Brain-Biomarker Changes in Body Fluids of Patients with Parkinson’s Disease
Abstract
1. Introduction
1.1. PD
1.2. PD Biomarkers: General Information
2. Brain-Biomarker Changes in Body Fluids of PD Patients
2.1. Neurofilament Light Protein
2.2. Substance P
2.3. S100 Calcium-Binding Protein A10
2.4. Neurotensin
2.5. Chromogranins
2.6. VGF
2.7. Glial Fibrillary Acidic Protein
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Przedborski, S. The Two-Century Journey of Parkinson Disease Research. Nat. Rev. Neurosci. 2017, 18, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Tysnes, O.-B.; Storstein, A. Epidemiology of Parkinson’s Disease. J. Neural. Transm. 2017, 124, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.G.; Postuma, R. Premotor and Nonmotor Features of Parkinson’s Disease. Curr. Opin. Neurol. 2014, 27, 434–441. [Google Scholar] [CrossRef]
- Siderowf, A.; Lang, A.E. Premotor Parkinson’s Disease: Concepts and Definitions. Mov. Disord. 2012, 27, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Hess, C.W.; Okun, M.S. Diagnosing Parkinson Disease. Contin. Lifelong Learn. Neurol. 2016, 22, 1047–1063. [Google Scholar] [CrossRef]
- Joutsa, J.; Gardberg, M.; Röyttä, M.; Kaasinen, V. Diagnostic Accuracy of Parkinsonism Syndromes by General Neurologists. Park. Relat. Disord. 2014, 20, 840–844. [Google Scholar] [CrossRef]
- Poewe, W.; Wenning, G. The Differential Diagnosis of Parkinson’s Disease. Eur. J. Neurol. 2002, 9, 23–30. [Google Scholar] [CrossRef]
- del Rey, N.L.-G.; García-Cabezas, M.Á. Cytology, Architecture, Development, and Connections of the Primate Striatum: Hints for Human Pathology. Neurobiol. Dis. 2023, 176, 105945. [Google Scholar] [CrossRef]
- Afifi, A.K. Topical Review: Basal Ganglia: Functional Anatomy and Physiology. Part 2. J. Child. Neurol. 1994, 9, 352–361. [Google Scholar] [CrossRef]
- Emamzadeh, F.N.; Surguchov, A. Parkinson’s Disease: Biomarkers, Treatment, and Risk Factors. Front. Neurosci. 2018, 12, 612. [Google Scholar] [CrossRef]
- Lotankar, S.; Prabhavalkar, K.S.; Bhatt, L.K. Biomarkers for Parkinson’s Disease: Recent Advancement. Neurosci. Bull. 2017, 33, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, L.; Xie, J.; Shi, L. The Emerging Role of Neuropeptides in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 646726. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, T.; Lehéricy, S.; Chiu, S.Y.; Strafella, A.P.; Stoessl, A.J.; Vaillancourt, D.E. Emerging Neuroimaging Biomarkers Across Disease Stage in Parkinson Disease. JAMA Neurol. 2021, 78, 1262. [Google Scholar] [CrossRef] [PubMed]
- Farrer, M.J. Genetics of Parkinson Disease: Paradigm Shifts and Future Prospects. Nat. Rev. Genet. 2006, 7, 306–318. [Google Scholar] [CrossRef]
- Brooks, J.; Ding, J.; Simon-Sanchez, J.; Paisan-Ruiz, C.; Singleton, A.B.; Scholz, S.W. Parkin and PINK1 Mutations in Early-Onset Parkinson’s Disease: Comprehensive Screening in Publicly Available Cases and Control. J. Med. Genet. 2009, 46, 375–381. [Google Scholar] [CrossRef]
- Wittke, C.; Petkovic, S.; Dobricic, V.; Schaake, S.; Arzberger, T.; Compta, Y.; Englund, E.; Ferguson, L.W.; Gelpi, E.; Roeber, S.; et al. Genotype–Phenotype Relations for the Atypical Parkinsonism Genes: MDSGene Systematic Review. Mov. Disord. 2021, 36, 1499–1510. [Google Scholar] [CrossRef]
- Lesage, S.; Houot, M.; Mangone, G.; Tesson, C.; Bertrand, H.; Forlani, S.; Anheim, M.; Brefel-Courbon, C.; Broussolle, E.; Thobois, S.; et al. Genetic and Phenotypic Basis of Autosomal Dominant Parkinson’s Disease in a Large Multi-Center Cohort. Front. Neurol. 2020, 11, 682. [Google Scholar] [CrossRef]
- Billingsley, K.J.; Ding, J.; Jerez, P.A.; Illarionova, A.; Levine, K.; Grenn, F.P.; Makarious, M.B.; Moore, A.; Vitale, D.; Reed, X.; et al. Genome-Wide Analysis of Structural Variants in Parkinson Disease. Ann. Neurol. 2023, 93, 1012–1022. [Google Scholar] [CrossRef]
