Nanoform of Phospholipid Composition: Investigation of the Morphological Features by Atomic Force Microscopy
Abstract
:1. Introduction
2. Results
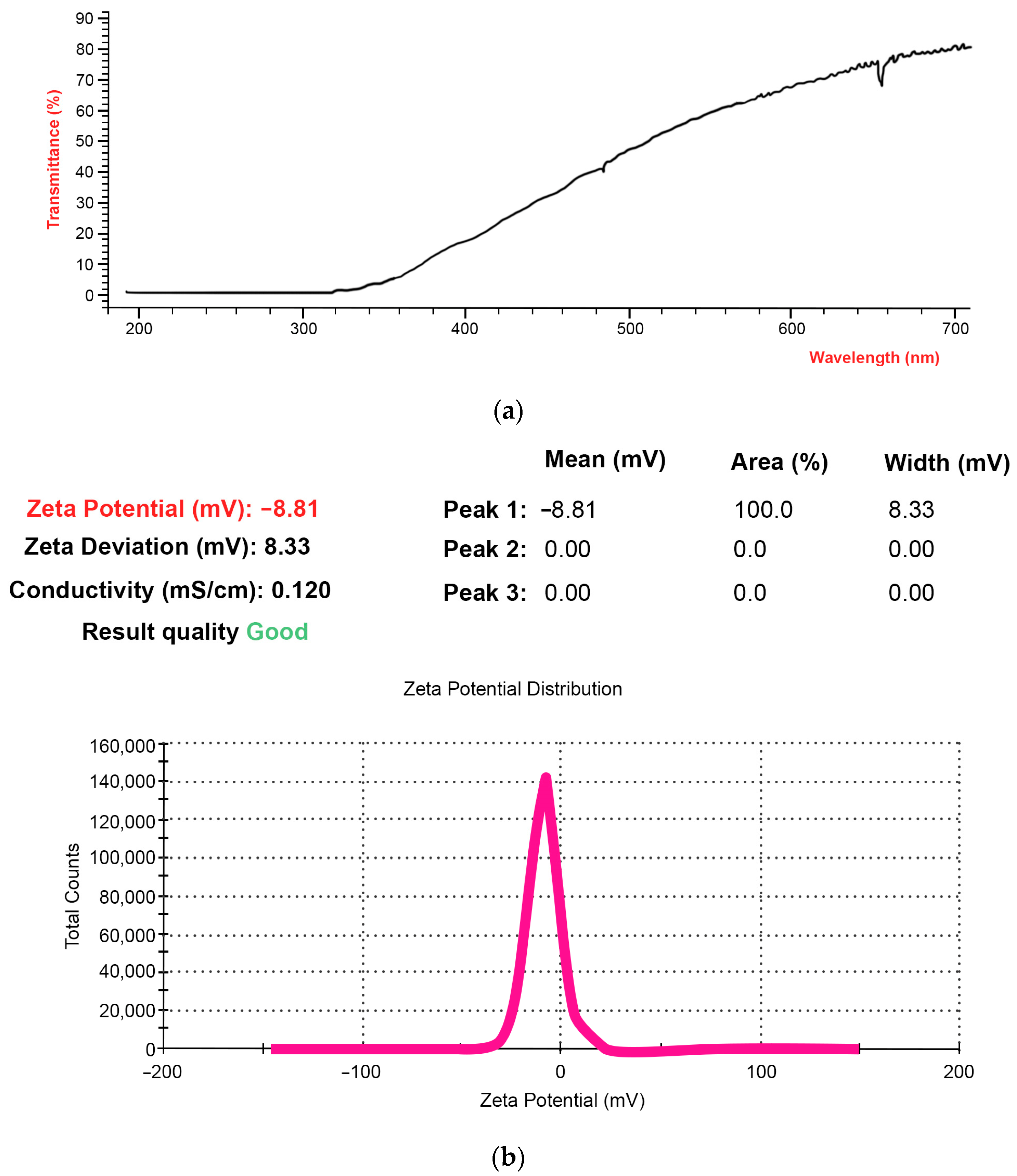
2.1. NFPh Characterization by Macroscopic Methods
2.2. Characteristics of NFPh Measured by DLS
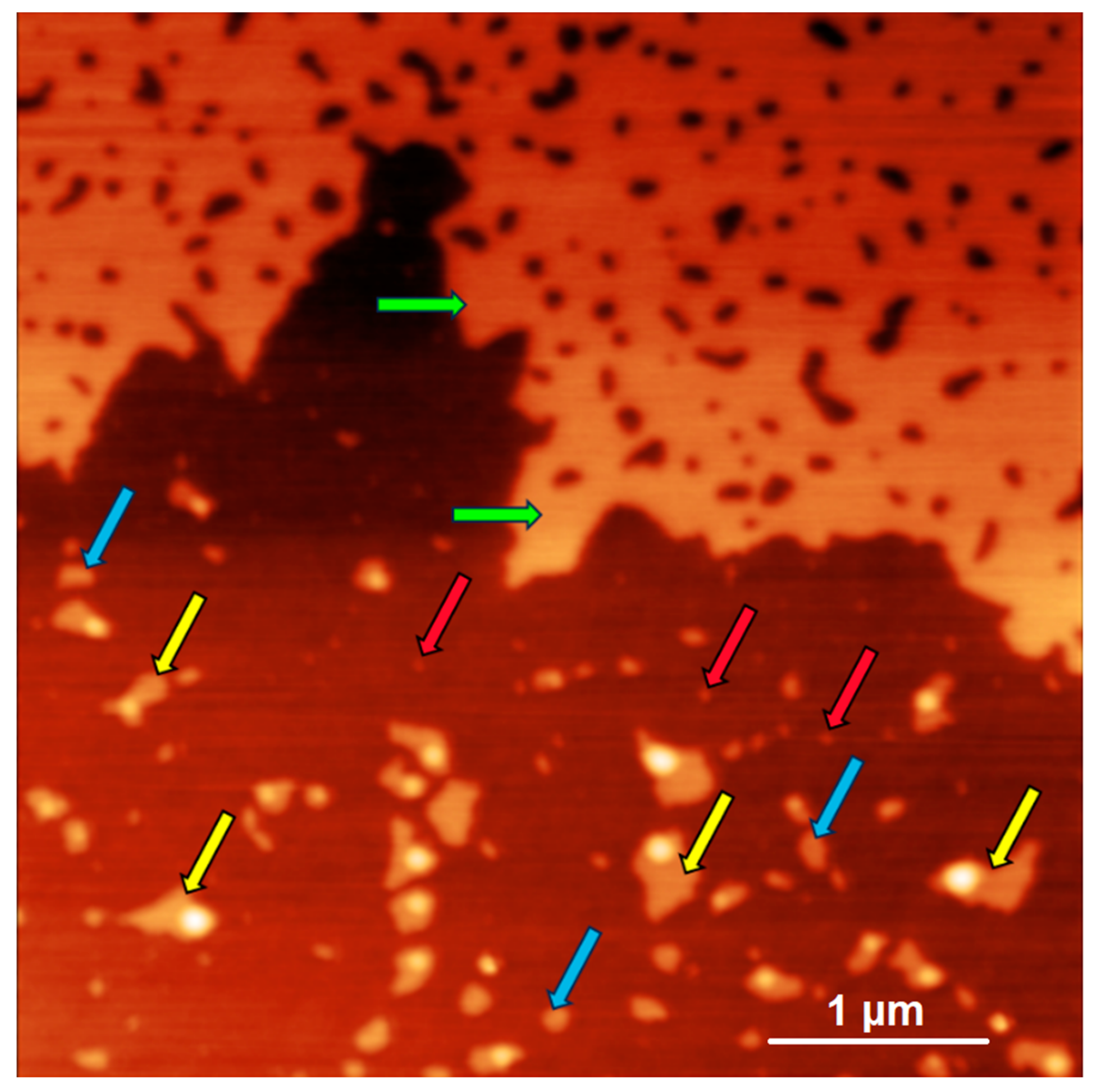
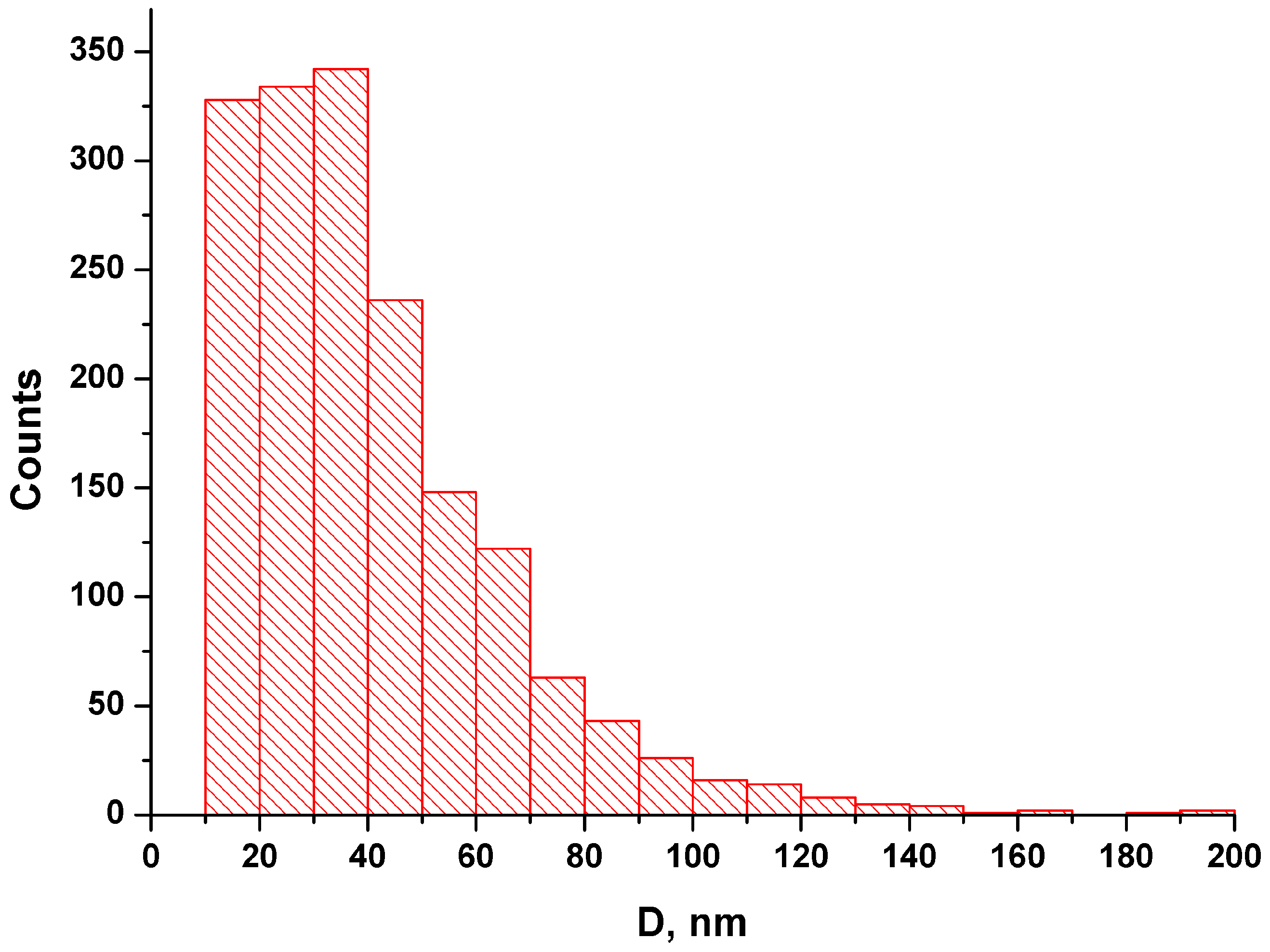
2.3. Characteristics of NFPh Measured by AFM
3. Discussion
4. Materials and Methods
4.1. Preparation of the NFPh
4.2. NFPh Characterization by Macroscopic Methods
4.3. Atomic Force Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yanar, F.; Carugo, D.; Zhang, X. Hybrid Nanoplatforms Comprising Organic Nanocompartments Encapsulating Inorganic Nanoparticles for Enhanced Drug Delivery and Bioimaging Applications. Molecules 2023, 28, 5694. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, X.; Xu, H.; Jiao, P.; Zhao, L.-X.; Su, G. Nanomaterial-Based Drug Delivery Systems for Pain Treatment and Relief: From the Delivery of a Single Drug to Co-Delivery of Multiple Therapeutics. Pharmaceutics 2023, 15, 2309. [Google Scholar] [CrossRef]
- Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and Drug Delivery: A Mini Review. Int. Nano Lett. 2014, 4, 94. [Google Scholar] [CrossRef]
- Mashaghi, S.; Jadidi, T.; Koenderink, G.; Mashaghi, A. Lipid Nanotechnology. Int. J. Mol. Sci. 2013, 14, 4242–4282. [Google Scholar] [CrossRef]
- Mahmoudi, M. Debugging Nano–Bio Interfaces: Systematic Strategies to Accelerate Clinical Translation of Nanotechnologies. Trends Biotechnol. 2018, 36, 755–769. [Google Scholar] [CrossRef]
