Effects of L-Cysteine and γ-Aminobutyric Acid Treatment on Postharvest Quality and Antioxidant Activity of Loquat Fruit during Storage
Abstract
1. Introduction
2. Results
2.1. Effects of L-Cys and GABA Treatments on Weight Loss Rate, Browning Index, Decay Index, and Respiration Intensity
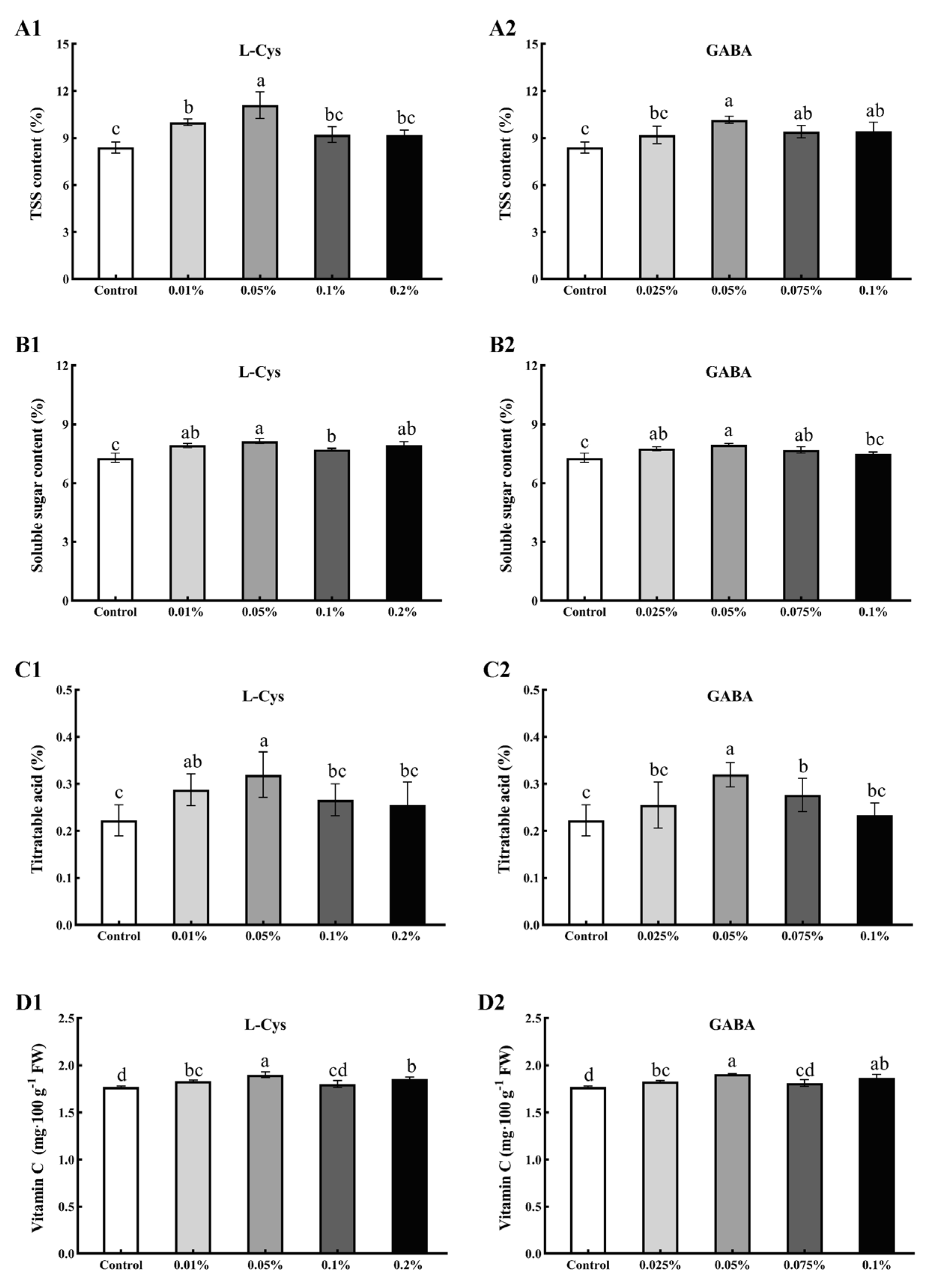
2.2. Effects of L-Cys and GABA Treatments on TSS, SS, TA, and Vitamin C Content
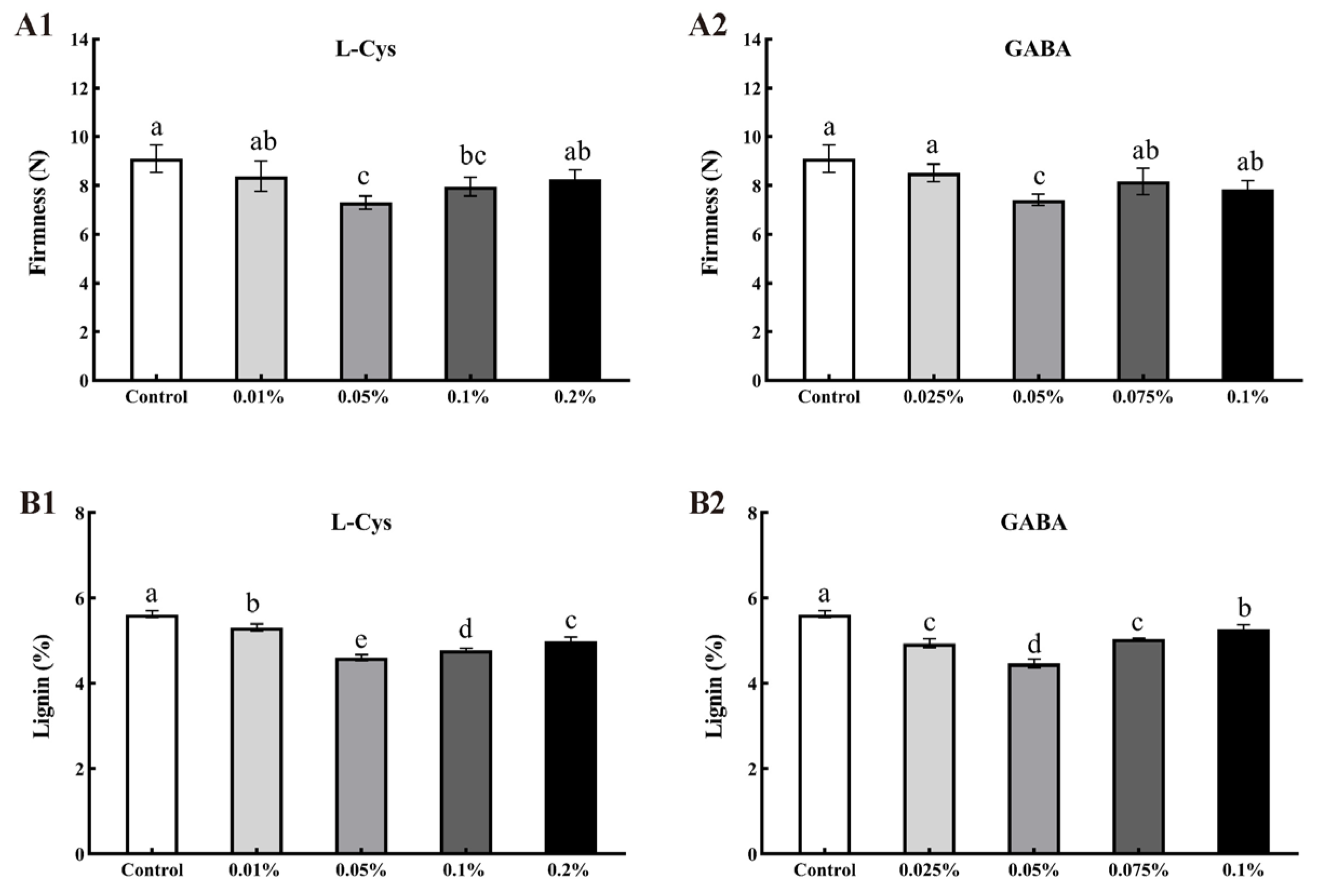
2.3. Effects of L-Cys and GABA Treatments on Firmness and Lignin Content of Loquat Fruit after Harvest
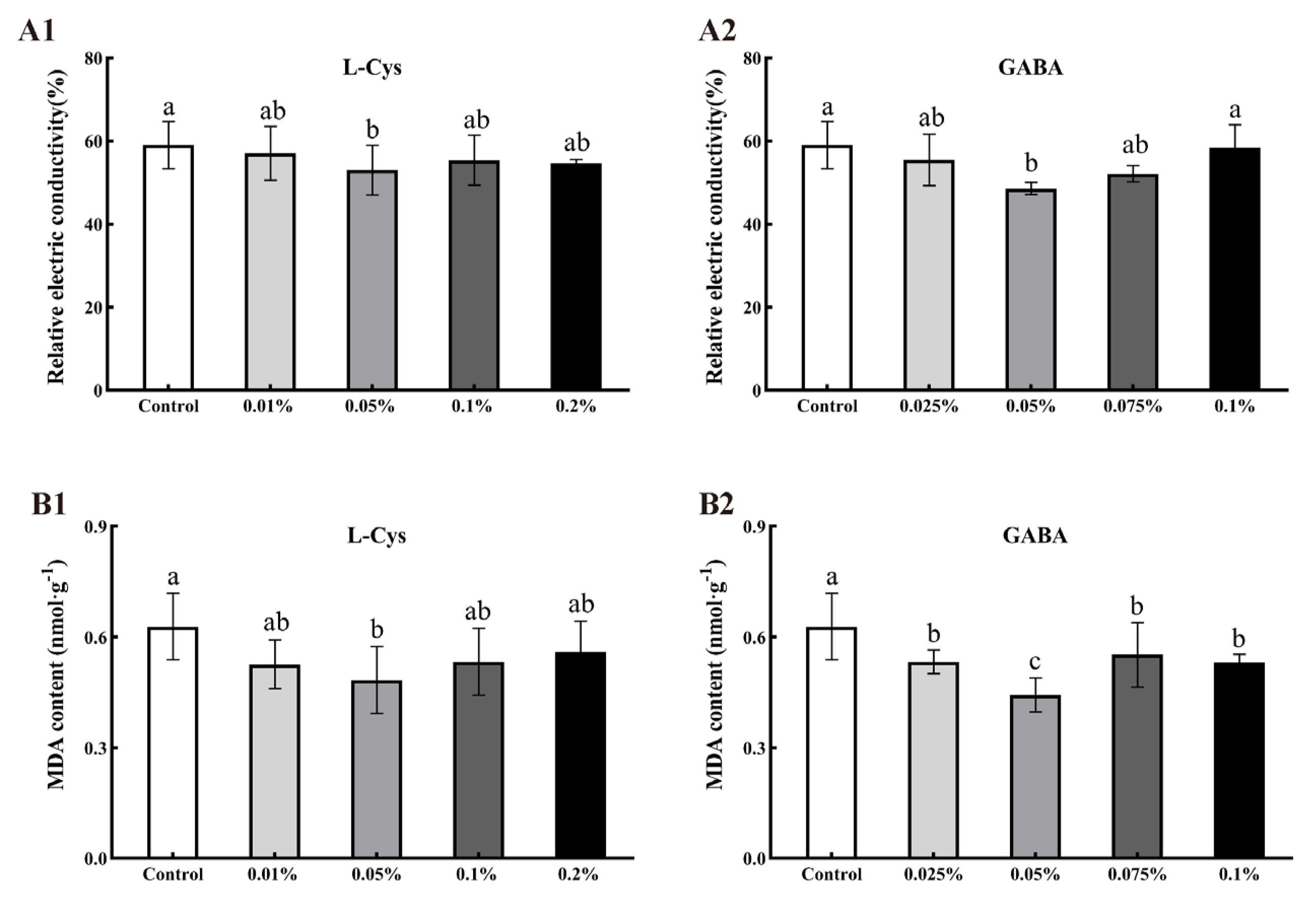
2.4. Effects of L-Cys and GABA on Relative Electric Conductivity and MDA Content of Loquat Fruit after Harvest
2.5. Effects of L-Cys and GABA Treatments on Total Phenols, Total Flavonoids, Flavanols, and Carotenoids in Loquat Fruit after Harvest
2.6. Effects of L-Cys and GABA on DPPH and FRAP of Loquat Fruit after Harvest
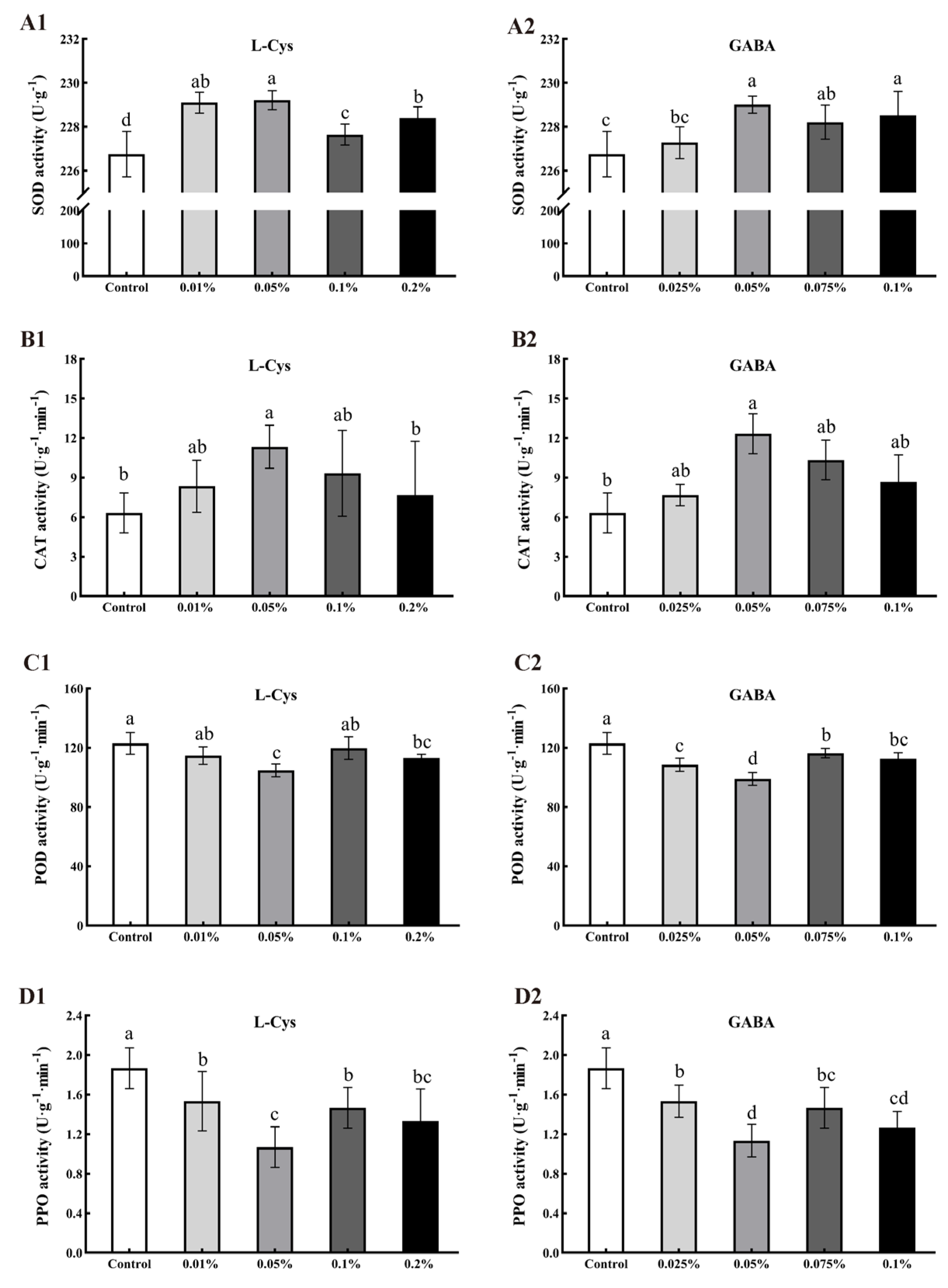
2.7. Effects of L-Cys and GABA on SOD, CAT, POD, and PPO Activity of Loquat Fruit after Harvest
2.8. PCA
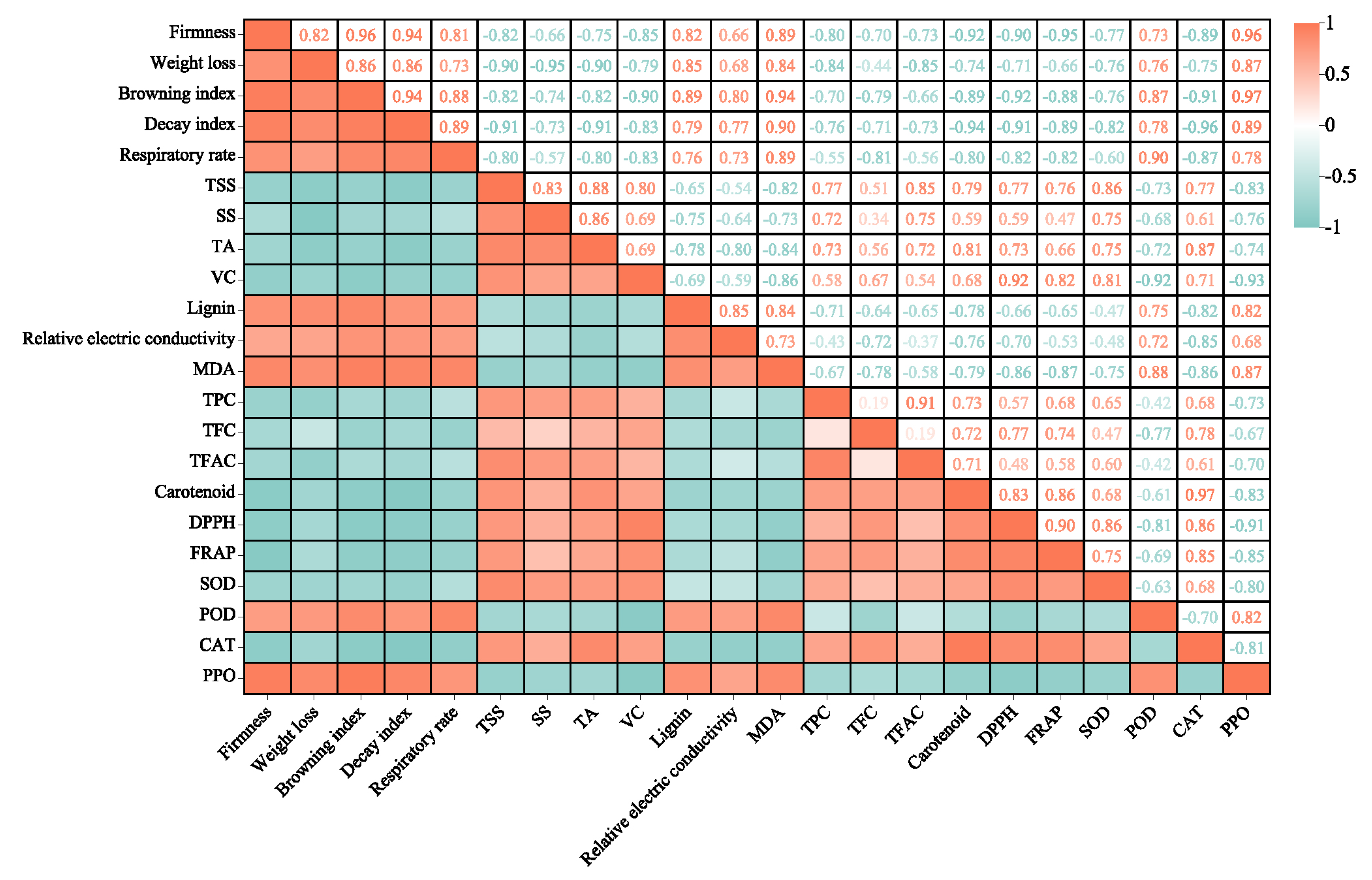
2.9. Analysis of Relationships
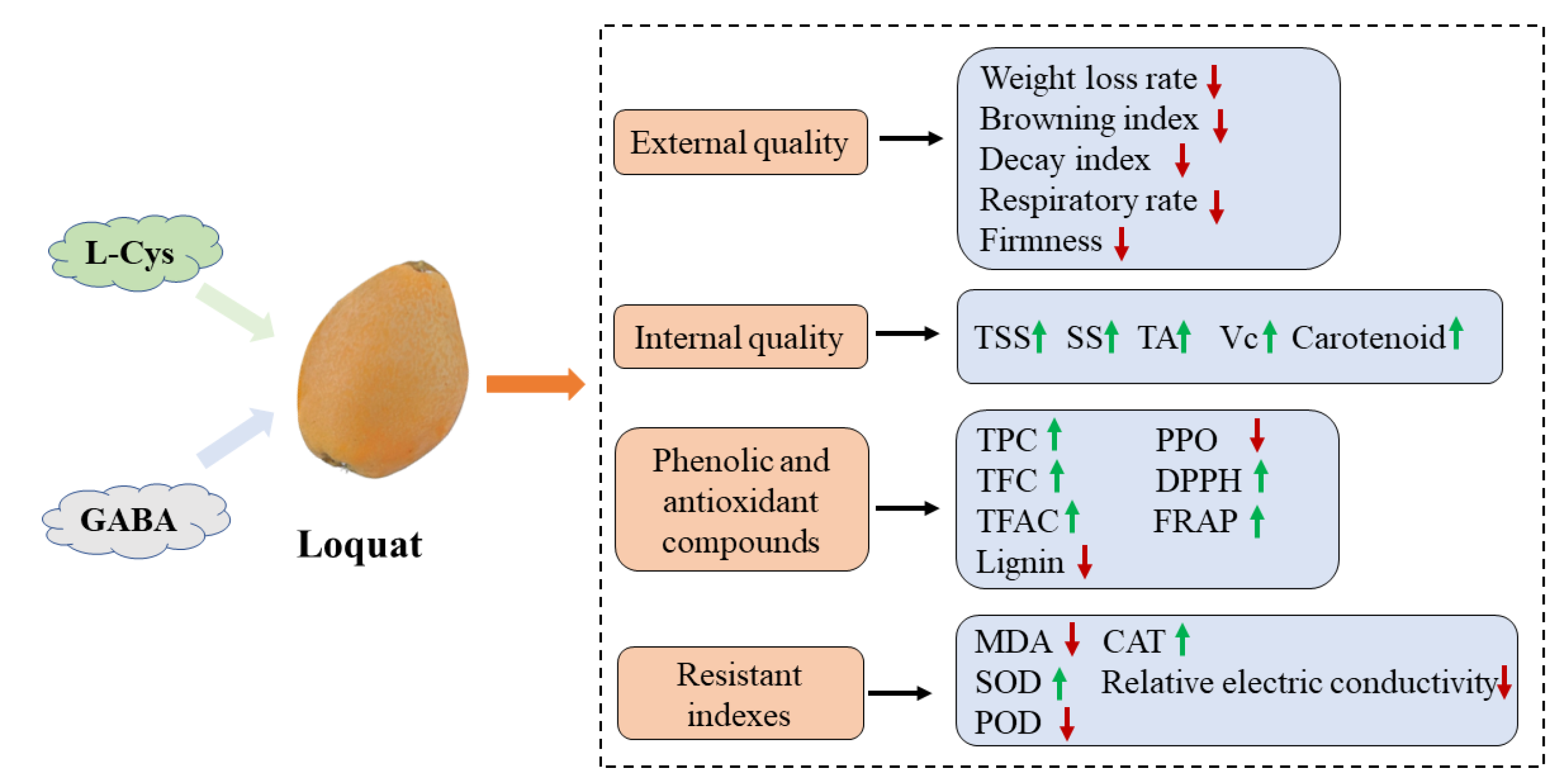
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Determination of Weight Loss Rate, Browning Index, Decay Index, and Respiratory Rate
4.3. Determination of Total Soluble Solids (TSS), Soluble Sugar (SS), Titratable Acid (TA) and Vitamin C (Vc) Content
4.4. Determination of Fruit Firmness and Lignin
4.5. Determination of Relative Electric Conductivity and MDA
4.6. Determination of Total Phenolic Content
4.7. Determination of Flavonoid Content
4.8. Determination of Total Flavanol Content
4.9. Determination of Carotenoids
4.10. Determination of DPPH and FRAP
4.11. Determination of Superoxide Dismutase (SOD), Catalase (CAT), Peroxidase (POD), and Polyphenol Oxidase (PPO) Activity
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; Zhang, W.; Xu, C.; Li, X. Biological Activities of Extracts from Loquat (Eriobotrya japonica Lindl.): A Review. Int. J. Mol. Sci. 2016, 17, 1983. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Minas, I.S.; Kourdoulas, P.M.; Vicente, A.R.; Manganaris, G.A. Phytochemical content, antioxidants and cell wall metabolism of two loquat (Eriobotrya japonica) cultivars under different storage regimes. Food Chem. 2014, 155, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Petriccione, M.; Pasquariello, M.S.; Mastrobuoni, F.; Zampella, L.; Di, P.D.; Scortichini, M. Influence of a chitosan coating on the quality and nutraceutical traits of loquat fruit during postharvest life. Sci. Hortic. 