A Genome-Wide Analysis of the Pentatricopeptide Repeat Protein Gene Family in Two Kiwifruit Species with an Emphasis on the Role of RNA Editing in Pathogen Stress
Abstract
:1. Introduction
2. Results
2.1. Identification and Synteny Analysis of PPR Members in Two Kiwifruit Species

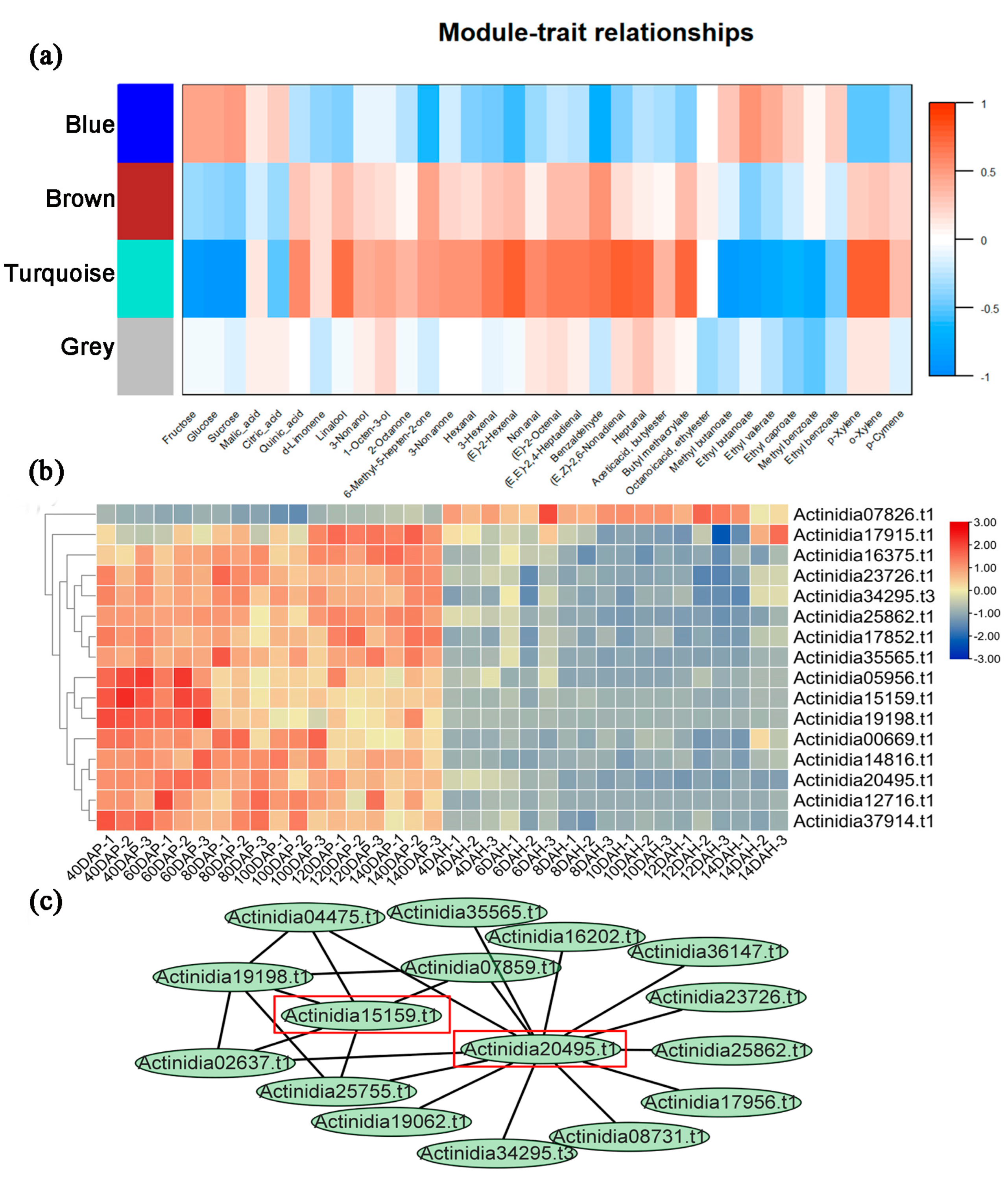
2.2. PPRs Play Roles in Fruit Development and Ripening
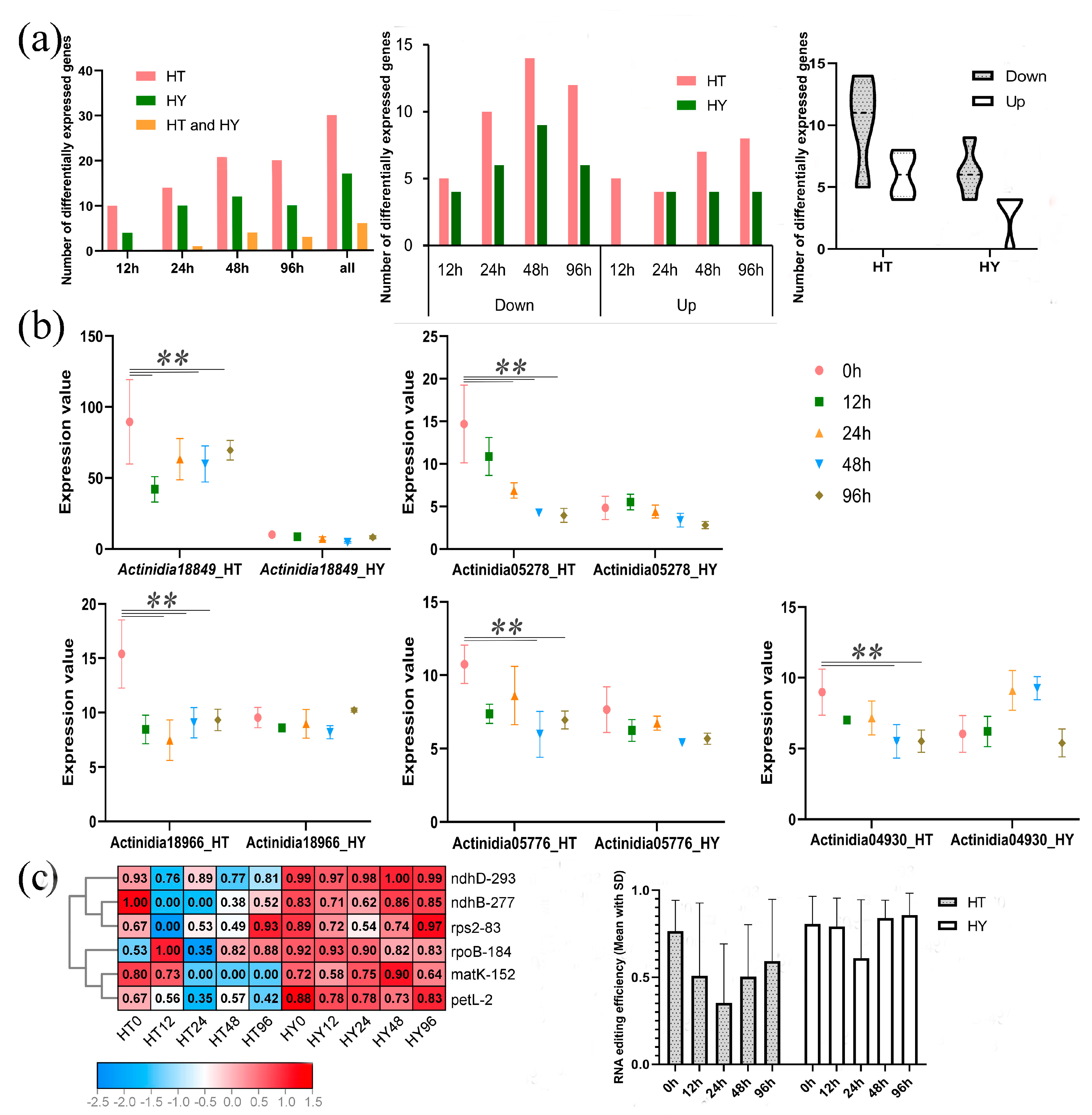
2.3. Different PPR Expression and RNA Editing in Response to Psa Infection in Two Kiwifruit Species
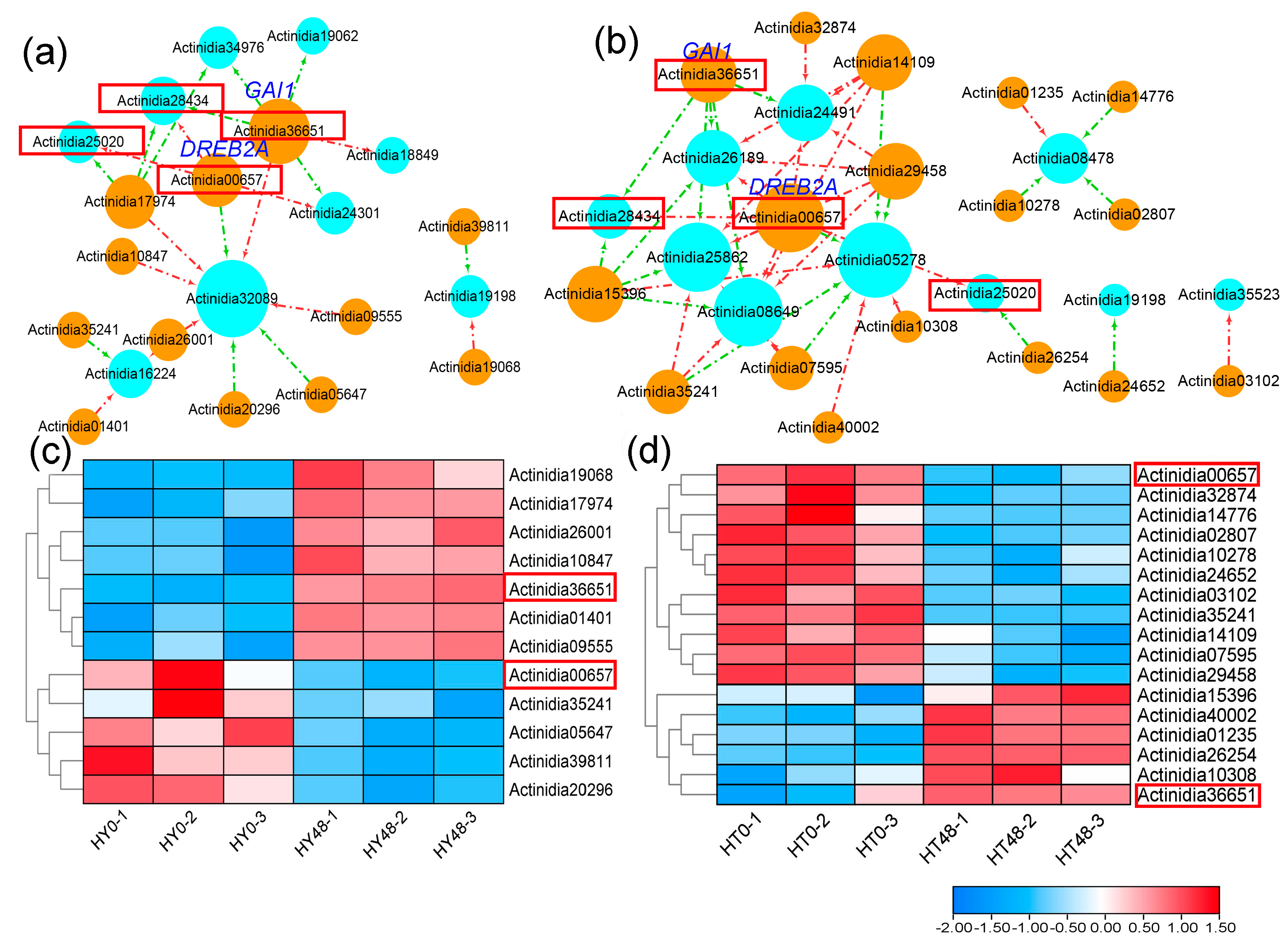
2.4. Upstream Transcription Factors Associated with PPRs in Kiwifruit
3. Discussion
4. Methods and Materials
4.1. Genome-Wide Identification of PPRs in Ach and Ace
4.2. Subcellular Localization and Physical Localization
4.3. Synteny Analysis of PPRs in Two Kiwifruit Species
4.4. Expression Analysis of PPR Genes in Ach and Ace
4.5. Gene Co-Expression Network Analysis
4.6. Identification of RNA Editing Sites
4.7. Identification of Upstream Regulatory Transcription Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Park, Y.S.; Im, M.H.; Ham, K.S.; Kang, S.G.; Park, Y.K.; Namiesnik, J.; Leontowicz, H.; Leontowicz, M.; Katrich, E.; Gorinstein, S. Nutritional and pharmaceutical properties of bioactive compounds in organic and conventional growing kiwifruit. Plant Foods Hum. Nutr. 2013, 68, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Di Cecco, I.; Arena, S.; Scaloni, A.; Scortichini, M. Proteomic changes in Actinidia chinensis shoot during systemic infection with a pandemic Pseudomonas syringae pv. actinidiae strain. J. Proteom. 2013, 78, 461–476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, H.; Xia, H.; Zhong, C.; Li, L.; Zeng, C. Genomic characterization of two nickie-like bacteriophages that infect the kiwifruit canker phytopathogen Pseudomonas syringae pv. actinidiae. Arch. Virol. 2022, 167, 1713–1715. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Dai, D.; Lv, L.; Ahmed, T.; Chen, L.; Wang, Y.; An, Q.; Sun, G.; Li, B. Advancements in the Use of Bacteriophages to Combat the Kiwifruit Canker Phytopathogen Pseudomonas syringae pv. actinidiae. Viruses 2022, 14, 2704. [Google Scholar] [CrossRef]