- Jia, Y.; Jiang, Y.; He, Y.; Zhang, W.; Zou, J.; Magar, K.T.; Boucetta, H.; Teng, C.; He, W. Approved Nanomedicine against Diseases. Pharmaceutics 2023, 15, 774. [Google Scholar] [CrossRef]
- Boulaiz, H.; Alvarez, P.J.; Ramirez, A.; Marchal, J.A.; Prados, J.; Rodríguez-Serrano, F.; Perán, M.; Melguizo, C.; Aranega, A. Nanomedicine: Application Areas and Development Prospects. Int. J. Mol. Sci. 2011, 12, 3303–3321. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef]
- Tallury, P.; Malhotra, A.; Byrne, L.M.; Santra, S. Nanobioimaging and Sensing of Infectious Diseases. Adv. Drug Deliv. Rev. 2010, 62, 424–437. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key Principles and Methods for Studying the Endocytosis of Biological and Nanoparticle Therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef]
- Flores, A.M.; Ye, J.; Jarr, K.-U.; Hosseini-Nassab, N.; Smith, B.R.; Leeper, N.J. Nanoparticle Therapy for Vascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 635–646. [Google Scholar] [CrossRef]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.-O. Microbe-Mediated Biosynthesis of Nanoparticles: Applications and Future Prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition Design and Medical Application of Liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Tereshkina, Y.A.; Torkhovskaya, T.I.; Tikhonova, E.G.; Kostryukova, L.V.; Sanzhakov, M.A.; Korotkevich, E.I.; Khudoklinova, Y.Y.; Orlova, N.A.; Kolesanova, E.F. Nanoliposomes as Drug Delivery Systems: Safety Concerns. J. Drug Target. 2022, 30, 313–325. [Google Scholar] [CrossRef]
- Storm, G.; Oussoren, C.; Peeters, P.A.; Barenholz, Y. Liposome Technology; CRC Press: Boca Raton, FL, USA, 1993; Volume 3. [Google Scholar]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, Characterisation, Preparation, and Recent Innovation in Clinical Applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef]