- Havelund, J.; Heegaard, N.; Færgeman, N.; Gramsbergen, J. Biomarker Research in Parkinson’s Disease Using Metabolite Profiling. Metabolites 2017, 7, 42. [Google Scholar] [CrossRef]
- Hamid, Z.; Basit, A.; Pontis, S.; Piras, F.; Assogna, F.; Bossù, P.; Pontieri, F.E.; Stefani, A.; Spalletta, G.; Franceschi, P.; et al. Gender Specific Decrease of a Set of Circulating N-Acylphosphatidyl Ethanolamines (NAPEs) in the Plasma of Parkinson’s Disease Patients. Metabolomics 2019, 15, 74. [Google Scholar] [CrossRef]
- Jang, Y.; Pletnikova, O.; Troncoso, J.C.; Pantelyat, A.Y.; Dawson, T.M.; Rosenthal, L.S.; Na, C.H. Mass Spectrometry–Based Proteomics Analysis of Human Substantia Nigra from Parkinson’s Disease Patients Identifies Multiple Pathways Potentially Involved in the Disease. Mol. Cell. Proteom. 2023, 22, 100452. [Google Scholar] [CrossRef]
- Ganguly, U.; Singh, S.; Pal, S.; Prasad, S.; Agrawal, B.K.; Saini, R.V.; Chakrabarti, S. Alpha-Synuclein as a Biomarker of Parkinson’s Disease: Good, but Not Good Enough. Front. Aging Neurosci. 2021, 13, 702639. [Google Scholar] [CrossRef]
- Du, T.; Wang, L.; Liu, W.; Zhu, G.; Chen, Y.; Zhang, J. Biomarkers and the Role of α-Synuclein in Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 645996. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Idowu, A.; Rosenthal, L.; Mao, X. Parkinson’s Disease Fluid Biomarkers for Differential Diagnosis of Atypical Parkinsonian Syndromes. Clin. Transl. Discov. 2023, 3, e150. [Google Scholar] [CrossRef]
- Kang, J.-H.; Mollenhauer, B.; Coffey, C.S.; Toledo, J.B.; Weintraub, D.; Galasko, D.R.; Irwin, D.J.; Van Deerlin, V.; Chen-Plotkin, A.S.; Caspell-Garcia, C.; et al. CSF Biomarkers Associated with Disease Heterogeneity in Early Parkinson’s Disease: The Parkinson’s Progression Markers Initiative Study. Acta Neuropathol. 2016, 131, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.O.; Geren, B.B. The fibrous structure of the nerve axon in relation to the localization of “neurotubules”. J. Exp. Med. 1950, 91, 499–504. [Google Scholar] [CrossRef]
- Lee, M.K.; Cleveland, D.W. Neuronal Intermediate Filaments. Annu. Rev. Neurosci. 1996, 19, 187–217. [Google Scholar] [CrossRef]
- Yuan, A.; Rao, M.V.; Veeranna; Nixon, R.A. Neurofilaments at a Glance. J. Cell Sci. 2012, 125, 3257–3263. [Google Scholar] [CrossRef]
- Sainio, M.T.; Rasila, T.; Molchanova, S.M.; Järvilehto, J.; Torregrosa-Muñumer, R.; Harjuhaahto, S.; Pennonen, J.; Huber, N.; Herukka, S.-K.; Haapasalo, A.; et al. Neurofilament Light Regulates Axon Caliber, Synaptic Activity, and Organelle Trafficking in Cultured Human Motor Neurons. Front. Cell Dev. Biol. 2022, 9, 820105. [Google Scholar] [CrossRef]
- Goldman, J.E.; Yen, S.-H.; Chiu, F.-C.; Peress, N.S. Lewy Bodies of Parkinson’s Disease Contain Neurofilament Antigens. Science 1983, 221, 1082–1084. [Google Scholar] [CrossRef]
- Marques, T.M.; van Rumund, A.; Oeckl, P.; Kuiperij, H.B.; Esselink, R.A.J.; Bloem, B.R.; Otto, M.; Verbeek, M.M. Serum NFL Discriminates Parkinson Disease from Atypical Parkinsonisms. Neurology 2019, 92, e1479–e1486. [Google Scholar] [CrossRef] [PubMed]
- Hansson, O.; Janelidze, S.; Hall, S.; Magdalinou, N.; Lees, A.J.; Andreasson, U.; Norgren, N.; Linder, J.; Forsgren, L.; Constantinescu, R.; et al. Blood-Based NfL: A Biomarker for Differential Diagnosis of Parkinsonian Disorder. Neurology 2017, 88, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Li, C.-H.; Yang, K.-C.; Lin, F.-J.; Wu, C.-C.; Chieh, J.-J.; Chiu, M.-J. Blood NfL: A Biomarker for Disease Severity and Progression in Parkinson Disease. Neurology 2019, 93, e1104–e1111. [Google Scholar] [CrossRef] [PubMed]
- Oosterveld, L.P.; Verberk, I.M.W.; Majbour, N.K.; El-Agnaf, O.M.; Weinstein, H.C.; Berendse, H.W.; Teunissen, C.E.; Berg, W.D.J. CSF or Serum Neurofilament Light Added to α-Synuclein Panel Discriminates Parkinson’s from Controls. Mov. Disord. 2020, 35, 288–295. [Google Scholar] [CrossRef]
