The Antitumor Effect of the DNA Polymerase Alpha Inhibitor ST1926 in Glioblastoma: A Proteomics Approach
Abstract
1. Introduction
2. Results
2.1. POLA1 Levels Are Elevated in GBM Tissues and Are Reduced in GBM Cells upon ST1926 Treatment
2.2. ST1926 Inhibits Cell Growth of GBM Cell Lines
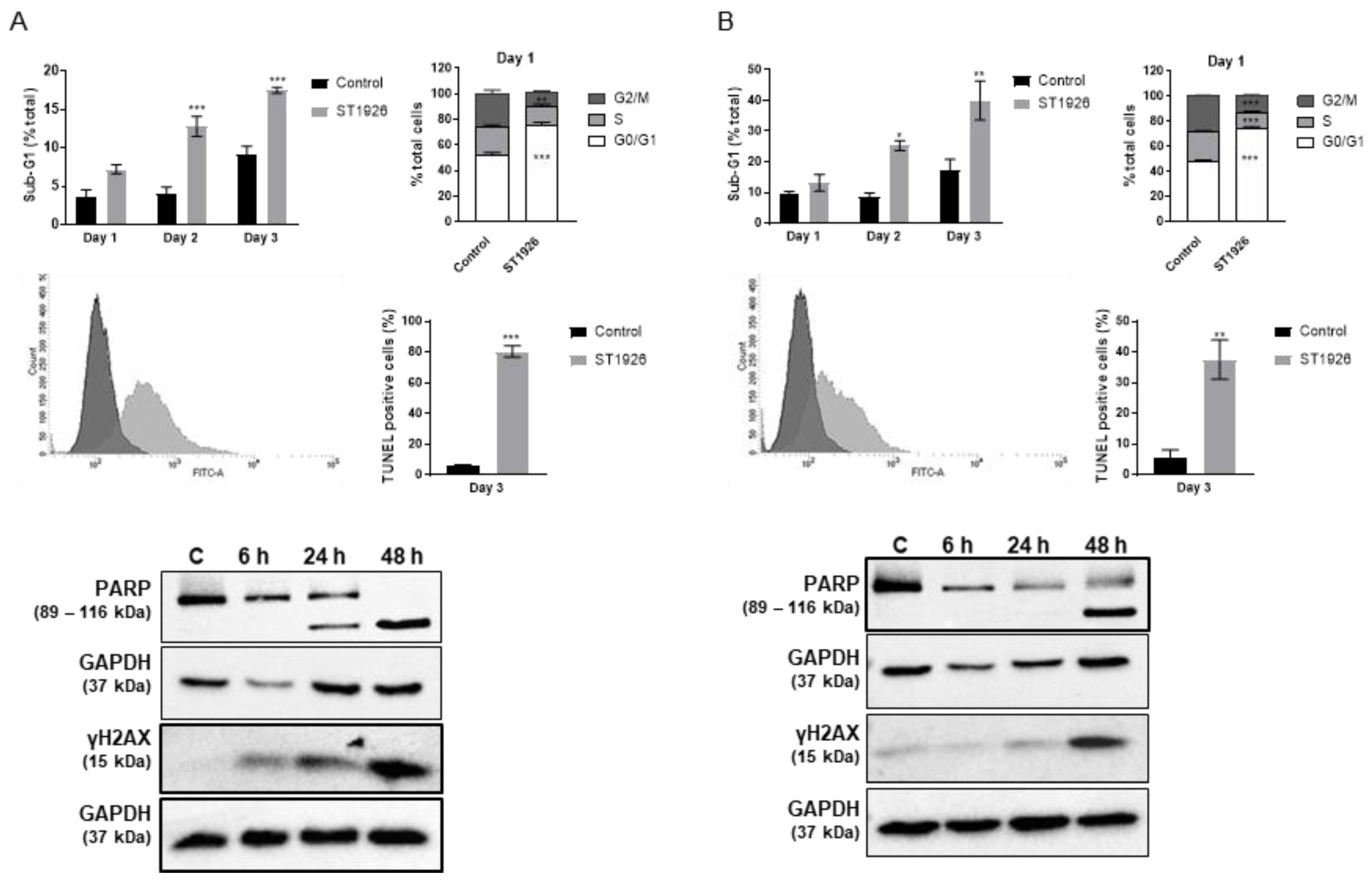
2.3. ST1926 Treatment of GBM Cells Results in G0/G1 Cell Cycle Arrest, Apoptosis, and DNA Damage
2.4. Proteomics Analysis of ST1926-Treated GBM Cells by LC/MS-MS
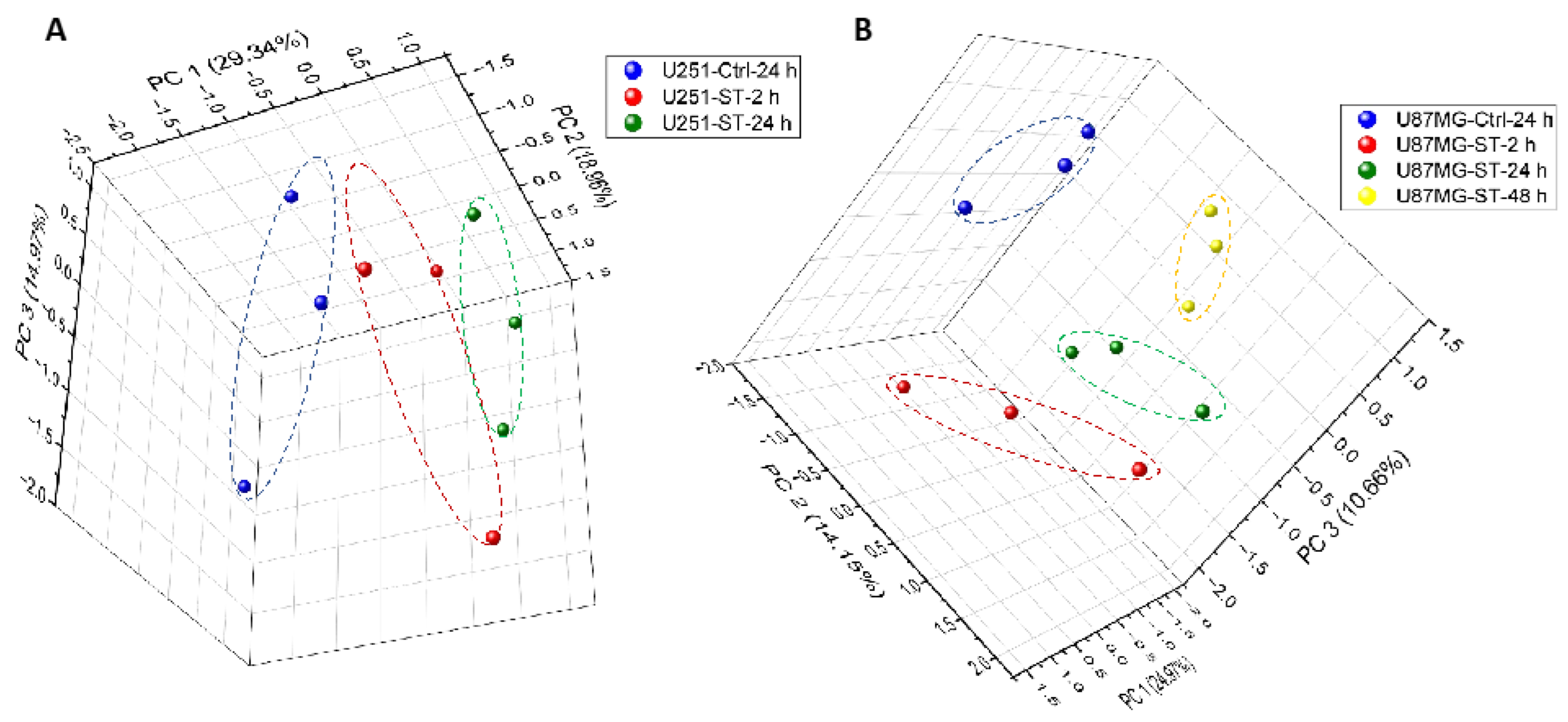
2.4.1. Protein Expression in GBM Cell Lines after Treating with ST1926 Using Principal Component Analysis
2.4.2. Protein Expression in GBM Cell Lines after Treating with ST1926 Using Volcano Plots
2.4.3. Protein Expression in GBM Cell Lines after Treating with ST1926 Using Hierarchical Heatmap Clustering
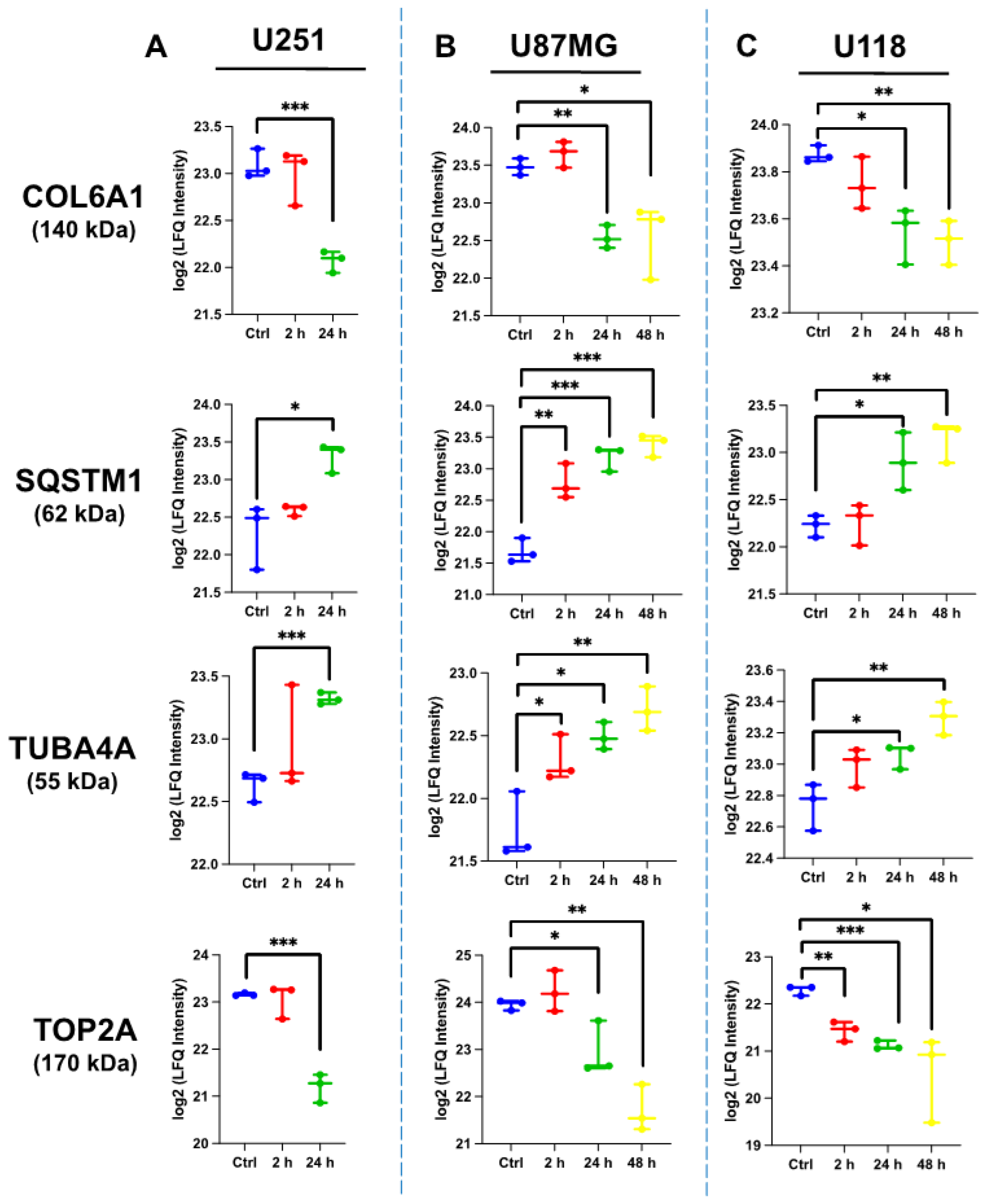
2.4.4. Comparative Protein Expression upon ST1926 Treatment of GBM Cell Lines
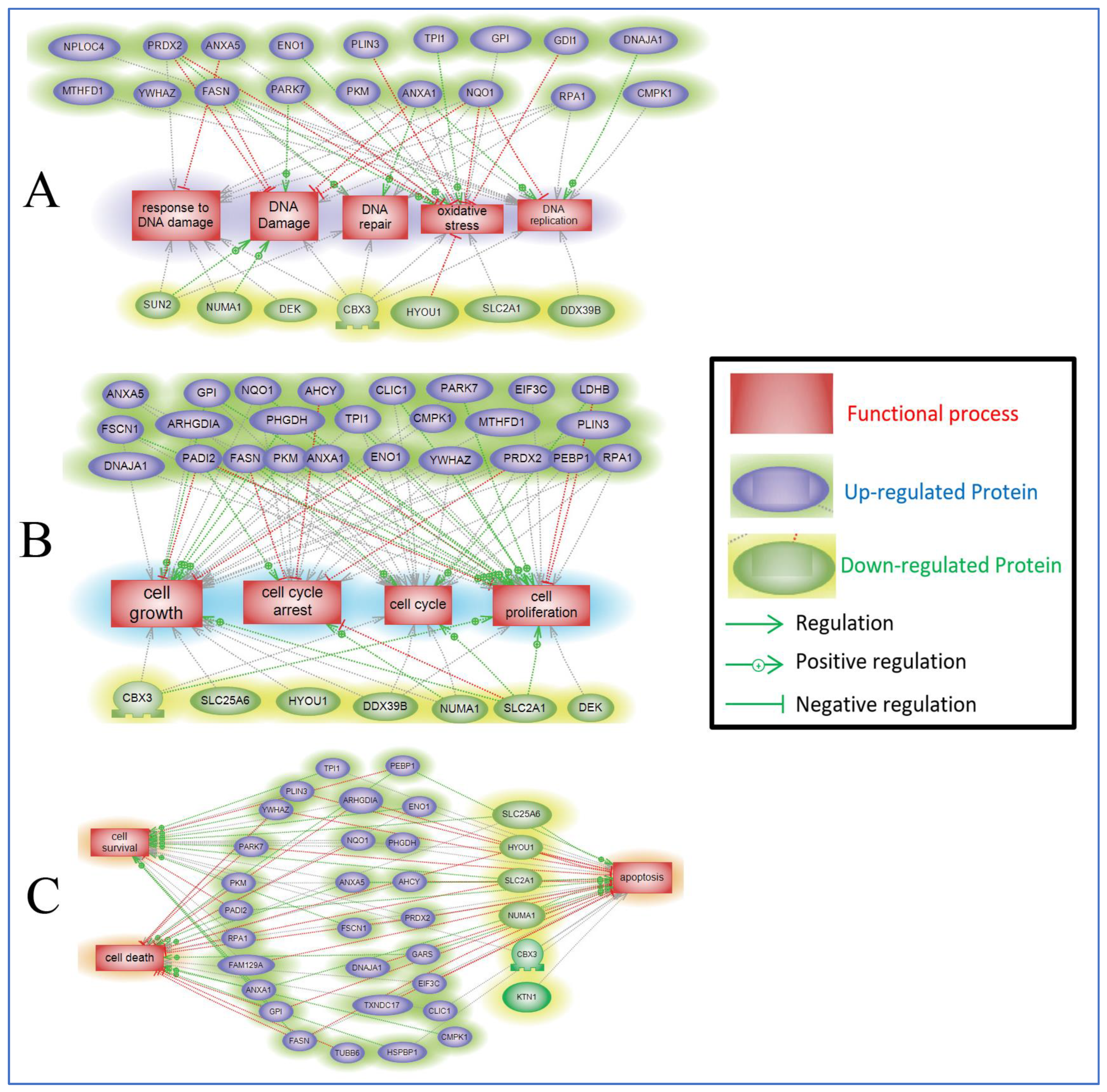
2.5. Protein Pathway Analysis of the Differentially Expressed Proteins in ST1926-Treated GBM Cells
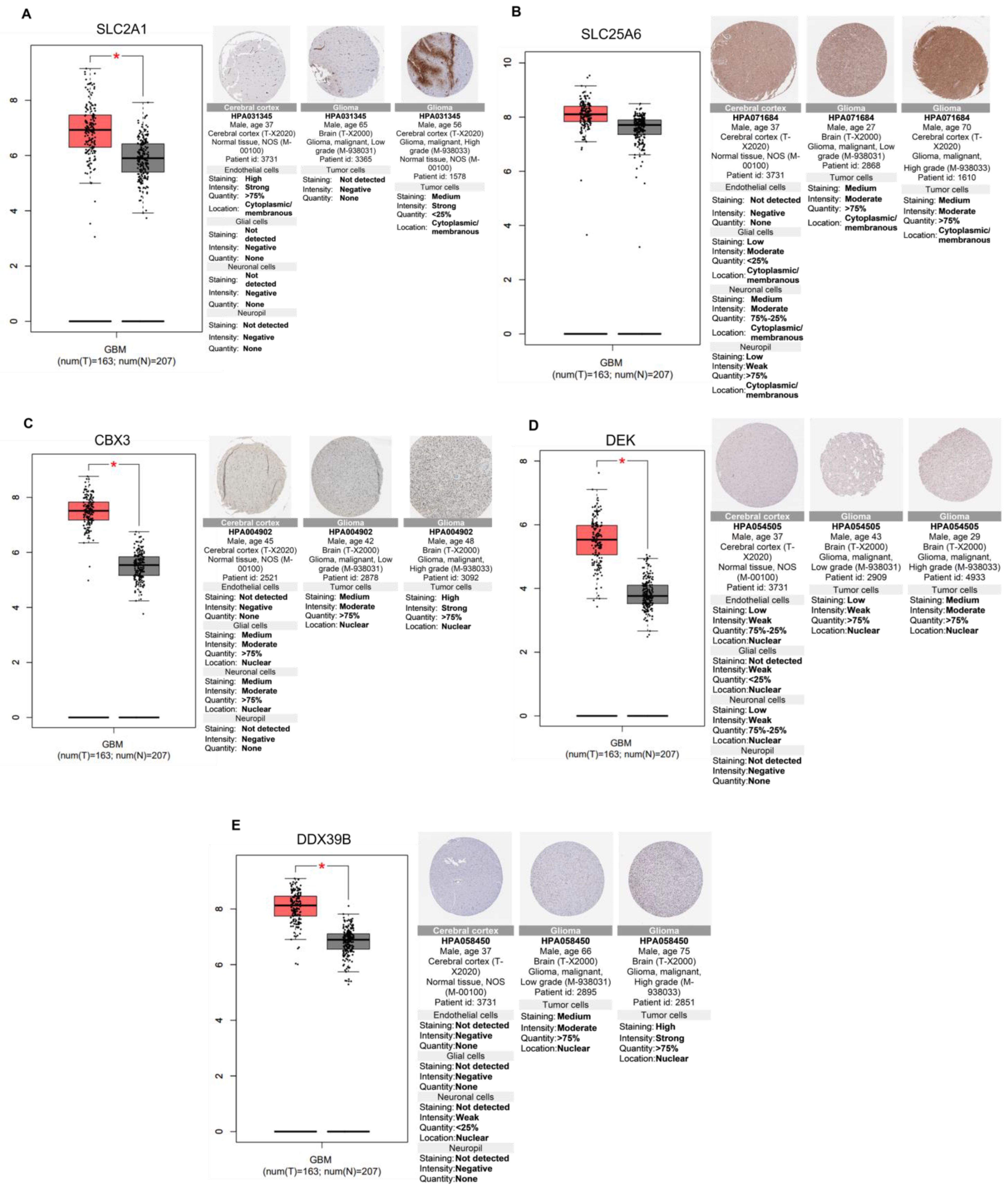
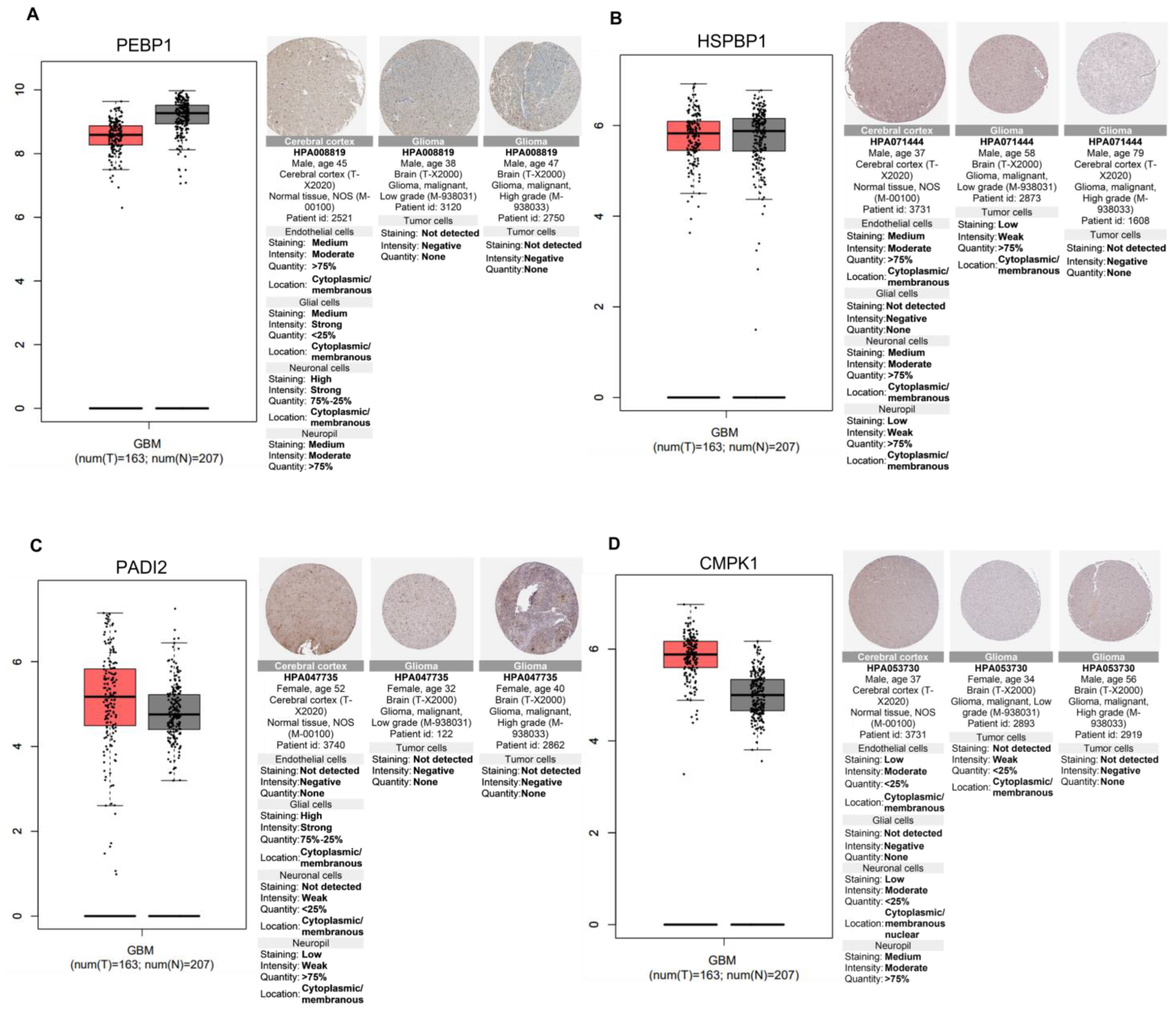
2.6. Relevance of Differentially Altered Genes by ST1926 Treatment between Normal Brain and Glioblastoma Tissues
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Drug Preparation
4.3. Cell Viability MTT Assay
4.4. Cell Cycle Analysis
4.5. TUNEL Assay
4.6. Cell Collections for Proteins Profiling
4.7. Proteomics Analysis
4.7.1. Cell Lysis and Protein Extraction
4.7.2. Protein Digestion
4.7.3. Liquid Chromatography–Mass Spectrometry (LC-MS/MS) Proteomics Analysis
4.7.4. Protein Identification and Quantification
4.8. Western Blot Analysis
4.9. Bioinformatic Analysis
4.9.1. Pathway Analysis
4.9.2. Principal Component Analysis (PCA)
4.9.3. Volcano Plot
4.9.4. Hierarchical Heatmap Clustering
4.9.5. In Silico Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro-Oncol. 2013, 15 (Suppl. 2), ii1–ii56. [Google Scholar] [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef]
- Chen, Y.F.; Shih, P.C.; Kuo, H.M.; Yang, S.N.; Lin, Y.Y.; Chen, W.F.; Tzou, S.J.; Liu, H.T.; Chen, N.F. TP3, an antimicrobial peptide, inhibits infiltration and motility of glioblastoma cells via modulating the tumor microenvironment. Cancer Med. 2020, 9, 3918–3931. [Google Scholar] [CrossRef]