- Tikhonova, E.G.; Sanzhakov, M.A.; Tereshkina, Y.A.; Kostryukova, L.V.; Khudoklinova, Y.Y.; Orlova, N.A.; Bobrova, D.V.; Ipatova, O.M. Drug Transport System Based on Phospholipid Nanoparticles: Production Technology and Characteristics. Pharmaceutics 2022, 14, 2522. [Google Scholar] [CrossRef]
- Ewert, K.K.; Scodeller, P.; Simón-Gracia, L.; Steffes, V.M.; Wonder, E.A.; Teesalu, T.; Safinya, C.R. Cationic Liposomes as Vectors for Nucleic Acid and Hydrophobic Drug Therapeutics. Pharmaceutics 2021, 13, 1365. [Google Scholar] [CrossRef]
- Audouy, S.A.L.; de Leij, L.F.M.H.; Hoekstra, D.; Molema, G. In Vivo Characteristics of Cationic Liposomes as Delivery Vectors for Gene Therapy. Pharm. Res. 2002, 19, 1599–1605. [Google Scholar] [CrossRef]
- Salama, L.; Pastor, E.R.; Stone, T.; Mousa, S.A. Emerging Nanopharmaceuticals and Nanonutraceuticals in Cancer Management. Biomedicines 2020, 8, 347. [Google Scholar] [CrossRef]
- Farjadian, F.; Ghasemi, A.; Gohari, O.; Roointan, A.; Karimi, M.; Hamblin, M.R. Nanopharmaceuticals and Nanomedicines Currently on the Market: Challenges and Opportunities. Nanomedicine 2019, 14, 93–126. [Google Scholar] [CrossRef]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (Part 1): Products on the Market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef]
- Ehmann, F.; Sakai-Kato, K.; Duncan, R.; Pérez De La Ossa, D.H.; Pita, R.; Vidal, J.-M.; Kohli, A.; Tothfalusi, L.; Sanh, A.; Tinton, S.; et al. Next-Generation Nanomedicines and Nanosimilars: EU Regulators’ Initiatives Relating to the Development and Evaluation of Nanomedicines. Nanomedicine 2013, 8, 849–856. [Google Scholar] [CrossRef]