2015, 197, 287–296. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ. Preserving postharvest storage quality of fresh loquat fruits by using different bio-materials. J. Food Sci. Technol. 2020, 57, 3004–3012. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, P.; Huang, Y.; Shan, T.; Wang, L.; Li, Y.; Zheng, Y. Effect of hot water combined with glycine betaine alleviates chilling injury in cold-stored loquat fruit. Postharvest Biol. Technol. 2016, 118, 141–147. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Rui, H.; Tang, S. Effect of 1-methylcyclopropene treatment on chilling injury, fatty acid and cell wall polysaccharide composition in loquat fruit. J. Agric. Food Chem. 2009, 57, 8439–8443. [Google Scholar] [CrossRef]
- Wang, J.; Yuan, H.; Wu, Y.; Yu, J.; Ali, B.; Zhang, J.; Tang, Z.; Xie, J.; Lyu, J.; Liao, W. Application of 5-aminolevulinic acid promotes ripening and accumulation of primary and secondary metabolites in postharvest tomato fruit. Front. Nutr. 2022, 9, 1036843. [Google Scholar] [CrossRef]
- Sun, J.; Chen, H.; Chen, Y.; Lin, M.; Hung, Y.C.; Jiang, Y.; Lin, H. ε-Poly-l-Lysine Enhances Fruit Disease Resistance in Postharvest Longans (Dimocarpus longan Lour.) by Modulating Energy Status and ATPase Activity. Foods 2022, 11, 773. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, J.L.; Zhao, Y.J.; Li, C.Y.; Xiang, C.B. L-Cysteine inhibits root elongation through auxin/PLETHORA and SCR/SHR pathway in Arabidopsis thaliana. J. Integr. Plant Biol. 2015, 57, 186–197. [Google Scholar] [CrossRef]
- Jiang, J.; Jiang, L.; Luo, H.; Yu, Z. Establishment of a statistical model for browning of fresh-cut lotus root during storage. Postharvest Biol. Technol. 2014, 92, 164–171. [Google Scholar] [CrossRef]
- Ali, H.M.; El-Gizawy, A.M.; El-Bassiouny, R.E.; Saleh, M.A. Browning inhibition mechanisms by cysteine, ascorbic acid and citric acid, and identifying PPO-catechol-cysteine reaction products. J. Food Sci. Technol. 2015, 52, 3651–3659. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, Y.; Meng, X.; Liu, B. Full inhibition of Whangkeumbae pear polyphenol oxidase enzymatic browning reaction by l-cysteine. Food Chem. 2018, 266, 1–8. [Google Scholar] [CrossRef]
- Wu, X.T.; Guo, X.N.; Zhu, K.X. Inhibition of L-Cysteine on the Browning of Fresh Wet Noodles. Foods 2021, 10, 156. [Google Scholar] [CrossRef]
- Pace, B.; Capotorto, I.; Ventura, M.; Cefola, M. Evaluation of L-Cysteine as Anti-Browning Agent in Fresh-Cut Lettuce Processing. J. Food Process. Preserv. 2015, 39, 985–993. [Google Scholar] [CrossRef]