- Fiorillo, A.; Frezza, D.; Di Lallo, G.; Visconti, S. A Phage Therapy Model for the Prevention of Pseudomonas syringae pv. actinidiae Infection of Kiwifruit Plants. Plant Dis. 2023, 107, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Bentolila, S.; Hanson, M.R. The Unexpected Diversity of Plant Organelle RNA Editosomes. Trends Plant Sci. 2016, 21, 962–973. [Google Scholar] [CrossRef]
- Bentolila, S.; Oh, J.; Hanson, M.R.; Bukowski, R. Comprehensive high-resolution analysis of the role of an Arabidopsis gene family in RNA editing. PLoS Genet 2013, 9, e1003584. [Google Scholar] [CrossRef]
- Oldenkott, B.; Yamaguchi, K.; Tsuji-Tsukinoki, S.; Knie, N.; Knoop, V. Chloroplast RNA editing going extreme: More than 3400 events of C-to-U editing in the chloroplast transcriptome of the lycophyte Selaginella uncinata. RNA 2014, 20, 1499–1506. [Google Scholar] [CrossRef]
- Giege, P.; Brennicke, A. RNA editing in Arabidopsis mitochondria effects 441 C to U changes in ORFs. Proc. Natl. Acad. Sci. USA 1999, 96, 15324–15329. [Google Scholar] [CrossRef]
- Wang, D.; Meng, S.; Su, W.; Bao, Y.; Lu, Y.; Yin, W.; Liu, C.; Xia, X. Genome-Wide Analysis of Multiple Organellar RNA Editing Factor Family in Poplar Reveals Evolution and Roles in Drought Stress. Int. J. Mol. Sci. 2019, 20, 1425. [Google Scholar] [CrossRef]
- Chateigner-Boutin, A.L.; Small, I. Plant RNA editing. RNA Biol. 2010, 7, 213–219. [Google Scholar] [CrossRef]
- Xiong, J.; Tao, T.; Luo, Z.; Yan, S.; Liu, Y.; Yu, X.; Liu, G.; Xia, H.; Luo, L. RNA Editing Responses to Oxidative Stress between a Wild Abortive Type Male-Sterile Line and Its Maintainer Line. Front. Plant Sci. 2017, 8, 2023. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.M. Salinity effects on nad3 gene RNA editing of wild barley mitochondria. Mol. Biol. Rep. 2020, 47, 3857–3865. [Google Scholar] [CrossRef]