- Bahadur, S.; Sahu, A.K.; Baghel, P.; Saha, S. Current promising treatment strategy for glioblastoma multiform: A review. Oncol. Rev. 2019, 13, 417. [Google Scholar] [CrossRef]
- Binder, Z.A.; O’Rourke, D.M. Glioblastoma: The Current State of Biology and Therapeutic Strategies. Cancer Res. 2022, 82, 769–772. [Google Scholar] [CrossRef]
- Das, A.; Banik, N.L.; Ray, S.K. Retinoids for the Treatment of Glioblastoma. In Glioblastoma; Springer: New York, NY, USA, 2009; pp. 265–281. [Google Scholar]
- Abdel-Samad, R.; Aouad, P.; Darwiche, N. Natural and synthetic retinoids in preclinical colorectal cancer models. Anti-Cancer Drugs 2019, 30, 655–669. [Google Scholar] [CrossRef]
- Basma, H.; Ghayad, S.E.; Rammal, G.; Mancinelli, A.; Harajly, M.; Ghamloush, F.; Dweik, L.; El-Eit, R.; Zalzali, H.; Rabeh, W. The synthetic retinoid ST 1926 as a novel therapeutic agent in rhabdomyosarcoma. Int. J. Cancer 2016, 138, 1528–1537. [Google Scholar] [CrossRef]
- Di Francesco, A.M.; Meco, D.; Torella, A.R.; Barone, G.; D’Incalci, M.; Pisano, C.; Carminati, P.; Riccardi, R. The novel atypical retinoid ST1926 is active in ATRA resistant neuroblastoma cells acting by a different mechanism. Biochem. Pharmacol. 2007, 73, 643–655. [Google Scholar] [CrossRef]
- Zuco, V.; Benedetti, V.; De Cesare, M.; Zunino, F. Sensitization of ovarian carcinoma cells to the atypical retinoid ST1926 by the histone deacetylase inhibitor, RC307: Enhanced DNA damage response. Int. J. Cancer 2010, 126, 1246–1255. [Google Scholar] [CrossRef]
- Bahmad, H.F.; Samman, H.; Monzer, A.; Hadadeh, O.; Cheaito, K.; Abdel-Samad, R.; Hayar, B.; Pisano, C.; Msheik, H.; Liu, Y.N. The synthetic retinoid ST1926 attenuates prostate cancer growth and potentially targets prostate cancer stem-like cells. Mol. Carcinog. 2019, 58, 1208–1220. [Google Scholar] [CrossRef]
- Abdel-Samad, R.; Aouad, P.; Gali-Muhtasib, H.; Sweidan, Z.; Hmadi, R.; Kadara, H.; D’Andrea, E.L.; Fucci, A.; Pisano, C.; Darwiche, N. Mechanism of action of the atypical retinoid ST1926 in colorectal cancer: DNA damage and DNA polymerase α. Am. J. Cancer Res. 2018, 8, 39. [Google Scholar]
