Efficacy of Green Synthesized Nanoparticles in Photodynamic Therapy: A Therapeutic Approach
Abstract
1. Introduction
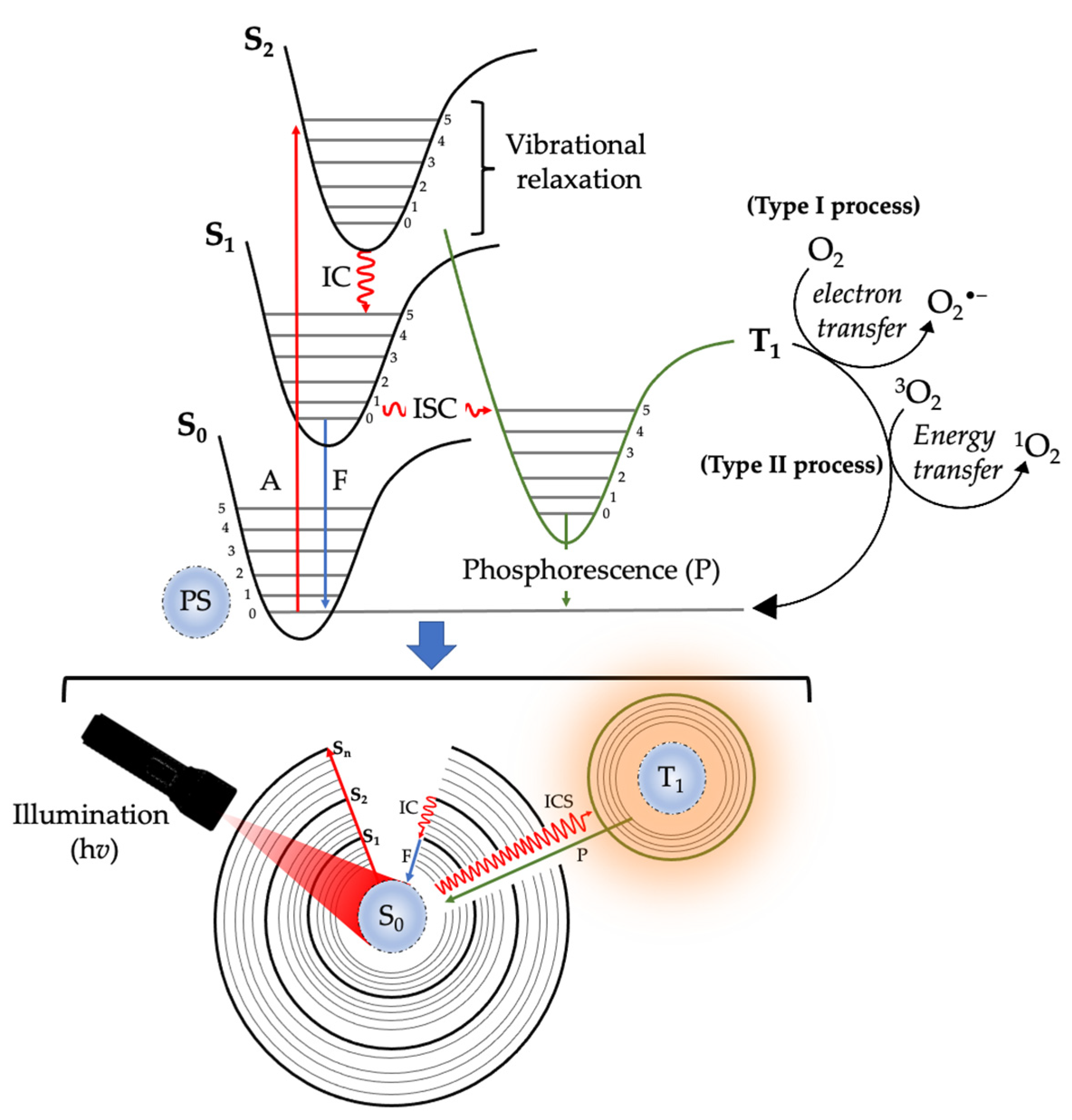
2. Photodynamic Therapy
2.1. Classification of Photosensitizers Used in PDT
| PS Name | Class | Chemical Structure | Application | Wavelength | Ref. |
|---|---|---|---|---|---|
| Hematoporphyrin (HpD) | Photofrin | 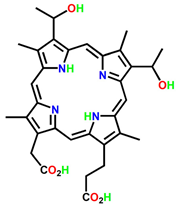 | Brain, cervical, endobronchial, oesophageal, bladder and gastric cancers | 30 nm | [21] |
| Benzoporphyrin derivative monoacid (BPD-MA) | Verteporfin | 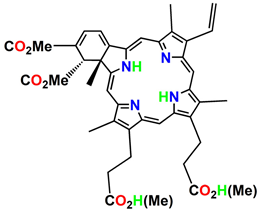 | Basal cell carcinoma | 689 nm | [23] |
| Temoporfin (m-THPC) | Foscan |  | Head and neck, prostate, and pancreatic cancers | 652 nm | [39] |
| 5-aminolevulinic acid (5-ALA) | Levulan | 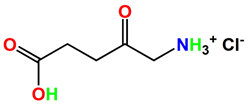 | Basal cell carcinoma, brain, head and neck and bladder and gynaecological cancers | 375–635 nm | [27] |
| 3-[1′-hexyloxyethyl]-2-devinylpyropheophorbide-a (HPPH) | Photochlor | 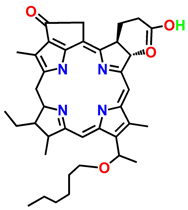 | Basal cell carcinoma | 665 nm | [40] |
| Gold Nano clustered Hyaluronan Nano-Assemblies | Nano assemblies |  | Orthotopic breast tumour model | - | [28] |
| Chlorin E6 (Ce6) + Upconversion nanoparticles | Nanoparticles |  | THP-1 macrophages | 980–405 nm | [29] |
| ICG-loaded nanospheres coated with chitosan | Nanoparticles |  | Coronavirus disease 2019, Ebola, and AIDS | 800–805 nm | [41] |
2.2. Application of Phytochemicals in PDT
| Medicinal Plants | Phytochemicals | Application | Ref. |
|---|---|---|---|
| Dicoma anomala | Sesquiterpenes | Breast, lung, prostate cancers | [70] |
| Aloe vera | Aloe emodin, Emodin | Head and neck cancer, glioblastoma, colon, breast cancer, and gastric carcinoma | [63,71] |
| Curcuma longa | Curcumin | Prostate, colorectal, breast, pancreatic, and head and neck cancers | [72,73] |
| Ipomoea mauritiana | Scopoletin | Lung, liver, skin and breast cancer | [74,75,76] |
| Berberis aristate | Berberine | Breast and colorectal cancer | [77,78] |
| Ficus religiosa | Furanocoumarin | Breast and ovarian cancer | [79,80] |
| Rubia cordifolia | Rubiadin | Human cervical, and larynx cancer | [69,81,82,83] |
2.2.1. Nanotechnology
| Nanoparticle Name | Structure | Size | Excitation Wavelength (nm) | Application | Ref. |
|---|---|---|---|---|---|
| Mesoporous silica nanoparticles (MSNs) |  | 30–300 nm | 804–815 nm | Drug delivery, bioceramics, pharmaceutics, and biomedicine | [109,110,111] |
| Liposomes |  | 0.025–2.5 μm | - | Pharmaceutics, and drug delivery | [112] |
| Micelle |  | 5–200 nm | 420 nm | Cancer treatment, skin treatment, eye treatment, head and neck cancer treatment, and drug delivery | [95,113] |
| Noncationic polymer nanoparticles |  | 1–1000 nm | - | Drug delivery, theragnostic and bioimaging | [114] |
| Nanosphere |  | <100 nm | 615 nm | Drug delivery | [100,115] |
| Gold nanoparticles (AuNPs) |  | <100 nm | 740 nm | Drug delivery | [100,116] |
| Nanorods |  | 10–120 nm | 700–800 nm | Electronic, optical, magnetic, and micromechanical devices | [117] |
| Solid lipid nanoparticles (SLNs) |  | 50–1000 nm | 495–515 nm | Drug delivery | [103,118] |
| Upconversion nanoparticles (UCNPs) |  | - | 970 nm | Bio-photonics, and bioimaging | [104,119] |
| Polyamide-amine (PAMAM) |  | 10–1000 nm | 450 nm | Targeted therapy, diagnostic therapy, and drug delivery | [108,120,121] |
| Silver nanoparticles (AgNPs) |  | 1–100 nm | 239–314 nm | Drug delivery, cosmetics, health-care, anti-fungal, anti-bacterial and anti-inflammatory | [108,122,123] |
2.2.2. Therapeutic Applications of Nanotechnology
3. Green Nanotechnology
3.1. Synthesis and Characterization
3.1.1. Synthesis Methods
3.1.2. Characterization Methods
Transmission Electron Microscopy (TEM)
Scanning Electron Microscopy (SEM)
Atomic Force Microscopy (AFM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cancer Tomorrow; World Health Organization: Geneva, Switzerland, 2020; Available online: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=23&single_unit=500&populations=710&sexes=2 (accessed on 30 April 2023).
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef]
- Hortobagyi, G.N. Treatment of Breast Cancer. N. Engl. J. Med. 1998, 339, 974–984. [Google Scholar] [CrossRef]
- Whelan, T.; Levine, M. Radiation Therapy and Tamoxifen: Concurrent or Sequential? That Is the Question. JCO 2005, 23, 1–4. [Google Scholar] [CrossRef]
