Proteomic Modulation in TGF-β-Treated Cholangiocytes Induced by Curcumin Nanoparticles
Abstract
1. Introduction
2. Results
2.1. CurNPs Synthesis and Characterization
2.2. CurNPs Dispersion Characterization
2.3. Sample Collection
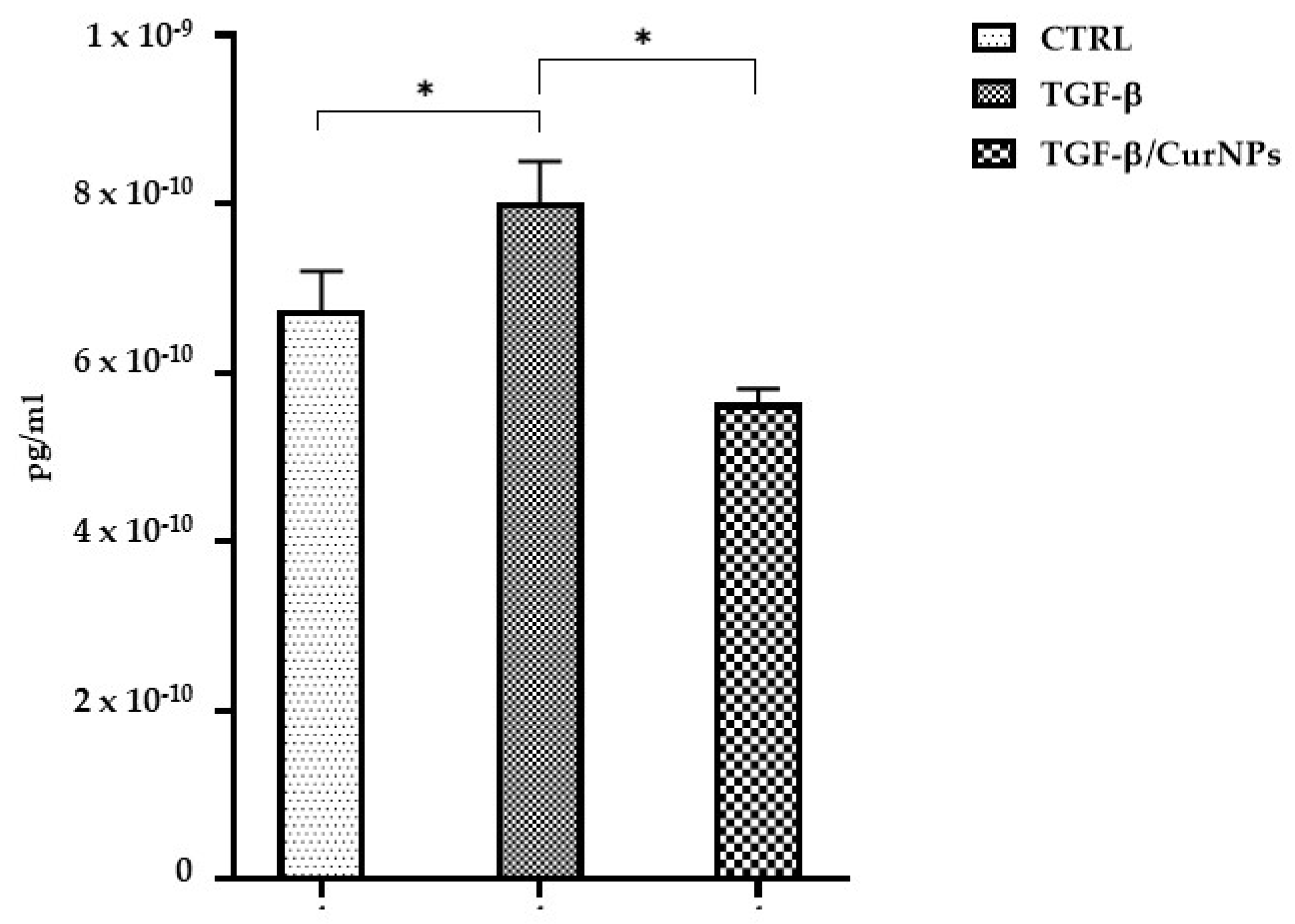
2.4. IL-6 Dosage
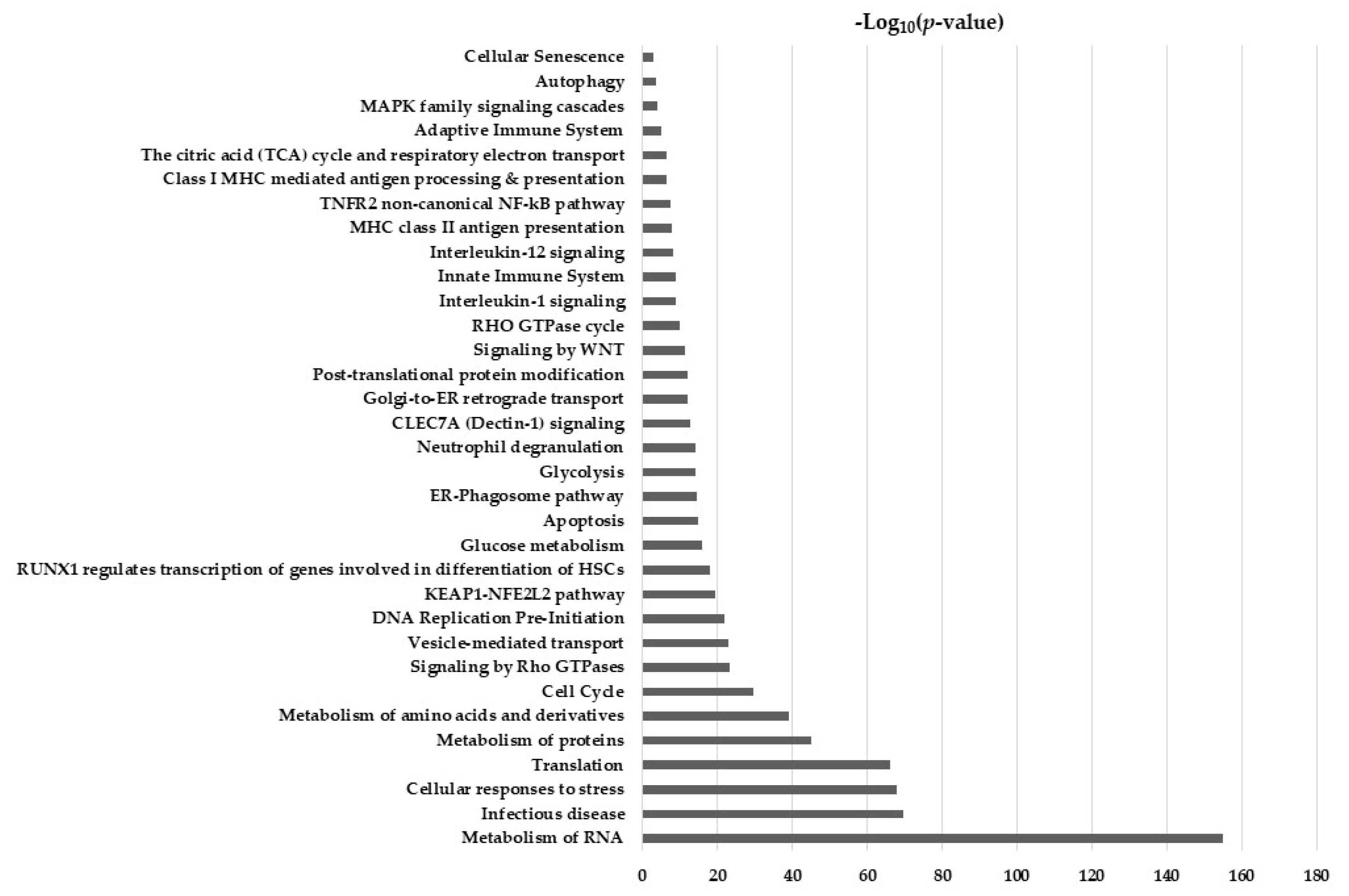
2.5. Hypothesis Free Proteomics and Bioinformatic Analysis
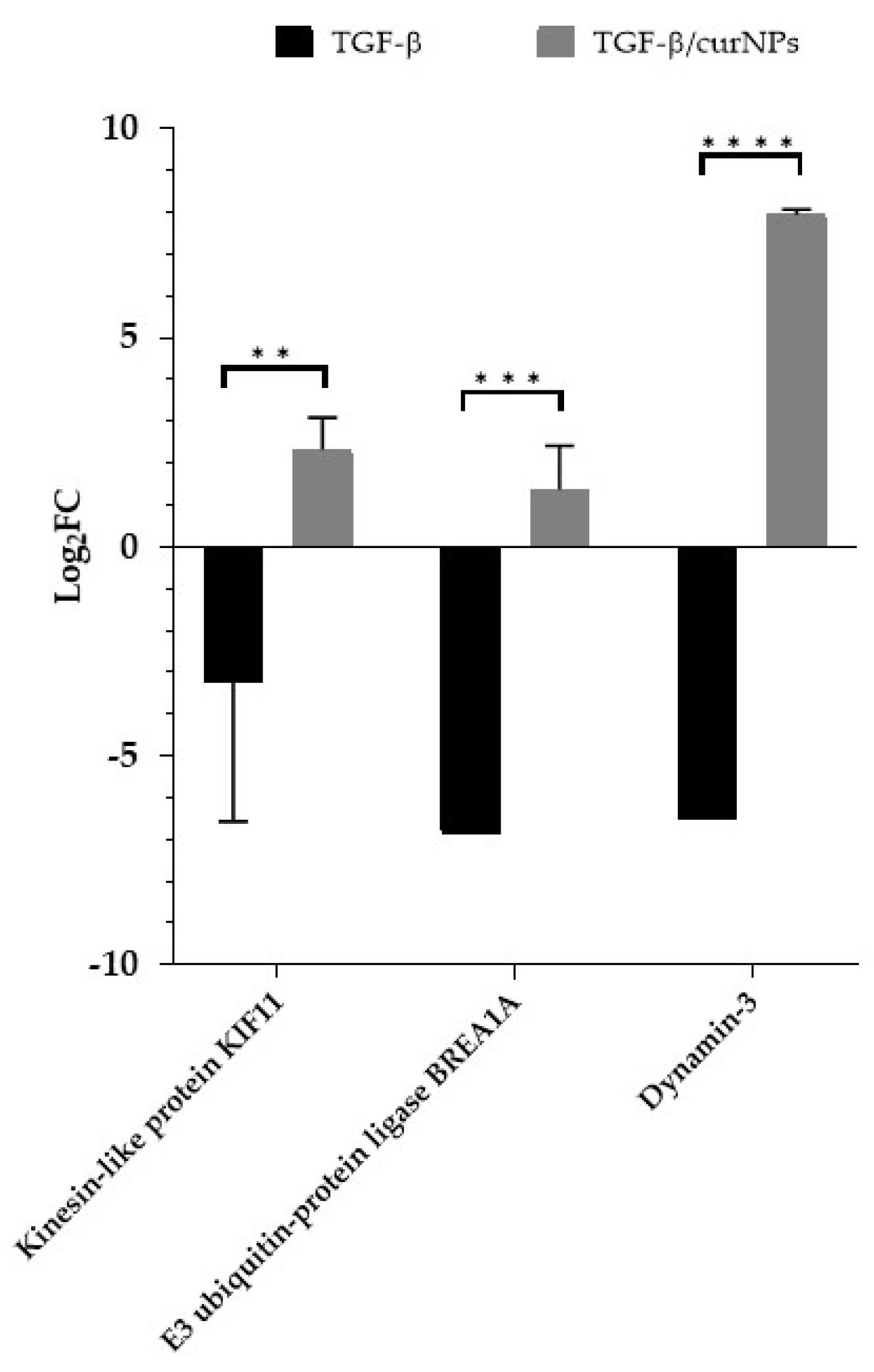
2.6. Comparative Analysis of TGF-β and TGF-β/CurNPs Dysregulated Proteins
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. CurNPs Synthesis
4.3. CurNPs Stability in Aqueous Media
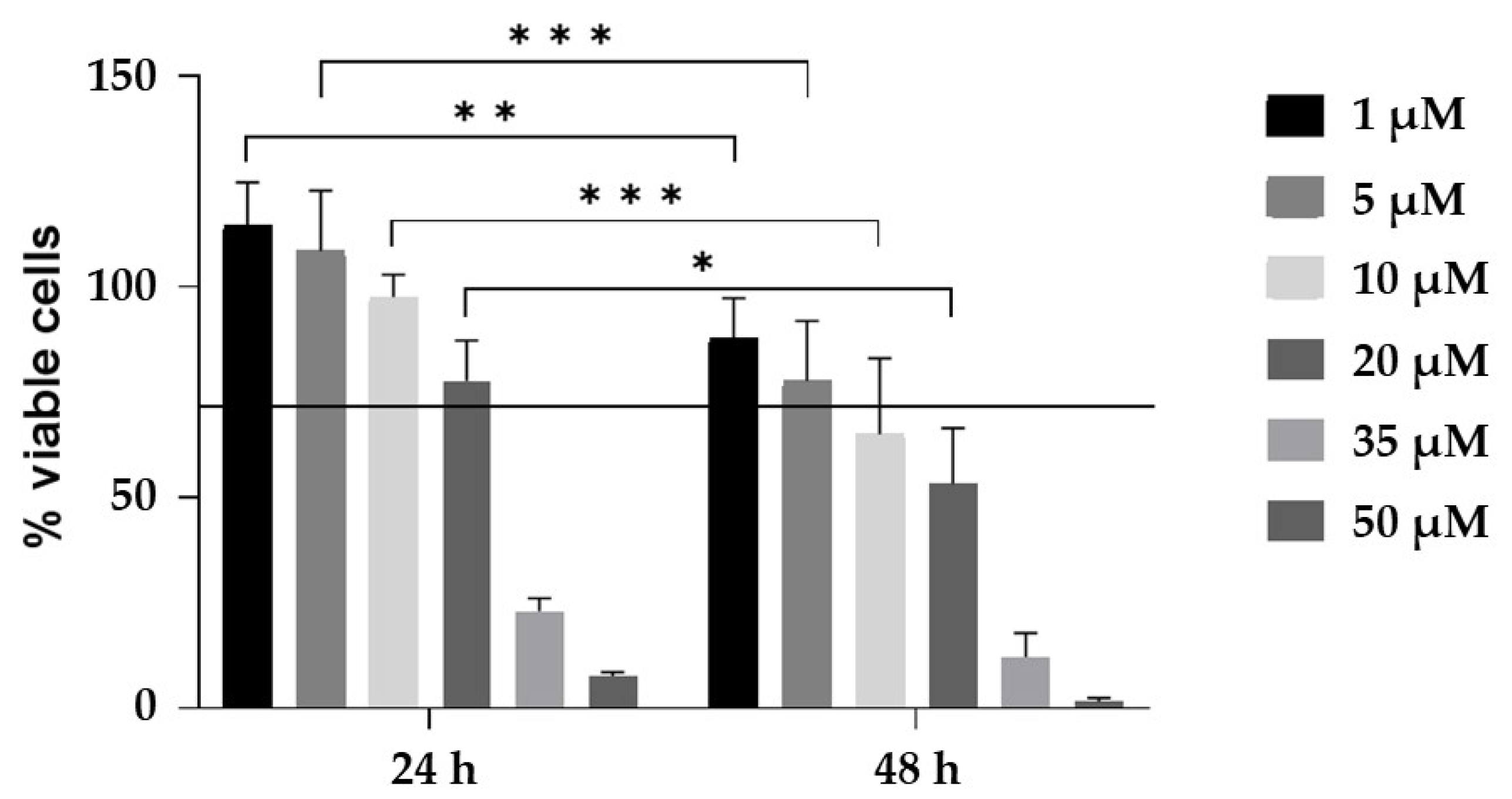
4.4. CurNPs Cytotoxicity Assay
4.5. Sample Preparation for Mass Spectrometry-Based Proteomics
4.6. LC-MS/MS Analysis
4.7. IL-6 Dosage
4.8. Data Processing and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lv, G.-P.; Hu, D.-J.; Zhou, Y.-Q.; Zhang, Q.-W.; Zhao, J.; Li, S.-P. Preparation and Application of Standardized Typical Volatile Components Fraction from Turmeric (Curcuma Longa L.) by Supercritical Fluid Extraction and Step Molecular Distillation. Molecules 2018, 23, 1831. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.-S.; Chen, T.-H.; Weng, L.; Huang, L.; Lai, D.; Weng, C.-F. Pharmacological Properties and Underlying Mechanisms of Curcumin and Prospects in Medicinal Potential. Biomed. Pharmacother. 2021, 141, 111888. [Google Scholar] [CrossRef] [PubMed]
- Priyadarsini, K. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Nikezić, A.V.V.; Bondžić, A.M.; Vasić, V.M. Drug Delivery Systems Based on Nanoparticles and Related Nanostructures. Eur. J. Pharm. Sci. 2020, 151, 105412. [Google Scholar] [CrossRef]