- De, L.; Yuan, T.; Yong, Z. ST1926 inhibits glioma progression through regulating mitochondrial complex II. Biomed. Pharmacother. 2020, 128, 110291. [Google Scholar] [CrossRef]
- Shai, R.; Shi, T.; Kremen, T.J.; Horvath, S.; Liau, L.M.; Cloughesy, T.F.; Mischel, P.S.; Nelson, S.F. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene 2003, 22, 4918–4923. [Google Scholar] [CrossRef]
- Sun, L.; Hui, A.-M.; Su, Q.; Vortmeyer, A.; Kotliarov, Y.; Pastorino, S.; Passaniti, A.; Menon, J.; Walling, J.; Bailey, R. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell 2006, 9, 287–300. [Google Scholar] [CrossRef]
- Murat, A.; Migliavacca, E.; Gorlia, T.; Lambiv, W.L.; Shay, T.; Hamou, M.-F.; De Tribolet, N.; Regli, L.; Wick, W.; Kouwenhoven, M.C. Stem cell–related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J. Clin. Oncol. 2008, 26, 3015–3024. [Google Scholar] [CrossRef]
- Bastiancich, C.; Danhier, P.; Préat, V.; Danhier, F. Anticancer drug-loaded hydrogels as drug delivery systems for the local treatment of glioblastoma. J. Control. Release 2016, 243, 29–42. [Google Scholar] [CrossRef]
- Khasraw, M.; Fujita, Y.; Lee-Chang, C.; Balyasnikova, I.V.; Najem, H.; Heimberger, A.B. New Approaches to Glioblastoma. Annu. Rev. Med. 2022, 73, 279–292. [Google Scholar] [CrossRef]
- Tang, X.-H.; Gudas, L.J. Retinoids, retinoic acid receptors, and cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Bayon, Y.; Lopez-Hernandez, F.J.; Piedrafita, F.J. Retinoids in combination therapies for the treatment of cancer: Mechanisms and perspectives. Drug Resist. Updates 2002, 5, 162–175. [Google Scholar] [CrossRef]
- Dallavalle, S.; Zunino, F. Synthetic retinoids as potential antitumour agents. Expert Opin. Ther. Pat. 2005, 15, 1625–1635. [Google Scholar] [CrossRef]
- Garattini, E.; Parrella, E.; Diomede, L.; Gianni’, M.; Kalac, Y.; Merlini, L.; Simoni, D.; Zanier, R.; Ferrara, F.F.; Chiarucci, I. ST1926, a novel and orally active retinoid-related molecule inducing apoptosis in myeloid leukemia cells: Modulation of intracellular calcium homeostasis. Blood 2004, 103, 194–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cincinelli, R.; Dallavalle, S.; Merlini, L.; Penco, S.; Pisano, C.; Carminati, P.; Giannini, G.; Vesci, L.; Gaetano, C.; Illy, B. A novel atypical retinoid endowed with proapoptotic and antitumor activity. J. Med. Chem. 2003, 46, 909–912. [Google Scholar] [CrossRef]