- Li, Y.; Liu, B.; Peng, Y.; Liu, C.; Li, C. Exogenous GABA alleviates alkaline stress in Malus hupehensis by regulating the accumulation of organic acids. Sci. Hortic. 2019, 261, 108982. [Google Scholar] [CrossRef]
- Oh, S.J.; Kim, H.S.; Lim, S.T.; Reddy, C.K. Enhanced accumulation of gamma-aminobutyric acid in rice bran using anaerobic incubation with various additives. Food Chem. 2019, 271, 187–192. [Google Scholar] [CrossRef]
- Shang, H.; Cao, S.; Yang, Z.; Cai, Y.; Zheng, Y. Effect of exogenous γ-aminobutyric acid treatment on proline accumulation and chilling injury in peach fruit after long-term cold storage. J. Agric. Food Chem. 2011, 59, 1264–1268. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, Z.; Huang, X.; Yang, K.; Du, R. Effect of exogenous γ-aminobutyric acid (GABA) treatment on chilling injury and antioxidant capacity in banana peel. Sci. Hortic. 2014, 168, 132–137. [Google Scholar] [CrossRef]
- Liu, Q.; Li, X.; Jin, S.; Dong, W.; Zhang, Y.; Chen, W.; Shi, L.; Cao, S.; Yang, Z. γ-Aminobutyric acid treatment induced chilling tolerance in postharvest kiwifruit (Actinidia chinensis cv. Hongyang) via regulating ascorbic acid metabolism. Food Chem. 2023, 404, 134661. [Google Scholar] [CrossRef]
- Ali, S.; Khan, A.S.; Malik, A.U.; Nawaz, A.; Shahid, M. Postharvest application of antibrowning chemicals modulates oxidative stress and delays pericarp browning of controlled atmosphere stored litchi fruit. J. Food Biochem. 2019, 43, e12746. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Lin, B.; Lin, H.; Lin, M.; Chen, J.; Lin, Y. γ-Aminobutyric acid treatment reduces chilling injury and improves quality maintenance of cold-stored Chinese olive fruit. Food Chem. X 2022, 13, 100208. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhu, N.; Zhu, C.; Wu, D.; Chen, K. Morphology and cell wall composition changes in lignified cells from loquat fruit during postharvest storage-ScienceDirect. Postharvest Biol. Technol. 2019, 157, 110975. [Google Scholar] [CrossRef]
- Öz, A.T.; Kafkas, E.; Bozdoğan, A. Combined effects of oxalic acid treatment with modified atmosphere packaging on postharvest quality of loquat fruit during storage. Turk. J. Agric. For. 2016, 40, 3. [Google Scholar] [CrossRef]
- Ramos-Ruiz, R.; Felix, M.; Knauf-Beiter, G. The effects of GABA in plants. Cogent Food Agric. 2019, 5, 1670553. [Google Scholar] [CrossRef]
- Ngaffo, M.F.; Duan, W.; Cisse, E.H.M.; Chen, T.; Xu, X. Alleviation of Postharvest Chilling Injury of Carambola Fruit by γ-aminobutyric Acid: Physiological, Biochemical, and Structural Characterization. Front. Nutr. 2021, 8, 752583. [Google Scholar] [CrossRef]