- Felice, F.; Piras, A.M.; Rocchiccioli, S.; Barsotti, M.C.; Santoni, T.; Pucci, A.; Burchielli, S.; Chiellini, F.; Ucciferri, N.; Solaro, R.; et al. Endothelial Progenitor Cell Secretome Delivered by Novel Polymeric Nanoparticles in Ischemic Hindlimb. Int. J. Pharm. 2018, 542, 82–89. [Google Scholar] [CrossRef]
- Mohammed, E.; El-Beih, N.; El-Hussieny, E.; El-Ahwany, E.; Hassan, M.; Zoheiry, M. Effects of Free and Nanoparticulate Curcumin on Chemically Induced Liver Carcinoma in an Animal Model. Arch. Med. Sci. 2021, 17, 218–227. [Google Scholar] [CrossRef]
- Atia, M.M.; Abdel-Tawab, H.S.; Mostafa, A.M.; Mobarak, S.A. Nanocurcumin and Curcumin Prevent N, N’-Methylenebisacrylamide-Induced Liver Damage and Promotion of Hepatic Cancer Cell Growth. Sci. Rep. 2022, 12, 8319. [Google Scholar] [CrossRef]
- Elzoheiry, A.; Ayad, E.; Omar, N.; Elbakry, K.; Hyder, A. Anti-Liver Fibrosis Activity of Curcumin/Chitosan-Coated Green Silver Nanoparticles. Sci. Rep. 2022, 12, 18403. [Google Scholar] [CrossRef]
- Wang, J.; Pan, W.; Wang, Y.; Lei, W.; Feng, B.; Du, C.; Wang, X. Enhanced Efficacy of Curcumin with Phosphatidylserine-Decorated Nanoparticles in the Treatment of Hepatic Fibrosis. Drug Deliv. 2018, 25, 1–11. [Google Scholar] [CrossRef]
- Tie, Y.; Tang, F.; Peng, D.; Zhang, Y.; Shi, H. TGF-Beta Signal Transduction: Biology, Function and Therapy for Diseases. Mol. Biomed. 2022, 3, 45. [Google Scholar] [CrossRef]
- Meng, X.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The Master Regulator of Fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Santos-Laso, A.; Munoz-Garrido, P.; Felipe-Agirre, M.; Bujanda, L.; Banales, J.M.; Perugorria, M.J. New Advances in the Molecular Mechanisms Driving Biliary Fibrosis and Emerging Molecular Targets. CDT 2017, 18, 908–920. [Google Scholar] [CrossRef]