- Aouad, P.; Saikali, M.; Abdel-Samad, R.; Fostok, S.; El-Houjeiri, L.; Pisano, C.; Talhouk, R.; Darwiche, N. Antitumor activities of the synthetic retinoid ST1926 in two-dimensional and three-dimensional human breast cancer models. Anti-Cancer Drugs 2017, 28, 757–770. [Google Scholar] [CrossRef]
- El Hajj, H.; Khalil, B.; Ghandour, B.; Nasr, R.; Shahine, S.; Ghantous, A.; Abdel-Samad, R.; Sinjab, A.; Hasegawa, H.; Jabbour, M. Preclinical efficacy of the synthetic retinoid ST1926 for treating adult T-cell leukemia/lymphoma. Blood J. Am. Soc. Hematol. 2014, 124, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; Vesci, L.; Foderà, R.; Ferrara, F.F.; Rossi, C.; De Cesare, M.; Zuco, V.; Pratesi, G.; Supino, R.; Zunino, F. Antitumor activity of the combination of synthetic retinoid ST1926 and cisplatin in ovarian carcinoma models. Ann. Oncol. 2007, 18, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Maier, D.; Merlo, A.; Tada, M.; Sawamura, Y.; Diserens, A.C.; Van Meir, E.G. Frequent Co-Alterations of TP53, p16/CDKN2A, p14ARF, PTEN Tumor Suppressor Genes in Human Glioma Cell Lines. Brain Pathol. 1999, 9, 469–479. [Google Scholar] [CrossRef]
- Wang, T.-J.; Huang, M.-S.; Hong, C.-Y.; Tse, V.; Silverberg, G.D.; Hsiao, M. Comparisons of tumor suppressor p53, p21, and p16 gene therapy effects on glioblastoma tumorigenicity in situ. Biochem. Biophys. Res. Commun. 2001, 287, 173–180. [Google Scholar] [CrossRef]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105. [Google Scholar]
- Yang, F.; Zou, Y.; Gong, Q.; Chen, J.; Li, W.D.; Huang, Q. From astrocytoma to glioblastoma: A clonal evolution study. FEBS Open Bio 2020, 10, 744–751. [Google Scholar] [CrossRef]
- Zuco, V.; Zanchi, C.; Cassinelli, G.; Lanzi, C.; Supino, R.; Pisano, C.; Zanier, R.; Giordano, V.; Garattini, E.; Zunino, F. Induction of apoptosis and stress response in ovarian carcinoma cell lines treated with ST1926, an atypical retinoid. Cell Death Differ. 2004, 11, 280–289. [Google Scholar] [CrossRef]