- Mollenhauer, B.; Dakna, M.; Kruse, N.; Galasko, D.; Foroud, T.; Zetterberg, H.; Schade, S.; Gera, R.G.; Wang, W.; Gao, F.; et al. Validation of Serum Neurofilament Light Chain as a Biomarker of Parkinson’s Disease Progression. Mov. Disord. 2020, 35, 1999–2008. [Google Scholar] [CrossRef]
- Liu, Y.; Dou, K.; Xue, L.; Li, X.; Xie, A. Neurofilament Light as a Biomarker for Motor Decline in Parkinson’s Disease. Front. Neurosci. 2022, 16, 959261. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, C.; Zhang, Q.; Shen, T.; Sun, J. Serum NFL Discriminates Parkinson Disease from Essential Tremor and Reflect Motor and Cognition Severity. BMC Neurol. 2022, 22, 39. [Google Scholar] [CrossRef]
- Niemann, L.; Lezius, S.; Maceski, A.; Leppert, D.; Englisch, C.; Schwedhelm, E.; Zeller, T.; Gerloff, C.; Kuhle, J.; Choe, C. Serum Neurofilament Is Associated with Motor Function, Cognitive Decline and Subclinical Cardiac Damage in Advanced Parkinson’s Disease (MARK-PD). Park. Relat. Disord. 2021, 90, 44–48. [Google Scholar] [CrossRef]
- Chen, J.-H.; Chan, L.; Chung, C.-C.; Bamodu, O.A.; Hong, C.-T. Blood Neurofilament Light Chain in Parkinson’s Disease: Comparability between Parkinson’s Progression Markers Initiative (PPMI) and Asian Cohorts. J. Clin. Med. 2021, 10, 5085. [Google Scholar] [CrossRef]
- Shi, J.; Qin, X.; Chang, X.; Wang, H.; Guo, J.; Zhang, W. Neurofilament Markers in Serum and Cerebrospinal Fluid of Patients with Amyotrophic Lateral Sclerosis. J. Cell Mol. Med. 2022, 26, 583–587. [Google Scholar] [CrossRef]
- Zhu, N.; Santos-Santos, M.; Illán-Gala, I.; Montal, V.; Estellés, T.; Barroeta, I.; Altuna, M.; Arranz, J.; Muñoz, L.; Belbin, O.; et al. Plasma Glial Fibrillary Acidic Protein and Neurofilament Light Chain for the Diagnostic and Prognostic Evaluation of Frontotemporal Dementia. Transl. Neurodegener. 2021, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Leuzy, A.; Mattsson-Carlgren, N.; Palmqvist, S.; Janelidze, S.; Dage, J.L.; Hansson, O. Blood-based Biomarkers for Alzheimer’s Disease. EMBO Mol. Med. 2022, 14, e14408. [Google Scholar] [CrossRef] [PubMed]
- Disanto, G.; Barro, C.; Benkert, P.; Naegelin, Y.; Schädelin, S.; Giardiello, A.; Zecca, C.; Blennow, K.; Zetterberg, H.; Leppert, D.; et al. Serum Neurofilament Light: A Biomarker of Neuronal Damage in Multiple Sclerosis. Ann. Neurol. 2017, 81, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.S.; von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and Their Receptors: Contributions to Physiological Control and the Mechanisms of Disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.M.; Leeman, S.E.; Niall, H.D. Amino-Acid Sequence of Substance P. Nat. New Biol. 1971, 232, 86–87. [Google Scholar] [CrossRef]
- Graefe, S.B.; Mohiuddin, S.S. Biochemistry, Substance P. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554583/?report=printable (accessed on 1 June 2023).
- Chang, C.-T.; Jiang, B.-Y.; Chen, C.-C. Ion Channels Involved in Substance P-Mediated Nociception and Antinociception. Int. J. Mol. Sci. 2019, 20, 1596. [Google Scholar] [CrossRef]
- Nisbet, A.P.; Foster, O.J.F.; Kingsbury, A.; Eve, D.J.; Daniel, S.E.; Marsden, C.D.; Lees, A.J. Preproenkephalin and Preprotachykinin Messenger RNA Expression in Normal Human Basal Ganglia and in Parkinson’s Disease. Neuroscience 1995, 66, 361–376. [Google Scholar] [CrossRef]
- Bolam, J.P.; Smith, Y. The GABA and Substance P Input to Dopaminergic Neurones in the Substantia Nigra of the Rat. Brain Res. 1990, 529, 57–78. [Google Scholar] [CrossRef]
- Shannon, K.M.; Keshavarzian, A.; Dodiya, H.B.; Jakate, S.; Kordower, J.H. Is Alpha-Synuclein in the Colon a Biomarker for Premotor Parkinson’s Disease? Evidence from 3 Cases. Mov. Disord. 2012, 27, 716–719. [Google Scholar] [CrossRef]
- Smith, R.L.; Baker, H.; Greer, C.A. Immunohistochemical Analyses of the Human Olfactory Bulb. J. Comp. Neurol. 1993, 333, 519–530. [Google Scholar] [CrossRef]