- Conklin, K.A. Chemotherapy-Associated Oxidative Stress: Impact on Chemotherapeutic Effectiveness. Integr. Cancer Ther. 2004, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.J.; Visvanathan, K.; Wolff, A.C. Long Term Side Effects of Adjuvant Chemotherapy in Patients with Early Breast Cancer. Breast 2015, 24 (Suppl. S2), S149–S153. [Google Scholar] [CrossRef] [PubMed]
- Farkona, S.; Diamandis, E.P.; Blasutig, I.M. Cancer Immunotherapy: The Beginning of the End of Cancer? BMC Med. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Juarranz, A.; Jaén, P.; Sanz-Rodríguez, F.; Cuevas, J.; González, S. Photodynamic Therapy of Cancer. Basic Principles and Applications. Clin. Transl. Oncol. 2008, 10, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.B.; Brown, E.A.; Walker, I. The Present and Future Role of Photodynamic Therapy in Cancer Treatment. Lancet Oncol. 2004, 5, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Spring, B.Q.; Bryan Sears, R.; Zheng, L.Z.; Mai, Z.; Watanabe, R.; Sherwood, M.E.; Schoenfeld, D.A.; Pogue, B.W.; Pereira, S.P.; Villa, E.; et al. A Photoactivable Multi-Inhibitor Nanoliposome for Tumour Control and Simultaneous Inhibition of Treatment Escape Pathways. Nat. Nanotechnol. 2016, 11, 378–387. [Google Scholar] [CrossRef]
- Thomas, C.; MacGill, R.S.; Miller, G.C.; Pardini, R.S. Photoactivation of Hypericin Generates Singlet Oxygen in Mitochondria and Inhibits Succinoxidase. Photochem. Photobiol. 1992, 55, 47–53. [Google Scholar] [CrossRef]
- Allison, R.R.; Downie, G.H.; Cuenca, R.; Hu, X.-H.; Childs, C.J.; Sibata, C.H. Photosensitizers in Clinical PDT. Photodiagnosis Photodyn. Ther. 2004, 1, 27–42. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Gomer, C.J.; Henderson, B.W.; Jori, G.; Kessel, D.; Korbelik, M.; Moan, J.; Peng, Q. Photodynamic Therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Toratani, S.; Tani, R.; Kanda, T.; Koizumi, K.; Yoshioka, Y.; Okamoto, T. Photodynamic Therapy Using Photofrin and Excimer Dye Laser Treatment for Superficial Oral Squamous Cell Carcinomas with Long-Term Follow Up. Photodiagnosis Photodyn. Ther. 2016, 14, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Selbo, P.K.; Weyergang, A.; Dietze, A.; Prasmickaite, L.; Bonsted, A.; Engesaeter, B.Ø.; Angell-Petersen, E.; Warloe, T.; Frandsen, N.; et al. Porphyrin-Related Photosensitizers for Cancer Imaging and Therapeutic Applications. J. Microsc. 2005, 218, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in Photodynamic Therapy: Part One-Photosensitizers, Photochemistry and Cellular Localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef]
- McCaughan, J.S., Jr.; Hicks, W.; Laufman, L.; May, E.; Roach, R. Palliation of Esophageal Malignancy with Photoradiation Therapy. Cancer 1984, 54, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Balchum, O.J.; Doiron, D.R.; Huth, G.C. Photoradiation Therapy of Endobronchial Lung Cancers Employing the Photodynamic Action of Hematoporphvrin Derivative. Lasers Surg. Med. 1984, 4, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.C.; Pottier, R.H.; Pross, D.C. Photodynamic Therapy with Endogenous Protoporphyrin IX: Basic Principles and Present Clinical Experience. J. Photochem. Photobiol. B 1990, 6, 143–148. [Google Scholar] [CrossRef]
- Maiolino, S.; Moret, F.; Conte, C.; Fraix, A.; Tirino, P.; Ungaro, F.; Sortino, S.; Reddi, E.; Quaglia, F. Hyaluronan-Decorated Polymer Nanoparticles Targeting the CD44 Receptor for the Combined Photo/Chemo-Therapy of Cancer. Nanoscale 2015, 7, 5643–5653. [Google Scholar] [CrossRef]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular Effectors of Multiple Cell Death Pathways Initiated by Photodynamic Therapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2007, 1776, 86–107. [Google Scholar] [CrossRef]
- Lee, H.M.; Jeong, Y.-I.; Kim, D.H.; Kwak, T.W.; Chung, C.-W.; Kim, C.H.; Kang, D.H. Ursodeoxycholic Acid-Conjugated Chitosan for Photodynamic Treatment of HuCC-T1 Human Cholangiocarcinoma Cells. Int. J. Pharm. 2013, 454, 74–81. [Google Scholar] [CrossRef]
- Lin, J.; Wang, S.; Huang, P.; Wang, Z.; Chen, S.; Niu, G.; Li, W.; He, J.; Cui, D.; Lu, G.; et al. Photosensitizer-Loaded Gold Vesicles with Strong Plasmonic Coupling Effect for Imaging-Guided Photothermal/Photodynamic Therapy. ACS Nano 2013, 7, 5320–5329. [Google Scholar] [CrossRef]
- Hasan, T.; Ortel, B.; Moor, A.C.E.; Pogue, B.W. Photodynamic Therapy of Cancer. In Holland-Frei Cancer Medicine, 6th ed.; BC Decker: Chicago, IL, USA, 2003. [Google Scholar]