- Cai, J.; Chen, T.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Metabolic Dynamics During Loquat Fruit Ripening and Postharvest Technologies. Front. Plant Sci. 2019, 10, 619. [Google Scholar] [CrossRef]
- Liu, R.; Tian, S.; Qin, G.Z.; Wang, Y.Y. Molecular basis of 1-methylcyclopropene regulating organic acid metabolism in apple fruit during storage. Postharvest Biol. Technol. 2016, 117, 57–63. [Google Scholar] [CrossRef]
- Ubeed, H.M.S.A.; Wills, R.B.H.; Bowyer, M.C.; Golding, J.B. Inhibition of postharvest senescence of green leafy vegetables by exogenous D-cysteine and L-cysteine as precursors of hydrogen sulphide. J. Hortic. Sci. Biotechnol. 2019, 94, 620–626. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Sohail, M.; Wills, R.B.H.; Bowyer, M.C.; Pristijono, P. Beneficial impact of exogenous arginine, cysteine and methionine on postharvest senescence of broccoli. Food Chem. 2021, 338, 128055. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, X.; Sun, C.; Li, X.; Chen, K. Phenolic composition from different loquat (Eriobotrya japonica Lindl.) cultivars grown in China and their antioxidant properties. Molecules 2015, 20, 542–555. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Pirzad, F.; Tahanian, H.; Aghdam, M.S. Influence of Gum Arabic Enriched with GABA Coating on Oxidative Damage of Walnut Kernels. Food Technol. Biotechnol. 2019, 57, 554–560. [Google Scholar] [CrossRef]
- Preczenhak, A.P.; Orsi, B.; Lima, G.P.P.; Tezotto-Uliana, J.V.; Minatel, I.O.; Kluge, R.A. Cysteine enhances the content of betalains and polyphenols in fresh-cut red beet. Food Chem. 2019, 286, 600–607. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Jiao, C.; Yin, X.; Fei, Z.; Wu, Q.; Chen, K. Transcriptome analysis provides insights into the regulation of metabolic processes during postharvest cold storage of loquat (Eriobotrya japonica) fruit. Hortic. Res. 2019, 6, 49. [Google Scholar] [CrossRef]
- Cai, C.; Xu, C.J.; Li, X.; Ferguson, I.; Chen, K.S. Accumulation of lignin in relation to change in activities of lignification enzymes in loquat fruit flesh after harvest. Postharvest Biol. Technol. 2006, 40, 163–169. [Google Scholar] [CrossRef]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef]
- Fang, L.; Jiang, B.; Zhang, T. Effect of combined high pressure and thermal treatment on kiwifruit peroxidase. Food Chem. 2008, 109, 802–807. [Google Scholar] [CrossRef]