- Lindefors, N.; Brodin, E.; Tossman, U.; Segovia, J.; Ungerstedt, U. Tissue Levels and in Vivo Release of Tachykinins and GABA in Striatum and Substantia Nigra of Rat Brain after Unilateral Striatal Dopamine Denervation. Exp. Brain Res. 1989, 74, 527–534. [Google Scholar] [CrossRef]
- Salin, P.; Hajji, M.D.; Goff, L. Bilateral 6-Hydroxydopamine-Induced Lesion of the Nigrostriatal Dopamine Pathway Reproduces the Effects of Unilateral Lesion on Substance P but Not on Enkephalin Expression in Rat Basal Ganglia. Eur. Neurosci. Assoc. 1996, 8, 17461–17757. [Google Scholar] [CrossRef]
- Taylor, M.D.; De Ceballos, M.L.; Rose, S.; Jenner, P.; Marsden, C.D. Effects of a Unilateral 6-Hydroxydopamine Lesion and Prolonged L-3,4-Dihydroxyphenylalanine Treatment on Peptidergic Systems in Rat Basal Ganglia. Eur. J. Pharmacol. 1992, 219, 183. [Google Scholar] [CrossRef] [PubMed]
- Radke, J.M.; Cumming, P.; Vincent, S.R. Effects of MPTP Poisoning on Central Somatostatin and Substance P Levels in the Mouse. Eur. J. Pharmacol. 1987, 134, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Meredith, G.E.; Rademacher, D.J. MPTP Mouse Models of Parkinson’s Disease: An Update. J. Park. Dis. 2011, 1, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Clevens, R.A.; Beal, M.F. Substance P-like Immunoreactivity in Brains with Pathological Features of Parkinson’s and Alzheimer’s Diseases. Brain Res. 1989, 486, 387–390. [Google Scholar] [CrossRef]
- Tenovuo, O.; Rinne, U.K.; Viljanen, M.K. Substance P Immunoreactivity in the Post-Mortem Parkinsonian Brain. Brain Res. 1984, 303, 113–116. [Google Scholar] [CrossRef]
- Mauborgne, A.; Javoy-Agid, F.; Legrand, J.C.; Agid, Y.; Cesselin, F. Decrease of Substance P-like Immunoreactivity in the Substantia Nigra and Pallidum of Parkinsonian Brains. Brain Res. 1983, 268, 167–170. [Google Scholar] [CrossRef]
- Sivam, S.P. Dopamine Dependent Decrease in Enkephalin and Substance P Levels in Basal Ganglia Regions of Postmortem Parkinsonian Brains. Neuropeptides 1991, 18, 201–207. [Google Scholar] [CrossRef]
- Fernandez, A.; De Ceballos, M.L.; Jenner, P.; Marsden, C.D. Striatal Neuropeptide Levels in Parkinson’s Disease Patients. Neurosci. Lett. 1992, 145, 171–174. [Google Scholar] [CrossRef]
- Ebihara, S.; Saito, H.; Kanda, A.; Nakajoh, M.; Takahashi, H.; Arai, H.; Sasaki, H. Impaired Efficacy of Cough in Patients with Parkinson Disease. Chest 2003, 124, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Schirinzi, T.; Maftei, D.; Ralli, M.; Greco, A.; Mercuri, N.B.; Lattanzi, R.; Severini, C. Serum Substance P Is Increased in Parkinson’s Disease and Correlates with Motor Impairment. Mov. Disord. 2022, 37, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Marenholz, I.; Heizmann, C.W.; Fritz, G. S100 Proteins in Mouse and Man: From Evolution to Function and Pathology (Including an Update of the Nomenclature). Biochem. Biophys. Res. Commun. 2004, 322, 1111–1122. [Google Scholar] [CrossRef]
- Svenningsson, P.; Greengard, P. P11 (S100A10)—An Inducible Adaptor Protein That Modulates Neuronal Functions. Curr. Opin. Pharmacol. 2007, 7, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Green, H.; Zhang, X.; Tiklova, K.; Volakakis, N.; Brodin, L.; Berg, L.; Greengard, P.; Perlmann, T.; Svenningsson, P. Alterations of P11 in Brain Tissue and Peripheral Blood Leukocytes in Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2017, 114, 2735–2740. [Google Scholar] [CrossRef]
- Gałecka, M.; Bliźniewska-Kowalska, K.; Gałecki, P.; Szemraj, J.; Orzechowska, A. Expression of P11 in Patients with Depression. J. Clin. Med. 2022, 11, 5743. [Google Scholar] [CrossRef]
- Aarsland, D.; Påhlhagen, S.; Ballard, C.G.; Ehrt, U.; Svenningsson, P. Depression in Parkinson Disease—Epidemiology, Mechanisms and Management. Nat. Rev. Neurol. 2012, 8, 35–47. [Google Scholar] [CrossRef]
