Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses
Abstract
1. Copper Properties and Applications
| Application | References |
|---|---|
| Agriculture and Livestock | |
| Fungicide, algicide, nutritional supplement | [8,9,20,21,22] |
| Building | |
| Electricity, wiring, plumbing, cooling, roofing | [1,9] |
| Industrial | |
| Water treatment, dye manufacturing, oil refining, wood preservation | [8,9,23,24] |
| Health | |
| Intrauterine devices, dental crowns, antimicrobial surfaces, face masks | [12,25,26,27] |
| Others | |
| Photoluminescence | [7] |
| Textile | [25,28] |
| Food Industry | [9] |
| Carbon–copper Nanotubes | [29] |
| Transformation of solar energy, batteries, and gas sensors | [30,31] |
2. Use of Copper as an Antimicrobial
| Application | Function | Type of Copper | References |
|---|---|---|---|
| Copper surfaces | Norovirus inactivation | Cu-alloy surfaces | [73] |
| Copper surfaces | Elimination of vancomycin-resistant Enterococci | Cu0 and Cu-alloy surfaces | [39,74] |
| Antimicrobial surfaces in spaceflight | Prevention of S. cohnii and E. coli infections | Cu0 and CuO/Cu2O | [75] |
| Surfaces in pediatric intensive care unit | Prevention of infections associated with hospitals | Cu0 surfaces | [55,76] |
| Copper-doped silica xerogels | Avoidance of skin infections | Cu ions | [56] |
| Wound dressings | Prevention of S. aureus and K. pneumoniae infections | CuO/Cu2O NPs | [25] |
| Application of nanotechnology in textiles, latex gloves, and polymers | Prevention of microorganisms in medical supplies | CuO/Cu2O NPs | [8,25,30] |
| Antiviral filters | Dialysis pumps, blood banks and air filters | CuO/Cu2O NPs | [34,57,58] |
| Cotton fibers | Prevention of bacteria, fungi and viruses | Cu ions and NPs | [54] |
| Surfaces with nanoparticle inlay | Antimicrobial activity | Cu0 NPs | [31,59] |
| Office, kindergarten, retirement home, hospital facilities | Antimicrobial activity | Cu/brass surfaces | [60] |
| Face masks | Antiviral activity against influenza A | CuO/Cu2O NPs | [25] |
| Dental materials | Bacterial plaque prevention | CuO/Cu2O NPs | [64,65,66,67] |
| Prostheses | Antibacterial 3D filaments | Cu-NPs | [77] |
| Polyester surface-coating paint | Antimicrobial activity | Cu-NPs | [68] |
| Wound dressing | Antibacterial activity | Bacterial celullose/Cu-NPs | [78] |
| Nanostructures | Antimicrobial– Antibiofilm activity | Cu-NPs/Carbon nanotubes | [79] |
| 3D face masks, connector for ventilators | Antiviral activity against SARS-CoV-2 | Cu-NPs | [80] |
| Antibacterial nanomaterials | Multidrug-resistant bacterial treatment | CuS NPs/ Graphene oxide nanosheets | [81] |
| Antibacterial coatings | Antibacterial activity Antibiofilm activity | CuS nanoparticles | [82] |
| Poultry industry | Antibiofilm formation by Salmonella enteritidis | Cu surface | [40] |
| Nanostructured copper surface/Prototypes for air and water cleaning | Antiviral activity against SARS-CoV-2 | Copper surface and copper nanoparticles | [70] |
| Transparent surface coating | Antiviral activity against Influenza A | Cu-NPs– Graphene | [71] |
| Spray coating | Antiviral activity against SARS-CoV-2 | Cu-NPs | [72] |
| Nanocomposite surfaces | Inhibition of SARS-CoV-2 and HuNV viruses | Cu–Ag nanocomposite surfaces | [69] |
| Textile coating | SARS-CoV-2 inactivation | Hybrid alginate–copper sulfate | [49] |
| Photothermal treatment | Inhibition of bacteria in infected skin | CuS NPs | [83] |
3. Copper Toxicity Mechanisms
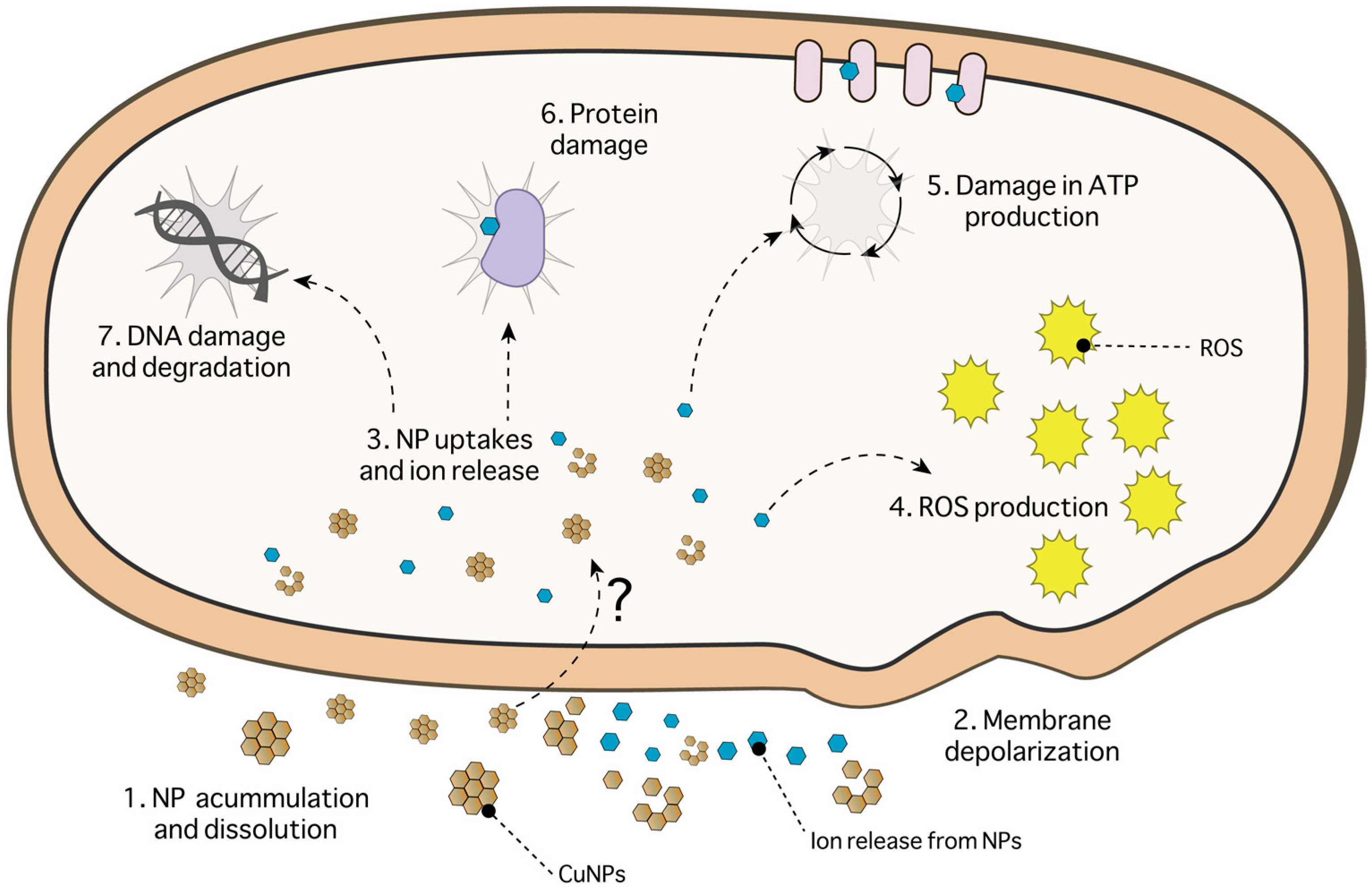
3.1. Copper-Based Nanoparticles
3.2. Copper Surfaces
4. Antiviral Effect of Copper Nanoparticles and Surfaces
4.1. Copper Nanoparticles as an Antiviral Agent
4.2. Antiviral Effect of Copper Surfaces

| Virus | Disease | Nucleic Acid Type | Family | Copper Type | Proposed Mechanism | References |
|---|---|---|---|---|---|---|
| Enveloped | ||||||
| Influenza A | Respiratory tract infection | Single-stranded RNA | Orthomyxoviridae | Cu surface | RNA damage | [15] |
| CuI NPs | Viral protein degradation | [128] | ||||
| Hepatitis C virus (HCV) | Hepatic cirrhosis and hepatocellular carcinomaoma | Single-stranded RNA | Flaviviridae | CuO NPs | Blockage of attachment and entry stages | [125] |
| Hepatitis B virus (HBV) | Acute and chronic viral hepatitis | Double-stranded DNA | Hepadnaviridae | Cu2S NPs | [47] | |
| Herpes Simplex Virus (HSV-1) | Labial lesions, encephalitis, and peripheral nervous system disorders | Double-stranded DNA | Herpesviridae | CuO NPs | Inactivation via oxidation of viral proteins, degradation of viral genome | [124] |
| Human Immunodeficiency Virus (HIV) | Acquired immunodeficiency syndrome | Single-stranded RNA | Retroviridae | Cu ions | HIV-1 protease neutralization | [134] |
| CuO filters | HIV-1 protease inactivation, nucleic acid denaturation | [58] | ||||
| Cupric ions | Nucleic acid degradation | [135] | ||||
| Respiratory Syncytial Virus (RSV) | Respiratory illness | Single-stranded RNA | Paramyxoviridae | CuO NPs | Viral neutralization | [57] |
| Cytomegalovirus (CMV) | Mononucleosis | Double-stranded DNA | Herpesviridae | CuO NPs | Viral neutralization | [57] |
| Bronchitis Virus | Inflammation of the bronchial tubes | Single-stranded RNA | Coronaviridae | Cu ions | Virus inactivation | [136] |
