Theoretical Analysis of Riboflavin Adsorption on Hexagonal Boron Nitride for Drug Delivery Applications: Unveiling the Influence of Point Defects
Abstract
1. Introduction
2. Results and Discussion
2.1. Molecular Geometry and Binding Energies
2.2. Charge Analysis
2.3. Orbital Analysis
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, T.D.; Whitehead, K.A.; Mitragotri, S. Materials for Oral Delivery of Proteins and Peptides. Nat. Rev. Mater. 2020, 5, 127–148. [Google Scholar] [CrossRef]
- Rao, P.N.; Crippin, J.; Levine, E.; Hunt, J.; Baliga, S.; Balart, L.; Anthony, L.; Mulekar, M.; Raj, M.H.G. Elevation of Serum Riboflavin Carrier Protein in Hepatocellular Carcinoma. Hepatol. Res. 2006, 35, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.; Ouhtit, A.; Gaur, R.; Fernando, A.; Schwarzenberger, P.; Su, J.; Ismail, M.F.; El-Sayyad, H.I.; Karande, A.; Elmageed, Z.A.; et al. Biochemical Characterization of Riboflavin Carrier Protein (RCP) in Prostate Cancer. Front. Biosci.-Landmark 2009, 14, 3634–3640. [Google Scholar] [CrossRef] [PubMed]
- Pliszka, M.; Szablewski, L. Glucose Transporters as a Target for Anticancer Therapy. Cancers 2021, 13, 4184. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, J.; Liu, Y.; Zhang, X.; Tang, Y. Constructing Transferrin Receptor Targeted Drug Delivery System by Using Doxorubicin Hydrochloride and Vanadocene Dichloride. Bioorg. Med. Chem. Lett. 2011, 21, 5982–5986. [Google Scholar] [CrossRef]
- Koo, H.; Huh, M.S.; Sun, I.-C.; Yuk, S.H.; Choi, K.; Kim, K.; Kwon, I.C. In Vivo Targeted Delivery of Nanoparticles for Theranosis. Acc. Chem. Res. 2011, 44, 1018–1028. [Google Scholar] [CrossRef]
- Han, J.; Zou, H.Y.; Gao, M.X.; Huang, C.Z. A Graphitic Carbon Nitride Based Fluorescence Resonance Energy Transfer Detection of Riboflavin. Talanta 2016, 148, 279–284. [Google Scholar] [CrossRef]
- Kundu, A.; Nandi, S.; Layek, R.K.; Nandi, A.K. Fluorescence Resonance Energy Transfer from Sulfonated Graphene to Riboflavin: A Simple Way to Detect Vitamin B2. ACS Appl. Mater. Interfaces 2013, 5, 7392–7399. [Google Scholar] [CrossRef]