- Sun, M.; Yang, X.L.; Zhu, Z.P.; Xu, Q.Y.; Wu, K.X.; Kang, Y.J.; Wang, H.; Xiong, A.S. Comparative transcriptome analysis provides insight into nitric oxide suppressing lignin accumulation of postharvest okra (Abelmoschus esculentus L.) during cold storage. Plant Physiol. Biochem. 2021, 167, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Xu, C.J.; Shan, L.L.; Li, X.; Zhou, C.H.; Zhang, W.S.; Ferguson, I.; Chen, K.S. Low temperature conditioning reduces postharvest chilling injury in loquat fruit. Postharvest Biol. Technol. 2006, 41, 252–259. [Google Scholar] [CrossRef]
- Zhan, L.; Hu, J.; Lim, L.T.; Pang, L.; Li, Y.; Shao, J. Light exposure inhibiting tissue browning and improving antioxidant capacity of fresh-cut celery (Apium graveolens var. dulce). Food Chem. 2013, 141, 2473–2478. [Google Scholar] [CrossRef] [PubMed]
- Shekari, A.; Hassani, R.N.; Aghdam, M.S. Exogenous application of GABA retards cap browning in Agaricus bisporus and its possible mechanism. Postharvest Biol. Technol. 2021, 174, 111434. [Google Scholar] [CrossRef]
- Wojtaszek, G. Mechanisms for the generation of reactive oxygen species in plant defence—A broad perspective. Physiol. Mol. Plant Pathol. 1997, 51, 347–366. [Google Scholar] [CrossRef]
- James, M.L. Chilling injury in plants. Annu. Rev. Plant Physiol. 2003, 24, 445–466. [Google Scholar] [CrossRef]
- Lin, D.; Yan, R.; Xing, M.; Liao, S.; Chen, J.; Gan, Z. Fucoidan treatment alleviates chilling injury in cucumber by regulating ROS homeostasis and energy metabolism. Front. Plant Sci. 2022, 13, 1107687. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Jin, P.; Rui, H. Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 2009, 115, 1458–1463. [Google Scholar] [CrossRef]
- Cao, S.F.; Wang, X.Q.; Yang, Z.F.; Ma, S.J.; Zheng, Y.H. Effects of methyl jasmonate treatment on quality and decay in cold-stored loquat fruit. Acta Hortic. 2007, 750, 425–430. [Google Scholar] [CrossRef]
- Chen, J.; Hu, Y.; Wang, J.; Hu, H.; Cui, H. Combined effect of ozone treatment and modified atmosphere packaging on antioxidant defense system of fresh-cut green peppers. J. Food Process. Preserv. 2016, 40, 1145–1150. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Yilmaz, B.; Pateiro, M.; Kumar, M.; Domínguez, R.; Shariati, M.A.; Hano, C.; Lorenzo, J.M. Valorization of by-products from Prunus genus fruit processing: Opportunities and applications. Crit. Rev. Food Sci. Nutr. 2022, 14, 1–16. [Google Scholar] [CrossRef]