- McBride, G. Studies Expand Potential Uses of Photodynamic Therapy. JNCI J. Natl. Cancer Inst. 2002, 94, 1740–1742. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic Therapy (PDT) of Cancer: From Local to Systemic Treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Otvagin, V.F.; Kuzmina, N.S.; Kudriashova, E.S.; Nyuchev, A.V.; Gavryushin, A.E.; Fedorov, A.Y. Conjugates of Porphyrinoid-Based Photosensitizers with Cytotoxic Drugs: Current Progress and Future Directions toward Selective Photodynamic Therapy. J. Med. Chem. 2022, 65, 1695–1734. [Google Scholar] [CrossRef] [PubMed]
- Wentrup, R.; Winkelmann, N.; Mitroshkin, A.; Prager, M.; Voderholzer, W.; Schachschal, G.; Jürgensen, C.; Büning, C. Photodynamic Therapy Plus Chemotherapy Compared with Photodynamic Therapy Alone in Hilar Nonresectable Cholangiocarcinoma. Gut Liver 2016, 10, 470–475. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal Complexes in Cancer Therapy—An Update from Drug Design Perspective. Drug Des. Devel. Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Muhammad, N.; Guo, Z. Metal-Based Anticancer Chemotherapeutic Agents. Curr. Opin. Chem. Biol. 2014, 19, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.J.; Vernon, D.I.; Brown, S.B. Photodynamic Therapy Effect of M-THPC (Foscan) In Vivo: Correlation with Pharmacokinetics. Br. J. Cancer 2003, 89, 398–404. [Google Scholar] [CrossRef]
- Kessel, D.; Luo, Y.; Deng, Y.; Chang, C.K. The Role of Subcellular Localization in Initiation of Apoptosis by Photodynamic Therapy. Photochem. Photobiol. 1997, 65, 422–426. [Google Scholar] [CrossRef]
- Kushibiki, T.; Hirasawa, T.; Okawa, S.; Ishihara, M. Responses of Cancer Cells Induced by Photodynamic Therapy. J. Healthc. Eng. 2013, 4, 87–108. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151543-6. [Google Scholar]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Montazeri, G.R.; Sepehri, Y. The Evolution of Botanical and Herbal Medicine in Islamic Civilization. OJE 2019, 09, 35–42. [Google Scholar] [CrossRef]
- Foresto, E.; Gilardi, P.; Ibarra, L.E.; Cogno, I.S. Light-Activated Green Drugs: How We Can Use Them in Photodynamic Therapy and Mass-Produce Them with Biotechnological Tools. Phytomed. Plus 2021, 1, 100044. [Google Scholar] [CrossRef]
- Falzon, C.C.; Balabanova, A. Phytotherapy: An Introduction to Herbal Medicine. Prim. Care Clin. Off. Pract. 2017, 44, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Gomer, C. Avastin Enhances Photodynamic Therapy Treatment of Kaposi’s Sarcoma in a Mouse Tumor Model. JEP(T) 2006, 25, 251–260. [Google Scholar] [CrossRef]
- Chota, A.; George, B.P.; Abrahamse, H. Dicoma Anomala Enhances Phthalocyanine Mediated Photodynamic Therapy in MCF-7 Breast Cancer Cells. Front. Pharmacol. 2022, 13, 1486. [Google Scholar] [CrossRef]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin Photoprotection and Inhibition of Photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.C.-L. Nuclear EGFR and Integrator/Super Elongation Complex Concurrently Binds to Immediate Early Genes for Gene Transactivation. J. Cancer 2018, 9, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Tiwari, V.K. Natural Products: An Evolving Role in Future Drug Discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Goel, A.; Aggarwal, B.B. Curcumin, the Golden Spice From Indian Saffron, Is a Chemosensitizer and Radiosensitizer for Tumors and Chemoprotector and Radioprotector for Normal Organs. Nutr. Cancer 2010, 62, 919–930. [Google Scholar] [CrossRef]
- Matsabisa, M.G.; Chukwuma, C.I.; Chaudhary, S.K.; Kumar, C.S.; Baleni, R.; Javu, M.; Oyedemi, S.O. Dicoma Anomala (Sond.) Abates Glycation and DPP-IV Activity and Modulates Glucose Utilization in Chang Liver Cells and 3T3-L1 Adipocytes. S. Afr. J. Bot. 2020, 128, 182–188. [Google Scholar] [CrossRef]
- Maroyi, A. Dicoma anomala Sond.: A review of its botany, ethnomedicine, phytochemistry and pharmacology. Asian J. Pharm. Clin. Res. 2018, 11, 70–77. [Google Scholar] [CrossRef]
- Steenkamp, V.; Gouws, M.C. Cytotoxicity of Six South African Medicinal Plant Extracts Used in the Treatment of Cancer. S. Afr. J. Bot. 2006, 72, 630–633. [Google Scholar] [CrossRef]
- Tripathy, S.; Rademan, S.; Matsabisa, M.G. Effects of Silver Nanoparticle from Dicoma Anomala Sond. Root Extract on MCF-7 Cancer Cell Line and NF54 Parasite Strain: An In Vitro Study. Biol. Trace Elem. Res. 2020, 195, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Chota, A.; George, B.P.; Abrahamse, H. Potential Treatment of Breast and Lung Cancer Using Dicoma Anomala, an African Medicinal Plant. Molecules 2020, 25, 4435. [Google Scholar] [CrossRef] [PubMed]