| Junin Virus | Argentine hemorrhagic fever | Single-stranded RNA | Arenaviridae | Copper ions + peroxide | Inactivation by ROS | [135] |
| Human coronavirus 229E (HuCoV-229E) | Respiratory illness | Single-stranded RNA | Coronaviridae | Cu Surfaces and Alloys | Capsid damage, loss of surface spikes and viral genome inactivation | [133] |
| Non-enveloped | ||||||
| Human norovirus (HuNoV) | Acute gastroenteritis | Single-stranded RNA | Caliciviridae | Au/CuS NPs | Capsid protein degradation, capsid damage | [126] |
| Dry Copper Surfaces and Alloys | RNA degradation and capsid damage | [73,131] | ||||
| Bacteriophages MS2 and F2 | - | Single-stranded RNA | Leviviridae | Cupric ions | Phage inactivation | [137] |
| Bacteriophage R17 | - | Single-stranded RNA | Leviviridae | Cupric ions | Degradation of phage genome | [138] |
| Bacteriophage Qß | - | Single-stranded RNA | Leviviridae | Particles Cu2O, CuO and Ag | ROS, leached copper ions and surface contact | [139] |
| Poliovirus | Poliomyelitis, paralysis | Single-stranded RNA | Picornaviridae | Cu ions | Capsid proteins and RNA attack | [140] |
| Cu ions | Inactivation of viral RNA | [141] | ||||
| Adenovirus Type I (HAdV) | Keratoconjunctivitis | Double-stranded DNA | Adenoviridae | CuO NPs | Viral neutralization | [57] |
| Rhinovirus 2 (HRV-2) | Respiratory illness | Single-stranded RNA | Picornaviridae | CuO NPs | Viral neutralization | [57] |
4.3. Copper and SARS-CoV-2
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barceloux, D.G. Copper. J. Toxicol. Clin. Toxicol. 1999, 37, 217–230. [Google Scholar] [CrossRef]
- Flemming, C.A.; Trevors, J.T. Copper Toxicity and Chemistry in the Environment: A Review. Water Air Soil Pollut. 1989, 44, 143–158. [Google Scholar] [CrossRef]
- Hong, R.; Kang, T.Y.; Michels, C.A.; Gadura, N. Membrane Lipid Peroxidation in Copper Alloy-Mediated Contact Killing of Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 1776–1784. [Google Scholar] [CrossRef]
- Puig, S.; Thiele, D.J. Molecular Mechanisms of Copper Uptake and Distribution. Curr. Opin. Chem. Biol. 2002, 6, 171–180. [Google Scholar] [CrossRef]
- Stevenson, J.; Barwinska-Sendra, A.; Tarrant, E.; Waldron, K.J. Mechanism of Action and Applications of the Antimicrobial Properties of Copper. Microb. Pathog. Strateg. Combat. Sci. Technol. Educ. 2013, 2, 468–479. [Google Scholar]
- Solioz, M. Copper and Bacteria: Evolution, Homeostasis and Toxicity; SpringerBriefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-94438-8. [Google Scholar]
- Xu, C.X.; Sun, X.W.; Zhang, X.H.; Ke, L.; Chua, S.J. Photoluminescent Properties of Copper-Doped Zinc Oxide Nanowires. Nanotechnology 2004, 15, 856–861. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper as a Biocidal Tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef]
- Elguindi, J.; Hao, X.; Lin, Y.; Alwathnani, H.A.; Wei, G.; Rensing, C. Advantages and Challenges of Increased Antimicrobial Copper Use and Copper Mining. Appl. Microbiol. Biotechnol. 2011, 91, 237–249. [Google Scholar] [CrossRef]
- Edmonds, M.S.; Izquierdo, O.A.; Baker, D.H. Feed Additive Studies with Newly Weaned Pigs: Efficacy of Supplemental Copper, Antibiotics and Organic Acids. J. Anim. Sci. 1985, 60, 462–469. [Google Scholar] [CrossRef]
- Scaletti, R.W.; Trammell, D.S.; Smith, B.A.; Harmon, R.J. Role of Dietary Copper in Enhancing Resistance to Escherichia coli Mastitis. J. Dairy Sci. 2003, 86, 1240–1249. [Google Scholar] [CrossRef]
- Casey, A.L.; Adams, D.; Karpanen, T.J.; Lambert, P.A.; Cookson, B.D.; Nightingale, P.; Miruszenko, L.; Shillam, R.; Christian, P.; Elliott, T.S.J. Role of Copper in Reducing Hospital Environment Contamination. J. Hosp. Infect. 2010, 74, 72–77. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Use of Copper Cast Alloys to Control Escherichia coli O157 Cross-Contamination during Food Processing. Appl. Environ. Microbiol. 2006, 72, 4239–4244. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Potential Use of Copper Surfaces to Reduce Survival of Epidemic Meticillin-Resistant Staphylococcus Aureus in the Healthcare Environment. J. Hosp. Infect. 2006, 63, 289–297. [Google Scholar] [CrossRef]
- Noyce, J.O.; Michels, H.; Keevil, C.W. Inactivation of Influenza A Virus on Copper versus Stainless Steel Surfaces. Appl. Environ. Microbiol. 2007, 73, 2748–2750. [Google Scholar] [CrossRef]
- Wheeldon, L.J.; Worthington, T.; Lambert, P.A.; Hilton, A.C.; Lowden, C.J.; Elliott, T.S.J. Antimicrobial Efficacy of Copper Surfaces against Spores and Vegetative Cells of Clostridium Difficile: The Germination Theory. J. Antimicrob. Chemother. 2008, 62, 522–525. [Google Scholar] [CrossRef]
- Wilks, S.A.; Michels, H.T.; Keevil, C.W. Survival of Listeria Monocytogenes Scott A on Metal Surfaces: Implications for Cross-Contamination. Int. J. Food Microbiol. 2006, 111, 93–98. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B.; Msagati, T.A.M. Application of Spinel Ferrite Nanoparticles in Water and Wastewater Treatment: A Review. Sep. Purif. Technol. 2017, 188, 399–422. [Google Scholar] [CrossRef]
- Blanc, D.S.; Carrara, P.; Zanetti, G.; Francioli, P. Water Disinfection with Ozone, Copper and Silver Ions, and Temperature Increase to Control Legionella: Seven Years of Experience in a University Teaching Hospital. J. Hosp. Infect. 2005, 60, 69–72. [Google Scholar] [CrossRef]