- Khaydukov, E.V.; Mironova, K.E.; Semchishen, V.A.; Generalova, A.N.; Nechaev, A.V.; Khochenkov, D.A.; Stepanova, E.V.; Lebedev, O.I.; Zvyagin, A.V.; Deyev, S.M.; et al. Riboflavin Photoactivation by Upconversion Nanoparticles for Cancer Treatment. Sci. Rep. 2016, 6, 35103. [Google Scholar] [CrossRef]
- Rao, P.N.; Levine, E.; Myers, M.O.; Prakash, V.; Watson, J.; Stolier, A.; Kopicko, J.J.; Kissinger, P.; Raj, S.G.; Raj, M.H.G. Elevation of Serum Riboflavin Carrier Protein in Breast Cancer. Cancer Epidemiol. Biomarkers Prev. 1999, 8, 985–990. [Google Scholar]
- Bareford, L.M.; Phelps, M.A.; Foraker, A.B.; Swaan, P.W. Intracellular Processing of Riboflavin in Human Breast Cancer Cells. Mol. Pharm. 2008, 5, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Bareford, L.M.; Avaritt, B.R.; Ghandehari, H.; Nan, A.; Swaan, P.W. Riboflavin-Targeted Polymer Conjugates for Breast Tumor Delivery. Pharm. Res. 2013, 30, 1799–1812. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, Y.; Eremin, S.A.; Rybakov, V.B.; Zhang, T.; Xu, Z.; Ren, L.; He, X.; Meng, M.; Xi, R. Indirect Competitive Immunoassay for Detection of Vitamin B2 in Foods and Pharmaceuticals. J. Agric. Food Chem. 2013, 61, 7048–7054. [Google Scholar] [CrossRef]
- Petteys, B.J.; Frank, E.L. Rapid Determination of Vitamin B2 (Riboflavin) in Plasma by HPLC. Clin. Chim. Acta 2011, 412, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yang, X.; Wang, C.; Yuan, H.; Xiao, D. Determination of Riboflavin in Urine and Beverages by Capillary Electrophoresis with In-Column Optical Fiber Laser-Induced Fluorescence Detection. J. Chromatogr. B 2007, 856, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, B.; Liu, J.; Zhang, P.; Li, Z.; Luan, Y. Green Fabricated Reduced Graphene Oxide: Evaluation of Its Application as Nano-Carrier for PH-Sensitive Drug Delivery. Int. J. Pharm. 2015, 496, 984–992. [Google Scholar] [CrossRef]
- Iannazzo, D.; Pistone, A.; Celesti, C.; Triolo, C.; Patané, S.; Giofré, S.V.; Romeo, R.; Ziccarelli, I.; Mancuso, R.; Gabriele, B.; et al. A Smart Nanovector for Cancer Targeted Drug Delivery Based on Graphene Quantum Dots. Nanomaterials 2019, 9, 282. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Wu, X.-Y.; Yu, Y.-Y.; Sun, D.-Z.; Jia, H.-H.; Yong, Y.-C. Facile In-Situ Fabrication of Graphene/Riboflavin Electrode for Microbial Fuel Cells. Electrochim. Acta 2017, 232, 439–444. [Google Scholar] [CrossRef]