- Zhang, A.; Jiang, X.; Zhang, F.; Wang, T.; Zhang, X. Dynamic response of RNA editing to temperature in grape by RNA deep sequencing. Funct. Integr. Genom. 2020, 20, 421–432. [Google Scholar] [CrossRef]
- Zhang, A.; Xiong, Y.; Fang, J.; Liu, K.; Peng, H.; Zhang, X. Genome-wide identification and expression analysis of peach multiple organellar RNA editing factors reveals the roles of RNA editing in plant immunity. BMC Plant Biol. 2022, 22, 583. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Fang, J.; Jiang, X.; Wang, T.; Liu, K.; Peng, H.; Zhang, X.; Zhang, A. Genome-Wide Analysis of Multiple Organellar RNA Editing Factor (MORF) Family in Kiwifruit (Actinidia chinensis) Reveals Its Roles in Chloroplast RNA Editing and Pathogens Stress. Plants 2022, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Andrade, J.; Ramirez, V.; Lopez, A.; Vera, P. Mediated plastid RNA editing in plant immunity. PLoS Pathog. 2013, 9, e1003713. [Google Scholar] [CrossRef]
- Takenaka, M.; Zehrmann, A.; Verbitskiy, D.; Kugelmann, M.; Hartel, B.; Brennicke, A. Multiple organellar RNA editing factor (MORF) family proteins are required for RNA editing in mitochondria and plastids of plants. Proc. Natl. Acad. Sci. USA 2012, 109, 5104–5109. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, W.; Hedtke, B.; Zhong, L.; Liu, L.; Peng, L.; Lu, C.; Grimm, B.; Lin, R. Tetrapyrrole biosynthetic enzyme protoporphyrinogen IX oxidase 1 is required for plastid RNA editing. Proc. Natl. Acad. Sci. USA 2014, 111, 2023–2028. [Google Scholar] [CrossRef]
- Barkan, A.; Small, I. Pentatricopeptide repeat proteins in plants. Annu. Rev. Plant Biol. 2014, 65, 415–442. [Google Scholar] [CrossRef]
- Cheng, S.F.; Gutmann, B.; Zhong, X.; Ye, Y.T.; Fisher, M.F.; Bai, F.Q.; Castleden, I.; Song, Y.; Song, B.; Huang, J.Y.; et al. Redefining the structural motifs that determine RNA binding and RNA editing by pentatricopeptide repeat proteins in land plants. Plant J. 2016, 85, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Huang, Z.Q.; Ma, B.; Yang, Y.Z.; Xiu, Z.H.; Wang, L.; Tan, B.C. Maize