- Muzi-Falconi, M.; Giannattasio, M.; Foiani, M.; Plevani, P. The DNA Polymerase _-Primase Complex: Multiple Functions and Interactions. Sci. World J. 2003, 3, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Goralski, M.; Capota, E.; Padrick, S.B.; Kim, J.; Xie, Y.; Nijhawan, D. The antitumor toxin CD437 is a direct inhibitor of DNA polymerase α. Nat. Chem. Biol. 2016, 12, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Guda, M.R.; Labak, C.M.; Omar, S.I.; Asuthkar, S.; Airala, S.; Tuszynski, J.; Tsung, A.J.; Velpula, K.K. GLUT1 and TUBB4 in glioblastoma could be efficacious targets. Cancers 2019, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- Rochette, L.; Meloux, A.; Zeller, M.; Malka, G.; Cottin, Y.; Vergely, C. Mitochondrial SLC25 carriers: Novel targets for cancer therapy. Molecules 2020, 25, 2417. [Google Scholar] [CrossRef]
- Clémençon, B.; Babot, M.; Trézéguet, V. The mitochondrial ADP/ATP carrier (SLC25 family): Pathological implications of its dysfunction. Mol. Asp. Med. 2013, 34, 485–493. [Google Scholar] [CrossRef]
- Ma, C.; Nie, X.G.; Wang, Y.L.; Liu, X.H.; Liang, X.; Zhou, Q.L.; Wu, D.P. CBX3 predicts an unfavorable prognosis and promotes tumorigenesis in osteosarcoma. Mol. Med. Rep. 2019, 19, 4205–4212. [Google Scholar] [CrossRef]
- Niu, H.; Chen, P.; Fan, L.; Sun, B. Comprehensive pan-cancer analysis on CBX3 as a prognostic and immunological biomarker. BMC Med. Genom. 2022, 15, 29. [Google Scholar] [CrossRef]
- Zhao, S.-P.; Wang, F.; Yang, M.; Wang, X.-Y.; Jin, C.-L.; Ji, Q.-K.; Li, S.; Zhao, X.-L. CBX3 promotes glioma U87 cell proliferation and predicts an unfavorable prognosis. J. Neuro-Oncol. 2019, 145, 35–48. [Google Scholar] [CrossRef]
- Riveiro-Falkenbach, E.; Soengas, M.S. Control of tumorigenesis and chemoresistance by the DEK oncogene. Clin. Cancer Res. 2010, 16, 2932–2938. [Google Scholar] [CrossRef]
- Feng, T.; Liu, Y.; Li, C.; Li, Z.; Cai, H. DEK proto-oncogene is highly expressed in astrocytic tumors and regulates glioblastoma cell proliferation and apoptosis. Tumor Biol. 2017, 39, 1010428317716248. [Google Scholar] [CrossRef]