- Sharifi, R.; Samaraweera, M.; Gascón, J.A.; Papadimitrakopoulos, F. Thermodynamics of the Quasi-Epitaxial Flavin Assembly around Various-Chirality Carbon Nanotubes. J. Am. Chem. Soc. 2014, 136, 7452–7463. [Google Scholar] [CrossRef]
- Kalachikova, P.M.; Goldt, A.E.; Khabushev, E.M.; Eremin, T.V.; Zatsepin, T.S.; Obraztsova, E.D.; Larionov, K.V.; Antipina, L.Y.; Sorokin, P.B.; Nasibulin, A.G. Single-Step Extraction of Small-Diameter Single-Walled Carbon Nanotubes in the Presence of Riboflavin. Beilstein J. Nanotechnol. 2022, 13, 1564–1571. [Google Scholar] [CrossRef]
- Ju, S.-Y.; Doll, J.; Sharma, I.; Papadimitrakopoulos, F. Selection of Carbon Nanotubes with Specific Chiralities Using Helical Assemblies of Flavin Mononucleotide. Nat. Nanotechnol. 2008, 3, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Ayán-Varela, M.; Paredes, J.I.; Guardia, L.; Villar-Rodil, S.; Munuera, J.M.; Díaz-González, M.; Fernández-Sánchez, C.; Martínez-Alonso, A.; Tascón, J.M.D. Achieving Extremely Concentrated Aqueous Dispersions of Graphene Flakes and Catalytically Efficient Graphene-Metal Nanoparticle Hybrids with Flavin Mononucleotide as a High-Performance Stabilizer. ACS Appl. Mater. Interfaces 2015, 7, 10293–10307. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Chapter 2—Functionalization of Boron Nitride Nanotubes for Applications in Nanobiomedicine. In Boron Nitride Nanotubes in Nanomedicine; Ciofani, G., Mattoli, V., Eds.; Micro and Nano Technologies; William Andrew Publishing: Boston, FL, USA, 2016; pp. 17–40. ISBN 978-0-323-38945-7. [Google Scholar]
- Gao, Z.; Zhi, C.; Bando, Y.; Golberg, D.; Serizawa, T. Noncovalent Functionalization of Disentangled Boron Nitride Nanotubes with Flavin Mononucleotides for Strong and Stable Visible-Light Emission in Aqueous Solution. ACS Appl. Mater. Interfaces 2011, 3, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Bando, Y.; Shen, G.; Zhi, C.; Golberg, D. Single-Source Precursor for Chemical Vapour Deposition of Collapsed Boron Nitride Nanotubes. Nanotechnology 2006, 17, 5882–5888. [Google Scholar] [CrossRef]