- Balogun, F.O.; Ashafa, A.O.T. Aqueous Root Extracts of Dicoma Anomala (Sond.) Extenuates Postprandial Hyperglycaemia In Vitro and Its Modulation on the Activities of Carbohydrate-Metabolizing Enzymes in Streptozotocin-Induced Diabetic Wistar Rats. S. Afr. J. Bot. 2017, 112, 102–111. [Google Scholar] [CrossRef]
- ESHUN, K.; HE, Q. Aloe Vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef]
- Medhin, L.B.; Sibhatu, D.B.; Seid, M.; Ferej, F.M.; Mohamedkassm, N.; Berhane, Y.; Kaushek, A.; Humida, M.E.; Gasmalbari, E. Comparative Antimicrobial Activities of the Gel, Leaf and Anthraquinone Fractionates of Four Aloe Species (Aloe Camperi, Aloe Elegans, Aloe Eumassawana and Aloe Scholleri). Adv. Microbiol. 2019, 9, 139–150. [Google Scholar] [CrossRef]
- Reynolds, T.; Dweck, A.C. Aloe Vera Leaf Gel: A Review Update. J. Ethnopharmacol. 1999, 68, 3–37. [Google Scholar] [CrossRef]
- Muniyandi, K.; George, B.; Parimelazhagan, T.; Abrahamse, H. Role of Photoactive Phytocompounds in Photodynamic Therapy of Cancer. Molecules 2020, 25, 4102. [Google Scholar] [CrossRef]
- Rathi, B.; Sahu, J.; Koul, S.; Kosha, R.L. Detailed Pharmacognostical Studies on Berberis Aristata DC Plant. Anc. Sci. Life 2013, 32, 234–240. [Google Scholar] [CrossRef]
- Komal, S.; Ranjan, B.; Neelam, C.; Birendra, S.; Saini, N. Berberis Aristata: A Review. Int. J. Res. Ayurveda Pharm. 2011, 2, 383–388. [Google Scholar]
- Pullaiah, T. Medicinal Plants in India, Regency Publication. New Delhi 2002, 1, 1–4. [Google Scholar]
- CT, S.; M, D.; AR, S.; KR, L.; Balachandran, I. Characterization of Coumarins from Ipomoea Mauritiana Jacq by LC-APCI-MS/MS Analysis and Evaluation of Its Anti-Amnesic Activity. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 24. [Google Scholar] [CrossRef]
- Sulaiman, C.; Geetha, S.P.; Indira, B. Identification of Phenolic Antioxidants in Ipomoea Mauritiana Jacq. Using Spectrophotometric and Mass Spectroscopic Studies. Avicenna J. Phytomed. 2014, 4, 89–96. [Google Scholar]
- Rao, G.M.M.; Rao, C.V.; Pushpangadan, P.; Shirwaikar, A. Hepatoprotective Effects of Rubiadin, a Major Constituent of Rubia Cordifolia Linn. J. Ethnopharmacol. 2006, 103, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.V.; van der Merwe, M.M.; van Brummelen, A.C.; Pillay, P.; Crampton, B.G.; Mmutlane, E.M.; Parkinson, C.; van Heerden, F.R.; Crouch, N.R.; Smith, P.J.; et al. In Vitro Anti-Plasmodial Activity of Dicoma Anomala Subsp. Gerrardii (Asteraceae): Identification of Its Main Active Constituent, Structure-Activity Relationship Studies and Gene Expression Profiling. Malar. J. 2011, 10, 295. [Google Scholar] [CrossRef]
- Manirakiza, A.; Irakoze, L.; Manirakiza, S. Aloe and Its Effects on Cancer: A Narrative Literature Review. East Afr. Health Res. J. 2021, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [PubMed]
- Tung, B.T.; Nham, D.T.; Hai, N.T.; Thu, D.K. Chapter 10—Curcuma Longa, the Polyphenolic Curcumin Compound and Pharmacological Effects on Liver. In Dietary Interventions in Liver Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 125–134. ISBN 978-0-12-814466-4. [Google Scholar]
- Lodia, S.; Kansala, L. Antioxidant activity of rubia cordifolia against lead toxicity. Int. J. Pharm. Sci. Res. IJPSR 2012, 3, 2224–2232. [Google Scholar]
- Beheshti, F.; Shabani, A.A.; Akbari Eidgahi, M.R.; Kookhaei, P.; Vazirian, M.; Safavi, M. Anticancer Activity of Ipomoea Purpurea Leaves Extracts in Monolayer and Three-Dimensional Cell Culture. Evid.-Based Complement. Altern. Med. 2021, 2021, e6666567. [Google Scholar] [CrossRef]
- Kashyap, P.; Ram, H.; Shukla, S.D.; Kumar, S. Scopoletin: Antiamyloidogenic, Anticholinesterase, and Neuroprotective Potential of a Natural Compound Present in Argyreia Speciosa Roots by In Vitro and In Silico Study. J. Exp. Neurosci. 2020, 15, 2633105520937693. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Li, K.; Guan, F.; Yao, F.; Yu, Y.; Zhang, M.; Hatch, G.M.; Chen, L. Berberine Pretreatment Confers Cardioprotection Against Ischemia–Reperfusion Injury in a Rat Model of Type 2 Diabetes. J. Cardiovasc. Pharm. Ther. 2016, 21, 486–494. [Google Scholar] [CrossRef]
- Zhao, Y.; Roy, S.; Wang, C.; Goel, A. A Combined Treatment with Berberine and Andrographis Exhibits Enhanced Anti-Cancer Activity through Suppression of DNA Replication in Colorectal Cancer. Pharmaceuticals 2022, 15, 262. [Google Scholar] [CrossRef]
- Ahmed, S.; Khan, H.; Aschner, M.; Mirzae, H.; Küpeli Akkol, E.; Capasso, R. Anticancer Potential of Furanocoumarins: Mechanistic and Therapeutic Aspects. Int. J. Mol. Sci. 2020, 21, 5622. [Google Scholar] [CrossRef]