- Banik, S.; Pérez-de-Luque, A. In Vitro Effects of Copper Nanoparticles on Plant Pathogens, Beneficial Microbes and Crop Plants. Span. J. Agric. Res. 2017, 15, e1005. [Google Scholar] [CrossRef]
- Bishop, W.M.; Villalon, G.V.; Willis, B.E. Assessing Copper Adsorption, Internalization, and Desorption Following Algaecide Application to Control Lyngbya Wollei from Lake Gaston, NC/VA, USA. Water Air Soil Pollut. 2018, 229, 152. [Google Scholar] [CrossRef]
- Gopi, R.; Avasthe, R.K.; Kalita, H.; Yadav, A.; Das, S.K.; Rai, D. Eco-Friendly Management of Tomato Late Blight Using Botanicals, Bio-Control Agents, Compost Tea and Copper Fungicides. Indian J. Agric. Sci. 2020, 90, 35–39. [Google Scholar] [CrossRef]
- de Arquer, F.P.G.; Talapin, D.V.; Klimov, V.I.; Arakawa, Y.; Bayer, M.; Sargent, E.H. Semiconductor Quantum Dots: Technological Progress and Future Challenges. Science 2021, 373, eaaz8541. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Borkow, G.; Okon-Levy, N.; Gabbay, J. Copper Oxide Impregnated Wound Dressing: Biocidal and Safety Studies. Wounds 2010, 22, 301–310. [Google Scholar]
- Lucas, L.C.; Lemons, J.E. Biodegradation of Restorative Metallic Systems. Adv. Dent. Res. 1992, 6, 32–37. [Google Scholar] [CrossRef]
- Turner, R.J. Metal-Based Antimicrobial Strategies. Microb. Biotechnol. 2017, 10, 1062–1065. [Google Scholar] [CrossRef]
- McArthur, J.V.; Tuckfield, R.C.; Baker-Austin, C. Antimicrobial Textiles. In Antibiotic Resistance; Coates, A.R.M., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 211, pp. 135–152. ISBN 978-3-642-28950-7. [Google Scholar]
- Chai, G.; Sun, Y.; Sun, J.; Chen, Q. Mechanical Properties of Carbon Nanotube–Copper Nanocomposites. J. Micromech. Microeng. 2008, 18, 035013. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the Antibacterial Mechanism of CuO Nanoparticles: Revealing the Route of Induced Oxidative Stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Elmi, A.; Nezhadi, S.H. Copper Nanoparticles as Antibacterial Agents. J. Mol. Pharm. Org. Process. Res. 2018, 6, 1. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Hans, M.; Támara, J.C.; Mathews, S.; Bax, B.; Hegetschweiler, A.; Kautenburger, R.; Solioz, M.; Mücklich, F. Laser Cladding of Stainless Steel with a Copper–Silver Alloy to Generate Surfaces of High Antimicrobial Activity. Appl. Surf. Sci. 2014, 320, 195–199. [Google Scholar] [CrossRef]
- Borkow, G. Using Copper to Fight Microorganisms. CCB 2012, 6, 93–103. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of Copper Oxides in Contact Killing of Bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact Killing and Antimicrobial Properties of Copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef]
- Arendsen, L.P.; Thakar, R.; Sultan, A.H. The Use of Copper as an Antimicrobial Agent in Health Care, Including Obstetrics and Gynecology. Clin. Microbiol. Rev. 2019, 32, e00125-18. [Google Scholar] [CrossRef]
- San, K.; Long, J.; Michels, C.A.; Gadura, N. Antimicrobial Copper Alloy Surfaces Are Effective against Vegetative but Not Sporulated Cells of Gram-positive Bacillus subtilis. MicrobiologyOpen 2015, 4, 753–763. [Google Scholar] [CrossRef]
- Warnes, S.L.; Keevil, C.W. Mechanism of Copper Surface Toxicity in Vancomycin-Resistant Enterococci Following Wet or Dry Surface Contact. Appl. Environ. Microbiol. 2011, 77, 6049–6059. [Google Scholar] [CrossRef]
- Pontin, K.P.; Borges, K.A.; Furian, T.Q.; Carvalho, D.; Wilsmann, D.E.; Cardoso, H.R.P.; Alves, A.K.; Chitolina, G.Z.; Salle, C.T.P.; de Souza Moraes, H.L.; et al. Antimicrobial Activity of Copper Surfaces against Biofilm Formation by Salmonella Enteritidis and Its Potential Application in the Poultry Industry. Food Microbiol. 2021, 94, 103645. [Google Scholar] [CrossRef]
- Warnes, S.L.; Highmore, C.J.; Keevil, C.W. Horizontal Transfer of Antibiotic Resistance Genes on Abiotic Touch Surfaces: Implications for Public Health. mBio 2012, 3, e00489-12. [Google Scholar] [CrossRef]
- Santo, C.E.; Lam, E.W.; Elowsky, C.G.; Quaranta, D.; Domaille, D.W.; Chang, C.J.; Grass, G. Bacterial Killing by Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 794–802. [Google Scholar] [CrossRef]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial Properties of a Novel Copper-Based Composite Coating with Potential for Use in Healthcare Facilities. Antimicrob. Resist. Infect. Control 2019, 8, 3. [Google Scholar] [CrossRef]
- Macomber, L.; Imlay, J.A. The Iron-Sulfur Clusters of Dehydratases Are Primary Intracellular Targets of Copper Toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef]
- Hans, M.; Mathews, S.; Mücklich, F.; Solioz, M. Physicochemical Properties of Copper Important for Its Antibacterial Activity and Development of a Unified Model. Biointerphases 2015, 11, 018902. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like Copper Redox Chemistry Revisited: Hydrogen Peroxide and Superoxide Mediation of Copper-Catalyzed Oxidant Production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Guo, Z.; Huang, X.; Li, Z.; Shi, J.; Zhai, X.; Hu, X.; Zou, X. Employing CuInS2quantum Dots Modified with Vancomycin for Detecting: Staphylococcus Aureus and Iron(Iii). Anal. Methods 2021, 13, 1517–1526. [Google Scholar] [CrossRef]