- Dai, P.; Xue, Y.; Wang, X.; Weng, Q.; Zhang, C.; Jiang, X.; Tang, D.; Wang, X.; Kawamoto, N.; Ide, Y.; et al. Pollutant Capturing SERS Substrate: Porous Boron Nitride Microfibers with Uniform Silver Nanoparticle Decoration. Nanoscale 2015, 7, 18992–18997. [Google Scholar] [CrossRef]
- Gudz, K.Y.; Permyakova, E.S.; Matveev, A.T.; Bondarev, A.V.; Manakhov, A.M.; Sidorenko, D.A.; Filippovich, S.Y.; Brouchkov, A.V.; Golberg, D.V.; Ignatov, S.G.; et al. Pristine and Antibiotic-Loaded Nanosheets/Nanoneedles-Based Boron Nitride Films as a Promising Platform to Suppress Bacterial and Fungal Infections. ACS Appl. Mater. Interfaces 2020, 12, 42485–42498. [Google Scholar] [CrossRef]
- Xue, L.; Lu, B.; Wu, Z.-S.; Ge, C.; Wang, P.; Zhang, R.; Zhang, X.-D. Synthesis of Mesoporous Hexagonal Boron Nitride Fibers with High Surface Area for Efficient Removal of Organic Pollutants. Chem. Eng. J. 2014, 243, 494–499. [Google Scholar] [CrossRef]
- Li, Q.; Yang, T.; Yang, Q.; Wang, F.; Chou, K.-C.; Hou, X. Porous Hexagonal Boron Nitride Whiskers Fabricated at Low Temperature for Effective Removal of Organic Pollutants from Water. Ceram. Int. 2016, 42, 8754–8762. [Google Scholar] [CrossRef]
- Maiti, K.; Thanh, T.D.; Sharma, K.; Hui, D.; Kim, N.H.; Lee, J.H. Highly Efficient Adsorbent Based on Novel Cotton Flower-like Porous Boron Nitride for Organic Pollutant Removal. Compos. Part B Eng. 2017, 123, 45–54. [Google Scholar] [CrossRef]
- Wang, J.; Hao, J.; Liu, D.; Qin, S.; Chen, C.; Yang, C.; Liu, Y.; Yang, T.; Fan, Y.; Chen, Y.; et al. Flower Stamen-like Porous Boron Carbon Nitride Nanoscrolls for Water Cleaning. Nanoscale 2017, 9, 9787–9791. [Google Scholar] [CrossRef]
- Ciofani, G. Potential Applications of Boron Nitride Nanotubes as Drug Delivery Systems. Expert Opin. Drug Deliv. 2010, 7, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Emanet, M.; Şen, Ö.; Çulha, M. Evaluation of Boron Nitride Nanotubes and Hexagonal Boron Nitrides as Nanocarriers for Cancer Drugs. Nanomedicne 2017, 12, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Sharker, S.M.; Alam, M.A.; Shill, M.C.; Rahman, G.S.; Reza, H.M. Functionalized HBN as Targeted Photothermal Chemotherapy for Complete Eradication of Cancer Cells. Int. J. Pharm. 2017, 534, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukova, I.V.; Zhitnyak, I.Y.; Kovalskii, A.M.; Matveev, A.T.; Lebedev, O.I.; Li, X.; Gloushankova, N.A.; Golberg, D.; Shtansky, D.V. Boron Nitride Nanoparticles with a Petal-Like Surface as Anticancer Drug-Delivery Systems. ACS Appl. Mater. Interfaces 2015, 7, 17217–17225. [Google Scholar] [CrossRef]