- Shaikh, A.; Patel, M.; Padul, M. Anticancer Potential of Ficus Religiosa (Linn) Against Human Breast Cancer Cell Line MDA-MB-231 and Its Phytoconstituents by HR-LCMS. Res. Sq. 2020, 1–17. [Google Scholar] [CrossRef]
- Rumie Vittar, N.B.; Comini, L.; Fernadez, I.M.; Agostini, E.; Nuñez-Montoya, S.; Cabrera, J.L.; Rivarola, V.A. Photochemotherapy Using Natural Anthraquinones: Rubiadin and Soranjidiol Sensitize Human Cancer Cell to Die by Apoptosis. Photodiagnosis Photodyn. Ther. 2014, 11, 182–192. [Google Scholar] [CrossRef]
- Patel, P.R.; Nagar, D.; Patel, R.C.; Rathod, D.K.; Patel, V. In Vitro Anticancer Activity of Rubia Cordifolia against Hela and HEP2 Cell Lines. Int. J. Pharm. Pharm. Sci. 2011, 3, 70–71. [Google Scholar]
- Peng, Z.; Fang, G.; Peng, F.; Pan, Z.; Su, Z.; Tian, W.; Li, D.; Hou, H. Effects of Rubiadin Isolated from Prismatomeris Connata on Anti-Hepatitis B Virus Activity In Vitro. Phytother. Res. 2017, 31, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wang, Z.; Shen, W.; Liang, R.; Yan, D.; Wei, M. Recent Advances in Innovative Strategies for Enhanced Cancer Photodynamic Therapy. Theranostics 2021, 11, 3278–3300. [Google Scholar] [CrossRef]
- Shen, J.; Shafiq, M.; Ma, M.; Chen, H. Synthesis and Surface Engineering of Inorganic Nanomaterials Based on Microfluidic Technology. Nanomaterials 2020, 10, 1177. [Google Scholar] [CrossRef]
- Ebbesen, M.; Jensen, T.G. Nanomedicine: Techniques, Potentials, and Ethical Implications. J. Biomed. Biotechnol. 2006, 2006, 51516. [Google Scholar] [CrossRef]
- Saini, R.; Saini, S.; Sharma, S. Nanotechnology: The Future Medicine. J. Cutan. Aesthet. Surg. 2010, 3, 32–33. [Google Scholar] [CrossRef]
- Feynman, R.P. There’s Plenty of Room at the Bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Peng, F.; Su, Y.; Zhong, Y.; Fan, C.; Lee, S.-T.; He, Y. Silicon Nanomaterials Platform for Bioimaging, Biosensing, and Cancer Therapy. Acc. Chem. Res. 2014, 47, 612–623. [Google Scholar] [CrossRef]
- Zhou, Y.; Quan, G.; Wu, Q.; Zhang, X.; Niu, B.; Wu, B.; Huang, Y.; Pan, X.; Wu, C. Mesoporous Silica Nanoparticles for Drug and Gene Delivery. Acta Pharm. Sin. B 2018, 8, 165–177. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- Jain, V.; Kumar, H.; Anod, H.V.; Chand, P.; Gupta, N.V.; Dey, S.; Kesharwani, S.S. A Review of Nanotechnology-Based Approaches for Breast Cancer and Triple-Negative Breast Cancer. J. Control. Release 2020, 326, 628–647. [Google Scholar] [CrossRef]
- Kim, B.; Park, J.-H.; Sailor, M.J. Rekindling RNAi Therapy: Materials Design Requirements for In Vivo SiRNA Delivery. Adv. Mater. 2019, 31, e1903637. [Google Scholar] [CrossRef]
- Chemin, M.; Brun, P.-M.; Lecommandoux, S.; Sandre, O.; Meins, J.-F.L. Hybrid Polymer/Lipid Vesicles: Fine Control of the Lipid and Polymer Distribution in the Binary Membrane. Soft Matter 2012, 8, 2867–2874. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric Micelle Stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Tapeinos, C.; Efthimiadou, E.K.; Boukos, N.; Charitidis, C.A.; Koklioti, M.; Kordas, G. Microspheres as Therapeutic Delivery Agents: Synthesis and Biological Evaluation of PH Responsiveness. J. Mater. Chem. B 2012, 1, 194–203. [Google Scholar] [CrossRef]
- Aibani, N.; Khan, T.N.; Callan, B. Liposome Mimicking Polymersomes; A Comparative Study of the Merits of Polymersomes in Terms of Formulation and Stability. Int. J. Pharm. X 2020, 2, 100040. [Google Scholar] [CrossRef]
- Bardhan, R.; Mukherjee, S.; Mirin, N.A.; Levit, S.D.; Nordlander, P.; Halas, N.J. Nanosphere-in-a-Nanoshell: A Simple Nanomatryushka. J. Phys. Chem. C 2010, 114, 7378–7383. [Google Scholar] [CrossRef]
- Liao, H.; Hafner, J.H. Gold Nanorod Bioconjugates. Chem. Mater. 2005, 17, 4636–4641. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Akombaetwa, N.; Ilangala, A.B.; Thom, L.; Memvanga, P.B.; Witika, B.A.; Buya, A.B. Current Advances in Lipid Nanosystems Intended for Topical and Transdermal Drug Delivery Applications. Pharmaceutics 2023, 15, 656. [Google Scholar] [CrossRef]
- Mu, H.; Holm, R. Solid Lipid Nanocarriers in Drug Delivery: Characterization and Design. Expert Opin. Drug Deliv. 2018, 15, 771–785. [Google Scholar] [CrossRef]
- Kumar, M.; Kakkar, V.; Mishra, A.K.; Chuttani, K.; Kaur, I.P. Intranasal Delivery of Streptomycin Sulfate (STRS) Loaded Solid Lipid Nanoparticles to Brain and Blood. Int. J. Pharm. 2014, 461, 223–233. [Google Scholar] [CrossRef]
- Yang, D.; Ma, P.; Hou, Z.; Cheng, Z.; Li, C.; Lin, J. Current Advances in Lanthanide Ion (Ln3+)-Based Upconversion Nanomaterials for Drug Delivery. Chem. Soc. Rev. 2015, 44, 1416–1448. [Google Scholar] [CrossRef]