- Wilks, S.A.; Michels, H.; Keevil, C.W. The Survival of Escherichia coli O157 on a Range of Metal Surfaces. Int. J. Food Microbiol. 2005, 105, 445–454. [Google Scholar] [CrossRef]
- Bataglioli, R.A.; Rocha Neto, J.B.M.; Calais, G.B.; Lopes, L.M.; Tsukamoto, J.; de Moraes, A.P.; Arns, C.W.; Beppu, M.M. Hybrid Alginate–Copper Sulfate Textile Coating for Coronavirus Inactivation. J. Am. Ceram. Soc. 2022, 105, 1748–1752. [Google Scholar] [CrossRef]
- Xu, W.; Hou, C.; Mao, Y.; Yang, L.; Tamaddon, M.; Zhang, J.; Qu, X.; Liu, C.; Su, B.; Lu, X. Characteristics of Novel Ti–10Mo-XCu Alloy by Powder Metallurgy for Potential Biomedical Implant Applications. Bioact. Mater. 2020, 5, 659–666. [Google Scholar] [CrossRef]
- Xiao, X.; Han, X.; Wang, L.-G.; Long, F.; Ma, X.-L.; Xu, C.-C.; Ma, X.-B.; Wang, C.-X.; Liu, Z.-Y. Anaerobically Photoreductive Degradation by CdS Nanocrystal: Biofabrication Process and Bioelectron-Driven Reaction Coupled with Shewanella Oneidensis MR-1. Biochem. Eng. J. 2020, 154, 107466. [Google Scholar] [CrossRef]
- Rubilar, O.; Rai, M.; Tortella, G.; Diez, M.C.; Seabra, A.B.; Durán, N. Biogenic Nanoparticles: Copper, Copper Oxides, Copper Sulphides, Complex Copper Nanostructures and Their Applications. Biotechnol. Lett. 2013, 35, 1365–1375. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial Properties of Nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.; Chand, N.; Bajpai, S.K. Copper Alginate-Cotton Cellulose (CACC) Fibers with Excellent Antibacterial Properties. J. Eng. Fibers Fabr. 2009, 4, 155892500900400. [Google Scholar] [CrossRef]
- Schmidt, M.G.; von Dessauer, B.; Benavente, C.; Benadof, D.; Cifuentes, P.; Elgueta, A.; Duran, C.; Navarrete, M.S. Copper Surfaces Are Associated with Significantly Lower Concentrations of Bacteria on Selected Surfaces within a Pediatric Intensive Care Unit. Am. J. Infect. Control 2016, 44, 203–209. [Google Scholar] [CrossRef]
- Wu, X.; Ye, L.; Liu, K.; Wang, W.; Wei, J.; Chen, F.; Liu, C. Antibacterial Properties of Mesoporous Copper-Doped Silica Xerogels. Biomed. Mater. 2009, 4, 045008. [Google Scholar] [CrossRef]
- Borkow, G.; Sidwell, R.W.; Smee, D.F.; Barnard, D.L.; Morrey, J.D.; Lara-Villegas, H.H.; Shemer-Avni, Y.; Gabbay, J. Neutralizing Viruses in Suspensions by Copper Oxide-Based Filters. Antimicrob. Agents Chemother. 2007, 51, 2605–2607. [Google Scholar] [CrossRef] [PubMed]
- Borkow, G.; Lara, H.H.; Covington, C.Y.; Nyamathi, A.; Gabbay, J. Deactivation of Human Immunodeficiency Virus Type 1 in Medium by Copper Oxide-Containing Filters. Antimicrob. Agents Chemother. 2008, 52, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, M.; Zodrow, K.R.; Genggeng, Q.; Kang, Y.; Giannelis, E.P.; Elimelech, M. Surface Functionalization of Thin-Film Composite Membranes with Copper Nanoparticles for Antimicrobial Surface Properties. Environ. Sci. Technol. 2014, 48, 384–393. [Google Scholar] [CrossRef]
- Inkinen, J.; Mäkinen, R.; Keinänen-Toivola, M.M.; Nordström, K.; Ahonen, M. Copper as an Antibacterial Material in Different Facilities. Lett. Appl. Microbiol. 2017, 64, 19–26. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Antibiofilm Peptides against Oral Biofilms. J. Oral Microbiol. 2017, 9, 1327308. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Kumar, K.D.; Akbari-Fakhrabadi, A.; Mangalaraja, R.V.; Amalraj, J. Chitosan Capped Copper Oxide/Copper Nanoparticles Encapsulated Microbial Resistant Nanocomposite Films. Int. J. Biol. Macromol. 2019, 128, 499–508. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Diez, A.M.d.R.; González, J.A.; Pérez, C.J.; Orrego, M.; Piehl, L.; Teves, S.; Copello, G.J. Antimicrobial Activity of Starch Hydrogel Incorporated with Copper Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 16280–16288. [Google Scholar] [CrossRef]
- Renné, W.G.; Lindner, A.; Mennito, A.S.; Agee, K.A.; Pashley, D.H.; Willett, D.; Sentelle, D.; Defee, M.; Schmidt, M.; Sabatini, C. Antibacterial Properties of Copper Iodide-Doped Glass Ionomer-Based Materials and Effect of Copper Iodide Nanoparticles on Collagen Degradation. Clin. Oral Investig. 2017, 21, 369–379. [Google Scholar] [CrossRef]
- Argueta-Figueroa, L.; Scougall-Vilchis, R.J.; Morales-Luckie, R.A.; Olea-Mejía, O.F. An Evaluation of the Antibacterial Properties and Shear Bond Strength of Copper Nanoparticles as a Nanofiller in Orthodontic Adhesive. Aust. Orthod. J. 2015, 31, 42–48. [Google Scholar] [CrossRef]
- ALGhanem, A.; Fernandes, G.; Visser, M.; Dziak, R.; Renné, W.G.; Sabatini, C. Biocompatibility and Bond Degradation of Poly-Acrylic Acid Coated Copper Iodide-Adhesives. Dent. Mater. 2017, 33, e336–e347. [Google Scholar] [CrossRef]
- Gosau, M.; Haupt, M.; Thude, S.; Strowitzki, M.; Schminke, B.; Buergers, R. Antimicrobial Effect and Biocompatibility of Novel Metallic Nanocrystalline Implant Coatings. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 1571–1579. [Google Scholar] [CrossRef]
- Palza, H.; Nuñez, M.; Bastías, R.; Delgado, K. In Situ Antimicrobial Behavior of Materials with Copper-Based Additives in a Hospital Environment. Int. J. Antimicrob. Agents 2018, 51, 912–917. [Google Scholar] [CrossRef]