- Fabregat, I.; Caballero-Díaz, D. Transforming Growth Factor-β-Induced Cell Plasticity in Liver Fibrosis and Hepatocarcinogenesis. Front. Oncol. 2018, 8, 357. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, M.; Luo, Z.; Wen, Z.; Yan, X. The Dichotomous Role of TGF-β in Controlling Liver Cancer Cell Survival and Proliferation. J. Genet. Genom. 2020, 47, 497–512. [Google Scholar] [CrossRef]
- Dewidar, B.; Soukupova, J.; Fabregat, I.; Dooley, S. Meindl-Beinker TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef]
- Tang, L.-Y.; Heller, M.; Meng, Z.; Yu, L.-R.; Tang, Y.; Zhou, M.; Zhang, Y.E. Transforming Growth Factor-β (TGF-β) Directly Activates the JAK1-STAT3 Axis to Induce Hepatic Fibrosis in Coordination with the SMAD Pathway. J. Biol. Chem. 2017, 292, 4302–4312. [Google Scholar] [CrossRef]
- Ceccherini, E.; Di Giorgi, N.; Michelucci, E.; Signore, G.; Tedeschi, L.; Vozzi, F.; Rocchiccioli, S.; Cecchettini, A. Biological Effects of Transforming Growth Factor Beta in Human Cholangiocytes. Biology 2022, 11, 566. [Google Scholar] [CrossRef]
- Farzaei, M.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nabavi, S.; Rahimi, R.; Abdollahi, M. Curcumin in Liver Diseases: A Systematic Review of the Cellular Mechanisms of Oxidative Stress and Clinical Perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Devi, A.; Chennakesavulu, S.; Suresh, C.; Reddy, A.B.M. Nutraceuticals and Their Role in Human Health and Disease. In Functional Food and Human Health; Rani, V., Yadav, U.C.S., Eds.; Springer Singapore: Singapore, 2018; pp. 375–403. ISBN 9789811311222. [Google Scholar]