- Lin, Y.; Li, N.; Lin, H.; Lin, M.; Chen, Y.; Wang, H.; Ritenour, M.A.; Lin, Y. Effects of chitosan treatment on the storability and quality properties of longan fruit during storage. Food Chem. 2020, 306, 125627. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Van, M.M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Bioanal. Chem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Femenia, A.; Garcia-Conesa, M.; Simal, S.; Rosselló, C. Characterisation of the cell walls of loquat (Eriobotrya japonica L.) fruit tissues. Carbohydr. Polym. 1998, 35, 169–177. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Banin, S.O.; Razavi, F.; Rabiei, V.; Gohari, G. Postharvest application of L-cysteine to prevent enzymatic browning of “Stanley” plum fruit during cold storage. J. Food Process. Preserv. 2022, 44, e14788. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, J.; Tang, Y.; Wang, M.; Tian, K.; Wang, Y.; Luo, X.; Deng, Q. Changes in Phenolic Compounds and Antioxidant Activity during Development of ‘Qiangcuili’ and ‘Cuihongli’ Fruit. Foods 2022, 11, 3198. [Google Scholar] [CrossRef]
- Wang, X.K. Principles and Techniques of Plant Physiological and Biochemical Experiments, 2nd ed.; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 28–30. [Google Scholar] [CrossRef]
- Benzie, I.S.J. Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluidsand Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods Enzymol. 1999, 229, 15–27. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria×anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Siragusa, S.; Fontana, C.A.; Cappa, F.; Caputo, L.; Cocconcelli, P.S.; Gobbetti, M.; Angelis, M.D. Disruption of the gene encoding glutamate dehydrogenase affects growth, amino acids catabolism and survival of Lactobacillus plantarum UC1001. Int. Dairy J. 2011, 21, 59–68. [Google Scholar] [CrossRef]
- Wang, J.; Yang, X.H.; Mujumdar, A.S.; Wang, D.; Zhao, J.H.; Fang, X.M.; Zhang, Q.; Xie, L.; Gao, Z.J.; Xiao, H.W. Effects of various blanching methods on weight loss, enzymes inactivation, phytochemical contents, antioxidant capacity, ultrastructure and drying kinetics of red bell pepper (Capsicum annuum L.). LWT-Food Sci. Technol. 2017, 77, 337–347. [Google Scholar] [CrossRef]



| Storage Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatments | Concentration | 0 Day | 7 Days | 14 Days | 21 Days | 28 Days | 35 Days | 40 Days | |
| Weight loss rate (%) | Control | Water | 0 | 0.77 ± 0.23a | 1.21 ± 0.18a | 1.62 ± 0.10a | 2.54 ± 0.12a | 3.51 ± 0.05a | 4.74 ± 0.12a |
| L-Cys | 0.01% | 0 | 0.58 ± 0.10a | 0.94 ± 0.04a | 1.40 ± 0.08ab | 1.75 ± 0.08cd | 3.15 ± 0.11abc | 4.29 ± 0.20bc | |
| 0.05% | 0 | 0.45 ± 0.09a | 0.87 ± 0.09a | 1.10 ± 0.02c | 1.56 ± 0.10d | 2.94 ± 0.06bc | 3.91 ± 0.05c | ||
| 0.1% | 0 | 0.54 ± 0.05a | 1.11 ± 0.06a | 1.37 ± 0.04b | 2.15 ± 0.13b | 3.10 ± 0.14abc | 4.32 ± 0.10abc | ||