- Mosselhy, D.A.; Kareinen, L.; Kivistö, I.; Virtanen, J.; Loikkanen, E.; Ge, Y.; Maunula, L.; Sironen, T. Inhibition of SARS-CoV-2 Alpha Variant and Murine Noroviruses on Copper-Silver Nanocomposite Surfaces. Nanomaterials 2022, 12, 1037. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Riduan, S.N.; Armugam, A.; Ong, J.T.; Hon, P.Y.; Abdad, M.Y.; Vasoo, S.; Ang, B.S.; Zhang, Y. Nanostructured Copper Surface Kills ESKAPE Pathogens and Viruses in Minutes. ChemMedChem 2021, 16, 3553–3558. [Google Scholar] [CrossRef] [PubMed]
- Das Jana, I.; Kumbhakar, P.; Banerjee, S.; Gowda, C.C.; Kedia, N.; Kuila, S.K.; Banerjee, S.; Das, N.C.; Das, A.K.; Manna, I.; et al. Copper Nanoparticle–Graphene Composite-Based Transparent Surface Coating with Antiviral Activity against Influenza Virus. ACS Appl. Nano Mater. 2021, 4, 352–362. [Google Scholar] [CrossRef]
- Foffa, I.; Losi, P.; Quaranta, P.; Cara, A.; Al Kayal, T.; D’Acunto, M.; Presciuttini, G.; Pistello, M.; Soldani, G. A Copper Nanoparticles-Based Polymeric Spray Coating: Nanoshield against Sars-Cov-2. J. Appl. Biomater. Funct. Mater. 2022, 20, 22808000221076330. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Summersgill, E.N.; Keevil, C.W. Inactivation of Murine Norovirus on a Range of Copper Alloy Surfaces Is Accompanied by Loss of Capsid Integrity. Appl. Environ. Microbiol. 2015, 81, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.V.; Vidal, A.R.; Durán, T.C. Aplicación de la capacidad bactericida del cobre en la práctica médica. Rev. Méd. Chile 2012, 140, 1325–1332. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Hans, M.; Hein, C.; Mancinelli, R.L.; Mücklich, F.; Wirth, R.; Rettberg, P.; Hellweg, C.E.; Moeller, R. Pure and Oxidized Copper Materials as Potential Antimicrobial Surfaces for Spaceflight Activities. Astrobiology 2017, 17, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- von Dessauer, B.; Navarrete, M.S.; Benadof, D.; Benavente, C.; Schmidt, M.G. Potential Effectiveness of Copper Surfaces in Reducing Health Care–Associated Infection Rates in a Pediatric Intensive and Intermediate Care Unit: A Nonrandomized Controlled Trial. Am. J. Infect. Control 2016, 44, e133–e139. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.M. 3D Printed Antibacterial Prostheses. Appl. Sci. 2018, 8, 1651. [Google Scholar] [CrossRef]
- He, W.; Huang, X.; Zheng, Y.; Sun, Y.; Xie, Y.; Wang, Y.; Yue, L. In Situ Synthesis of Bacterial Cellulose/Copper Nanoparticles Composite Membranes with Long-Term Antibacterial Property. J. Biomater. Sci. Polym. Ed. 2018, 29, 2137–2153. [Google Scholar] [CrossRef]
- Seo, Y.; Hwang, J.; Lee, E.; Kim, Y.J.; Lee, K.; Park, C.; Choi, Y.; Jeon, H.; Choi, J. Engineering Copper Nanoparticles Synthesized on the Surface of Carbon Nanotubes for Anti-Microbial and Anti-Biofilm Applications. Nanoscale 2018, 10, 15529–15544. [Google Scholar] [CrossRef]
- Zuniga, J.M.; Cortes, A. The Role of Additive Manufacturing and Antimicrobial Polymers in the COVID-19 Pandemic. Expert Rev. Med. Devices 2020, 17, 477–481. [Google Scholar] [CrossRef]
- Wang, W.; Li, B.; Yang, H.; Lin, Z.; Chen, L.; Li, Z.; Ge, J.; Zhang, T.; Xia, H.; Li, L.; et al. Efficient Elimination of Multidrug-Resistant Bacteria Using Copper Sulfide Nanozymes Anchored to Graphene Oxide Nanosheets. Nano Res. 2020, 13, 2156–2164. [Google Scholar] [CrossRef]
- Gargioni, C.; Borzenkov, M.; D’Alfonso, L.; Sperandeo, P.; Polissi, A.; Cucca, L.; Dacarro, G.; Grisoli, P.; Pallavicini, P.; D’Agostino, A.; et al. Self-Assembled Monolayers of Copper Sulfide Nanoparticles on Glass as Antibacterial Coatings. Nanomaterials 2020, 10, 352. [Google Scholar] [CrossRef]
- Wang, Z.; Hou, Z.; Wang, P.; Chen, F.; Luo, X. CuS-PNIPAm Nanoparticles with the Ability to Initiatively Capture Bacteria for Photothermal Treatment of Infected Skin. Regen. Biomater. 2022, 9, rbac026. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, Mechanism of Action, and Cytotoxicity of Copper-Based Nanoparticles: A Review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef]
- Giannousi, K.; Lafazanis, K.; Arvanitidis, J.; Pantazaki, A.; Dendrinou-Samara, C. Hydrothermal Synthesis of Copper Based Nanoparticles: Antimicrobial Screening and Interaction with DNA. J. Inorg. Biochem. 2014, 133, 24–32. [Google Scholar] [CrossRef]
- Thekkae Padil, V.V.; Černík, M. Green Synthesis of Copper Oxide Nanoparticles Using Gum Karaya as a Biotemplate and Their Antibacterial Application. Int. J. Nanomed. 2013, 8, 889–898. [Google Scholar] [CrossRef]
- Haldorai, Y.; Shim, J.-J. Novel Chitosan-TiO2 Nanohybrid: Preparation, Characterization, Antibacterial, and Photocatalytic Properties. Polym. Compos. 2014, 35, 327–333. [Google Scholar] [CrossRef]
- Lv, Q.; Zhang, B.; Xing, X.; Zhao, Y.; Cai, R.; Wang, W.; Gu, Q. Biosynthesis of Copper Nanoparticles Using Shewanella Loihica PV-4 with Antibacterial Activity: Novel Approach and Mechanisms Investigation. J. Hazard. Mater. 2018, 347, 141–149. [Google Scholar] [CrossRef]