- Carraway, R.; Leeman, S.E. The Isolation of a New Hypotensive Peptide, Neurotensin, from Bovine Hypothalami. J. Biol. Chem. 1973, 248, 6854–6861. [Google Scholar] [CrossRef]
- St-Gelais, F.; Jomphe, C.; Trudeau, L.-E. The Role of Neurotensin in Central Nervous System Pathophysiology: What Is the Evidence? J. Psychiatry Neurosci. 2006, 31, 229–245. [Google Scholar]
- Vankova, M.; Arluison, M.; Leviel, V.; Tramu, G. Afferent Connections of the Rat Substantia Nigra Pars Lateralis with Special Reference to Peptide-Containing Neurons of the Amygdalo-Nigral Pathway. J. Chem. Neuroanat. 1992, 5, 39–50. [Google Scholar] [CrossRef]
- Fernandez, A.; de Ceballos, M.L.; Rose, S.; Jenner, P.; Marsden, C.D. Alterations in Peptide Levels in Parkinson’s Disease and Incidental Lewy Body Disease. Brain 1996, 119, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Bissette, G.; Nemeroff, C.B.; Decker, M.W.; Kizer, J.S.; Agid, Y.; Javoy-Agid, F. Alterations in Regional Brain Concentrations of Neurotensin and Bombesin in Parkinson’s Disease. Ann. Neurol. 1985, 17, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Schimpff, R.-M. Increased Plasma Neurotensin Concentrations in Patients with Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 2001, 70, 784–786. [Google Scholar] [CrossRef] [PubMed]
- Eiden, L.E.; Huttner, W.B.; Mallet, J.; O’Connor, D.T.; Winkler, H.; Zanini, A. A Nomenclature Proposal for the Chromogranin/Secretogranin Proteins. Neuroscience 1987, 21, 1019–1021. [Google Scholar] [CrossRef]
- Estevez-Herrera, J.; Pardo, M.R.; Dominguez, N.; Pereda, D.; Machado, J.D.; Borges, R. The Role of Chromogranins in the Secretory Pathway. Biomol. Concepts 2013, 4, 605–609. [Google Scholar] [CrossRef]
- Feldman, S.A.; Eiden, L.E. The Chromogranins: Their Roles in Secretion from Neuroendocrine Cells and as Markers for Neuroendocrine Neoplasia. Endocr. Pathol. 2003, 14, 3–24. [Google Scholar] [CrossRef]
- Huttner, W.B.; Gerdes, H.H.; Rosa, P. The Granin-(Chromogranin/Secretogranin) Family. Trends Biochem. Sci. 1991, 16, 27–30. [Google Scholar] [CrossRef]
- Schafer, M.K.-H.; Mahata, S.K.; Stroth, N.; Eiden, L.E.; Weihe, E. Cellular Distribution of Chromogranin A in Excitatory, Inhibitory, Aminergic and Peptidergic Neurons of the Rodent Central Nervous System. Regul. Pept. 2010, 165, 36–44. [Google Scholar] [CrossRef]
- Nishimura, M.; Tomimoto, H.; Suenaga, T.; Nakamura, S.; Namba, Y.; Ikeda, K.; Akiguchi, I.; Kimura, J. Synaptophysin and Chromogranin A Immunoreactivities of Lewy Bodies in Parkinson’s Disease Brains. Brain Res. 1994, 634, 339–344. [Google Scholar] [CrossRef]
- Yasuhara, O.; Kawamata, T.; Aimi, Y.; McGeer, E.G.; McGeer, P.L. Expression of Chromogranin A in Lesions in the Central Nervous System from Patients with Neurological Diseases. Neurosci. Lett. 1994, 170, 13–16. [Google Scholar] [CrossRef]
- Xu, D.; Wei, L.; Li, H.; Zhang, W. Serum Levels of Chromogranins and Secretogranins Correlate with the Progress and Severity of Parkinson’s Disease. Kaohsiung J. Med. Sci. 2019, 35, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Eder, U.; Leitner, B.; Kirchmair, R.; Pohl, P.; Jobst, K.A.; Smith, A.D.; Mélly, J.; Benzer, A.; Riederer, P.; Reichmann, H.; et al. Levels and Proteolytic Processing of Chromogranin A and B and Secretogranin II in Cerebrospinal Fluid in Neurological Diseases. J. Neural. Transm. 1998, 105, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Levi, A.; Eldridge, J.D.; Paterson, B.M. Molecular Cloning of a Gene Sequence Regulated by Nerve Growth Factor. Science 1985, 229, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Canu, N.; Possenti, R.; Ricco, A.S.; Rocchi, M.; Levi, A. Cloning, Structural Organization Analysis, and Chromosomal Assignment of the Human Gene for the Neurosecretory Protein VGF. Genomics 1997, 45, 443–446. [Google Scholar] [CrossRef] [PubMed]