- Senior, J.H. Fate and Behavior of Liposomes in Vivo: A Review of Controlling Factors. Crit. Rev. Ther. Drug Carr. Syst. 1987, 3, 123–193. [Google Scholar]
- Ipatova, O.M. Phosphogliv: Mekhanismy Deistviya i Primenenie v Klinike (Phosphogliv: Mechanisms of Action and Clinical Application); Izd. GU NII Biomeditsinskoi Khimii RAMN (Publishing of State Research Institute of Biomedical Chemistry of RAMS): Moscow, Russia, 2005. [Google Scholar]
- Grabielle-Madelmont, C.; Lesieur, S.; Ollivon, M. Characterization of Loaded Liposomes by Size Exclusion Chromatography. J. Biochem. Biophys. Methods 2003, 56, 189–217. [Google Scholar] [CrossRef]
- Moon, M.H.; Giddings, J.C. Size Distribution of Liposomes by Flow Field-Flow Fractionation. J. Pharm. Biomed. Anal. 1993, 11, 911–920. [Google Scholar] [CrossRef]
- Crommelin, D.J.A.; Metselaar, J.M.; Storm, G. Liposomes: The Science and the Regulatory Landscape. In Non-Biological Complex Drugs: The Science and the Regulatory Landscape; Crommelin, D.J.A., de Vlieger, J.S.B., Eds.; AAPS Advances in the Pharmaceutical Sciences Series; Springer International Publishing: Cham, Switzerland, 2015; pp. 77–106. ISBN 978-3-319-16241-6. [Google Scholar]
- Prima, G.D.; Librizzi, F.; Carrotta, R. Light Scattering as an Easy Tool to Measure Vesicles Weight Concentration. Membranes 2020, 10, 222. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome Production by Microfluidics: Potential and Limiting Factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef]