- Zhang, Y.; Alexander, P.B.; Wang, X.-F. TGF-β Family Signaling in the Control of Cell Proliferation and Survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef] [PubMed]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; ten Dijke, P. The IT-LIVER Consortium TGF-β Signalling and Liver Disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Demetris, A.J.; Specht, S.; Nozaki, I.; Lunz, J.G.; Stolz, D.B.; Murase, N.; Wu, T. Small Proline-Rich Proteins (SPRR) Function as SH3 Domain Ligands, Increase Resistance to Injury and Are Associated with Epithelial–Mesenchymal Transition (EMT) in Cholangiocytes. J. Hepatol. 2008, 48, 276–288. [Google Scholar] [CrossRef]
- Nozaki, I.; Lunz, J.G.; Specht, S.; Stolz, D.B.; Taguchi, K.; Subbotin, V.M.; Murase, N.; Demetris, A.J. Small Proline-Rich Proteins 2 Are Noncoordinately Upregulated by IL-6/STAT3 Signaling after Bile Duct Ligation. Lab. Investig. 2005, 85, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Konishi, A.; Shimizu, S.; Hirota, J.; Takao, T.; Fan, Y.; Matsuoka, Y.; Zhang, L.; Yoneda, Y.; Fujii, Y.; Skoultchi, A.I.; et al. Involvement of Histone H1.2 in Apoptosis Induced by DNA Double-Strand Breaks. Cell 2003, 114, 673–688. [Google Scholar] [CrossRef]
- Funayama, R.; Saito, M.; Tanobe, H.; Ishikawa, F. Loss of Linker Histone H1 in Cellular Senescence. J. Cell Biol. 2006, 175, 869–880. [Google Scholar] [CrossRef]