- Trani, E.; Giorgi, A.; Canu, N.; Amadoro, G.; Rinaldi, A.M.; Halban, P.A.; Ferri, G.L.; Possenti, R.; Schininà, M.E.; Levi, A. Isolation and Characterization of VGF Peptides in Rat Brain. Role of PC1/3 and PC2 in the Maturation of VGF Precursor. J. Neurochem. 2002, 81, 565–574. [Google Scholar] [CrossRef]
- Brancia, C.; Noli, B.; Boido, M.; Boi, A.; Puddu, R.; Borghero, G.; Marrosu, F.; Bongioanni, P.; Orrù, S.; Manconi, B.; et al. VGF Protein and Its C-Terminal Derived Peptides in Amyotrophic Lateral Sclerosis: Human and Animal Model Studies. PLoS ONE 2016, 11, e0164689. [Google Scholar] [CrossRef]
- D’Amato, F.; Noli, B.; Angioni, L.; Cossu, E.; Incani, M.; Messana, I.; Manconi, B.; Solinas, P.; Isola, R.; Mariotti, S.; et al. VGF Peptide Profiles in Type 2 Diabetic Patients’ Plasma and in Obese Mice. PLoS ONE 2015, 10, e0142333. [Google Scholar] [CrossRef]
- Lewis, J.E.; Brameld, J.M.; Jethwa, P.H. Neuroendocrine Role for VGF. Front. Endocrinol. 2015, 6, 3. [Google Scholar] [CrossRef]
- Brancia, C.; Noli, B.; Boido, M.; Pilleri, R.; Boi, A.; Puddu, R.; Marrosu, F.; Vercelli, A.; Bongioanni, P.; Ferri, G.-L.; et al. TLQP Peptides in Amyotrophic Lateral Sclerosis: Possible Blood Biomarkers with a Neuroprotective Role. Neuroscience 2018, 380, 152–163. [Google Scholar] [CrossRef]
- Corda, G.; Noli, B.; Manconi, B.; Brancia, C.; Pellegrini, M.; Naro, F.; Olianas, A.; Ferri, G.-L.; Cocco, C. TLQP-21 Changes in Response to a Glucose Load. Tissue Cell 2021, 68, 101471. [Google Scholar] [CrossRef]
- Noli, B.; Brancia, C.; Corda, G.; Ferri, G.-L.; Cocco, C. Dynamic of TLQP-Peptides upon Fasting. Tissue Cell 2020, 65, 101368. [Google Scholar] [CrossRef]
- D’Amato, F.; Cocco, C.; Noli, B.; Cabras, T.; Messana, I.; Ferri, G.-L. VGF Peptides upon Osmotic Stimuli: Changes in Neuroendocrine Regulatory Peptides 1 and 2 in the Hypothalamic–Pituitary-Axis and Plasma. J. Chem. Neuroanat. 2012, 44, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.-J.; Leweke, F.M.; Oxley, D.; Wang, L.; Harris, N.; Koethe, D.; Gerth, C.W.; Nolden, B.M.; Gross, S.; Schreiber, D.; et al. Disease Biomarkers in Cerebrospinal Fluid of Patients with First-Onset Psychosis. PLoS Med. 2006, 3, e428. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.T.-J.; Leweke, F.M.; Tsang, T.M.; Koethe, D.; Kranaster, L.; Gerth, C.W.; Gross, S.; Schreiber, D.; Ruhrmann, S.; Schultze-Lutter, F.; et al. CSF Metabolic and Proteomic Profiles in Patients Prodromal for Psychosis. PLoS ONE 2007, 2, e756. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Chen, S.; Lu, N.; Yue, Y.; Yin, Y.; Zhang, Y.; Jiang, W.; Liang, J.; Yuan, Y. Reduced Serum VGF Levels Were Reversed by Antidepressant Treatment in Depressed Patients. World J. Biol. Psychiatry 2017, 18, 586–591. [Google Scholar] [CrossRef]
- Li, X.; Ge, H.; Zhou, D.; Wu, X.; Qi, G.; Chen, Z.; Yu, C.; Zhang, Y.; Yu, H.; Wang, C. Reduced Serum VGF Levels Are Linked with Suicide Risk in Chinese Han Patients with Major Depressive Disorder. BMC Psychiatry 2020, 20, 225. [Google Scholar] [CrossRef]
- van Steenoven, I.; Noli, B.; Cocco, C.; Ferri, G.L.; Oeckl, P.; Otto, M.; Koel-Simmelink, M.J.A.; Bridel, C.; van der Flier, W.M.; Lemstra, A.W.; et al. VGF Peptides in Cerebrospinal Fluid of Patients with Dementia with Lewy Bodies. Int. J. Mol. Sci. 2019, 20, 4674. [Google Scholar] [CrossRef]
- van Steenoven, I.; Koel-Simmelink, M.J.A.; Vergouw, L.J.M.; Tijms, B.M.; Piersma, S.R.; Pham, T.V.; Bridel, C.; Ferri, G.-L.; Cocco, C.; Noli, B.; et al. Identification of Novel Cerebrospinal Fluid Biomarker Candidates for Dementia with Lewy Bodies: A Proteomic Approach. Mol. Neurodegener. 2020, 15, 36. [Google Scholar] [CrossRef]
- Boiten, W.; van Steenoven, I.; Xiao, M.-F.; Worley, P.; Noli, B.; Cocco, C.; Ferri, G.-L.; Lemstra, A.; Teunissen, C. Pathologically Decreased CSF Levels of Synaptic Marker NPTX2 in DLB Are Correlated with Levels of Alpha-Synuclein and VGF. Cells 2020, 10, 38. [Google Scholar] [CrossRef]