PPR-E proteins mediate RNA C-to-U editing in mitochondria by recruiting the trans deaminase PCW1. Plant Cell 2023, 35, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, B.; Royan, S.; Schallenberg-Rudinger, M.; Lenz, H.; Castleden, I.R.; McDowell, R.; Vacher, M.A.; Tonti-Filippini, J.; Bond, C.S.; Knoop, V.; et al. The Expansion and Diversification of Pentatricopeptide Repeat RNA-Editing Factors in Plants. Mol. Plant 2020, 13, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Zhao, P.; Sun, J.; Zhao, Y.; Zhang, Y.; Yang, Q.; Wang, W.; Chen, Z.; Mai, T.; Zou, Y.; et al. Research Progress of PPR Proteins in RNA Editing, Stress Response, Plant Growth and Development. Front. Genet. 2021, 12, 765580. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.J.; Xiu, Z.H.; Meeley, R.; Tan, B.C. Empty Pericarp5 Encodes a Pentatricopeptide Repeat Protein That Is Required for Mitochondrial RNA Editing and Seed Development in Maize. Plant Cell 2013, 25, 868–883. [Google Scholar] [CrossRef]
- Kotera, E.; Tasaka, M.; Shikanai, T. A pentatricopeptide repeat protein is essential for RNA editing in chloroplasts. Nature 2005, 433, 326–330. [Google Scholar] [CrossRef]
- Lee, K.; Kang, H. Engineering of pentatricopeptide repeat proteins in organellar gene regulation. Front. Plant Sci. 2023, 14, 1144298. [Google Scholar] [CrossRef]
- Best, C.; Mizrahi, R.; Edris, R.; Tang, H.; Zer, H.; Colas des Francs-Small, C.; Finkel, O.M.; Zhu, H.; Small, I.D.; Ostersetzer-Biran, O. MSP1 encodes an essential RNA-binding pentatricopeptide repeat factor required for nad1 maturation and complex I biogenesis in Arabidopsis mitochondria. New Phytol. 2023, 238, 2375–2392. [Google Scholar] [CrossRef]
- Zheng, S.; Dong, J.; Lu, J.; Li, J.; Jiang, D.; Yu, H.; Ye, S.; Bu, W.; Liu, Z.; Zhou, H.; et al. A cytosolic pentatricopeptide repeat protein is essential for tapetal plastid development by regulating OsGLK1 transcript levels in rice. New Phytol. 2022, 234, 1678–1695. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Liu, X.Y.; Tang, J.J.; Wang, Y.; Xu, C.; Tan, B.C. GRP23 plays a core role in E-type editosomes via interacting with MORFs and atypical PPR-DYWs in Arabidopsis mitochondria. Proc. Natl. Acad. Sci. USA 2022, 119, e2210978119. [Google Scholar] [CrossRef]