- Masyuk, A.I.; Masyuk, T.V.; Splinter, P.L.; Huang, B.Q.; Stroope, A.J.; LaRusso, N.F. Cholangiocyte Cilia Detect Changes in Luminal Fluid Flow and Transmit Them Into Intracellular Ca2+ and CAMP Signaling. Gastroenterology 2006, 131, 911–920. [Google Scholar] [CrossRef]
- Maroni, L.; Haibo, B.; Ray, D.; Zhou, T.; Wan, Y.; Meng, F.; Marzioni, M.; Alpini, G. Functional and Structural Features of Cholangiocytes in Health and Disease. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 368–380. [Google Scholar] [CrossRef]
- Gradilone, S.A.; Masyuk, A.I.; Splinter, P.L.; Banales, J.M.; Huang, B.Q.; Tietz, P.S.; Masyuk, T.V.; LaRusso, N.F. Cholangiocyte Cilia Express TRPV4 and Detect Changes in Luminal Tonicity Inducing Bicarbonate Secretion. Proc. Natl. Acad. Sci. USA 2007, 104, 19138–19143. [Google Scholar] [CrossRef]
- LaRusso, N.F.; Masyuk, T.V. The Role of Cilia in the Regulation of Bile Flow. Dig. Dis. 2011, 29, 6–12. [Google Scholar] [CrossRef]
- Ciliopathy Working Group; Shaheen, R.; Szymanska, K.; Basu, B.; Patel, N.; Ewida, N.; Faqeih, E.; Al Hashem, A.; Derar, N.; Alsharif, H.; et al. Characterizing the Morbid Genome of Ciliopathies. Genome Biol. 2016, 17, 242. [Google Scholar] [CrossRef]
- Cochran, J.C.; Sontag, C.A.; Maliga, Z.; Kapoor, T.M.; Correia, J.J.; Gilbert, S.P. Mechanistic Analysis of the Mitotic Kinesin Eg5. J. Biol. Chem. 2004, 279, 38861–38870. [Google Scholar] [CrossRef]
- Zalenski, A.A.; Majumder, S.; De, K.; Venere, M. An Interphase Pool of KIF11 Localizes at the Basal Bodies of Primary Cilia and a Reduction in KIF11 Expression Alters Cilia Dynamics. Sci. Rep. 2020, 10, 13946. [Google Scholar] [CrossRef]
- Masyuk, T.V.; Masyuk, A.I.; LaRusso, N.F. TGR5 in the Cholangiociliopathies. Dig. Dis. 2015, 33, 420–425. [Google Scholar] [CrossRef]