- Cocco, C.; Corda, G.; Lisci, C.; Noli, B.; Carta, M.; Brancia, C.; Manca, E.; Masala, C.; Marrosu, F.; Solla, P.; et al. VGF Peptides as Novel Biomarkers in Parkinson’s Disease. Cell Tissue Res. 2020, 379, 93–107. [Google Scholar] [CrossRef]
- Cocco, C.; D’Amato, F.; Noli, B.; Ledda, A.; Brancia, C.; Bongioanni, P.; Ferri, G.L. Distribution of VGF Peptides in the Human Cortex and Their Selective Changes in Parkinson’s and Alzheimer’s Diseases. J. Anat. 2010, 217, 683–693. [Google Scholar] [CrossRef]
- Henderson-Smith, A.; Corneveaux, J.J.; De Both, M.; Cuyugan, L.; Liang, W.S.; Huentelman, M.; Adler, C.; Driver-Dunckley, E.; Beach, T.G.; Dunckley, T.L. Next-Generation Profiling to Identify the Molecular Etiology of Parkinson Dementia. Neurol. Genet. 2016, 2, e75. [Google Scholar] [CrossRef] [PubMed]
- Rotunno, M.S.; Lane, M.; Zhang, W.; Wolf, P.; Oliva, P.; Viel, C.; Wills, A.-M.; Alcalay, R.N.; Scherzer, C.R.; Shihabuddin, L.S.; et al. Cerebrospinal Fluid Proteomics Implicates the Granin Family in Parkinson’s Disease. Sci. Rep. 2020, 10, 2479. [Google Scholar] [CrossRef] [PubMed]
- Virreira Winter, S.; Karayel, O.; Strauss, M.T.; Padmanabhan, S.; Surface, M.; Merchant, K.; Alcalay, R.N.; Mann, M. Urinary Proteome Profiling for Stratifying Patients with Familial Parkinson’s Disease. EMBO Mol. Med. 2021, 13, e13257. [Google Scholar] [CrossRef] [PubMed]
- Karayel, O.; Virreira Winter, S.; Padmanabhan, S.; Kuras, Y.I.; Vu, D.T.; Tuncali, I.; Merchant, K.; Wills, A.M.; Scherzer, C.R.; Mann, M. Proteome Profiling of Cerebrospinal Fluid Reveals Biomarker Candidates for Parkinson’s Disease. Cell Rep. Med. 2022, 3, 100661. [Google Scholar] [CrossRef]
- Noli, B.; Sanna, F.; Brancia, C.; D’Amato, F.; Manconi, B.; Vincenzoni, F.; Messana, I.; Melis, M.R.; Argiolas, A.; Ferri, G.-L.; et al. Profiles of VGF Peptides in the Rat Brain and Their Modulations after Phencyclidine Treatment. Front. Cell Neurosci. 2017, 11, 158. [Google Scholar] [CrossRef]
- Brenner, M. Role of GFAP in CNS Injuries. Neurosci. Lett. 2014, 565, 7–13. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in Health and Disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Kurosaki, R.; Watanabe, H.; Michimata, M.; Matsubara, M.; Imai, Y.; Araki, T. Cerebral Alterations in a MPTP-Mouse Model of Parkinson’s Disease—An Immunocytochemical Study. J. Neural. Transm. 2003, 110, 1129–1144. [Google Scholar] [CrossRef]
- Gomide, V.C.; Silveira, G.A.; Chadi, G. Transient and Widespread Astroglial Activation in the Brain after a Striatal 6-Ohda-Induced Partial Lesion of the Nigrostriatal System. Int. J. Neurosci. 2005, 115, 99–117. [Google Scholar] [CrossRef]
- Banati, R.B.; Daniel, S.E.; Blunt, S.B. Glial Pathology but Absence of Apoptotic Nigral Neurons in Long-Standing Parkinson’s Disease. Mov. Disord. 1998, 13, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Ang, L.-C.; Williams, B.; Furukawa, Y.; Fitzmaurice, P.; Guttman, M.; Boileau, I.; Hornykiewicz, O.; Kish, S.J. Low Levels of Astroglial Markers in Parkinson’s Disease: Relationship to α-Synuclein Accumulation. Neurobiol. Dis. 2015, 82, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, H.J.; Vidyadhara, D.J.; Mahadevan, A.; Philip, M.; Parmar, S.K.; Manohari, S.G.; Shankar, S.K.; Raju, T.R.; Alladi, P.A. Aging Causes Morphological Alterations in Astrocytes and Microglia in Human Substantia Nigra Pars Compacta. Neurobiol. Aging 2015, 36, 3321–3333. [Google Scholar] [CrossRef] [PubMed]
- Damier, P.; Hirsch, E.C.; Zhang, P.; Agid, Y.; Javoy-Agid, F. Glutathione Peroxidase, Glial Cells and Parkinson’s Disease. Neuroscience 1993, 52, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Han, L.; Li, S.; Hu, T.; Xu, Z.; Fan, Y.; Liang, X.; Yu, H.; Wu, J.; Wang, J. Plasma GFAP in Parkinson’s Disease with Cognitive Impairment and Its Potential to Predict Conversion to Dementia. NPJ Park. Dis. 2023, 9, 23. [Google Scholar] [CrossRef]