- Lu, K.; Li, C.; Guan, J.; Liang, W.H.; Chen, T.; Zhao, Q.Y.; Zhu, Z.; Yao, S.; He, L.; Wei, X.D.; et al. The PPR-Domain Protein SOAR1 Regulates Salt Tolerance in Rice. Rice 2022, 15, 62. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, D.; Li, Z.A.; Molloy, D.P.; Luo, Z.F.; Su, Y.; Li, H.O.; Liu, Q.; Wang, R.Z.; Xiao, L.T. The PPR protein RARE1-mediated editing of chloroplast accD transcripts is required for fatty acid biosynthesis and heat tolerance in Arabidopsis. Plant Commun. 2022, 4, 100461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, C.; Wang, Y.; He, M.; Li, Z.; Shen, L.; Li, Q.; Zhu, L.; Ren, D.; Hu, J.; et al. OsPPR11 encoding P-type PPR protein that affects group II intron splicing and chloroplast development. Plant Cell Rep. 2023, 42, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [PubMed]
- Boos, F.; Mühlhaus, T.; Herrmann, J.M. Detection of Internal Matrix Targeting Signal-like Sequences (iMTS-Ls) in Mitochondrial Precursor Proteins Using the TargetP Prediction Tool. Bio-Protocol 2018, 8, e2474. [Google Scholar] [CrossRef]
- Small, I.; Peeters, N.; Legeai, F.; Lurin, C. Predotar: A tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 2004, 4, 1581–1590. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Wang, R.; Shu, P.; Zhang, C.; Zhang, J.; Chen, Y.; Zhang, Y.; Du, K.; Xie, Y.; Li, M.; Ma, T.; et al. Integrative analyses of metabolome and genome-wide transcriptome reveal the regulatory network governing flavor formation in kiwifruit (Actinidia chinensis). New Phytol. 2022, 233, 373–389. [Google Scholar] [CrossRef]
- Song, Y.; Sun, L.; Lin, M.; Chen, J.; Qi, X.; Hu, C.; Fang, J. Comparative transcriptome analysis of resistant and susceptible kiwifruits in response to Pseudomonas syringae pv. actinidiae during early infection. PLoS ONE 2019, 14, e0211913. [Google Scholar] [CrossRef]
