; Dubrovsky, A.; Saranceva, E.; Abdurashitov, A.; Khorovodov, A.; Terskov, A.; Mamedova, A.; et al. Pilot study of transcranial photobiomodulation of lymphatic clearance of beta-amyloid from the mouse brain: Breakthrough strategies for non-pharmacologic therapy of Alzheimer’s disease. Biomed. Opt. Exp. 2019, 10, 4003–4017. [Google Scholar] [CrossRef] [PubMed]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Klimova, M.; Dubrovsky, A.; Shirokov, A.; Fomin, A.; Terskov, A.; Agranovich, I.; Mamedova, A.; Khorovodov, A.; et al. Photostimulation of cerebral and peripheral lymphatic functions. Transl. Biophotonics 2020, 2, e201900036. [Google Scholar] [CrossRef]
- Sokolovski, S.; Zolotovskaya, S.; Goltsov, A.; Pourreyron, C.; South, A.; Rafailov, E. Infrared laser pulse triggers increased singlet oxygen production in tumour cells. Sci. Rep. 2013, 3, 3484. [Google Scholar] [CrossRef]
- Khokhlova, A.; Zolotovskii, I.; Sokolovski, S.; Saenko, Y.; Rafailov, E.; Stoliarov, D.; Pogodina, E.; Svetukhin, V.; Sibirny, V.; Fotiadi, A. The light-oxygen effect in biological cells enhanced by highly localized surface plasmon-polaritons. Sci. Rep. 2019, 9, 18435. [Google Scholar] [CrossRef] [PubMed]
- Khokhlova, A.; Zolotovskii, I.; Stoliarov, D.; Vorsina, S.; Liamina, D.; Pogodina, E.; Fotiadi, A.; Sokolovski, S.; Saenko, Y.; Rafailov, E. The Photobiomodulation of Vital Parameters of the Cancer Cell Culture by Low Dose of Near-IR Laser Irradiation. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 7201510. [Google Scholar] [CrossRef]
- Anquez, F.; El Yazidi-Belkoura, I.; Randoux Pierre, S.; Courtade, S. Cancerous Cell Death from Sensitizer Free Photoactivation of Singlet Oxygen. Photochem. Photobiol. 2012, 88, 167–174. [Google Scholar] [CrossRef]
- Shi, L.; Sordillo, L.; Rodríguez-Contreras, A.; Alfano, R. Transmission in near-infrared optical windows for deep brain imaging. J. Biophot. 2016, 9, 38–43. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Terskov, A.; Khorovodov, A.; Telnova, V.; Blokhina, I.; Saranceva, E.; Kurths, J. Photodynamic Opening of the Blood–Brain Barrier and the Meningeal Lymphatic System: The New Niche in Immunotherapy for Brain Tumors. Pharmaceutics 2022, 14, 2612. [Google Scholar] [CrossRef]
- Hoffmann, H.; Dionne, V. Temperature dependence of ion permeation at the endplate channel. J. Gen. Physiol. 1983, 81, 687–703. [Google Scholar] [CrossRef]
- Moser, E.; Mathiesen, I.; Andersen, P. Association between brain temperature and dentate field potentials in exploring and swimming rats. Science 1993, 259, 1324–1326. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Aaronson, S.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [PubMed]
- Osifchin, N.; Jiang, D.; Ohtani-Fujita, N.; Fujita, T.; Carroza, M.; Kim, S.; Sakai, T.; Robbins, P. Identification of a p53 binding site in the human retinoblastoma susceptibility gene promoter. J. Biol. Chem. 1994, 269, 6383–6389. [Google Scholar] [CrossRef] [PubMed]
- Owen-Schaub, L.; Zhang, W.; Cusack, J.; Angelo, L.; Santee, S.; Fujiwara, T.; Roth, J.; Deisseroth, A.; Zhang, W.-W.; Kruzel, E.; et al. Wild-type human p53 and a temperature-sensitive mutant induce Fas/APO-1 expression. Mol. Cell. Biol. 1995, 371, 3032–3040. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, G.; Balzan, R. Oxidative stress and programmed cell death in yeast. Front. Oncol. 2012, 2, 64. [Google Scholar] [CrossRef] [PubMed]