- Meghana, S.; Kabra, P.; Chakraborty, S.; Padmavathy, N. Understanding the Pathway of Antibacterial Activity of Copper Oxide Nanoparticles. RSC Adv. 2015, 5, 12293–12299. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Song, H.; Lu, J.; Yuan, Z.; Guo, J. Copper Nanoparticles and Copper Ions Promote Horizontal Transfer of Plasmid-Mediated Multi-Antibiotic Resistance Genes across Bacterial Genera. Environ. Int. 2019, 129, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, D.K.; Subramaniyan, S.B.; Tharunya, M.P.; Veerappan, A. Antimicrobial Lipid Capped Copper Sulfide Nanoparticles Display Enhanced Bactericidal Effect against Carbapenem-Resistant Acinetobacter Baumannii. Mater. Lett. 2022, 306, 130985. [Google Scholar] [CrossRef]
- Ahmed, K.B.A.; Subramaniyan, S.B.; Banu, S.F.; Nithyanand, P.; Veerappan, A. Jacalin-Copper Sulfide Nanoparticles Complex Enhance the Antibacterial Activity against Drug Resistant Bacteria via Cell Surface Glycan Recognition. Colloids Surf. B Biointerfaces 2018, 163, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Midander, K.; Cronholm, P.; Karlsson, H.L.; Elihn, K.; Möller, L.; Leygraf, C.; Wallinder, I.O. Surface Characteristics, Copper Release, and Toxicity of Nano- and Micrometer-Sized Copper and Copper(II) Oxide Particles: A Cross-Disciplinary Study. Small 2009, 5, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of Antibacterial Activity of Copper Nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; ul Hasan, M.M. Investigations into the Antibacterial Behavior of Copper Nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Ruparelia, J.P.; Chatterjee, A.K.; Duttagupta, S.P.; Mukherji, S. Strain Specificity in Antimicrobial Activity of Silver and Copper Nanoparticles. Acta Biomater. 2008, 4, 707–716. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The Effect of Charge at the Surface of Silver Nanoparticles on Antimicrobial Activity against Gram-Positive and Gram-Negative Bacteria: A Preliminary Study. J. Nanomater. 2015, 2015, 53. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A Review on the Biosynthesis of Metal and Metal Salt Nanoparticles by Microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef]
- Gold, K.; Slay, B.; Knackstedt, M.; Gaharwar, A.K. Antimicrobial Activity of Metal and Metal-Oxide Based Nanoparticles. Adv. Therap. 2018, 1, 1700033. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and Their Antimicrobial Properties against Pathogens Including Bacteria, Fungi, Parasites and Viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Ninganagouda, S.; Rathod, V.; Singh, D.; Hiremath, J.; Singh, A.K.; Mathew, J.; ul-Haq, M. Growth Kinetics and Mechanistic Action of Reactive Oxygen Species Released by Silver Nanoparticles from Aspergillus niger on Escherichia coli. BioMed Res. Int. 2014, 2014, 753419. [Google Scholar] [CrossRef]
- Kaweeteerawat, C.; Chang, C.H.; Roy, K.R.; Liu, R.; Li, R.; Toso, D.; Fischer, H.; Ivask, A.; Ji, Z.; Zink, J.I.; et al. Cu Nanoparticles Have Different Impacts in Escherichia coli and Lactobacillus brevis than Their Microsized and Ionic Analogues. ACS Nano 2015, 9, 7215–7225. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Silva, R.G.; Morris, B.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Surface Charge-Dependent Toxicity of Silver Nanoparticles. Environ. Sci. Technol. 2011, 45, 283–287. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed; Oves; Khan; Habib; Memic, A. Antimicrobial Activity of Metal Oxide Nanoparticles against Gram-Positive and Gram-Negative Bacteria: A Comparative Study. IJN 2012, 7, 6003. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Sarkar, R.K.; Chattopadhyay, A.P.; Aich, P.; Chakraborty, R.; Basu, T. A Simple Robust Method for Synthesis of Metallic Copper Nanoparticles of High Antibacterial Potency against E. coli. Nanotechnology 2012, 23, 085103. [Google Scholar] [CrossRef]
- Jiang, S.; Lee, J.-H.; Kim, M.-G.; Myung, N.V.; Fredrickson, J.K.; Sadowsky, M.J.; Hur, H.-G. Biogenic Formation of As-S Nanotubes by Diverse Shewanella Strains. Appl. Environ. Microbiol. 2009, 75, 6896–6899. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver Nanoparticles as Antimicrobial Agent: A Case Study on E. Coli as a Model for Gram-Negative Bacteria. J. Colloid. Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- van de Lagemaat, M.; Grotenhuis, A.; van de Belt-Gritter, B.; Roest, S.; Loontjens, T.J.A.; Busscher, H.J.; van der Mei, H.C.; Ren, Y. Comparison of Methods to Evaluate Bacterial Contact-Killing Materials. Acta Biomater. 2017, 59, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Molteni, C.; Abicht, H.K.; Solioz, M. Killing of Bacteria by Copper Surfaces Involves Dissolved Copper. Appl. Environ. Microbiol. 2010, 76, 4099–4101. [Google Scholar] [CrossRef]
- Warnes, S.L.; Green, S.M.; Michels, H.T.; Keevil, C.W. Biocidal Efficacy of Copper Alloys against Pathogenic Enterococci Involves Degradation of Genomic and Plasmid DNAs. Appl. Environ. Microbiol. 2010, 76, 5390–5401. [Google Scholar] [CrossRef]
- Warnes, S.L.; Caves, V.; Keevil, C.W. Mechanism of Copper Surface Toxicity in Escherichia coli O157:H7 and Salmonella Involves Immediate Membrane Depolarization Followed by Slower Rate of DNA Destruction Which Differs from That Observed for Gram-Positive Bacteria: Rapid Death of Gram-Negative Bacteria on Dry Copper Surfaces. Environ. Microbiol. 2012, 14, 1730–1743. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.G.; Beveridge, T.J.; Fyfe, W.S. Iron-Silica Crystallite Nucleation by Bacteria in a Geothermal Sediment. Nature 1986, 320, 609–611. [Google Scholar] [CrossRef]