- Zhitnyak, I.Y.; Bychkov, I.N.; Sukhorukova, I.V.; Kovalskii, A.M.; Firestein, K.L.; Golberg, D.; Gloushankova, N.A.; Shtansky, D.V. Effect of BN Nanoparticles Loaded with Doxorubicin on Tumor Cells with Multiple Drug Resistance. ACS Appl. Mater. Interfaces 2017, 9, 32498–32508. [Google Scholar] [CrossRef]
- Türkez, H.; Arslan, M.E.; Sönmez, E.; Açikyildiz, M.; Tatar, A.; Geyikoğlu, F. Synthesis, Characterization and Cytotoxicity of Boron Nitride Nanoparticles: Emphasis on Toxicogenomics. Cytotechnology 2019, 71, 351–361. [Google Scholar] [CrossRef]
- Kıvanç, M.; Barutca, B.; Koparal, A.T.; Göncü, Y.; Bostancı, S.H.; Ay, N. Effects of Hexagonal Boron Nitride Nanoparticles on Antimicrobial and Antibiofilm Activities, Cell Viability. Mater. Sci. Eng. C 2018, 91, 115–124. [Google Scholar] [CrossRef]
- Antipina, L.Y.; Kotyakova, K.Y.; Tregubenko, M.V.; Shtansky, D.V. Experimental and Theoretical Study of Sorption Capacity of Hexagonal Boron Nitride Nanoparticles: Implication for Wastewater Purification from Antibiotics. Nanomaterials 2022, 12, 3157. [Google Scholar] [CrossRef]
- Farzad, F.; Hashemzadeh, H. Probing the Effect of Polyethene Glycol on the Adsorption Mechanisms of Gem on the Hexagonal Boron Nitride as a Highly Efficient Polymer-Based Drug Delivery System: DFT, Classical MD and Well-Tempered Metadynamics Simulations. J. Mol. Graph. Model. 2020, 98, 107613. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Antipina, L.Y.; Kovalskii, A.M.; Zhitnyak, I.Y.; Gudz, K.Y.; Polcak, J.; Sorokin, P.B.; Manakhov, A.M.; Shtansky, D.V. Experimental and Theoretical Study of Doxorubicin Physicochemical Interaction with BN(O) Drug Delivery Nanocarriers. J. Phys. Chem. C 2018, 122, 26409–26418. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Antipina, L.Y.; Kiryukhantsev-Korneev, P.; Kovalskii, A.M.; Polcak, J.; Manakhov, A.; Gudz, K.Y.; Sorokin, P.B.; Shtansky, D. Plasma Surface Polymerized and Biomarker Conjugated Boron Nitride Nanoparticles for Cancer-Specific Therapy: Experimental and Theoretical Study. Nanomaterials 2019, 9, 1658. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, 1133–1138. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Salzner, U.; Baer, R. Koopmans’ Springs to Life. J. Chem. Phys. 2009, 131, 231101. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, T.; Song, J.-W.; Suzuki, S.; Hirao, K. On Koopmans’ Theorem in Density Functional Theory. J. Chem. Phys. 2010, 133, 174101. [Google Scholar] [CrossRef]
- Chong, D.P.; Gritsenko, O.V.; Baerends, E.J. Interpretation of the Kohn–Sham Orbital Energies as Approximate Vertical Ionization Potentials. J. Chem. Phys. 2002, 116, 1760–1772. [Google Scholar] [CrossRef]
- Almbladh, C.-O.; von Barth, U. Exact Results for the Charge and Spin Densities, Exchange-Correlation Potentials, and Density-Functional Eigenvalues. Phys. Rev. B 1985, 31, 3231–3244. [Google Scholar] [CrossRef]
- Imamura, Y.; Tashiro, M.; Katouda, M.; Hada, M. Extrapolation of Polymer Gap by Combining Cluster and Periodic Boundary Condition Calculations with Hückel Theory. Chem. Phys. Lett. 2018, 707, 44–48. [Google Scholar] [CrossRef]
- Saikia, N.; Pati, S.K.; Deka, R.C. First Principles Calculation on the Structure and Electronic Properties of BNNTs Functionalized with Isoniazid Drug Molecule. Appl. Nanosci. 2012, 2, 389–400. [Google Scholar] [CrossRef]
- Khare, K.P.; Kathal, R.; Shukla, N.; Srivastava, R.; Srivastava, A. 6 Thioguanine Sensing Using Poly Pyrrole: DFT Study. AIP Conf. Proc. 2021, 2352, 020072. [Google Scholar] [CrossRef]
- Yang, J.; Kim, D.; Hong, J.; Qian, X. Magnetism in Boron Nitride Monolayer: Adatom and Vacancy Defect. Surf. Sci. 2010, 604, 1603–1607. [Google Scholar] [CrossRef]
- Chettri, B.; Patra, P.K.; Vu, T.V.; Nguyen, C.Q.; Lalrinkima; Yaya, A.; Obodo, K.O.; Tran, N.T.T.; Laref, A.; Rai, D.P. Induced Ferromagnetism in Bilayer Hexagonal Boron Nitride (h-BN) on Vacancy Defects at B and N Sites. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 126, 114436. [Google Scholar] [CrossRef]
- Troullier, N.; Martins, J.L. Efficient Pseudopotentials for Plane-Wave Calculations. Phys. Rev. B Condens. Matter 1991, 43, 1993–2006. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Dion, M.; Rydberg, H.; Schröder, E.; Langreth, D.C.; Lundqvist, B.I. Van Der Waals Density Functional for General Geometries. Phys. Rev. Lett. 2004, 92, 246401. [Google Scholar] [CrossRef]