- Kanno, T.; Yamada, T.; Iwabuki, H.; Tanaka, H.; Kuroda, S.; Tanizawa, K.; Kawai, T. Size Distribution Measurement of Vesicles by Atomic Force Microscopy. Anal. Biochem. 2002, 309, 196–199. [Google Scholar] [CrossRef]
- Hoo, C.M.; Starostin, N.; West, P.; Mecartney, M.L. A Comparison of Atomic Force Microscopy (AFM) and Dynamic Light Scattering (DLS) Methods to Characterize Nanoparticle Size Distributions. J. Nanopart. Res. 2008, 10, 89–96. [Google Scholar] [CrossRef]
- Sitterberg, J.; Özcetin, A.; Ehrhardt, C.; Bakowsky, U. Utilising Atomic Force Microscopy for the Characterisation of Nanoscale Drug Delivery Systems. Eur. J. Pharm. Biopharm. 2010, 74, 2–13. [Google Scholar] [CrossRef]
- Reviakine, I.; Brisson, A. Formation of Supported Phospholipid Bilayers from Unilamellar Vesicles Investigated by Atomic Force Microscopy. Langmuir 2000, 16, 1806–1815. [Google Scholar] [CrossRef]
- Schönherr, H.; Johnson, J.M.; Lenz, P.; Frank, C.W.; Boxer, S.G. Vesicle Adsorption and Lipid Bilayer Formation on Glass Studied by Atomic Force Microscopy. Langmuir 2004, 20, 11600–11606. [Google Scholar] [CrossRef]
- Attwood, S.J.; Choi, Y.; Leonenko, Z. Preparation of DOPC and DPPC Supported Planar Lipid Bilayers for Atomic Force Microscopy and Atomic Force Spectroscopy. Int. J. Mol. Sci. 2013, 14, 3514–3539. [Google Scholar] [CrossRef]
- Manaia, E.B.; Abuçafy, M.P.; Chiari-Andréo, B.G.; Silva, B.L.; Oshiro Junior, J.A.; Chiavacci, L.A. Physicochemical Characterization of Drug Nanocarriers. Int. J. Nanomed. 2017, 12, 4991–5011. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Long-Circulating and Target-Specific Nanoparticles: Theory to Practice. Pharmacol. Rev. 2001, 53, 283–318. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Seifert, U. Configurations of Fluid Membranes and Vesicles. Adv. Phys. 1997, 46, 13–137. [Google Scholar] [CrossRef]