- Zhang, H.; He, C.; Guo, X.; Fang, Y.; Lai, Q.; Wang, X.; Pan, X.; Li, H.; Qin, K.; Li, A. DDX39B contributes to the proliferation of colorectal cancer through direct binding to CDK6/CCND1. Cell Death Discov. 2022, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Li, A.; Lai, Q.; Ding, J.; Yan, Q.; Liu, S.; Li, Q. The DDX39B/FUT3/TGFβR-I axis promotes tumor metastasis and EMT in colorectal cancer. Cell Death Dis. 2021, 12, 74. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Yi, Y.; Qiu, S.-J.; Gao, Q.; Li, Y.-W.; Dai, C.-X.; Cai, M.-Y.; Ju, M.-J.; Zhou, J.; Zhang, B.-H. PEBP1 downregulation is associated to poor prognosis in HCC related to hepatitis B infection. J. Hepatol. 2010, 53, 872–879. [Google Scholar] [CrossRef]
- Gimenez, M.; Marie, S.K.N.; Oba-Shinjo, S.; Uno, M.; Izumi, C.; Oliveira, J.B.; Rosa, J.C. Quantitative proteomic analysis shows differentially expressed HSPB1 in glioblastoma as a discriminating short from long survival factor and NOVA1 as a differentiation factor between low-grade astrocytoma and oligodendroglioma. BMC Cancer 2015, 15, 481. [Google Scholar] [CrossRef]
- Beato, M.; Sharma, P. Peptidyl arginine deiminase 2 (PADI2)-mediated arginine citrullination modulates transcription in cancer. Int. J. Mol. Sci. 2020, 21, 1351. [Google Scholar] [CrossRef]
- Chu, H.; Han, N.; Xu, J. CMPK1 regulated by miR-130b attenuates response to 5-FU treatment in gastric cancer. Front. Oncol. 2021, 11, 637470. [Google Scholar] [CrossRef]
- Liu, N.Q.; De Marchi, T.; Timmermans, A.; Trapman-Jansen, A.M.; Foekens, R.; Look, M.P.; Smid, M.; van Deurzen, C.H.; Span, P.N.; Sweep, F.C. Prognostic significance of nuclear expression of UMP-CMP kinase in triple negative breast cancer patients. Sci. Rep. 2016, 6, 32027. [Google Scholar] [CrossRef]
- Souza, A.P.; Albuquerque, C.; Torronteguy, C.; Frasson, A.; Maito, F.; Pereira, L.; Duval da Silva, V.; Zerwes, F.; Raynes, D.; Guerriero, V. HspBP1 levels are elevated in breast tumor tissue and inversely related to tumor aggressiveness. Cell Stress Chaperones 2009, 14, 301–310. [Google Scholar] [CrossRef]
- Youn, C.K.; Lee, J.-H.; Hariharasudhan, G.; Kim, H.B.; Kim, J.; Lee, S.; Lim, S.-C.; Yoon, S.-P.; Park, S.-G.; Chang, I.-Y. HspBP1 is a dual function regulatory protein that controls both DNA repair and apoptosis in breast cancer cells. Cell Death Dis. 2022, 13, 309. [Google Scholar] [CrossRef]