- Pedersen, L.B.; Mogensen, J.B.; Christensen, S.T. Endocytic Control of Cellular Signaling at the Primary Cilium. Trends Biochem. Sci. 2016, 41, 784–797. [Google Scholar] [CrossRef]
- Perrais, D. Cellular and Structural Insight into Dynamin Function during Endocytic Vesicle Formation: A Tale of 50 Years of Investigation. Biosci. Rep. 2022, 42, BSR20211227. [Google Scholar] [CrossRef]
- Inokawa, Y.; Nomoto, S.; Hishida, M.; Hayashi, M.; Kanda, M.; Nishikawa, Y.; Takeda, S.; Fujiwara, M.; Koike, M.; Sugimoto, H.; et al. Dynamin 3: A New Candidate Tumor Suppressor Gene in Hepatocellular Carcinoma Detected by Triple Combination Array Analysis. OncoTargets Ther. 2013, 6, 1417–1424. [Google Scholar] [CrossRef]
- Chen, S.; Dai, X.; Li, H.; Gong, Y.; Zhao, Y.; Huang, H. Overexpression of Ring Finger Protein 20 Inhibits the Progression of Liver Fibrosis via Mediation of Histone H2B Lysine 120 Ubiquitination. Hum. Cell 2021, 34, 759–770. [Google Scholar] [CrossRef]
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte Pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef]
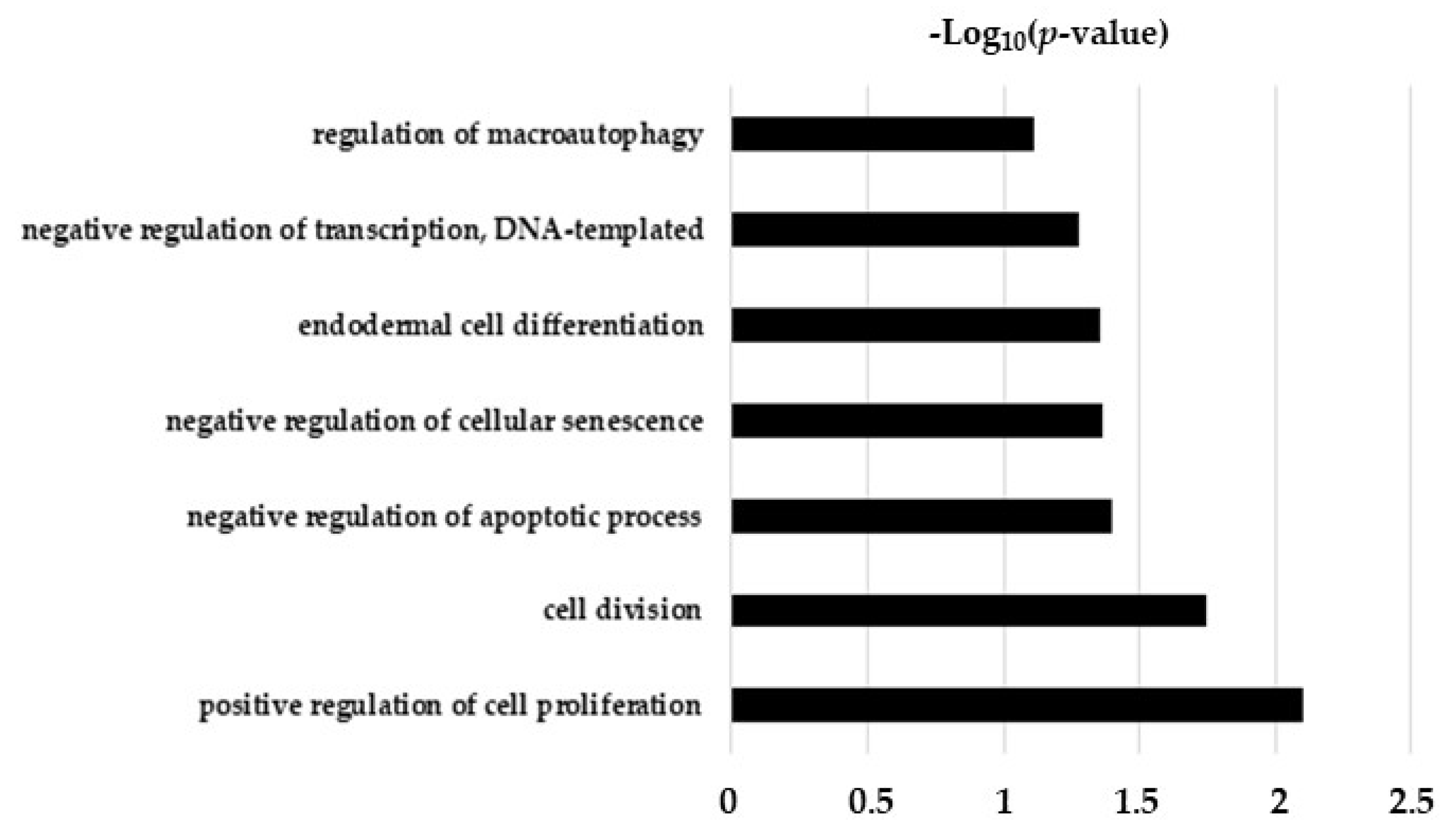
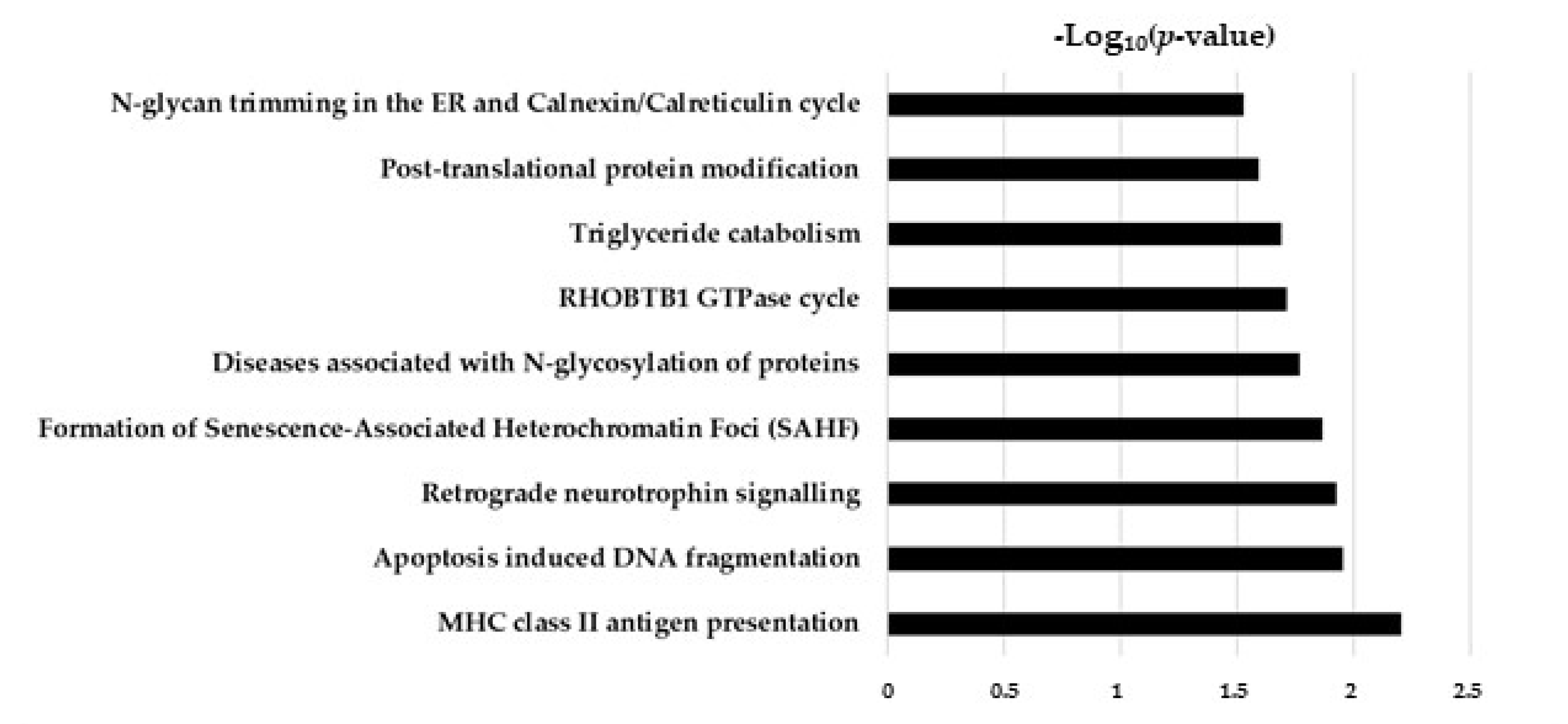

| Protein Name | Protein ID | FC | p-Value |
|---|---|---|---|
| Fibronectin | P02751 | 295.85 | 0.0045 |
| Cullin-3 | Q13618 | 13.69 | 0.0233 |
| Tensin-1 | Q9HBL0 | 7.15 | 0.008 |
| Haloacid dehalogenase-like hydrolase domain-containing protein 2 | Q9H0R4 | 6.05 | 0.0055 |
| Palladin | Q8WX93 | 5.03 | 0.001 |
| Bcl-2-like protein 12 | Q9HB09 | 4.77 | 0.0042 |
| Protein MTSS 1 | O43312 | 4.51 | 0.0026 |
| TSC22 domain family protein 1 | Q15714 | 4.16 | 0.0016 |
| Cytospin-A | Q69YQ0 | 3.19 | 0.0167 |