- Dupont, C.L.; Grass, G.; Rensing, C. Copper Toxicity and the Origin of Bacterial Resistance—New Insights and Applications. Metallomics 2011, 3, 1109. [Google Scholar] [CrossRef]
- Santo, C.E.; Morais, P.V.; Grass, G. Isolation and Characterization of Bacteria Resistant to Metallic Copper Surfaces. Appl. Environ. Microbiol. 2010, 76, 1341–1348. [Google Scholar] [CrossRef]
- Quaranta, D.; Krans, T.; Santo, C.E.; Elowsky, C.G.; Domaille, D.W.; Chang, C.J.; Grass, G. Mechanisms of Contact-Mediated Killing of Yeast Cells on Dry Metallic Copper Surfaces. Appl. Environ. Microbiol. 2011, 77, 416–426. [Google Scholar] [CrossRef]
- Peters, K.; Pazos, M.; Edoo, Z.; Hugonnet, J.E.; Martorana, A.M.; Polissi, A.; VanNieuwenhze, M.S.; Arthur, M.; Vollmer, W. Copper inhibits peptidoglycan LD-transpeptidases suppressing β-lactam resistance due to bypass of penicillin-binding proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10786–10791. [Google Scholar] [CrossRef]
- Nandakumar, R.; Santo, C.E.; Madayiputhiya, N.; Grass, G. Quantitative Proteomic Profiling of the Escherichia coli Response to Metallic Copper Surfaces. Biometals 2011, 24, 429–444. [Google Scholar] [CrossRef]
- Luo, J.; Hein, C.; Mücklich, F.; Solioz, M. Killing of Bacteria by Copper, Cadmium, and Silver Surfaces Reveals Relevant Physicochemical Parameters. Biointerphases 2017, 12, 020301. [Google Scholar] [CrossRef]
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact Killing of Bacteria on Copper Is Suppressed If Bacterial-Metal Contact Is Prevented and Is Induced on Iron by Copper Ions. Appl. Environ. Microbiol. 2013, 79, 2605–2611. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, M.; Solioz, M.; Edongué, H.; Arzt, E.; Schneider, A.S. Surface Structure Influences Contact Killing of Bacteria by Copper. MicrobiologyOpen 2014, 3, 327–332. [Google Scholar] [CrossRef]
- Tavakoli, A.; Hashemzadeh, M.S. Inhibition of Herpes Simplex Virus Type 1 by Copper Oxide Nanoparticles. J. Virol. Methods 2020, 275, 113688. [Google Scholar] [CrossRef]
- Hang, X.; Peng, H.; Song, H.; Qi, Z.; Miao, X.; Xu, W. Antiviral Activity of Cuprous Oxide Nanoparticles against Hepatitis C Virus in Vitro. J. Virol. Methods 2015, 222, 150–157. [Google Scholar] [CrossRef]
- Broglie, J.J.; Alston, B.; Yang, C.; Ma, L.; Adcock, A.F.; Chen, W.; Yang, L. Antiviral Activity of Gold/Copper Sulfide Core/Shell Nanoparticles against Human Norovirus Virus-Like Particles. PLoS ONE 2015, 10, e0141050. [Google Scholar] [CrossRef]
- Guo, X.; Sun, M.; Gao, R.; Qu, A.; Chen, C.; Xu, C.; Kuang, H.; Xu, L. Ultrasmall Copper (I) Sulfide Nanoparticles Prevent Hepatitis B Virus Infection. Angew. Chem. Int. Ed. 2021, 60, 13073–13080. [Google Scholar] [CrossRef]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel Antiviral Characteristics of Nanosized Copper(I) Iodide Particles Showing Inactivation Activity against 2009 Pandemic H1N1 Influenza Virus. Appl. Environ. Microbiol. 2012, 78, 951–955. [Google Scholar] [CrossRef]
- Shionoiri, N.; Sato, T.; Fujimori, Y.; Nakayama, T.; Nemoto, M.; Matsunaga, T.; Tanaka, T. Investigation of the Antiviral Properties of Copper Iodide Nanoparticles against Feline Calicivirus. J. Biosci. Bioeng. 2012, 113, 580–586. [Google Scholar] [CrossRef]
- Thurman, R.B.; Gerba, C.P.; Bitton, G. The Molecular Mechanisms of Copper and Silver Ion Disinfection of Bacteria and Viruses. Crit. Rev. Environ. Control 1989, 18, 295–315. [Google Scholar] [CrossRef]
- Warnes, S.L.; Keevil, C.W. Inactivation of Norovirus on Dry Copper Alloy Surfaces. PLoS ONE 2013, 8, e75017. [Google Scholar] [CrossRef] [PubMed]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Warnes, S.L.; Little, Z.R.; Keevil, C.W. Human Coronavirus 229E Remains Infectious on Common Touch Surface Materials. mBio 2015, 6, e01697-15. [Google Scholar] [CrossRef]
- Karlström, A.R.; Levine, R.L. Copper Inhibits the Protease from Human Immunodeficiency Virus 1 by Both Cysteine-Dependent and Cysteine-Independent Mechanisms. Proc. Natl. Acad. Sci. USA 1991, 88, 5552–5556. [Google Scholar] [CrossRef]
- Sagripanti, J.L.; Routson, L.B.; Lytle, C.D. Virus Inactivation by Copper or Iron Ions Alone and in the Presence of Peroxide. Appl. Environ. Microbiol. 1993, 59, 4374–4376. [Google Scholar] [CrossRef]
- Jordan, F.T.; Nassar, T.J. The Influence of Copper on the Survival of Infectious Bronchitis Vaccine Virus in Water. Vet. Rec. 1971, 89, 609–610. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Hiatt, C.W.; Haller, W. Mechanism of Inactivation of Bacteriophages by Metals. Biochim. Biophys. Acta 1964, 91, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Morgan, A.R.; Paranchych, W. Controlled Cleavage of Phage R17 RNA within the Virion by Treatment with Ascorbate and Copper (II). Can. J. Biochem. 1974, 52, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Minoshima, M.; Hashimoto, K. Highly Efficient Antiviral and Antibacterial Activities of Solid-State Cuprous Compounds. J. Hazard. Mater. 2012, 235–236, 265–270. [Google Scholar] [CrossRef]
- Yahya, M.T.; Straub, T.M.; Gerba, C.P. Inactivation of Coliphage MS-2 and Poliovirus by Copper, Silver, and Chlorine. Can. J. Microbiol. 1992, 38, 430–435. [Google Scholar] [CrossRef]