- Hayes, M.L.; Giang, K.; Mulligan, R.M. Molecular evolution of pentatricopeptide repeat genes reveals truncation in species lacking an editing target and structural domains under distinct selective pressures. BMC Evol. Biol. 2012, 12, 66. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, S.; Bi, C.; Mei, C.; Jiang, S.C.; Wang, X.F.; Lu, Z.J.; Zhang, D.P. Arabidopsis exoribonuclease USB1 interacts with the PPR-domain protein SOAR1 to negatively regulate abscisic acid signaling. J. Exp. Bot. 2020, 71, 5837–5851. [Google Scholar] [CrossRef]
- Liu, J.M.; Zhao, J.Y.; Lu, P.P.; Chen, M.; Guo, C.H.; Xu, Z.S.; Ma, Y.Z. The E-Subgroup Pentatricopeptide Repeat Protein Family in Arabidopsis thaliana and Confirmation of the Responsiveness PPR96 to Abiotic Stresses. Front. Plant Sci. 2016, 7, 1825. [Google Scholar] [CrossRef] [PubMed]
- Amirsadeghi, S.; Robson, C.A.; Vanlerberghe, G.C. The role of the mitochondrion in plant responses to biotic stress. Physiol. Plant. 2007, 129, 253–266. [Google Scholar] [CrossRef]
- Zsigmond, L.; Rigó, G.; Szarka, A.; Székely, G.; Otvös, K.; Darula, Z.; Medzihradszky, K.F.; Koncz, C.; Koncz, Z.; Szabados, L. Arabidopsis PPR40 connects abiotic stress responses to mitochondrial electron transport. Plant Physiol. 2008, 146, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Loiacono, F.V.; Walther, D.; Seeger, S.; Thiele, W.; Gerlach, I.; Karcher, D.; Schottler, M.A.; Zoschke, R.; Bock, R. Emergence of Novel RNA-Editing Sites by Changes in the Binding Affinity of a Conserved PPR Protein. Mol. Biol. Evol. 2022, 39, msac222. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Danecek, P.; McCarthy, S.A. BCFtools/csq: Haplotype-aware variant consequences. Bioinformatics 2017, 33, 2037–2039. [Google Scholar] [CrossRef]