- Tanimura, S.; Hirano, A.; Hashizume, J.; Yasunaga, M.; Kawabata, T.; Ozaki, K.-i.; Kohno, M. Anticancer drugs up-regulate HspBP1 and thereby antagonize the prosurvival function of Hsp70 in tumor cells. J. Biol. Chem. 2007, 282, 35430–35439. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Thul, P.J.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef] [PubMed]




| Cell Line | Conditions | Number of Up-Regulated Proteins | Number of Down-Regulated Proteins |
|---|---|---|---|
| U251 | Ctrl vs. 2 h | 47 | 30 |
| Ctrl vs. 24 h | 126 | 71 | |
| U118 | Ctrl vs. 2 h | 51 | 45 |
| Ctrl vs. 24 h | 36 | 35 | |
| Ctrl vs. 48 h | 83 | 53 | |
| U87MG | Ctrl vs. 2 h | 54 | 61 |
| Ctrl vs. 24 h | 88 | 79 | |
| Ctrl vs. 48 h | 156 | 126 |
| Common gene alteration among U251, U87MG, and U118 cells (4) |
| DNA topoisomerase 2-alpha (TOP2A) Sequestosome-1/p62 (SQSTM1) Tubulin alpha-4A chain (TUBA4A) Collagen alpha-1(VI) chain (COL6A1) |
| Common gene alteration between U251 and U87MG cells (39) |
| Kinectin (KTN1) Ribosome-binding protein 1(RRBP1) Importin subunit alpha-1 (KPNA) General transcription factor II-I (GTF2I) Proliferation marker protein Ki-67 (MKI67) Galectin-3-binding protein (LGALS3BP) DnaJ homolog subfamily A member (DNAJA1) Golgi apparatus protein 1(GLG1) Procollagen-lysine,2-oxoglutarate 5-dioxygenase 1 (PLOD1) S-phase kinase-associated protein 1 (SKP1) Laminin B2 chain (LAMB2) Staphylococcal nuclease domain-containing protein 1 (SND1) Nuclear mitotic apparatus protein 1 (NUMA1) Guanine nucleotide-binding protein G(s) subunit alpha isoforms short (GNAS) Chromobox protein homolog 1 (CBX1) Solute carrier family 25 member (SLC25A6) X-ray repair cross-complementing protein 5 (XRCC5) 60S ribosomal protein L11 (RPL11) Tryptophan-tRNA ligase (WARS1) 40S ribosomal protein S23 (RPS23) Y-box-binding protein 1 (YBX1) A-kinase anchor protein 12 (AKAP12) Spermine synthase (SMS) Heat shock cognate 71 kDa protein (HSPA8) Fatty acid synthase (FAS) Proliferation-associated protein 2G4 (PA2G4) Triosephosphate isomerase (TIM) Eukaryotic translation initiation factor 3 subunit C (EIF3C) Inosine-5′-monophosphate dehydrogenase 2 (IMPD 2) 14-3-3 protein beta/alpha (YWHAB) Perilipin-3 (PLIN3) Annexin A1 (ANXA1) ADP-ribosylation factor 1 (ARF1) Stathmin (STMN1) FUSE-binding protein 2 (FUBP2) 60S acidic ribosomal protein P2 (RPLP2) Hsc70-interacting protein (FAM10A1) Filamin-A (FLNA) Trifunctional purine biosynthetic protein adenosine-3 (GART) |