- Su, W.; Chen, H.B.; Li, S.H.; Wu, D.Y. Correlational Study of the Serum Levels of the Glial Fibrillary Acidic Protein and Neurofilament Proteins in Parkinson’s Disease Patients. Clin. Neurol. Neurosurg. 2012, 114, 372–375. [Google Scholar] [CrossRef]
- Mao, S.; Teng, X.; Li, Z.; Zu, J.; Zhang, T.; Xu, C.; Cui, G. Association of Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein Levels with Cognitive Decline in Parkinson’s Disease. Brain Res. 2023, 1805, 148271. [Google Scholar] [CrossRef]
- Lin, Y.-Q.; Chen, S.-D. RBD: A Red Flag for Cognitive Impairment in Parkinson’s Disease? Sleep Med. 2018, 44, 38–44. [Google Scholar] [CrossRef]
- Teng, X.; Mao, S.; Wu, H.; Shao, Q.; Zu, J.; Zhang, W.; Zhou, S.; Zhang, T.; Zhu, J.; Cui, G.; et al. The Relationship between Serum Neurofilament Light Chain and Glial Fibrillary Acidic Protein with the REM Sleep Behavior Disorder Subtype of Parkinson’s Disease. J. Neurochem. 2023, 165, 268–276. [Google Scholar] [CrossRef]
- Oeckl, P.; Halbgebauer, S.; Anderl-Straub, S.; Steinacker, P.; Huss, A.M.; Neugebauer, H.; von Arnim, C.A.F.; Diehl-Schmid, J.; Grimmer, T.; Kornhuber, J.; et al. Glial Fibrillary Acidic Protein in Serum Is Increased in Alzheimer’s Disease and Correlates with Cognitive Impairment. J. Alzheimer’s Dis. 2019, 67, 481–488. [Google Scholar] [CrossRef]
- Benussi, A.; Cantoni, V.; Rivolta, J.; Archetti, S.; Micheli, A.; Ashton, N.; Zetterberg, H.; Blennow, K.; Borroni, B. Classification Accuracy of Blood-Based and Neurophysiological Markers in the Differential Diagnosis of Alzheimer’s Disease and Frontotemporal Lobar Degeneration. Alzheimers Res. Ther. 2022, 14, 155. [Google Scholar] [CrossRef] [PubMed]
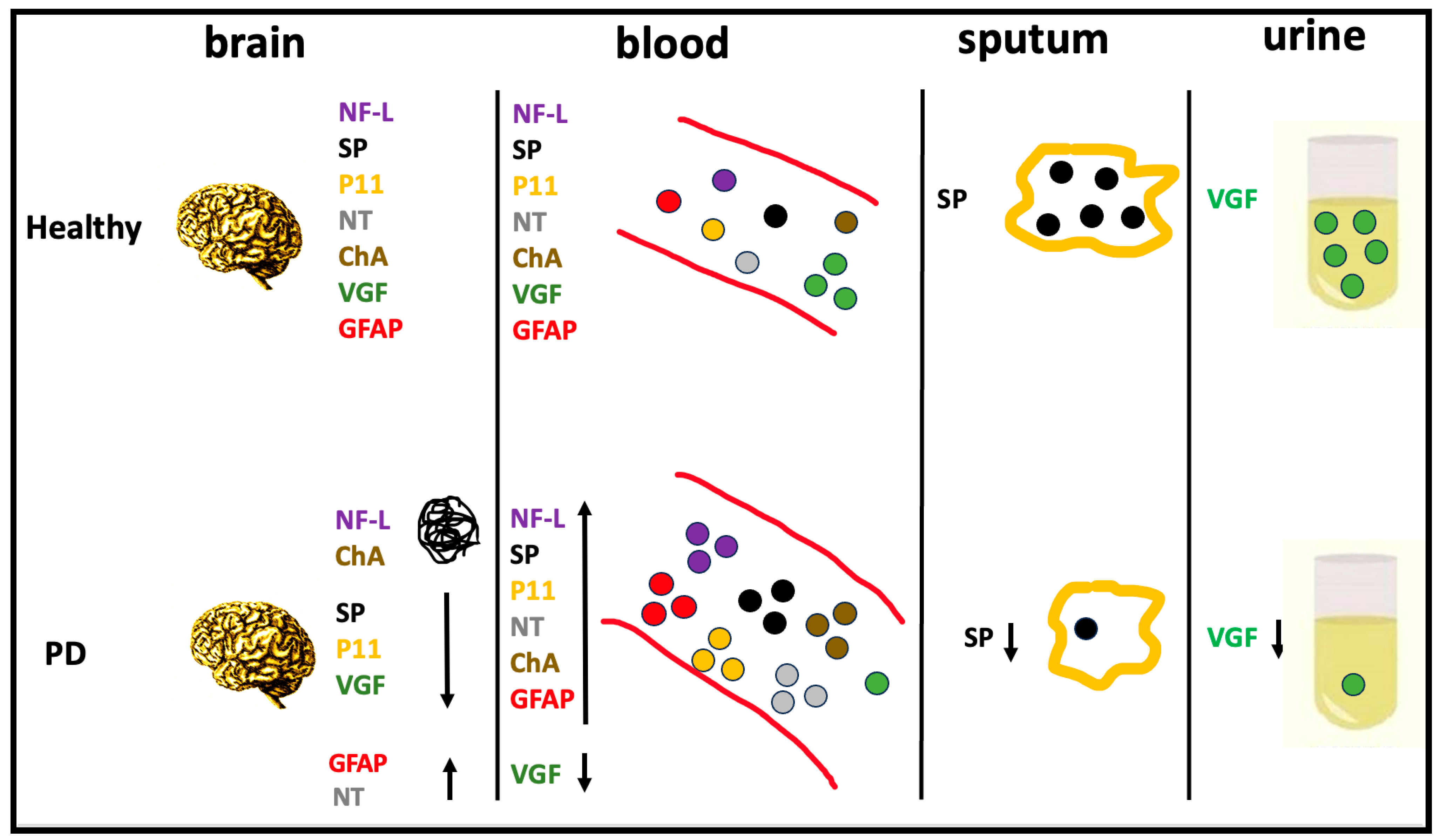
| Protein | Brain | Body Fluid | ||||||
|---|---|---|---|---|---|---|---|---|
| Method | Change | Sample | Phase | Method | Change | Sample | Phase | |
| NF-L | IHC [17] | * | SN | Advanced Early | SIMOA [21,22,23,24] ECLIA [25] | ↑ | Serum | Advanced Prodromic |
| ECLIA [20] | ↑ | Plasma | Advanced | |||||
| SP | RIA [44,45,46] | ↓ | SN | Advanced | ELISA [50] | ↑ | Serum | Advanced |
| RIA [45,47] | ↓ | Putamen | Advanced | ELISA [49] | ↓ | Sputum | Early Advanced | |
| RIA [48] | ↓ | Caudate | Advanced | |||||
| RIA [46] | ↓ | GP | Advanced | |||||
| P11 | qPCR [53] | ↓ | SN | Advanced | Flow cytometry [53] | ↑ | Plasma | Advanced |
| NT | HPLCRIA [58,59] | ↑ ↓ | SN Hyp. | Advanced | RIA [60] | ↑ | Plasma | Early Advanced |
| ChA | IHC [66,67] | * | SN cerebellum cerebrum | Advanced | ELISA [68] | ↑ | Serum | Early Intermediate Advanced |
| VGF | ELISA [87,88,89] | ↓ | Cortex | Advanced | ELISA [87] | ↓ | Plasma | Early Advanced |
| LC-MS/MS [91] | ↓ | Urine | with or without LRRK2 G2019S mutation | |||||
| GFAP | IHC [101] | ↑ | SN | Advanced | SIMOA [102] | ↑ | Plasma | Advanced |
| ELISA [103] [104] SIMOA [106] | ↑ | Serum | Advanced | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocco, C.; Manai, A.L.; Manca, E.; Noli, B. Brain-Biomarker Changes in Body Fluids of Patients with Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 10932. https://doi.org/10.3390/ijms241310932
Cocco C, Manai AL, Manca E, Noli B. Brain-Biomarker Changes in Body Fluids of Patients with Parkinson’s Disease. International Journal of Molecular Sciences. 2023; 24(13):10932. https://doi.org/10.3390/ijms241310932
Chicago/Turabian StyleCocco, Cristina, Antonio Luigi Manai, Elias Manca, and Barbara Noli. 2023. "Brain-Biomarker Changes in Body Fluids of Patients with Parkinson’s Disease" International Journal of Molecular Sciences 24, no. 13: 10932. https://doi.org/10.3390/ijms241310932
APA StyleCocco, C., Manai, A. L., Manca, E., & Noli, B. (2023). Brain-Biomarker Changes in Body Fluids of Patients with Parkinson’s Disease. International Journal of Molecular Sciences, 24(13), 10932. https://doi.org/10.3390/ijms241310932