| 0.2% | 0 | 0.65 ± 0.09a | 1.18 ± 0.09a | 1.43 ± 0.05ab | 2.22 ± 0.08b | 3.19 ± 0.15abc | 4.27 ± 0.12bc | ||
| GABA | 0.025% | 0 | 0.62 ± 0.04a | 1.03 ± 0.09a | 1.21 ± 0.11bc | 2.19 ± 0.13b | 3.28 ± 0.11ab | 4.39 ± 0.12ab | |
| 0.05% | 0 | 0.41 ± 0.06a | 0.89 ± 0.09a | 1.19 ± 0.03bc | 1.82 ± 0.01cd | 2.86 ± 0.11c | 4.10 ± 0.18bc | ||
| 0.075% | 0 | 0.66 ± 0.14a | 1.00 ± 0.14a | 1.38 ± 0.04b | 2.18 ± 0.06b | 3.05 ± 0.19bc | 4.44 ± 0.09ab | ||
| 0.1% | 0 | 0.64 ± 0.02a | 1.10 ± 0.11a | 1.36 ± 0.10b | 1.20 ± 0.03bc | 3.13 ± 0.13abc | 4.51 ± 0.09ab | ||
| Browning index (%) | Control | Water | 0 | 7.61 ± 1.18a | 11.11 ± 1.39a | 27.08 ± 2.08a | 44.17 ± 0.17a | 60.42 ± 5.51a | 71.00 ± 2.02a |
| L-Cys | 0.01% | 0 | 5.55 ± 2.78ab | 4.83 ± 0.35c | 18.89 ± 1.94bcd | 38.33 ± 0.83ab | 55.83 ± 1.27ab | 61.83 ± 2.04b | |
| 0.05% | 0 | 2.78 ± 1.47ab | 4.10 ± 0.54c | 15.56 ± 0.56d | 28.33 ± 1.67c | 38.89 ± 2.00c | 49.83 ± 0.63c | ||
| 0.1% | 0 | 6.93 ± 1.40ab | 7.50 ± 1.73abc | 19.16 ± 1.27bcd | 35.28 ± 1.21b | 53.06 ± 1.55ab | 59.28 ± 1.62b | ||
| 0.2% | 0 | 3.15 ± 1.61ab | 8.89 ± 0.56ab | 21.94 ± 1.55bc | 42.22 ± 1.47a | 52.78 ± 2.78ab | 59.17 ± 2.20b | ||
| GABA | 0.025% | 0 | 5.26 ± 1.57ab | 7.07 ± 1.26bc | 17.78 ± 1.11cd | 39.72 ± 1.21ab | 54.72 ± 2.90ab | 60.53 ± 2.22b | |
| 0.05% | 0 | 1.67 ± 1.67b | 5.56 ± 1.39bc | 15.56 ± 0.56d | 28.06 ± 1.55c | 38.33 ± 0.83c | 48.17 ± 1.41c | ||
| 0.075% | 0 | 3.04 ± 1.54ab | 6.67 ± 1.67bc | 20.00 ± 0.00bcd | 35.00 ± 2.89b | 46.67 ± 1.67bc | 58.33 ± 1.67b | ||
| 0.1% | 0 | 3.04 ± 1.54ab | 6.06 ± 0.97bc | 23.33 ± 1.67ab | 41.67 ± 4.41ab | 50.56 ± 4.44b | 57.72 ± 0.96b | ||
| Decay index (%) | Control | Water | 0 | 5.14 ± 0.60a | 12.44 ± 1.44a | 20.00 ± 2.89a | 30.28 ± 4.72a | 35.33 ± 2.17a | 49.36 ± 1.78a |
| L-Cys | 0.01% | 0 | 2.10 ± 1.20ab | 9.72 ± 1.39abc | 13.33 ± 0.83bc | 23.33 ± 2.93abc | 28.05 ± 3.74bc | 42.00 ± 1.75b | |
| 0.05% | 0 | 0.72 ± 0.72b | 6.93 ± 1.40c | 11.11 ± 2.00c | 20.56 ± 2.42 bc | 26.67 ± 1.67bc | 34.17 ± 2.20c | ||
| 0.1% | 0 | 0.72 ± 0.72b | 11.06 ± 0.82 ab | 14.72 ± 1.21abc | 23.33 ± 1.67abc | 30.83 ± 1.27abc | 42.67 ± 0.51ab | ||
| 0.2% | 0 | 2.39 ± 1.45ab | 9.61 ± 0.66abc | 14.17 ± 0.83abc | 23.33 ± 1.67abc | 32.78 ± 1.47ab | 44.50 ± 2.30ab | ||
| GABA | 0.025% | 0 | 4.45 ± 0.28a | 10.28 ± 1.21abc | 11.67 ± 0.83c | 25.28 ± 2.65abc | 31.11 ± 1.94abc | 45.61 ± 1.22ab | |
| 0.05% | 0 | 0.72 ± 0.72b | 7.55 ± 1.68bc | 10.83 ± 0.83c | 18.89 ± 1.11c | 25.00 ± 0.00c | 34.44 ± 0.56c | ||
| 0.075% | 0 | 3.33 ± 1.67ab | 10.00 ± 0.00abc | 16.67 ± 1.67abc | 28.44 ± 2.51ab | 30.00 ± 2.89abc | 40.83 ± 1.59bc | ||
| 0.1% | 0 | 3.05 ± 1.55ab | 11.58 ± 0.80a | 18.72 ± 3.20ab | 23.61 ± 1.39abc | 30.83 ± 1.27abc | 42.22 ± 1.47b | ||