| Common gene alteration between U251 and U118 cells (6) |
| Lysosome-associated membrane glycoprotein 2 (LAMP2) SUN domain-containing protein 2 (SUN2) Spliceosome RNA helicase (DDX39B) Nucleosome assembly protein 1-like 4 (NAP1L4) Histidine triad nucleotide-binding protein 1 (HINT1) Adenosylhomocysteinase (AHCY) |
| Common gene alteration between U87MG and U118 cells (10) |
| Endoplasmin (HSP90B1) Guanine nucleotide-binding protein G(i) subunit alpha-2 (GNAI2) Signal transducer and activator of transcription 3 (STAT3) X-ray repair cross-complementing protein 6 (XRCC6) Exportin-1 (XPO1) Transportin-3 (TNPO3) Catenin alpha-1 (CTNAA1) 4F2 cell-surface antigen heavy chain (SLC3A2) Vesicle-associated membrane protein-associated protein A (VAPA) Elongation factor 1-gamma (EF1G) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Baba, C.; Ayache, Z.; Goli, M.; Hayar, B.; Kawtharani, Z.; Pisano, C.; Kobeissy, F.; Mechref, Y.; Darwiche, N. The Antitumor Effect of the DNA Polymerase Alpha Inhibitor ST1926 in Glioblastoma: A Proteomics Approach. Int. J. Mol. Sci. 2023, 24, 14069. https://doi.org/10.3390/ijms241814069
El-Baba C, Ayache Z, Goli M, Hayar B, Kawtharani Z, Pisano C, Kobeissy F, Mechref Y, Darwiche N. The Antitumor Effect of the DNA Polymerase Alpha Inhibitor ST1926 in Glioblastoma: A Proteomics Approach. International Journal of Molecular Sciences. 2023; 24(18):14069. https://doi.org/10.3390/ijms241814069
Chicago/Turabian StyleEl-Baba, Chirine, Zeinab Ayache, Mona Goli, Berthe Hayar, Zeinab Kawtharani, Claudio Pisano, Firas Kobeissy, Yehia Mechref, and Nadine Darwiche. 2023. "The Antitumor Effect of the DNA Polymerase Alpha Inhibitor ST1926 in Glioblastoma: A Proteomics Approach" International Journal of Molecular Sciences 24, no. 18: 14069. https://doi.org/10.3390/ijms241814069
APA StyleEl-Baba, C., Ayache, Z., Goli, M., Hayar, B., Kawtharani, Z., Pisano, C., Kobeissy, F., Mechref, Y., & Darwiche, N. (2023). The Antitumor Effect of the DNA Polymerase Alpha Inhibitor ST1926 in Glioblastoma: A Proteomics Approach. International Journal of Molecular Sciences, 24(18), 14069. https://doi.org/10.3390/ijms241814069