| Phosphatidylcholine translocator ABCB4 | P21439 | 3.10 | 0.049 |
| Centromere protein F | P49454 | 3.04 | 0.0069 |
| Four and a half LIM domains protein 2 | Q14192 | 3.01 | 0.0036 |
| High mobility group protein HMGI-C | P52926 | 3.00 | 0.0074 |
| Protein PRRC1 | Q96M27 | 3.00 | 0.0102 |
| Zinc finger protein 746 | Q6NUN9 | 2.95 | 0.0376 |
| Protein FAM114A2 | Q9NRY5 | 2.94 | 0.0367 |
| Keratin, type II cytoskeletal 1 | P04264 | 2.90 | 0.0210 |
| Fos-related antigen 1 | P15407 | 2.85 | 0.0212 |
| Calpain small subunit 1 | P04632 | 2.66 | 0.0266 |
| SH3 and PX domain-containing protein 2A | Q5TCZ1 | 2.65 | 0.0075 |
| Transducin-like enhancer protein 3 | Q04726 | 2.62 | 0.0089 |
| Zinc finger protein Helios | Q9UKS7 | 2.60 | 0.0036 |
| Tetratricopeptide repeat protein 24 | A2A3L6 | 2.57 | 0.0036 |
| Moesin | P26038 | 2.51 | 0.0003 |
| Kinesin-like protein KIF11 * | P52732 | 0.41 | 0.0132 |
| Protein HEXIM1 | O94992 | 0.40 | 0.0228 |
| E3 ubiquitin-protein ligase BRE1A | Q5VTR2 | 0.30 | 0.0001 |
| Cornifin-A | P35321 | 0.25 | 0.0009 |
| Dynamin-3 | Q9UQ16 | 0.01 | <0.0001 |
| Protein Name | Protein ID | FC | p-Value |
|---|---|---|---|
| Dynamin-3 | Q9UQ16 | 248.1 | 0.0371 |
| Kinesin-like protein KIF11 | P52732 | 4.60 | 0.0081 |
| E3 ubiquitin-protein ligase BRE1A | Q5VTR2 | 3.93 | 0.0462 |
| Histone H1.2 | P16403 | 2.94 | 0.0062 |
| Nucleobindin-1 | Q02818 | 2.79 | 0.03 |
| Mannosyl-oligosaccharide glucosidase | Q13724 | 2.75 | 0.0018 |
| Thioredoxin domain-containing protein 15 | Q96J42 | 2.69 | 0.0086 |
| Bromodomain adjacent to zinc finger domain protein 1A | Q9NRL2 | 2.67 | 0.0485 |