- Lipowsky, R.; Seifert, U. Adhesion of Vesicles and Membranes. Mol. Cryst. Liq. Cryst. 1991, 202, 17–25. [Google Scholar] [CrossRef]
- Liang, X.; Mao, G.; Simon Ng, K.Y. Probing Small Unilamellar EggPC Vesicles on Mica Surface by Atomic Force Microscopy. Colloids Surf. B Biointerfaces 2004, 34, 41–51. [Google Scholar] [CrossRef]
- Xu, J.; Stevens, M.J.; Oleson, T.A.; Last, J.A.; Sahai, N. Role of Oxide Surface Chemistry and Phospholipid Phase on Adsorption and Self-Assembly: Isotherms and Atomic Force Microscopy. J. Phys. Chem. C 2009, 113, 2187–2196. [Google Scholar] [CrossRef]
- Aguiar, J.; Carpena, P.; Molina-Bolívar, J.A.; Carnero Ruiz, C. On the Determination of the Critical Micelle Concentration by the Pyrene 1:3 Ratio Method. J. Colloid Interface Sci. 2003, 258, 116–122. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T. Soybean Lecithin Fractionation and Functionality. J. Am. Oil Chem. Soc. 2003, 80, 319–326. [Google Scholar] [CrossRef]
- Egawa, H.; Furusawa, K. Liposome Adhesion on Mica Surface Studied by Atomic Force Microscopy. Langmuir 1999, 15, 1660–1666. [Google Scholar] [CrossRef]
- Németh, Z.; Csóka, I.; Semnani Jazani, R.; Sipos, B.; Haspel, H.; Kozma, G.; Kónya, Z.; Dobó, D.G. Quality by Design-Driven Zeta Potential Optimisation Study of Liposomes with Charge Imparting Membrane Additives. Pharmaceutics 2022, 14, 1798. [Google Scholar] [CrossRef] [PubMed]
- Critical Micelle Concentrations (CMCs)|Avanti Polar Lipids (En-US). Available online: https://avantilipids.com/tech-support/physical-properties/cmcs (accessed on 30 August 2023).