- Totsuka, A.; Ohtaki, K. The Effects of Amino Acids and Metals on the Infectivity of Poliovirus Ribonucleic Acid. Jpn. J. Microbiol. 1974, 18, 107–112. [Google Scholar] [CrossRef]
- Poggio, C.; Colombo, M.; Arciola, C.R.; Greggi, T.; Scribante, A.; Dagna, A. Copper-Alloy Surfaces and Cleaning Regimens against the Spread of SARS-CoV-2 in Dentistry and Orthopedics. From Fomites to Anti-Infective Nanocoatings. Materials 2020, 13, 3244. [Google Scholar] [CrossRef]
- Muller, M.P.; MacDougall, C.; Lim, M.; Ontario Agency for Health Protection and Promotion Public Health Ontario; Provincial Infectious Diseases Advisory Committee on Infection Prevention and Control. Provincial Infectious Diseases Advisory Committee on Infection Prevention and Control Antimicrobial Surfaces to Prevent Healthcare-Associated Infections: A Systematic Review. J. Hosp. Infect. 2016, 92, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Behzadinasab, S.; Chin, A.; Hosseini, M.; Poon, L.; Ducker, W.A. A Surface Coating That Rapidly Inactivates SARS-CoV-2. ACS Appl. Mater. Interfaces 2020, 12, 34723–34727. [Google Scholar] [CrossRef]
- Mantlo, E.; Rhodes, T.; Boutros, J.; Patterson-Fortin, L.; Evans, A.; Paessler, S. In Vitro Efficacy of a Copper Iodine Complex PPE Disinfectant for SARS-CoV-2 Inactivation. F1000Res 2020, 9, 674. [Google Scholar] [CrossRef] [PubMed]
- Paital, B.; Das, K.; Parida, S.K. Inter Nation Social Lockdown versus Medical Care against COVID-19, a Mild Environmental Insight with Special Reference to India. Sci. Total Environ. 2020, 728, 138914. [Google Scholar] [CrossRef] [PubMed]
- Cortes, A.A.; Zuñiga, J.M. The Use of Copper to Help Prevent Transmission of SARS-Coronavirus and Influenza Viruses. A General Review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115176. [Google Scholar] [CrossRef]
- Colorado, H.A.; Mendoza, D.E.; Lin, H.-T.; Gutierrez-Velasquez, E. Additive Manufacturing against the Covid-19 Pandemic: A Technological Model for the Adaptability and Networking. J. Mater. Res. Technol. 2022, 16, 1150–1164. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Kukushkina, E.A.; Hossain, S.I.; Picca, R.A.; Ditaranto, N.; Cioffi, N. Can Nanotechnology and Materials Science Help the Fight against SARS-CoV-2? Nanomaterials 2020, 10, 802. [Google Scholar] [CrossRef]
- Aboubakr, H.A.; Sharafeldin, T.A.; Goyal, S.M. Stability of SARS-CoV-2 and Other Coronaviruses in the Environment and on Common Touch Surfaces and the Influence of Climatic Conditions: A Review. Transbound. Emerg. Dis. 2021, 68, 296–312. [Google Scholar] [CrossRef]
- Kampf, G.; Todt, D.; Pfaender, S.; Steinmann, E. Persistence of Coronaviruses on Inanimate Surfaces and Their Inactivation with Biocidal Agents. J. Hosp. Infect. 2020, 104, 246–251. [Google Scholar] [CrossRef]
- Bisht, N.; Dwivedi, N.; Kumar, P.; Venkatesh, M.; Yadav, A.K.; Mishra, D.; Solanki, P.; Verma, N.K.; Lakshminarayanan, R.; Ramakrishna, S.; et al. Recent Advances in Copper and Copper-Derived Materials for Antimicrobial Resistance and Infection Control. Curr. Opin. Biomed. Eng. 2022, 24, 100408. [Google Scholar] [CrossRef]
- Airey, P.; Verran, J. Potential Use of Copper as a Hygienic Surface; Problems Associated with Cumulative Soiling and Cleaning. J. Hosp. Infect. 2007, 67, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Hewawaduge, C.; Senevirathne, A.; Jawalagatti, V.; Kim, J.W.; Lee, J.H. Copper-Impregnated Three-Layer Mask Efficiently Inactivates SARS-CoV2. Environ. Res. 2021, 196, 110947. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Ger, T.-R.; Uapipatanakul, B.; Huang, J.-C.; Chen, K.H.-C.; Hsiao, C.-D. Review of Copper and Copper Nanoparticle Toxicity in Fish. Nanomaterials 2020, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Reshma, V.G.; Mohanan, P.V. Quantum Dots: Applications and Safety Consequences. J. Lumin. 2019, 205, 287–298. [Google Scholar] [CrossRef]
- Tulinska, J.; Mikusova, M.L.; Liskova, A.; Busova, M.; Masanova, V.; Uhnakova, I.; Rollerova, E.; Alacova, R.; Krivosikova, Z.; Wsolova, L.; et al. Copper Oxide Nanoparticles Stimulate the Immune Response and Decrease Antioxidant Defense in Mice after Six-Week Inhalation. Front. Immunol. 2022, 13, 874253. [Google Scholar] [CrossRef]
- Wu, F.; Harper, B.J.; Crandon, L.E.; Harper, S.L. Assessment of Cu and CuO Nanoparticle Ecological Responses Using Laboratory Small-Scale Microcosms. Environ. Sci. Nano 2020, 7, 105–115. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023, 24, 10503. https://doi.org/10.3390/ijms241310503
Ramos-Zúñiga J, Bruna N, Pérez-Donoso JM. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. International Journal of Molecular Sciences. 2023; 24(13):10503. https://doi.org/10.3390/ijms241310503
Chicago/Turabian StyleRamos-Zúñiga, Javiera, Nicolás Bruna, and José M. Pérez-Donoso. 2023. "Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses" International Journal of Molecular Sciences 24, no. 13: 10503. https://doi.org/10.3390/ijms241310503
APA StyleRamos-Zúñiga, J., Bruna, N., & Pérez-Donoso, J. M. (2023). Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. International Journal of Molecular Sciences, 24(13), 10503. https://doi.org/10.3390/ijms241310503