- Wu, S.; Liu, W.; Aljohi, H.A.; Alromaih, S.A.; Alanazi, I.O.; Lin, Q.; Yu, J.; Hu, S. REDO: RNA Editing Detection in Plant Organelles Based on Variant Calling Results. J. Comput. Biol. 2018, 25, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Yang, D.C.; Meng, Y.Q.; Jin, J.; Gao, G. PlantRegMap: Charting functional regulatory maps in plants. Nucleic Acids Res. 2020, 48, D1104–D1113. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Lopes, C.T.; Fong, D.; Kucera, M.; Cheung, M.; Siper, M.C.; Huck, G.; Dong, Y.; Sumer, O.; Bader, G.D. Cytoscape.js 2023 update: A graph theory library for visualization and analysis. Bioinformatics 2023, 39, btad031. [Google Scholar] [CrossRef] [PubMed]

| Type | Actinidia chinensis (Ach) | Actinidia eriantha (Ace) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| P | 251 | 50.5% | 247 | 49.50% |
| E | 91 | 18.31% | 74 | 14.83% |
| E+ | 95 | 19.11% | 102 | 20.44% |
| DYW | 60 | 12.07% | 76 | 15.23% |
| All | 497 | / | 499 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Xiong, Y.; Liu, F.; Zhang, X. A Genome-Wide Analysis of the Pentatricopeptide Repeat Protein Gene Family in Two Kiwifruit Species with an Emphasis on the Role of RNA Editing in Pathogen Stress. Int. J. Mol. Sci. 2023, 24, 13700. https://doi.org/10.3390/ijms241813700
Zhang A, Xiong Y, Liu F, Zhang X. A Genome-Wide Analysis of the Pentatricopeptide Repeat Protein Gene Family in Two Kiwifruit Species with an Emphasis on the Role of RNA Editing in Pathogen Stress. International Journal of Molecular Sciences. 2023; 24(18):13700. https://doi.org/10.3390/ijms241813700
Chicago/Turabian StyleZhang, Aidi, Yuhong Xiong, Fang Liu, and Xiujun Zhang. 2023. "A Genome-Wide Analysis of the Pentatricopeptide Repeat Protein Gene Family in Two Kiwifruit Species with an Emphasis on the Role of RNA Editing in Pathogen Stress" International Journal of Molecular Sciences 24, no. 18: 13700. https://doi.org/10.3390/ijms241813700
APA StyleZhang, A., Xiong, Y., Liu, F., & Zhang, X. (2023). A Genome-Wide Analysis of the Pentatricopeptide Repeat Protein Gene Family in Two Kiwifruit Species with an Emphasis on the Role of RNA Editing in Pathogen Stress. International Journal of Molecular Sciences, 24(18), 13700. https://doi.org/10.3390/ijms241813700