| Fatty acid-binding protein, adipocyte | P15090 | 0.012 | 0.0001 |
| Ras-related protein Rab-15 | P59190 | 0.0014 | 0.0002 |
| Protein Name | Protein ID | FC | |
|---|---|---|---|
| TGF-β | TGF-β/CurNPs | ||
| E3 ubiquitin-protein ligase BRE1A | Q5VTR2 | 0.30 | 3.93 |
| Kinesin-like protein KIF11 | P52732 | 0.41 | 4.60 |
| Dynamin-3 | Q9UQ16 | 0.01 | 248.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccherini, E.; Signore, G.; Tedeschi, L.; Vozzi, F.; Di Giorgi, N.; Michelucci, E.; Cecchettini, A.; Rocchiccioli, S. Proteomic Modulation in TGF-β-Treated Cholangiocytes Induced by Curcumin Nanoparticles. Int. J. Mol. Sci. 2023, 24, 10481. https://doi.org/10.3390/ijms241310481
Ceccherini E, Signore G, Tedeschi L, Vozzi F, Di Giorgi N, Michelucci E, Cecchettini A, Rocchiccioli S. Proteomic Modulation in TGF-β-Treated Cholangiocytes Induced by Curcumin Nanoparticles. International Journal of Molecular Sciences. 2023; 24(13):10481. https://doi.org/10.3390/ijms241310481
Chicago/Turabian StyleCeccherini, Elisa, Giovanni Signore, Lorena Tedeschi, Federico Vozzi, Nicoletta Di Giorgi, Elena Michelucci, Antonella Cecchettini, and Silvia Rocchiccioli. 2023. "Proteomic Modulation in TGF-β-Treated Cholangiocytes Induced by Curcumin Nanoparticles" International Journal of Molecular Sciences 24, no. 13: 10481. https://doi.org/10.3390/ijms241310481
APA StyleCeccherini, E., Signore, G., Tedeschi, L., Vozzi, F., Di Giorgi, N., Michelucci, E., Cecchettini, A., & Rocchiccioli, S. (2023). Proteomic Modulation in TGF-β-Treated Cholangiocytes Induced by Curcumin Nanoparticles. International Journal of Molecular Sciences, 24(13), 10481. https://doi.org/10.3390/ijms241310481