- Román-Pérez, G.; Soler, J.M. Efficient Implementation of a van Der Waals Density Functional: Application to Double-Wall Carbon Nanotubes. Phys. Rev. Lett. 2009, 103, 096102. [Google Scholar] [CrossRef]
- Paszkowicz, W.; Pelka, J.B.; Knapp, M.; Szyszko, T.; Podsiadlo, S. Lattice Parameters and Anisotropic Thermal Expansion of Hexagonal Boron Nitride in the 10–297.5 K Temperature Range. Appl. Phys. A 2002, 75, 431–435. [Google Scholar] [CrossRef]
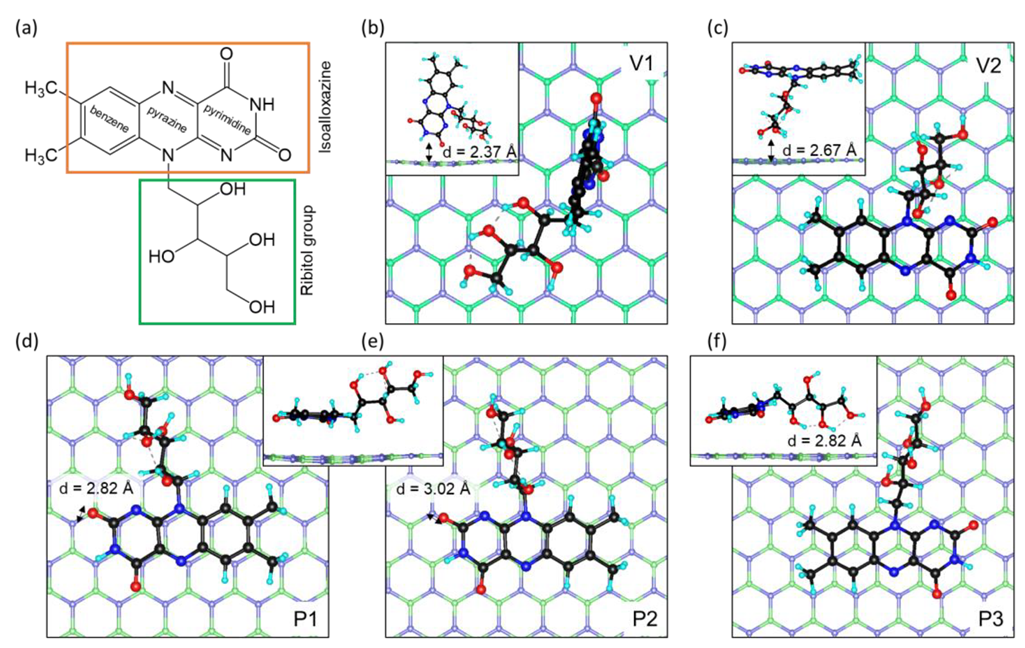
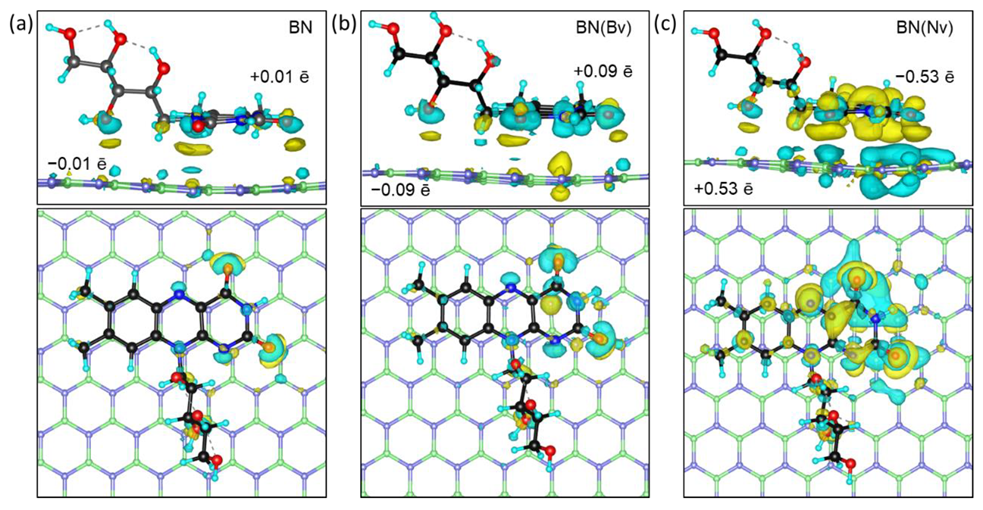
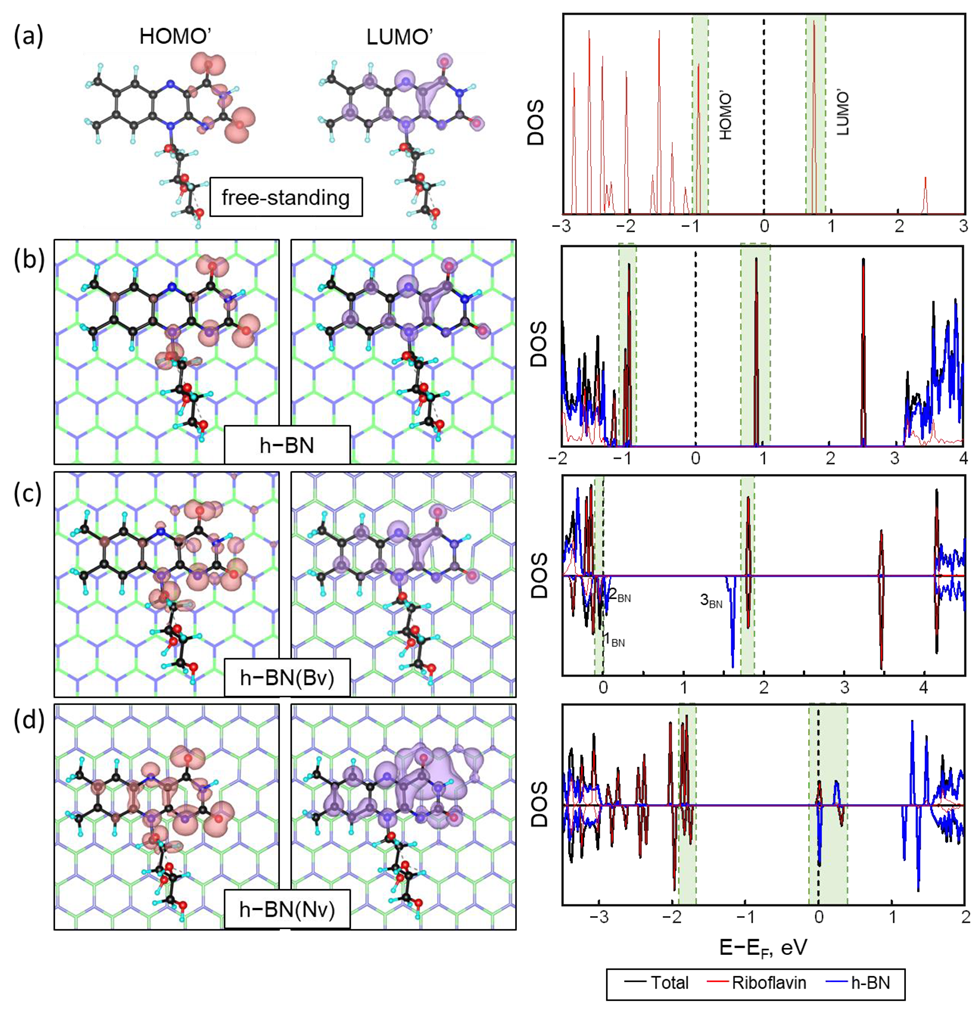
| Configuration | Carrier Types | ||
|---|---|---|---|
| BN | BN(Bv) | BN(Nv) | |
| V1 | −1.82 | −1.99 | −3.64 |
| V2 | −1.15 | −2.22 | −2.77 |
| P1 | −3.49 | −3.55 | −3.69 |
| P2 | −3.73 | −3.93 | −4.01 |
| P3 | −3.16 | −3.10 | −3.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antipina, L.Y.; Kotyakova, K.Y.; Sorokin, P.B. Theoretical Analysis of Riboflavin Adsorption on Hexagonal Boron Nitride for Drug Delivery Applications: Unveiling the Influence of Point Defects. Int. J. Mol. Sci. 2023, 24, 11648. https://doi.org/10.3390/ijms241411648
Antipina LY, Kotyakova KY, Sorokin PB. Theoretical Analysis of Riboflavin Adsorption on Hexagonal Boron Nitride for Drug Delivery Applications: Unveiling the Influence of Point Defects. International Journal of Molecular Sciences. 2023; 24(14):11648. https://doi.org/10.3390/ijms241411648
Chicago/Turabian StyleAntipina, Liubov Yu., Kristina Yu. Kotyakova, and Pavel B. Sorokin. 2023. "Theoretical Analysis of Riboflavin Adsorption on Hexagonal Boron Nitride for Drug Delivery Applications: Unveiling the Influence of Point Defects" International Journal of Molecular Sciences 24, no. 14: 11648. https://doi.org/10.3390/ijms241411648
APA StyleAntipina, L. Y., Kotyakova, K. Y., & Sorokin, P. B. (2023). Theoretical Analysis of Riboflavin Adsorption on Hexagonal Boron Nitride for Drug Delivery Applications: Unveiling the Influence of Point Defects. International Journal of Molecular Sciences, 24(14), 11648. https://doi.org/10.3390/ijms241411648