- Smith, R.; Tanford, C. The Critical Micelle Concentration of L-α-Dipalmitoylphosphatidylcholine in Water and Water/Methanol Solutions. J. Mol. Biol. 1972, 67, 75–83. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Tanford, C.; Stone, W.L. Interaction of L-Alpha-Didecanoyl Phosphatidylcholine with the AI Polypeptide of High Density Lipoprotein. Proc. Natl. Acad. Sci. USA 1977, 74, 3796–3799. [Google Scholar] [CrossRef]
- Zhang, H.; Dudley, E.G.; Harte, F. Critical Synergistic Concentration of Lecithin Phospholipids Improves the Antimicrobial Activity of Eugenol against Escherichia Coli. Appl. Environ. Microbiol. 2017, 83, e01583. [Google Scholar] [CrossRef]
- Thomas, A.H.; Catalá, Á.; Vignoni, M. Soybean Phosphatidylcholine Liposomes as Model Membranes to Study Lipid Peroxidation Photoinduced by Pterin. Biochim. Biophys. Acta (BBA) Biomembranes 2016, 1858, 139–145. [Google Scholar] [CrossRef]
- Nir, S.; Bentz, J.; Wilschut, J.; Duzgunes, N. Aggregation and Fusion of Phospholipid Vesicles. Prog. Surf. Sci. 1983, 13, 1–124. [Google Scholar] [CrossRef]
- Kucerka, N.; Pencer, J.; Sachs, J.N.; Nagle, J.F.; Katsaras, J. Curvature Effect on the Structure of Phospholipid Bilayers. Langmuir 2007, 23, 1292–1299. [Google Scholar] [CrossRef]
- Parente, R.A.; Nir, S.; Szoka, F.C. PH-Dependent Fusion of Phosphatidylcholine Small Vesicles. Induction by a Synthetic Amphipathic Peptide. J. Biol. Chem. 1988, 263, 4724–4730. [Google Scholar] [CrossRef] [PubMed]
- Nibali, V.C.; Maiti, S.; Saija, F.; Heyden, M.; Cassone, G. Electric-field induced entropic effects in liquid water. J. Chem. Phys. 2023, 158, 184501. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kraevsky, S.V.; Ivanova, I.A.; Kanashenko, S.L.; Shumov, I.D.; Ryazantsev, I.A.; Tereshkina, Y.A.; Kostryukova, L.V.; Romashova, Y.A.; Pleshakova, T.O. Nanoform of Phospholipid Composition: Investigation of the Morphological Features by Atomic Force Microscopy. Int. J. Mol. Sci. 2023, 24, 15338. https://doi.org/10.3390/ijms242015338
Kraevsky SV, Ivanova IA, Kanashenko SL, Shumov ID, Ryazantsev IA, Tereshkina YA, Kostryukova LV, Romashova YA, Pleshakova TO. Nanoform of Phospholipid Composition: Investigation of the Morphological Features by Atomic Force Microscopy. International Journal of Molecular Sciences. 2023; 24(20):15338. https://doi.org/10.3390/ijms242015338
Chicago/Turabian StyleKraevsky, Sergey V., Irina A. Ivanova, Sergey L. Kanashenko, Ivan D. Shumov, Ilya A. Ryazantsev, Yulia A. Tereshkina, Lyubov V. Kostryukova, Yulia A. Romashova, and Tatyana O. Pleshakova. 2023. "Nanoform of Phospholipid Composition: Investigation of the Morphological Features by Atomic Force Microscopy" International Journal of Molecular Sciences 24, no. 20: 15338. https://doi.org/10.3390/ijms242015338
APA StyleKraevsky, S. V., Ivanova, I. A., Kanashenko, S. L., Shumov, I. D., Ryazantsev, I. A., Tereshkina, Y. A., Kostryukova, L. V., Romashova, Y. A., & Pleshakova, T. O. (2023). Nanoform of Phospholipid Composition: Investigation of the Morphological Features by Atomic Force Microscopy. International Journal of Molecular Sciences, 24(20), 15338. https://doi.org/10.3390/ijms242015338