- Qi, R.; Zhang, H.; Xu, L.; Shen, W.; Chen, C.; Wang, C.; Cao, Y.; Wang, Y.; van Dongen, M.A.; He, B.; et al. G5 PAMAM Dendrimer versus Liposome: A Comparison Study on the In Vitro Transepithelial Transport and In Vivo Oral Absorption of Simvastatin. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1141–1151. [Google Scholar] [CrossRef]
- Menjoge, A.R.; Kannan, R.M.; Tomalia, D.A. Dendrimer-Based Drug and Imaging Conjugates: Design Considerations for Nanomedical Applications. Drug Discov. Today 2010, 15, 171–185. [Google Scholar] [CrossRef]
- Gu, J.; Fang, X.; Hao, J.; Sha, X. Reversal of P-Glycoprotein-Mediated Multidrug Resistance by CD44 Antibody-Targeted Nanocomplexes for Short Hairpin RNA-Encoding Plasmid DNA Delivery. Biomaterials 2015, 45, 99–114. [Google Scholar] [CrossRef]
- Prashob Peter, K.J. Multi-Functional Silver Nanoparticles for Drug Delivery: A Review. Int. J. Curr. Res. Rev. 2017, 9, 1–5. [Google Scholar]
- Vallet-Regí, M. Our Contributions to Applications of Mesoporous Silica Nanoparticles. Acta Biomater. 2022, 137, 44–52. [Google Scholar] [CrossRef]
- Kumar, L.; Pathak, Y. Nanocarriers for Drug-Targeting Brain Tumors; Elsevier Science: Amsterdam, The Netherlands, 2022; ISBN 978-0-323-90773-6. [Google Scholar]
- Palantavida, S.; Tang, R.; Sudlow, G.P.; Akers, W.J.; Achilefu, S.; Sokolov, I. Ultrabright NIR Fluorescent Mesoporous Silica Nanoparticles. J. Mater. Chem. B 2014, 2, 3107–3114. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Bose, A.; Roy Burman, D.; Sikdar, B.; Patra, P. Nanomicelles: Types, Properties and Applications in Drug Delivery. IET Nanobiotechnol. 2021, 15, 19–27. [Google Scholar] [CrossRef]
- Cohen, E.M.; Ding, H.; Kessinger, C.W.; Khemtong, C.; Gao, J.; Sumer, B.D. Polymeric Micelle Nanoparticles for Photodynamic Treatment of Head and Neck Cancer Cells. Otolaryngol.-Head Neck Surg. 2010, 143, 109–115. [Google Scholar] [CrossRef]
- Zhang, P.; Song, N.; Liu, S.; Li, Q.; Wang, Y.; Zhou, B. Tuning the Photoluminescence of Lanthanide Metal–Organic Framework Nanospheres through Ligand-Induced Phase Transition towards Sensing. J. Mater. Chem. C 2021, 9, 6208–6216. [Google Scholar] [CrossRef]
- Anzalone, A.; Gabriel, M.; Estrada, L.C.; Gratton, E. Spectral Properties of Single Gold Nanoparticles in Close Proximity to Biological Fluorophores Excited by 2-Photon Excitation. PLoS ONE 2015, 10, e0124975. [Google Scholar] [CrossRef] [PubMed]
- Ghassan, A.A.; Mijan, N.-A.; Taufiq-Yap, Y.H.; Ghassan, A.A.; Mijan, N.-A.; Taufiq-Yap, Y.H. Nanomaterials: An Overview of Nanorods Synthesis and Optimization. In Nanorods and Nanocomposites; IntechOpen: London, UK, 2019; pp. 1–24. ISBN 978-1-78984-469-6. [Google Scholar]
- Andreozzi, E.; Seo, J.W.; Ferrara, K.; Louie, A. A Novel Method to Label Solid Lipid Nanoparticles (SLNs) with 64Cu for Positron Emission Tomography (PET) Imaging. Bioconjug. Chem. 2011, 22, 808–818. [Google Scholar] [CrossRef]
- Pominova, D.V.; Romanishkin, I.D.; Proydakova, V.Y.; Grachev, P.V.; Moskalev, A.S.; Ryabova, A.V.; Makarov, V.I.; Linkov, K.G.; Kuznetsov, S.V.; Voronov, V.V.; et al. Optimization of Upconversion Luminescence Excitation Mode for Deeper In Vivo Bioimaging without Contrast Loss or Overheating. Methods Appl. Fluoresc. 2020, 8, 025006. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Zijian, Y.; Wei, D. Poly (Amido Amine)s with Different Branched Architecture: Synthesis, Reactivity and Their Application in Gene Delivery. Chin. J. Org. Chem. 2018, 38, 2713. [Google Scholar] [CrossRef]
- Wang, D.; Imae, T. Fluorescence Emission from Dendrimers and Its PH Dependence. J. Am. Chem. Soc. 2004, 126, 13204–13205. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, X.; Yuan, H.; Zhou, Z.; Xiao, D. Preparation of Silver Nanoparticle and Its Application to the Determination of Ct-DNA. Sensors 2007, 7, 708–718. [Google Scholar] [CrossRef]
- Galatage, S.T.; Hebalkar, A.S.; Dhobale, S.V.; Mali, O.R.; Kumbhar, P.S.; Nikade, S.V.; Killedar, S.G.; Galatage, S.T.; Hebalkar, A.S.; Dhobale, S.V.; et al. Silver Nanoparticles: Properties, Synthesis, Characterization, Applications and Future Trends. In Silver Micro-Nanoparticles-Properties, Synthesis, Characterization, and Applications; IntechOpen: London, UK, 2021; pp. 1–19. ISBN 978-1-83968-660-3. [Google Scholar]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug Targeting to Tumors: Principles, Pitfalls and (Pre-) Clinical Progress. J. Control. Release 2012, 161, 175–187. [Google Scholar] [CrossRef]