- Koukourakis, M.; Kalamida, D.; Giatromanolaki, A.; Zois, C.; Sivridis, E.; Pouliliou, S.; Mitrakas, A.; Gatter, K.; Harris, A. Autophagosome Proteins LC3A, LC3B and LC3C Have Distinct Subcellular Distribution Kinetics and Expression in Cancer Cell Lines. PLoS ONE 2015, 10, e0137675. [Google Scholar] [CrossRef] [PubMed]
- Latomanski, E.; Newton, H. Interaction between autophagic vesicles and the Coxiella-containing vacuole requires CLTC (clathrin heavy chain). Autophag 2018, 14, 1710–1725. [Google Scholar] [CrossRef]
- Zhang, X.; Ramírez, C.; Aryal, B.; Madrigal-Matute, J.; Liu, X.; Diaz, A.; Torrecilla-Parra, M.; Suárez, Y.; Cuervo, A.; Sessa, W.; et al. Cav-1 (Caveolin-1) Deficiency Increases Autophagy in the Endothelium and Attenuates Vascular Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. 2020, 40, 1510–1522. [Google Scholar] [CrossRef]
- Hablitz, L.; Vinitsky, H.; Sun, Q.; Stæger, F.; Sigurdsson, B.; Mortensen, K.; Lilius, T.; Nedergaard, M. Increased glymphatic influx is correlated with high EEG delta power and low heart rate in mice under anesthesia. Sci. Adv. 2019, 5, eaav5447. [Google Scholar] [CrossRef]
- Ahn, J.; Cho, H.; Kim, J.; Kim, S.; Ham, J.; Park, I.; Suh, S.; Hong, S.; Song, J.; Hong, Y.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Sokolovski, S.; Rafailov, E.; Abramov, A.; Angelova, P. Singlet oxygen stimulates mitochondrial bioenergetics in brain cells. Free Radic. Biol. Med. 2021, 163, 306–313. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, G. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Trejo-Solís, C.; Serrano-Garcia, N.; Escamilla-Ramírez, Á.; Castillo-Rodríguez, R.; Jimenez-Farfan, D.; Palencia, G.; Calvillo, M.; Alvarez-Lemus, M.; Flores-Nájera, A.; Cruz-Salgado, A.; et al. Autophagic and Apoptotic Pathways as Targets for Chemotherapy in Glioblastoma. Int. J. Mol. Sci. 2018, 19, 3773. [Google Scholar] [CrossRef] [PubMed]
- Jennewein, L.; Ronellenfitsch, M.; Antonietti, P.; Ilina, E.; Jung, J.; Stadel, D.; Flohr, L.; Zinke, J.; von Renesse, J.; Drott, U.; et al. Diagnostic and clinical relevance of the autophago-lysosomal network in human gliomas. Oncotarget 2016, 7, 20016–20032. [Google Scholar] [CrossRef]
- Li, Z.; Mbah, N.; Maltese, W. Vacuole-inducing compounds that disrupt endolysosomal trafficking stimulate production of exosomes by glioblastoma cells. Mol. Cell Biochem. 2018, 439, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bălașa, A.; Șerban, G.; Chinezu, R.; Hurghiș, C.; Tămaș, F.; Manu, D. The Involvement of Exosomes in Glioblastoma Development, Diagnosis, Prognosis, and Treatment. Brain Sci. 2020, 10, 553. [Google Scholar] [CrossRef] [PubMed]
- Naryzhny, S.; Volnitskiy, A.; Kopylov, A.; Zorina, E.; Kamyshinsky, R.; Bairamukov, V.; Garaeva, L.; Shlikht, A.; Shtam, T. Proteome of Glioblastoma-Derived Exosomes as a Source of Biomarkers. Biomedicines 2020, 8, 216. [Google Scholar] [CrossRef] [PubMed]
- Dremin, V.; Novikova, I.; Rafailov, E. Simulation of thermal field distribution in biological tissue and cell culture media irradiated with infrared wavelengths. Opt. Express 2022, 13, 230782022. [Google Scholar] [CrossRef]
- Sager, O.; Dincoglan, F.; Demiral, S.; Uysal, B.; Gamsiz, H.; Dirican, B.; Beyzadeoglu, M. A concise review of immunotherapy for glioblastoma. Neuroimmunol. Neuroinflamm. 2018, 5, 25. [Google Scholar] [CrossRef]
- Hwang, E.; Sayour, E.; Flores, C.; Grant, G.; Wechsler-Reya, R.; Hoang-Minh, L.B.; Kieran, M.W.; Salcido, J.; Prins, R.M.; Figg, J.W.; et al. The current landscape of immunotherapy for pediatric brain tumors. Nat. Cancer 2022, 3, 11–24. [Google Scholar] [CrossRef]