| Respiratory rate (mg·kg−1·h) | Control | Water | 83.24 ± 7.96 | 81.57 ± 1.58a | 79.81 ± 0.68a | 76.78 ± 1.07a | 73.45 ± 1.11a | 70.54 ± 0.72a | 67.05 ± 0.85a |
| L-Cys | 0.01% | 83.24 ± 7.96 | 79.00 ± 3.19abc | 78.04 ± 3.78ab | 75.54 ± 1.18a | 71.29 ± 1.22ab | 68.94 ± 0.60a | 66.03 ± 0.78ab | |
| 0.05% | 83.24 ± 7.96 | 73.83 ± 1.36bc | 72.67 ± 0.52ab | 68.26 ± 1.40b | 67.54 ± 0.69bc | 64.84 ± 0.34b | 63.04 ± 0.25c | ||
| 0.1% | 83.24 ± 7.96 | 78.36 ± 2.02abc | 72.64 ± 4.04ab | 76.16 ± 0.70a | 72.23 ± 1.24a | 69.50 ± 0.62a | 66.19 ± 1.62ab | ||
| 0.2% | 83.24 ± 7.96 | 79.30 ± 0.61abc | 79.62 ± 3.69aab | 74.60 ± 1.45a | 72.98 ± 1.20a | 70.31 ± 1.50a | 66.90 ± 0.85a | ||
| GABA | 0.025% | 83.24 ± 7.96 | 80.32 ± 2.86ab | 78.45 ± 1.19a | 74.28 ± 2.28a | 72.00 ± 1.20a | 70.55 ± 1.45a | 65.20 ± 0.49abc | |
| 0.05% | 83.24 ± 7.96 | 72.20 ± 2.01c | 70.44 ± 1.53b | 67.81 ± 1.47b | 66.10 ± 1.43b | 63.70 ± 1.10b | 62.33 ± 0.37bc | ||
| 0.075% | 83.24 ± 7.96 | 77.75 ± 1.94abc | 75.82 ± 0.62ab | 78.47 ± 2.46a | 72.60 ± 1.65a | 71.85 ± 0.65a | 65.62 ± 1.35ab | ||
| 0.1% | 83.24 ± 7.96 | 78.59 ± 2.67abc | 78.42 ± 0.44a | 75.68 ± 0.50a | 72.14 ± 1.71a | 70.70 ± 0.90a | 65.35 ± 1.19abc | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Pu, J.; Liu, H.; Wang, M.; Du, Y.; Tang, X.; Luo, X.; Wang, Y.; Deng, Q. Effects of L-Cysteine and γ-Aminobutyric Acid Treatment on Postharvest Quality and Antioxidant Activity of Loquat Fruit during Storage. Int. J. Mol. Sci. 2023, 24, 10541. https://doi.org/10.3390/ijms241310541
Zhang H, Pu J, Liu H, Wang M, Du Y, Tang X, Luo X, Wang Y, Deng Q. Effects of L-Cysteine and γ-Aminobutyric Acid Treatment on Postharvest Quality and Antioxidant Activity of Loquat Fruit during Storage. International Journal of Molecular Sciences. 2023; 24(13):10541. https://doi.org/10.3390/ijms241310541
Chicago/Turabian StyleZhang, Huifen, Jing Pu, Han Liu, Miao Wang, Ying Du, Xiaofu Tang, Xian Luo, Yongqing Wang, and Qunxian Deng. 2023. "Effects of L-Cysteine and γ-Aminobutyric Acid Treatment on Postharvest Quality and Antioxidant Activity of Loquat Fruit during Storage" International Journal of Molecular Sciences 24, no. 13: 10541. https://doi.org/10.3390/ijms241310541
APA StyleZhang, H., Pu, J., Liu, H., Wang, M., Du, Y., Tang, X., Luo, X., Wang, Y., & Deng, Q. (2023). Effects of L-Cysteine and γ-Aminobutyric Acid Treatment on Postharvest Quality and Antioxidant Activity of Loquat Fruit during Storage. International Journal of Molecular Sciences, 24(13), 10541. https://doi.org/10.3390/ijms241310541