- Ranganathan, R.; Madanmohan, S.; Kesavan, A.; Baskar, G.; Krishnamoorthy, Y.R.; Santosham, R.; Ponraju, D.; Rayala, S.K.; Venkatraman, G. Nanomedicine: Towards Development of Patient-Friendly Drug-Delivery Systems for Oncological Applications. IJN 2012, 7, 1043–1060. [Google Scholar] [CrossRef]
- Torchilin, V.P. Multifunctional Nanocarriers. Adv. Drug Deliv. Rev. 2006, 58, 1532–1555. [Google Scholar] [CrossRef]
- Gao, J.; Chen, K.; Xie, R.; Xie, J.; Yan, Y.; Cheng, Z.; Peng, X.; Chen, X. In Vivo Tumor-Targeted Fluorescence Imaging Using Near-Infrared Non-Cadmium Quantum Dots. Bioconjug. Chem. 2010, 21, 604–609. [Google Scholar] [CrossRef]
- Jokerst, J.V.; Thangaraj, M.; Kempen, P.J.; Sinclair, R.; Gambhir, S.S. Photoacoustic Imaging of Mesenchymal Stem Cells in Living Mice via Silica-Coated Gold Nanorods. ACS Nano 2012, 6, 5920–5930. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Shi, J. In Vivo Bio-Safety Evaluations and Diagnostic/Therapeutic Applications of Chemically Designed Mesoporous Silica Nanoparticles. Adv. Mater. 2013, 25, 3144–3176. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.; Chan, J.; Wang, A.; Langer, R.; Farokhzad, O. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Thomas, M.; Klibanov, A.M.; Langer, R. Exploring Polyethylenimine-Mediated DNA Transfection and the Proton Sponge Hypothesis. J. Gene Med. 2005, 7, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and Development of Polymers for Gene Delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Alsehli, M.; Al-Enizi, A.; Nafady, A. Recent Advances in Mesoporous Silica Nanoparticles for Targeted Drug Delivery Applications. Curr. Drug Deliv. 2022, 19, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous Silica Nanoparticles: Synthesis, Classification, Drug Loading, Pharmacokinetics, Biocompatibility, and Application in Drug Delivery. Expert Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, J. Mesoporous Silica Nanoparticle Based Nano Drug Delivery Systems: Synthesis, Controlled Drug Release and Delivery, Pharmacokinetics and Biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.Y.; Gao, J.; Wang, Z. Recent Advances in Photodynamic Therapy for Cancer and Infectious Diseases. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1560. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications. Adv. Healthc. Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef]
- Ahmed, H.; Gomte, S.S.; Prathyusha, E.; Prabakaran, A.; Agrawal, M.; Alexander, A.; Prabakaran, A.; Agrawal, M.; Alexander, A. Biomedical Applications of Mesoporous Silica Nanoparticles as a Drug Delivery Carrier. J. Drug Deliv. Sci. Technol. 2022, 76, 103729. [Google Scholar] [CrossRef]
- Torchilin, V.P. Micellar Nanocarriers: Pharmaceutical Perspectives. Pharm. Res. 2007, 24, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Xu, C.; Sun, X.; Chen, X. Nanoparticle Design Strategies for Enhanced Anticancer Therapy by Exploiting the Tumour Microenvironment. Chem. Soc. Rev. 2017, 46, 3830–3852. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tian, Y.; Zhai, S.; Liu, Y.; Chu, S.; Xiong, Z. The Progress of Research on the Application of Redox Nanomaterials in Disease Therapy. Front. Chem. 2023, 11, 1115440. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent Advances with Liposomes as Pharmaceutical Carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Labhasetwar, V. Nanotech Approaches to Drug Delivery and Imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Xu, C.-F.; Luo, Y.-L.; Lu, Z.-D.; Wang, J. Systemic Delivery of CRISPR/Cas9 with PEG-PLGA Nanoparticles for Chronic Myeloid Leukemia Targeted Therapy. Biomater. Sci. 2018, 6, 1592–1603. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-Viral Vectors for Gene-Based Therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Z.; Wientjes, M.G.; Au, J.L.-S. Delivery of SiRNA Therapeutics: Barriers and Carriers. AAPS J. 2010, 12, 492–503. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The Golden Age: Gold Nanoparticles for Biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold Nanoparticles: Opportunities and Challenges in Nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Mwakwari, S.C.; Sodji, Q.H.; Oyelere, A.K.; El-Sayed, M.A. Tamoxifen−Poly(Ethylene Glycol)−Thiol Gold Nanoparticle Conjugates: Enhanced Potency and Selective Delivery for Breast Cancer Treatment. Bioconjug. Chem. 2009, 20, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Fomina, N.; McFearin, C.; Sermsakdi, M.; Edigin, O.; Almutairi, A. UV and Near-IR Triggered Release from Polymeric Nanoparticles. J. Am. Chem. Soc. 2010, 132, 9540–9542. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Hernández, M.; del Pino, P.; Mitchell, S.G.; Moros, M.; Stepien, G.; Pelaz, B.; Parak, W.J.; Gálvez, E.M.; Pardo, J.; de la Fuente, J.M. Dissecting the Molecular Mechanism of Apoptosis during Photothermal Therapy Using Gold Nanoprisms. ACS Nano 2015, 9, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Liao, I.-C.; Liu, J.B.; Bursac, N.; Leong, K.W. Effect of Electromechanical Stimulation on the Maturation of Myotubes on Aligned Electrospun Fibers. Cell. Mol. Bioeng. 2008, 1, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A New Ageless Bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Warner, J.C.; Cannon, A.S.; Dye, K.M. Green Chemistry. Environ. Impact Assess. Rev. 2004, 24, 775–799. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Green Synthesis of Biogenic Metal Nanoparticles by Terrestrial and Aquatic Phototrophic and Heterotrophic Eukaryotes and Biocompatible Agents. Adv. Colloid Interface Sci. 2011, 169, 59–79. [Google Scholar] [CrossRef]
- Sharma, D.; Kanchi, S.; Bisetty, K. Biogenic Synthesis of Nanoparticles: A Review. Arab. J. Chem. 2019, 12, 3576–3600. [Google Scholar] [CrossRef]
- Brandis, A.S.; Salomon, Y.; Scherz, A. Chlorophyll Sensitizers in Photodynamic Therapy. In Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2006; pp. 461–483. ISBN 978-1-4020-4516-5. [Google Scholar]
- Thipe, V.C.; Karikachery, A.R.; Çakılkaya, P.; Farooq, U.; Genedy, H.H.; Kaeokhamloed, N.; Phan, D.-H.; Rezwan, R.; Tezcan, G.; Roger, E.; et al. Green Nanotechnology—An Innovative Pathway towards Biocompatible and Medically Relevant Gold Nanoparticles. J. Drug Deliv. Sci. Technol. 2022, 70, 103256. [Google Scholar] [CrossRef]
- Alivisatos, P. The Use of Nanocrystals in Biological Detection. Nat. Biotechnol. 2004, 22, 47–52. [Google Scholar] [CrossRef]
- Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M.; Mulvaney, P. Gold Nanorods: Synthesis, Characterization and Applications. Coord. Chem. Rev. 2005, 249, 1870–1901. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, e5102014. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Surrey, A.; Pohl, D.; Schultz, L.; Rellinghaus, B. Quantitative Measurement of the Surface Self-Diffusion on Au Nanoparticles by Aberration-Corrected Transmission Electron Microscopy. Nano Lett. 2012, 12, 6071–6077. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Greer, H.F. What Can Electron Microscopy Tell Us Beyond Crystal Structures? Eur. J. Inorg. Chem. 2016, 2016, 941–950. [Google Scholar] [CrossRef]
- Wen, S.; Liu, J.; Deng, J. Chapter 3—Methods for the Detection and Composition Study of Fluid Inclusions. In Fluid Inclusion Effect in Flotation of Sulfide Minerals; Wen, S., Liu, J., Deng, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–68. ISBN 978-0-12-819845-2. [Google Scholar]
- Rades, S.; Hodoroaba, V.-D.; Salge, T.; Wirth, T.; Lobera, M.P.; Labrador, R.H.; Natte, K.; Behnke, T.; Gross, T.; Unger, W.E.S. High-Resolution Imaging with SEM/T-SEM, EDX and SAM as a Combined Methodical Approach for Morphological and Elemental Analyses of Single Engineered Nanoparticles. RSC Adv. 2014, 4, 49577–49587. [Google Scholar] [CrossRef]
- Millan, M.C.; Gasque, M.; Garcia-Diego, F.J.; Curiel, J.; Ruiz, G. Determination of Punica Granatum L. Carpellary Membrane Elastic Properties Using Atomic Force Microscopy. In Recent Advances in Multidisciplinary Applied Physics; Méndez-Vilas, A., Ed.; Elsevier Science Ltd.: Oxford, UK, 2005; pp. 119–125. ISBN 978-0-08-044648-6. [Google Scholar]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahra, M.; Chota, A.; Abrahamse, H.; George, B.P. Efficacy of Green Synthesized Nanoparticles in Photodynamic Therapy: A Therapeutic Approach. Int. J. Mol. Sci. 2023, 24, 10931. https://doi.org/10.3390/ijms241310931
Zahra M, Chota A, Abrahamse H, George BP. Efficacy of Green Synthesized Nanoparticles in Photodynamic Therapy: A Therapeutic Approach. International Journal of Molecular Sciences. 2023; 24(13):10931. https://doi.org/10.3390/ijms241310931
Chicago/Turabian StyleZahra, Mehak, Alexander Chota, Heidi Abrahamse, and Blassan P. George. 2023. "Efficacy of Green Synthesized Nanoparticles in Photodynamic Therapy: A Therapeutic Approach" International Journal of Molecular Sciences 24, no. 13: 10931. https://doi.org/10.3390/ijms241310931
APA StyleZahra, M., Chota, A., Abrahamse, H., & George, B. P. (2023). Efficacy of Green Synthesized Nanoparticles in Photodynamic Therapy: A Therapeutic Approach. International Journal of Molecular Sciences, 24(13), 10931. https://doi.org/10.3390/ijms241310931