- Watkins, S.; Robel, S.; Kimbrough, I.; Robert, S.; Ellis-Davies, G.; Sontheimer, H. Disruption of astrocyte–vascular coupling and the blood–brain barrier by invading glioma cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef]
- Wu, C.; Lin, G.; Lin, Z.; Zhang, J.; Liu, S.; Zhou, C. Peritumoral edema shown by MRI predicts poor clinical outcome in glioblastoma. World J. Surg. Oncol. 2015, 13, 97. [Google Scholar] [CrossRef]
- Li, D.Y.; Liu, S.J.; Yu, T.T.; Liu, Z.; Sun, S.L.; Bragin, D.; Navolokin, N.; Kurths, J.; Glushkovskaya-Semyachkina, O.; Zhu, D. Photostimulation of lymphatic clearance of red blood cells from the mouse brain after intraventricular haemorrhage. bioRxiv 2020. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Shirokov, A.; Fomin, A.; Semyachkina-Glushkovskaya, O. Fluorescent Glioma Cell Line and Method for Production Thereof. Russian Federation. RU 2699754 C1: 09.09.2019 Bull. No. 25. Available online: https://patentimages.storage.googleapis.com/11/10/ae/0e47732ce1ccd1/RU2699754C1.pdf (accessed on 1 September 2023).
- Jia, Y.; Chen, L.; Chi, D.; Cong, D.; Zhou, P.; Jin, J.; Ji, H.; Liang, B.; Gao, S.; Hu, S. Photodynamic therapy combined with temozolomide inhibits C6 glioma migration and invasion and promotes mitochondrial-associated apoptosis by inhibiting sodium-hydrogen exchanger isoform 1. Photodiagnosis Photodyn. Ther. 2019, 26, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Shen, B.; Lin, M.; Tong, M.; Zheng, Y.; Jiang, X.; Yang, W.; Yuan, J.; Yao, Q.; Zhao, Y. Homing of ICG-loaded liposome inlaid with tumor cellular membrane to the homologous xenografts glioma eradicates the primary focus and prevents lung metastases through phototherapy. Biomater. Sci. 2018, 6, 2410–2425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, M.; Shen, L.; Hu, S. Combination of photodynamic therapy and temozolomide on glioma in a rat C6 glioma model. Photodiagnosis Photodyn. Ther. 2014, 11, 603–612. [Google Scholar] [CrossRef]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105–112. [Google Scholar]
- Brickner, A.G.; Koop, B.F.; Aronow, B.J.; Wiginton, D.A. Genomic sequence comparison of the human and mouse adenosine deaminase gene regions. Mamm. Genome 1999, 10, 95–101. [Google Scholar] [CrossRef]
- Lenting, K.; Verhaak, R.; Ter Laan, M.; Wesseling, P.; Leenders, W. Glioma: Experimental models and reality. Acta Neuropathol. 2017, 133, 263–282. [Google Scholar] [CrossRef]
- Bewick, V.; Cheek, L.; Ball, J. Statistics review 12: Survival analysis. Crit. Care 2004, 8, 389–394. [Google Scholar] [CrossRef]
- Bragin, D.; Kameneva, M.; Bragina, O.; Thomson, S.; Statom, G.; Lara, D.; Yang, Y.; Nemoto, E. Rheological effects of drag-reducing polymers improve cerebral blood flow and oxygenation after traumatic brain injury in rats. J. Cereb. Blood Flow. Metab. 2017, 37, 762–775. [Google Scholar] [CrossRef]
- Mirrett, S. Acridine Orange Stain. Infect. Control 1982, 3, 250–253. [Google Scholar] [CrossRef]
- Shirokov, A.A.; Budanova, A.A.; Burov, A.M.; Khlebtsov, B.N.; Krasov, A.I.; Shchyogolev, S.Y.; Matora, L.Y. Immunoelectron microscopy investigation of the cell surface of Azospirillum brasilense strains. Microbiology 2017, 86, 487–492. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, Q.; Tang, Q.; Hou, M.; Qi, H.; Chen, G.; Chen, W.; Zhang, J.; Chen, Y.; Xu, X. A simple method for isolating and culturing the rat brain microvascular endothelial cells. Microvasc. Res. 2013, 90, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Khilazheva, E.; Boytsova, E.; Pozhilenkova, E.; Solonchuk, Y.; Salmina, A. Obtaining a three-cell model of a neurovascular unit in vitro. Cell Tissue Res. 2015, 9, 447–451. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, M. Measurement of Reactive Oxygen Species (ROS) and Mitochondrial ROS in AMPK Knockout Mice Blood Vessels. Methods Mol. Biol. 2018, 1732, 507–517. [Google Scholar]
- Schneider, T.; Muthukumar, T.; Hinkelmann, B.; Franke, R.; Döring, M.; Jacob, C.; Sasse, F. Deciphering intracellular targets of organochalcogen based redox catalysts. MedChemComm 2012, 3, 784–787. [Google Scholar] [CrossRef]
- Huang, Y.; Furtmüller, G.; Tong, D.; Zhu, S.; Lee, W.; Brandacher, G.; Kang, J. MEMS-Based Handheld Fourier Domain Doppler Optical Coherence Tomography for Intraoperative Microvascular Anastomosis Imaging. PLoS ONE 2014, 9, e114215. [Google Scholar] [CrossRef]
- You, J.; Du, C.; Volkow, N.D.; Pan, Y. Optical coherence Doppler tomography for quantitative cerebral blood flowimaging. Biomed. Opt. Express 2014, 5, 3217–3230. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source plat-form for biological-image analysis. Nat. Methods 2019, 9, 676–682. [Google Scholar] [CrossRef]
- Mollanji, R.; Bozanovic-Sosic, R.; Zakharov, A.; Makarian, L.; Johnston, M. Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1593–R1599. [Google Scholar] [CrossRef]
- Devos, S.; Miller, T. Direct intraventricular delivery of drugs to the rodent central nervous system. J. Vis. Exp. 2013, 12, e50326. [Google Scholar]
- Koeller, K.; Henry, J. From the archives of the AFIP: Superficial gliomas: Radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics 2001, 21, 1533–1556. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Mackay, A.; Ismer, B.; Pickles, J.C.; Tatevossian, R.G.; Newman, S.; Bale, T.A.; Stoler, I.; Izquierdo, E.; Temelso, S.; et al. Infant High-Grade Gliomas Comprise Multiple Subgroups Characterized by Novel Targetable Gene Fusions and Favorable Outcomes. Cancer Discov. 2020, 10, 942–963. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semyachkina-Glushkovskaya, O.; Sokolovski, S.; Fedosov, I.; Shirokov, A.; Navolokin, N.; Bucharskaya, A.; Blokhina, I.; Terskov, A.; Dubrovski, A.; Telnova, V.; et al. Transcranial Photosensitizer-Free Laser Treatment of Glioblastoma in Rat Brain. Int. J. Mol. Sci. 2023, 24, 13696. https://doi.org/10.3390/ijms241813696
Semyachkina-Glushkovskaya O, Sokolovski S, Fedosov I, Shirokov A, Navolokin N, Bucharskaya A, Blokhina I, Terskov A, Dubrovski A, Telnova V, et al. Transcranial Photosensitizer-Free Laser Treatment of Glioblastoma in Rat Brain. International Journal of Molecular Sciences. 2023; 24(18):13696. https://doi.org/10.3390/ijms241813696
Chicago/Turabian StyleSemyachkina-Glushkovskaya, Oxana, Sergey Sokolovski, Ivan Fedosov, Alexander Shirokov, Nikita Navolokin, Alla Bucharskaya, Inna Blokhina, Andrey Terskov, Alexander Dubrovski, Valeria Telnova, and et al. 2023. "Transcranial Photosensitizer-Free Laser Treatment of Glioblastoma in Rat Brain" International Journal of Molecular Sciences 24, no. 18: 13696. https://doi.org/10.3390/ijms241813696
APA StyleSemyachkina-Glushkovskaya, O., Sokolovski, S., Fedosov, I., Shirokov, A., Navolokin, N., Bucharskaya, A., Blokhina, I., Terskov, A., Dubrovski, A., Telnova, V., Tzven, A., Tzoy, M., Evsukova, A., Zhlatogosrkaya, D., Adushkina, V., Dmitrenko, A., Manzhaeva, M., Krupnova, V., Noghero, A., ... Rafailov, E. (2023). Transcranial Photosensitizer-Free Laser Treatment of Glioblastoma in Rat Brain. International Journal of Molecular Sciences, 24(18), 13696. https://doi.org/10.3390/ijms241813696








